Abstract
Background
Visual masking paradigms assess the early part of visual information processing, which may reflect vulnerability measures for schizophrenia. We examined the neural substrates of visual backward performance in unaffected siblings of schizophrenia patients using functional magnetic resonance imaging (fMRI).
Methods
Twenty-one unaffected siblings of schizophrenia patients and 19 healthy controls performed a backward masking task, and three functional localizer tasks to identify three visual processing regions of interest (ROI): lateral occipital complex (LO), the motion-sensitive area (hMT+), and retinotopic areas. In the masking task, we systematically manipulated stimulus onset asynchronies (SOAs). We analyzed fMRI data in two complementary ways: an ROI approach for three visual areas and a whole brain analysis.
Results
The groups did not differ in behavioral performance. In the ROI analysis, both groups showed increased activation as SOA increased in LO only. The groups did not differ in activation levels of the three ROIs. In the whole brain analysis, controls showed increased activation as a function of SOA, compared to siblings, in several brain regions (i.e. anterior cingulate cortex, posterior cingulate cortex, inferior prefrontal cortex, inferior parietal lobule).
Conclusions
The study found; 1) area LO showed sensitivity to the masking effect in both groups; 2) siblings did not differ from controls in activation of LO; and 3) the groups differed significantly in several brain regions outside visual processing areas that have been closely related to attentional or re-entrant processes. These suggest that LO dysfunction may be a disease indicator rather than a risk indicator for schizophrenia.
Keywords: schizophrenia, unaffected siblings, early visual perception, fMRI, backward masking, lateral occipital complex
In a visual masking paradigm, the ability to identify a visual target is disrupted when a mask occurs briefly before or after the target (1, 2). If the mask follows the target, it is called backward masking. In general schizophrenia patients have more difficulty, compared with controls, in identifying the target in the presence of a visual mask (3, 4). Impaired backward masking performance may be a vulnerability marker for schizophrenia because deficits have been reported in patients in clinical remission (5, 6) and they show stability over 18-months in first-episode patients (7). In addition, some studies (8–10), but not others, (11, 12), have reported masking impairment in first-degree relatives of schizophrenia patients compared with healthy controls. Masking deficits have been observed in psychosis-prone individuals (13, 14). These studies suggest that visual masking deficits may be an indicator of genetic liability for schizophrenia, but some studies have shown impaired backward masking performance in patients with bipolar disorder (3, 15, 16) or learning disabilities (17) so the impairment is not limited to schizophrenia. To better understand the putative genetic nature of the visual masking deficit in schizophrenia, it is helpful to study people who are unaffected, but at risk for the disorder. In this study we explore the functional neuroanatomy of visual backward masking in unaffected siblings of schizophrenia patients.
There are two primary paths for processing visual information in backward masking paradigms: a feed-forward pathway that travels from retina to visual cortical areas, and a recurrent or reentrant pathway in which neural feedback from visual (or higher) cortical areas affect early components of visual processing (18–20). Though earlier research on visual backward masking emphasized feed-forward processing (1), recent studies suggest that backward masking may occur due to disrupted re-entrant or feedback signals that are necessary for conscious perception of a target (21–23). Further, there are at least two levels of re-entrant processes. One is a short re-entrant processing between striate and extrastriate cortex within the visual cortex (18, 24). The other is a re-entrant processing over longer distances between visual and higher brain regions (including frontal, parietal and cingulate cortices) (18–20). It remains to be determined whether schizophrenia patients show backward masking deficits due to impaired feed-forward processing, deficient re-entrant processing or a combination of both (25, 26).
Several studies have examined visual cortical areas during the backward masking task and suggested that the lateral occipital complex (LO), which is associated with object recognition (27), plays an important role in visual backward masking (28, 29). During a backward masking task, a target is initially processed but it fails to reach visual awareness especially when the mask follows a target very quickly. By examining differential activation of brain areas as a function of target visibility, one can identify brain regions that are important for visual backward masking performance. In a healthy sample, we previously found increased LO activation with increasing duration between target and mask (30). The same study also found similar sensitivity to the masking effect in several areas outside early visual cortical areas, including inferior parietal lobule and anterior cingulate cortex. These areas may be associated with reentrant processing of visual information or with effortful visual processing. In a subsequent study, we examined neural mechanisms associated with backward masking deficits in schizophrenia (31). Although schizophrenia patients showed sensitivity to target visibility in area LO, similar to that of healthy controls, they showed lower activations in LO compared to healthy controls. This study suggested that reduced LO activation may play an important role of understanding backward masking deficits in schizophrenia.
In the present study, we examined the neural substrates of visual backward masking performance in unaffected siblings of schizophrenia patients using functional magnetic resonance imaging (fMRI). If visual masking deficits in schizophrenia reflect a vulnerability to the illness, unaffected siblings would be expected to show differences in regional brain activity compared with controls. To our knowledge, this is the first study to investigate neural activity of backward masking performance in unaffected siblings of schizophrenia. We focused primarily on three key visual processing regions of interests (ROIs): LO, the human motion-sensitive area (hMT+) and the retinotopic area. We selected these three ROIs because they represent key early and middle visual processing regions and have well-established localizer tasks. After identifying three functionally defined ROIs with localizer tasks, we compared neural activation during the backward masking task between siblings and controls. To examine the masking effect systematically, we varied the stimulus-onset asynchronies (SOAs) between target and mask, which enabled us to create a range of masking effects (from strong to weak). We employed: 1) an ROI approach to determine whether siblings and controls differ in activation of key visual processing areas during visual masking, and 2) an exploratory whole-brain approach to determine whether siblings and controls show different response to the masking effect in areas outside of the key visual processing regions.
METHODS
Participants
Twenty-three (11 female) unaffected siblings of patients with schizophrenia and 19 (5 female) healthy controls participated in this study. All participants were part of a larger NIMH-funded study of early visual processing in schizophrenia (PI: M. F. G). Participants in the sibling group shared both biological parents with a patient who met diagnostic criteria of schizophrenia using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; 32). Probands of the siblings were recruited from the mental health clinics of the VA Greater Los Angeles Healthcare system and mental health clinics from the local community. Healthy control participants were recruited through flyers posted in the local community, newspaper advertisements in local newspapers, and website postings. The data from healthy controls were also included in an earlier study on neural activation patterns in schizophrenia using the same experimental procedure (31).
All participants received a diagnostic interview with SCID (32) and selected sections of the Structural Clinical Interview for DSM-IV Axis II disorders (33). Because siblings are harder to recruit than healthy controls, the parent study included siblings in the behavioral paradigms who were clinically affected. For the current fMRI component of the study, exclusion criteria for both groups of subjects were: 1) diagnosis of schizophrenia or other psychotic disorder or any substance abuse in the last six months, 2) any of the following Axis II disorders: avoidant, paranoid, schizoid, schizotypal, or borderline, 3) history of loss of consciousness for more than one hour, 4) any significant neurological disorder or head injury, or 5) insufficient fluency in English. In addition, healthy controls were excluded for recurrent episodes of major depression and history of substance dependence. Finally, to better separate the control and sibling groups, controls were excluded if they had a first-degree relative with schizophrenia or other psychotic disorder. All participants had normal or corrected to normal vision (of at least 20/30).
All SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC). All participants were evaluated for the capacity to give informed consent and provided written informed consent after all procedures were fully explained, according to procedures approved by the Institutional Review Boards at UCLA.
Design and Procedure
All participants completed six runs of the visual backward masking task followed by three localizer tasks (retinotopic areas, hMT+, and LO) in the MRI scanner. The entire scanning session lasted 60 minutes. The visual backward masking task was presented using E-prime software and the localizer tasks were presented with the Psychophysics Toolbox (34) for MATLAB. All tasks were presented with MR-compatible LCD goggles (Resonance Technology, Northridge, CA). These experimental procedures are described in detail elsewhere (31).
For the visual backward masking task, we employed a rapid event-related design and the trials were presented in a “permuted block design” to maximize both hemodynamic response function (HRF) estimation and signal detection power (35–37). The target was a square with a gap on one of three sides (up, down, or left) that appeared at the center of the screen. The mask was a composite square made up of four smaller squares, overlapping the area occupied by the target. The target subtended 5.7 degrees and the mask 10.2 degrees of visual angle. The beginning of each trial was signaled by two 100 ms flashes of a fixation point, followed by a 600-ms blank period (see Figure 1). Then, a target was presented for 26.6 ms, followed by a 53.3-ms mask at one of four possible SOAs: 26.6, 40, 80, 200 ms. The only component that varied from a trial to a trial was the SOA, resulting in a slight difference between the offset of a mask and the start of the next trial across trials depending on the SOA. Participants were instructed to identify the location of a gap in the target (up, bottom, or left) by pressing a corresponding button with their dominant hand. The visual backward masking tasks consisted of 6 runs, each with 30 5-second trials (i.e. 6 trials for each of the 4 SOAs and 6 null trials that included fixation but no stimuli).
Figure 1. Schematic diagram of a single trial in a visual backward masking task.
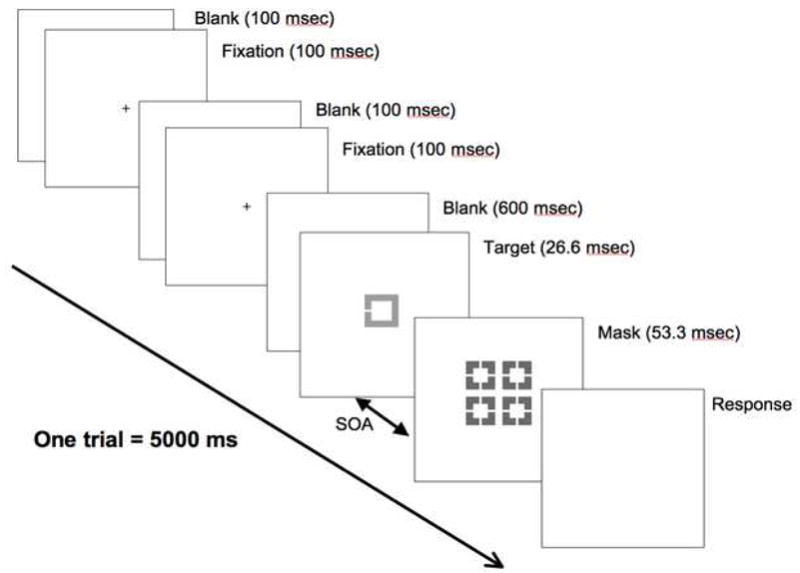
The beginning of each visual backward masking trial was signaled by two 100 ms flashes of a fixation point, followed by a 600-ms blank period. A target was briefly presented for 26.6 ms, followed by a 53.3-ms mask at one of the 4 possible SOAs (26.6, 40, 80, and 200 ms). After the mask disappeared, a blank screen was presented while subjects made responses. Since each trial lasted 5 seconds, there was a slight difference between the offset of a mask and the start of the next trial across trials depending on the SOA. On each trial, participants identified the location of a gap in a target (up, bottom, or left) by pressing a corresponding button with their dominant hand.
After the visual backward masking task, participants performed three functional localization tasks: retinotopic areas, and hMT+, and LO. Full descriptions of the three functional localizer tasks are provided elsewhere (31, 38) and are summarized briefly here. To identify retinotopic areas, participants viewed slowly rotating wedges of a contrast-reversing checkerboard (39). The wedge made 5 rotations, with one rotation every 30 s. The localizer task for the motion sensitive hMT+ consisted of alternating blocked presentations of moving rings and stationary rings, with each block presented for 15 s. There were 5 blocks each of moving and stationary rings. The LO localizer task consisted of alternating blocked presentations of pictures of abstract objects (i.e. sculptures) and scrambled pictures of objects, with each block containing 10 images presented for a total of 12.5 s (27, 40). There were 6 blocks each of abstract objects and scrambled objects.
fMRI data acquisition
All scanning was conducted on a 3T scanner (Siemens Allegra, Erlangen, Germany) located in the UCLA Ahmanson Lovelace Brain Mapping Center. For anatomical reference, a high-resolution echo planar axial T2-weighted series was obtained for each subject prior to functional scanning (TR = 6000 ms, TE = 54 ms, flip angle = 90 degrees, 30 axial slices, FOV 20 cm). A T2*-weighted gradient-echo sequence was used to detect blood-oxygen level-dependent (BOLD) signal (TR=2000 ms, TE=42 ms, flip angle=80 degrees, voxel size of 3.125 × 3.125 × 4.00 mm with a 1-mm gap), acquiring 24 slices parallel to the AC-PC plane.
fMRI data analysis
Data were analyzed using the FMRIB Software Library (FSL, 41). The pre-statistics processing included motion correction (42), non-brain removal (43), spatial smoothing using a Gaussian kernel of FWMH 5 mm, and high pass temporal filtering (Gaussian weighted LSF straight line fitting with sigma = 25.0 s). To facilitate multi-subject analyses, statistical images created for each subject were normalized into a standard space of Montreal Neurological Institute (MNI) coordinates. To examine neural activations associated with visual backward masking in unaffected siblings of schizophrenia patients, we approached the fMRI data analyses in two complementary ways: an ROI-based approach and a whole brain analysis.
For the ROI analysis we were interested in the activation patterns of the ROIs during the backward masking task. First, we identified the three key visual processing areas (retinotopic regions, hMT+, and LO) in each individual subject based on the localizer scans. Retinotopic regions were defined as those where activity was temporally correlated with a sinusoid at the stimulus modulation frequency at a level above a defined threshold (p < 0.001, uncorrected) (39). To identify hMT+, the blocked time series (moving versus stationary rings) were convolved with a model HRF and used as regressors in a multiple regression analysis. The contrast of moving rings versus stationary rings produced a statistical parametric map of t-values with a specified threshold (p < 0.001, uncorrected). Area hMT+ was identified based on contiguously activated voxels within the occipital cortex bilaterally. A similar approach was employed to identify LO. Specifically, blocks (abstract versus scrambled images) were modeled, and the contrast of abstract images greater than the scrambled images created a statistical parametric map of t values with a specified threshold (p < 0.001, uncorrected). LO was identified as a group of contiguously activated voxels within the lateral occipital cortex bilaterally.
Second, we modeled the hemodynamic responses at each SOA during the visual backward masking task using 7 finite impulse response (FIR) functions, one for each peristimulus time point (total window = 14 seconds) (44, 45). With fewer assumptions about the exact shape of the hemodynamic responses (44, 45), the FIR model can capture any shape of hemodynamic response and makes it possible to selectively average each trial type for an fast-event related design. After fitting the FIR function, response amplitude (i.e., percent signal change) was calculated by averaging event-related responses across trials, separately for each SOA. Third, we determined whether the early visual processing areas showed the expected masking effect (i.e., increased neural responses with longer SOA) by examining percent signal change using a repeated-measure ANOVA with group as a between-subject variable and time point and SOA as within-subject factors.
For the whole-brain analyses, fMRI data for each SOA were convolved with our model HRF and used as regressors in a multiple regression analysis. The 6 motion parameters were included as covariates of no interest to increase statistical sensitivity. The contrast of interest was a parametric change: increased activation as a function of increased SOA. After voxels were selected in this manner, we considered any group differences using a mixed-effects model of FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 only (46, 47). Statistical images were thresholded using the cluster threshold of z ≥ 3.2 and p ≤ 0.05, corrected for multiple comparisons using Gaussian random field theory (48).
RESULTS
Two siblings were excluded from analyses: one had excessive movement artifact and another showed chance-level performance (defined as at or below 33% accuracy) at the longest SOA. Therefore, 21 siblings of schizophrenia patients and 19 healthy controls were included in the following analyses.
Demographic information and performance data
Siblings of the schizophrenia patients were younger and had higher education compared to healthy controls (Table 1). Figure 2 shows behavioral performance of the visual backward masking task in the scanner. A repeated measures ANOVA with SOAs as a within-group factor and group as a between-group factor showed a significant main effect of SOA (F3, 114 = 313.72, p<0.001), but no SOA by Group effect (F3, 114 = .38, p=.76) and group effect (F1, 38 = .80, p=.37). Because siblings were younger than controls and a previous study found association between age and masking performance (49), we also performed an ANCOVA with age as a covariate, which did not change the findings. As expected, both groups showed improved performance as SOAs increased (i.e., masking effect became weaker and the target became more visible). Because both groups showed close to chance level performance for SOA 1 and SOA2, we combined the responses for these SOAs in all subsequent analyses.
Table 1.
Demographics of unaffected siblings of schizophrenia patients and healthy controls
| Unaffected siblings | Healthy controls | Statistics | |
|---|---|---|---|
| Age | 36.0 (10.3) | 42.7 (9.0) | t39 = 2.18, p < .01 |
| Education (yrs) | 15.9 (1.6) | 13.2 (1.3) | t38=−5.64, p <.001 |
| Gender (female/male) | 11/10 | 5/14 | |
| Racial breakdown | |||
| Caucasian | 9 | 16 | |
| Latino | 3 | 1 | |
| Asian | 1 | 0 | |
| African-American | 5 | 2 | |
| Other/unknown | 1 | 2 |
Values are given as mean (standard deviation).
Figure 2. Behavioral performance on a backward masking task.
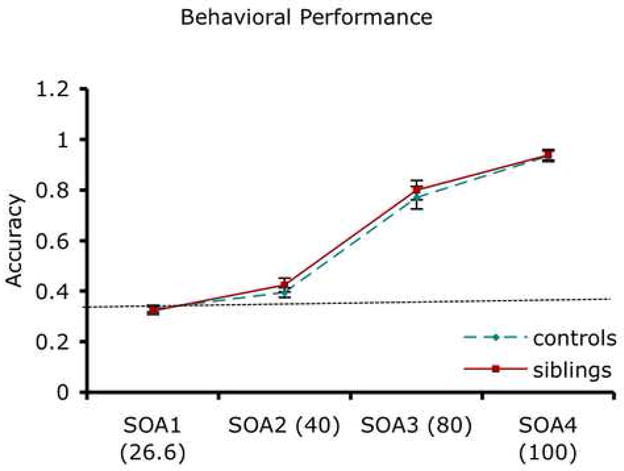
The mean (standard error, SE) performance of siblings and controls is shown for the 4 SOAs. Both groups showed increased accuracy with increasing SOAs (i.e., decreased masking effect) and the groups did not differ significantly at any SOA. Chance performance is 33% (indicated by a dotted line). Values are presented as mean (SE).
Figure 3 present the time-series of percent signal change for each ROI during the backward masking task. For retinotopic areas (A for controls and B for siblings) and hMT+ (C for controls and D for siblings) the main effect of time was significant (F6,210 = 54.61, p< 0.001 for retinotopic; F6,210 = 26.71, p<0.001 for hMT+). For LO (E for controls and F for siblings), we found a significant main effect of time (F6,198 = 33.92, p< 0.001) and a significant SOA by time interaction effect (F12,396 = 3.26, p< 0.01). The group effect was not significant. To further examine the SOA by time interaction for LO, a repeated measures ANOVA was performed with SOA as a within-subject factor for each time point, separately. We found a trend toward significant SOA effect (F2,68 = 2.44, p=.09) at time point 6 and a significant main effect for SOA (F2,68 = 8.22, p< 0.001) at time point 8. These findings indicate that across groups, LO activation increased as SOA became longer and the target became more visible.
Figure 3. Time series for the regions of interest.
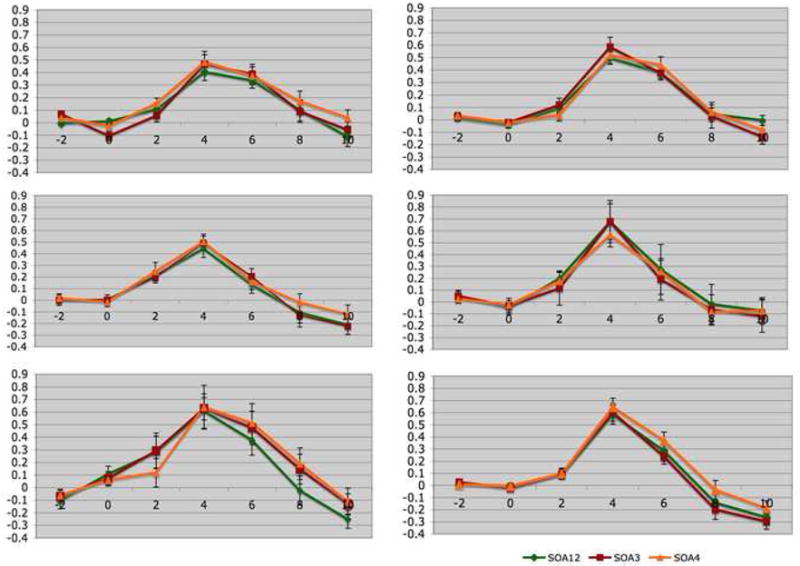
These figures show the time series of percent signal change for each region of interest in controls (the left panel) and siblings (the right panel). The abscissa reflects the time since target onset. A (controls) and B (siblings), Retinotopic areas. C (controls) and D (siblings), the human motion selective cortex (hMT+). E (controls) and F (siblings), the lateral occipital cortex (LO). Values are presented as mean (SE)
Whole-brain analyses
For the whole brain analyses, we were interested in regions in which groups differed in their sensitivity to the masking effect. Hence, we focused on areas that 1) showed a parametric increase of SOA 1,2 < SOA 3 < SOA 4 and 2) showed differences between siblings and controls (Table 2). Areas where controls showed increased parametric activations compared with siblings included anterior cingulate cortex, posterior cingulate cortex, inferior prefrontal cortex, inferior parietal lobule, precentral gyrus, and precuneus (see Figure 4). There was no region in which siblings showed more activation than controls.
Table 2.
Activated brain regions
| Hemisphere | Regions | Broadman’s area | MNI coordinates |
z statistics | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left | inferior frontal gyrus | BA46 | −50 | 32 | 2 | 4.34 |
| Left | inferior frontal gyrus | BA9 | −56 | 10 | 26 | 3.94 |
| Left | inferior parietal lobule | BA40 | −46 | −36 | 48 | 4.25 |
| Left | precentral gyrus | BA6 | −58 | 2 | 34 | 3.87 |
| anterior cingulate cortex | BA32 | 0 | −40 | 18 | 3.94 | |
| posterior cingulate cortex | BA30 | 2 | −50 | 18 | 3.67 | |
| precuneus | BA31 | −4 | −46 | 38 | 3.52 | |
MNI, Montreal Neurological Institute
Figure 4. Whole brain analyses.
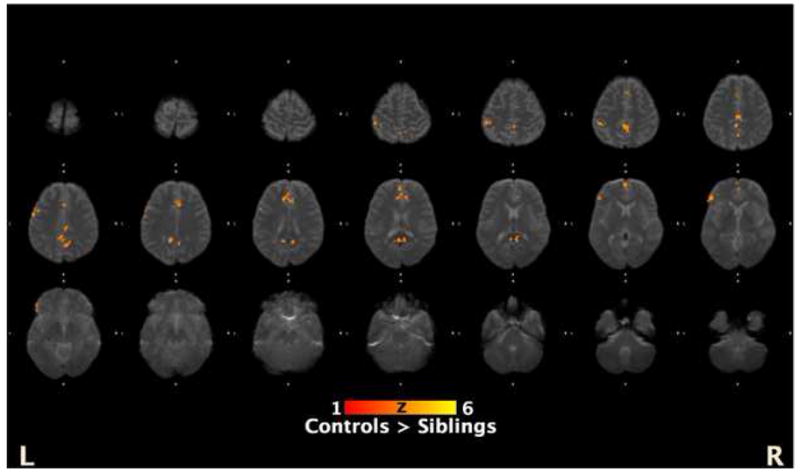
This figure shows the areas that controls showed increased activations compared with siblings from the exploratory whole brain analysis of regions that showed a parametric increase with increased SOAs (SOA 1,2 < SOA 3 < SOA 4). The coordinates of the regions are presented in Table 2.
DISCUSSION
Visual backward masking performance has characteristics suggesting it is a vulnerability marker for schizophrenia (7–12, 50, 51). Hence, we expected unaffected siblings to show differential patterns of neural activation as a function of a masking effect during backward masking compared to healthy controls. In the current study we used two complementary approaches to investigate neural activity associated with visual backward masking in unaffected siblings of schizophrenia patients. First, we used an ROI approach to examine the neural response for siblings and controls in three key visual processing areas: retinotopic areas, hMT+ and LO. Both groups showed an increase in LO activation with increased visibility of the target, but this pattern was not observed in hMT+ and retinotopic areas. The groups did not differ in any of these three areas. The modulation of LO activation as a function of the target visibility is consistent with our previous studies (30, 31). Second, we conducted exploratory whole brain analyses to examine neural activation to target visibility in areas outside the key visual processing ROIs. Several brain areas demonstrated significant group differences in a parametric increase of activation as a function of the target visibility, including the anterior cingulate cortex, posterior cingulate cortex, inferior prefrontal gyrus, precuneus, and inferior parietal lobule. Some of these regions, such as the anterior cingulate cortex and inferior parietal lobule, have shown sensitivity to masking effects in our previous study with healthy individuals (30). Current findings indicate that during a backward masking task, compared to controls, siblings utilize LO in a similar way but show reduced task-related activations in several polymodal brain regions.
We did not find a behavioral performance difference between siblings and controls in the scanner, in contrast to our previously published psychophysics studies (10, 51). There are several possible reasons for this lack of difference. First, the current backward masking task was designed principally to generate and detect neural activation and was not optimal for detecting group differences between siblings and controls. Specifically, it included stimuli that were much larger and of higher contrast than those used in our behavioral masking studies (10, 49, 51, 52), which may have overrode any subtle deficits that siblings may have shown. Second, while some studies find that siblings show impairment in backward masking (8–10), others do not (11, 12). Third, our sample was relatively small. On the other hand, the absence of performance difference provides an interpretative advantage for the fMRI findings because the group differences in regional brain activity were not confounded with performance level.
A closer examination of the brain regions that distinguish siblings from controls on a whole brain analysis suggest the specific cognitive and perceptual processes that may be closely related to impaired backward masking performance seen in siblings of schizophrenia patients. One explanation is that siblings of schizophrenia patients may have reduced attentional resources compared to healthy controls that could influence alertness, readiness to respond, or response selection. Most of areas that showed group differences in whole brain analyses are involved in attention. For example, inferior frontal gyrus and inferior parietal lobule are considered part of an attention network (53, 54), and the anterior cingulate cortex is frequently associated with attentional control or cognitive effort necessary to perform a task (55). In addition, the precuneus is involved in variety of cognitive tasks, including shifting attention to visual stimuli (56, 57). If siblings failed to use attentional resources effectively (e.g. have inefficient resource allocation or response selection), they would show decreased task-related activations of these regions in response to the target visibility during a backward masking task. Reduced task-related activation in siblings in this study may indicate other cognitive dysfunction (i.e., attention) that could affect early visual processing, instead of directly reflecting impaired early visual processing.
Another feature shared by several of these regions is their association with awareness of a visual perception (54, 58). A visual stimulus activates the visual system through either a cascade of feed-forward connections or re-entrant pathways that can be short or long. There is increasing support for the theory that visual masking, under most conditions, is mainly a result of disrupted re-entrant processes, rather than impaired feed-forward processes (59). Most of the brain regions that showed group differences in the current study (i.e., anterior cingulate cortex, posterior cingulate cortex, inferior prefrontal gyrus, precuneus, and inferior parietal lobule) have been implicated in awareness of visual stimuli or re-entrant processing of visual information (20, 60, 61). Hence, this pattern of results suggests that siblings of schizophrenia patients may not utilize these neural regions associated with re-entrant pathways as efficiently as controls do. The patterns of neural activation observed in schizophrenia patients from our previous study could be due to short reentrant processing, whereas the pattern observed in the current study with unaffected siblings might represent disrupted long re-entrant processing. A recent study with healthy controls and a specialized masking task showed that activation in LO during masking is primarily related to re-entrant processing (62). In contrast, unaffected siblings may have relatively spared LO but show differences in areas associated with long re-entrant processing, including inferior parietal lobule and the anterior cingulate cortex (18–20). However, this speculation does not explain why schizophrenia patients show deficits in a short, but not long, re-entrant processing.
In this study, we employed a visual backward masking, a task that is heavily dependent on LO activation (28–30). This study is distinct from previous studies on early visual processing in schizophrenia using fMRI, most of which focused on area hMT+ or V1 (63–65). The results from this study with unaffected siblings differ in some respects from our previous finding using fMRI to assess backward masking in schizophrenia (31). With schizophrenia patients, we found lower activation of LO compared to controls but did not find any differential activation patterns in whole brain analyses outside three key visual areas between patients and controls. The results from the current analyses are the reverse: no group difference in LO or other visual ROIs, but notable differences in activation with increasing visibility in other brain regions. One may argue that the absence of a behavioral difference could explain the lack of a group difference between siblings and controls in LO. However, we found blunted LO activation of patients in our previous study despite comparable behavioral performance. In addition, another study from our laboratory also showed increased extent of LO activation in patients using the LO activation task (38). Based on these findings, we speculate that LO differences between siblings and controls would not have emerged even if we had detected performance differences. However, this prediction needs confirmation with a different masking paradigm that yields performance differences. The absence of group differences in LO activation in the current study raises questions as to whether an aberrant LO is a disease indicator, rather than reflecting genetic vulnerability for schizophrenia. This view is consistent with a finding of impaired performance of patients but the normal performance of individuals at risk for schizophrenia in a perceptual organization task, which are strongly associated with intact object recognition (66). Hence, reduced attentional resources or re-entrant processing may be associated with vulnerability to schizophrenia, but that dysfunctional activation of LO may be a disease specific factor, instead of a risk factor.
Acknowledgments
Support for this study came from NIMH Grants MH43292 and MH065707 (PI: Michael F. Green, Ph.D). The authors wish to thank Poorang Nori, Alex Korb and Alisa Malin for assistance in data collection.
For generous support of the UCLA Brain Mapping Center, we also thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breitmeyer BG. Visual masking: An integrative approach. New York: Oxford University Press; 1984. [Google Scholar]
- 2.Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: A comparison, review, and update. Perception and Psychophysics. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- 3.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51(12):939–44. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 4.Green M, Walker E. Susceptibility to backward masking in schizophrenic patients with positive or negative symptoms. Am J Psychiatry. 1984;141(10):1273–5. doi: 10.1176/ajp.141.10.1273. [DOI] [PubMed] [Google Scholar]
- 5.Miller S, Saccuzzo D, Braff D. Information processing deficits in remitted schizophrenics. Journal of Abnormal Psychology. 1979;88:446–449. [PubMed] [Google Scholar]
- 6.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Backward masking in unmedicated schizophrenic patients in psychotic remission: Possible reflections of aberrant cortical oscillations. American Journal of Psychiatry. 1999;156:1367–1373. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Nuechterlein KH, Subotnik KL, Sugar CA, Ventura J, Gretchen-Doorly D, Kelly K, Green MF. Stability of visual masking performance in recent-onset schizophrenia: an 18-month longitudinal study. Schizophr Res. 2008;103(1–3):266–74. doi: 10.1016/j.schres.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biological Psychiatry. 2006;59:446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: A neurocognitive approach. Psychological Medicine. 2001;31:915–922. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- 10.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54(5):465–72. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 11.Lieb K, Denz E, Hess R, Schuttler R, Kornhuber HH, Schreiber H. Preattentive information processing as measured by backward masking and texton detection tasks in adolescents at high genetic risk for schizophrenia. Schizophrenia Research. 1996;21:171–182. doi: 10.1016/0920-9964(96)00025-4. [DOI] [PubMed] [Google Scholar]
- 12.Bedwell JS, Brown JM, Miller S. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophrenia Research. 2003;63:273–284. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- 13.Merritt RD, Balogh DW. Backward masking spatial frequency effects among hypothetically schizotypal individuals. Schizophr Bull. 1989;15(4):573–83. doi: 10.1093/schbul/15.4.573. [DOI] [PubMed] [Google Scholar]
- 14.Cadenhead KS, Perry W, Braff DL. The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biological Psychiatry. 1996;40:853–858. doi: 10.1016/0006-3223(95)00547-1. [DOI] [PubMed] [Google Scholar]
- 15.Fleming K, Green MF. Backward masking performance during and after manic episodes. J Abnorm Psychol. 1995;104(1):63–8. doi: 10.1037//0021-843x.104.1.63. [DOI] [PubMed] [Google Scholar]
- 16.MacQueen GM, Grof P, Alda M, Marriott M, Young LT, Duffy A. A pilot study of visual backward masking performance among affected versus unaffected offspring of parents with bipolar disorder. Bipolar Disord. 2004;6(5):374–8. doi: 10.1111/j.1399-5618.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 17.Boden C, Brodeur DA. Visual processing of verbal and nonverbal stimuli in adolescents with reading disabilities. J Learn Disabil. 1999;32(1):58–71. doi: 10.1177/002221949903200106. [DOI] [PubMed] [Google Scholar]
- 18.Haynes JD, Driver J, Rees G. Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron. 2005;46(5):811–21. doi: 10.1016/j.neuron.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Lamme VA. Why visual attention and awareness are different. Trends Cogn Sci. 2003;7(1):12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 20.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10(5):204–11. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Enns JT, Di Lollo V. What’s new in visual masking? Trends Cogn Sci. 2000;4(9):345–352. doi: 10.1016/s1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- 22.Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys. 2000;62(8):1572–95. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- 23.Di Lollo V, Enns JT, Rensink RA. Competition for consciousness among visual events: the psychophysics of reentrant visual processes. J Exp Psychol Gen. 2000;129(4):481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Walsh V. Fast back projections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292(5516):510–2. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- 25.Del Cul A, Dehaene S, Leboyer M. Preserved subliminal processing and impaired conscious access in schizophrenia. Arch Gen Psychiatry. 2006;63(12):1313–23. doi: 10.1001/archpsyc.63.12.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Modulation of attention during visual masking in schizophrenia. Am J Psychiatry. 2005;162(8):1533–5. doi: 10.1176/appi.ajp.162.8.1533. [DOI] [PubMed] [Google Scholar]
- 27.Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A. 1995;92(18):8135–9. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-sensitive activation correlate with recognition performance in humans. Nat Neurosci. 2000;3:387–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- 29.Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 30.Green MF, Glahn D, Engel SA, Nuechterlein KH, Sabb F, Strojwas M, Cohen MS. Regional brain activity associated with visual backward masking. Journal of Cognitive Neuroscience. 2005;17(1):13–23. doi: 10.1162/0898929052880011. [DOI] [PubMed] [Google Scholar]
- 31.Green MF, Lee J, Cohen MS, Engel S, Korb AS, Nuechterlein KH, Wynn JK, Glahn D. Functional neuroanatomy of visual masking deficits in schizophrenia. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2009.132. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition, in Biometrics Research Department. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- 33.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Avis II Personality Disorders, in Biometrics Research Department. New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- 34.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 35.Buxton R-B, Liu TT, Martinez A, Frank LR, Luh WM, Wong E-C. Sorting out event-related paradigms in fMRI: The distinction between detecting an actiation and estimating the hemodynamic response. Neuroimage. 2000;11:S457. [Google Scholar]
- 36.Liu TT. Efficiency, power, and entropy in event-related fMRI with multiple trial types II: design of experiments. NeuroImage. 2004;21(1):401–413. doi: 10.1016/j.neuroimage.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Liu TT, Frank LR. Efficiency, power, and entropy in event-related FMRI with multiple trial types. Part I: theory. Neuroimage. 2004;21(1):387–400. doi: 10.1016/j.neuroimage.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Wynn JK, Green MF, Engel SA, Korb A, Lee J, Glahn D, Nuechterlein KH, Cohen MS. Increased extent of object-selective cortex in schizophrenia. Psychiatry Res: Neuroimaging. 2008;164(2):97–105. doi: 10.1016/j.pscychresns.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex. 1997;7(2):181–92. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- 40.Grill-Spector K, Malach R. The human visual cortex. Annual Review of Neuroscience. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 43.Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):218–29. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- 45.Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):210–7. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- 46.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 47.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multi-level linear modeling for fMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Worsley KJ. Statistical analysis of activation images, in Functional MRI. In: Jezzard P, Matthews PM, Smith SM, editors. An Introduction to Methods. USA: Oxford University Press; 2001. [Google Scholar]
- 49.Green MF, Nuechterlein KH, Breitmeyer B, Tsuang J, Mintz J. Forward and backward visual masking in schizophrenia: influence of age. Psychol Med. 2003;33(5):887–95. doi: 10.1017/s003329170200716x. [DOI] [PubMed] [Google Scholar]
- 50.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenia patients: Evidence for a vulnerability indicator. Archives of General Psychiatry. 1997;54:465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 51.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59(5):446–51. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 52.Rassovsky Y, Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Paracontrast and metacontrast in schizophrenia: clarifying the mechanism for visual masking deficits. Schizophr Res. 2004;71(2–3):485–92. doi: 10.1016/j.schres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 54.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Current Opinion in Neurobiology. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 56.Serences JT, Schwartzbach J, Courtney SM, Golay X, Yantis S. Control of object-based attention in human cortex. Cereb Cortex. 2004;14:1246–1357. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- 57.Cavanna AE, Trimble MR. The precuneus: a reivew of its functional anatomy and behavioral correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 58.Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nature Review Neuroscience. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 59.Fahrenfort JJ, Scholte HS, Lamme VA. Masking disrupts reentrant processing in human visual cortex. J Cogn Neurosci. 2007;19(9):1488–97. doi: 10.1162/jocn.2007.19.9.1488. [DOI] [PubMed] [Google Scholar]
- 60.Kjaer TW, Nowak M, Kjaer KW, Lou AR, Lou HC. Precuneus-prefrontal activity during awareness of visual verbal stimuli. Conscious Cogn. 2001;10(3):356–65. doi: 10.1006/ccog.2001.0509. [DOI] [PubMed] [Google Scholar]
- 61.Lamme VA. Towards a true neural stance on consciousness. Trends Cogn Sci. 2006;10(11):494–501. doi: 10.1016/j.tics.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Carlson TA, Rauschenberger R, Verstraten FA. No representation without awareness in the lateral occipital cortex. Psychol Sci. 2007;18(4):298–302. doi: 10.1111/j.1467-9280.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 63.Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. Journal of International Neuroscience. 2009;8(2):175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS. Differential activaiton patterns of occipital and prefrontal cortices during motion procesing: Evidence from normal and schizophrnia brains. Cognitive, Affective, & Behavioral Neuroscience. 2009;8(3):293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guifolye DN, Jalbrazikowski M, Silipo G, Javitt DC. Magnocelluar pathway impairment in schizophrenia: Evidence from functional magnetic resonance imaging. Journal of Neuroscience. 2008;38(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silverstein S, Uhlhaas PJ, Essex B, Halpin S, Schall U, Carr V. Perceptual organization in first episode schizophrenia and ultra-high-risk states. Schizophr Res. 2006;83(1):41–52. doi: 10.1016/j.schres.2006.01.003. [DOI] [PubMed] [Google Scholar]


