Abstract
The Eph receptor tyrosine kinases and their ephrin ligands have intriguing expression patterns in cancer cells and tumor blood vessels, which suggest important roles for their bidirectional signals in multiple aspects of cancer development and progression. Eph gene mutations likely also contribute to cancer pathogenesis. Eph receptors and ephrins have been shown to affect the growth and migration/invasion of cancer cells in culture as well as tumor growth, invasiveness, angiogenesis, and metastasis in vivo. However, Eph signaling activities in cancer appear to be complex, and are characterized by puzzling dichotomies. The Eph receptors nevertheless represent promising new therapeutic targets in cancer.
Eph receptors and their ephrin ligands together form an important cell communication system with widespread roles in normal physiology and disease pathogenesis1. Links between Eph receptors and cancer date back to the first identified Eph family member2. EphA1 was cloned from a carcinoma cell line in a screen for novel oncogenic tyrosine kinases. The novel receptor was found to be upregulated in tumor versus normal tissues and its overexpression caused the oncogenic transformation of NIH3T3 fibroblasts2,3. The first Eph receptor-interacting (ephrin) ligand, EPHRIN-A1, was also identified from cancer cells a few years later4. The evidence implicating Eph receptors and ephrins in cancer is now extensive, and continues to grow.
The activities of the Eph system in cancer are complex, and intriguing in their paradoxical effects. For example, multiple Eph receptors and/or ephrins are present in essentially all types of cancer cells. However, not only increased but also decreased Eph expression has been linked to cancer progression. Consistent with this dichotomy, there is good evidence that Eph receptors and ephrins can both promote and inhibit tumorigenicity. The factors responsible for these divergent activities are only beginning to be uncovered.
Following a brief overview of the Eph and ephrin families and their bidirectional signaling mechanisms, the factors that regulate their expression and the remarkable multiplicity of their roles in cancer will be discussed, and the strategies under evaluation to target the Eph system for cancer therapy outlined. Other reviews provide more in depth information on Eph signaling mechanisms in development and adult physiology1,5–8.
Eph and ephrin families
In the human genome there are 9 EphA receptors, which promiscuously bind 5 glycosylphosphatidylinositol (GPI)-linked ephrin-A ligands, and 5 EphB receptors, which promiscuously bind 3 transmembrane ephrin-B ligands5. Exceptions are the EPHA4 and EPHB2 receptors, which can also bind ephrin-Bs and EPHRIN-A5, respectively, and EPHB4, which preferentially binds only EPHRIN-B2. Eph receptors typically interact with the cell surface-associated ephrins at sites of cell-cell contact (Fig. 1). In addition, soluble ephrin-As released from the cell surface retain the ability to activate EPHA24,9,10.
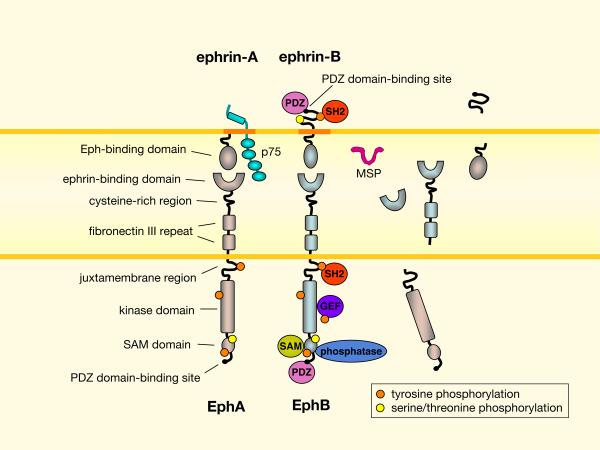
Fig. 1. Eph receptor and ephrin domain structure and signaling interactions.
Domain structures of Eph receptors and ephrins are shown. Additionally, alternative splicing or proteolysis can generate extracellular and intracellular domain fragments of Eph receptors and ephrins of both A and B classes. The major sperm protein (MSP) domain from VAMP (vesicle-associated membrane protein)-associated protein (VAP) proteins is another Eph ligand that can compete with ephrins for binding84. Eph receptor “forward” signaling involves ephrin-induced clustering, autophosphorylation, and association with signaling effectors containing SH2, PDZ, SAM and other protein interaction domains1,5. Some signaling proteins, such as certain guanine nucleotide exchange factors (GEFs) for Rho family GTPases, can constitutively associated with Eph receptors175. The activities of some effectors are modified by activated Eph receptors, for example through phosphorylation. Phosphotyrosine phosphatases bind Eph receptors and ephrins to dampen or terminate their activity through dephosphorylation. Eph receptors are also phosphorylated on serine/threonine residues176, which can have dramatic functional consequences72. The transmembrane ephrin-Bs mediate “reverse” signals, which involve Src-dependent tyrosine phosphorylation of their cytoplasmic segment and association with SH2 and PDZ domain-containing proteins5,6. EphB binding can also affect ephrin-B function by inducing serine phosphorylation, as shown in neurons7. The glycosylphosphatidylinositol (GPI)-linked ephrin-As also mediate reverse signals, through poorly understood signaling interactions that may occur in lipid rafts (dark orange). In neurons, ephrin-As can use the p75 nerve growth factor receptor as a signaling partner to activate the FYN Src family kinase177. Most domain names are illustrated on EphA/ephrin-A and signaling interactions are illustrated on EphB/ephrin-B, but each applies to the other.
Eph-ephrin complexes emanate bidirectional signals: forward signals that depend on Eph kinase activity propagate in the receptor-expressing cell and reverse signals that depend on Src family kinases propagate in the ephrin-expressing cell. Ephrin-dependent but kinase-independent Eph signals can also occur11–13. Eph signaling controls cell morphology, adhesion, migration and invasion by modifying the organization of the actin cytoskeleton and influencing the activities of integrins and intercellular adhesion molecules1,5. Recent work has also uncovered Eph effects on cell proliferation and survival as well as specialized cellular functions such as synaptic plasticity, insulin secretion, bone remodeling and immune function1.
Bidirectional signals can lead to removal of the adhesive Eph-ephrin complexes from cell contact sites through an unusual endocytic mechanism that involves their internalization, together with patches of the surrounding plasma membranes, into the receptor- or the ephrin-expressing cell5. This enables separation of the engaged cell surfaces to produce the characteristic Eph repulsive responses. Another mechanism allowing cell separation involves protease-mediated cleavage of the Eph or ephrin extracellular domains14–18. Internalization and cleavage result in degradation, which can profoundly downregulate Eph levels. However, in certain cellular contexts Eph-ephrin complexes persist at intercellular junctions and emanate prolonged bidirectional signals that favor adhesiveness. For example, the cell adhesion molecule E-CADHERIN promotes EPHA2/EPHRIN-A1 localization at epithelial cell junctions and the metalloprotease ADAM19 stabilizes EPHA4/EPHRIN-A5 at neuromuscular junctions independently of its proteolytic activity19–21. A combination of Eph-dependent repulsive and adhesive forces can drive the segregation of cell populations expressing different repertoires of Eph receptors and ephrins, which may include transformed and normal cells or divergent subpopulations of tumor cells5,22,23.
There is also increasing evidence that other signaling modalities beyond “conventional” bidirectional signaling contribute to the multiple activities of the Eph system in cancer. For example, an initial extracellular Eph or ephrin cleavage by metalloproteases followed by γ-secretase-mediated cleavage within the transmembrane segment releases intracellular domains that can generate distinctive signals14,16,24,25. Eph receptors and ephrins can also signal independently of each other, through crosstalk with other signaling systems, which produces yet more distinctive outcomes. In addition, they participate in feedback loops that may switch between different outputs depending on the state of other cellular signaling networks (Fig. 2).
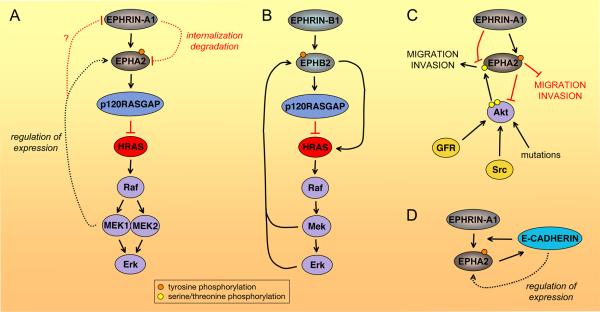
Fig. 2. Eph feedback loops.
(A) EPHA2/HRAS-Erk negative feedback loop. Activation of the HRAS-Erk pathway increases EPHA2 expression through MEK1 and decreases EPHRIN-A1 expression, although it is not known if this also occurs through MEK146,178,179. In turn, ephrin-dependent EPHA2 activation inhibits HRAS-Erk signaling and also downregulates EPHA2 levels by causing receptor internalization and degradation. (B) Positive and negative EPHB2/MAP kinase feedback loops. In a positive feedback loop, ephrin-B-dependent EPHB2 activation stimulates the HRAS-Erk pathway, and the increase in Mek and/or Erk activity in turn enables enhanced responsiveness of EPHB2 to ephrin-B stimulation through unknown mechanisms79. However, in a different cellular context EPHB2 can also inhibit the HRAS-Erk pathway5,180, which may in turn reduce EPHB2 activation by ephrin. Although not shown in the figure, EPHRIN-B1 stimulation can also downregulate EphB2 levels by causing internalization/degradation. (C) EPHA2/Akt negative feedback loop. The Akt kinase (activated by growth factor receptors (GFR), Src family kinases or mutations in upstream proteins or Akt itself) phosphorylates serine 897 in the carboxy-terminal tail of EPHA2 leading to increased EPHA2-dependent cell migration and invasion72. In turn, EPHRIN-A1-induced EPHA2 signaling inactivates Akt by causing its dephosphorylation at T308 and S473, thus decreasing EPHA2 phosphorylation at serine 897 and, consequently, cell migration and invasion. Other pathways downstream of EPHA2 can also inhibit migration and invasion. (D) EPHA2/E-CADHERIN positive feedback loop. E-CADHERIN expression increases EPHA2 expression, surface localization, interaction with EPHRIN-A1, and thus forward signaling19,20 (Box 1). In turn, EPHA2 signaling enhances E-CADHERIN-mediated adhesion. Dotted lines indicate regulation of protein levels rather than activity.
Eph and ephrin dysregulation in cancer
The Eph and ephrin families have grown in complexity during evolution, keeping pace with the increasingly more sophisticated tissue organization of higher organisms. Finely coordinated spatial and temporal regulation of Eph receptor and ephrin expression controls many processes that are critical for development and tissue homeostasis, including the formation of tissue boundaries, assembly of intricate neuronal circuits, remodeling of blood vessels, and organ size1,5. Multiple Eph receptors and/or ephrins are also expressed in both cancer cells and the tumor microenvironment, where they influence tumor properties by enabling aberrant cell-cell communication within and between tumor compartments26–32. Mutations dysregulating Eph function likely also play a role in cancer progression.
Expression in cancer cells
Many studies have correlated Eph and ephrin expression levels with cancer progression, metastatic spread and patient survival (Table 1). EPHA2, for example, is upregulated in a wide variety of cancers and its expression has been linked to increased malignancy and a poor clinical prognosis27,28,31,33. Furthermore, EPHA2 seems to be preferentially expressed in the malignant breast and prostate cancers with a basal phenotype34,35. EPHB4 is also widely expressed in cancer cells and its increased abundance has been correlated with cancer progression29,36,37. However, decreased Eph or ephrin levels in malignant cancer cell lines and tumor specimens have been reported as well. For example, EPHA1 is downregulated in advanced human skin and colorectal cancers38,39, EphB receptors in colorectal cancer23,40–42 and EPHRIN-A5 in glioblastomas43. Furthermore, EPHB6 expression is lower in metastatic than non-metastatic lung cancers44. Reconciling these discrepancies, recent studies show that an initial Eph receptor upregulation (due to activated oncogenic signaling pathways and other factors) can be followed by epigenetic silencing in more advanced stages due to promoter hypermethylation, as shown for several EphB receptors and EPHA1 in colorectal cancer23,39–41. Transcriptional repression, such as repression of EPHB2 by C-REL (a member of the nuclear factor-κB family) in colorectal cancer, may also play a role in Eph silencing45. Intriguingly, differential transcriptional regulation has been reported for EPHB2 and EPHB4 expression during colorectal cancer progression37. This was attributed to a switch in the association of β-catenin from the p300 coactivator (which induces EphB2) to the CBP coactivator (which induces EphB4). An inverse expression pattern has also been observed for EPHA2 versus ephrin-A expression in breast cancer cell lines, owing at least in part to feedback loops (Fig. 2A), and for several EphB receptors versus ephrin-Bs in early colorectal tumors and breast cancer cell lines23,46,47.
Table 1.
Examples of regulation of Eph receptor expression in cancer cells
| Mechanism | Eph/ephrin | Change | Cell type/Cancer type | Ref. |
|---|---|---|---|---|
| Frequent chromosomal abnormalities that may lead to altered Eph/ephrin expression a | ||||
| 1p36 loss | EPHA2 EPHA8, EPHB2 | down | various cancers | 48, 49, 69, 187–190b |
| 1q21-q22 gain | EPHRIN-A1, -A3, -A4 | up | various cancers | b |
| 2q36.1 loss | EPHA4 | down | cervical cancer | 191 |
| 3p11.2 loss | EPHA3 | down | lung and various other cancers | 187 b |
| 3q21-qter gain | EPHB3 | up | early stage squamous cell lung carcinoma | 50 |
| 5q21 loss | EPHRIN-A5 | down | myeloid cancers, prostate cancer | 187, 192c |
| 6q16.1 loss | EPHA7 | down | various cancers | 187, 192c |
| 7q22 loss | EPHB4 | down | myeloid cancers, colon cancer | 187 c |
| 7q22 gain | EPHB4 | up | various tumors and cancer cell lines | 187, 193, 194 |
| 7q33-35 loss | EPHB6, EPHA1 | down | myeloid cancers | 187 |
| 7q33-35 gain | EPHB6, EPHA1 | up | neuroblastoma, glioblastoma | 187 |
| 13q33 loss | EPHRIN-B2 | down | multiple myeloma, chronic lymphocytic leukemia, head and neck cancer | 187b,c |
| 17p13.1-p11.2 loss | EPHRIN-B3 | down | various cancers | b,c |
| 19p13.3 loss | EPHRIN-A2 | down | various cancers | 195 c |
|
| ||||
| Promoter hypermethylation | ||||
| EPHA1 | down | advanced colorectal cancer | 39 | |
| EPHA3 | down | leukemias, hematopoietic tumor cells | 196 | |
| EPHA7 | down | prostate, gastric, colorectal cancer | 197 – 199 | |
| soluble EPHA7 ectodomain | down | B-cell lymphomas | 200 | |
| EPHB2 | down | colorectal cancer | 40, 189, 201 | |
| EPHB4 | down | colorectal cancer | 41 | |
| EPHB6 | down | MDA-MB-231 breast cancer cells | 202 | |
|
| ||||
| mRNA stability | ||||
| nonsense-mediated mRNA decay | EPHB2 | down | prostate cancer | 49 |
| binding sites for RNA binding protein HuR in 3' UTR | EPHA2, EPHA4, EPHRIN-A2 | down | HeLa cervical cancer and U373MG glioma cells | 51 |
| microRNA-210 | EPHRIN-A3 | down | endothelial cells | 62, 203 |
|
| ||||
| Transcription | ||||
| Ras-MAP kinase (MEK1) | EPHA2 | up | breast cancer cells, activated B-Raf-transfected fibroblasts | 46, 179 |
| p53 | EPHA2, EPHB4, EPHRIN-A1 | up | various p53-transfected cell lines | 204 – 206 |
| Twist | EPHA4, EPHRIN-A4 | up | developing skull, Sézary's lymphoma? | 207, 208 |
| c-REL | EPHB2 | down | SW620 colon cancer cells | 45 |
| Wnt/β-catenin/TCF | EPHB2, EPHB3, EPHB4 | up | early colorectal cancer | 23, 209 |
| Wnt/β-catenin/p300/TCF | EPHB2 | up | early colorectal cancer | 37 |
| Wnt/β-catenin/CBP/TCF | EPHB4 | up | advanced colorectal cancer | 37 |
| estrogen | EPHB4, EPHRIN-B2 | up | mouse mammary epithelium | 210 |
| Ras/MAP kinase | EPHRIN-A1 | down | MCF10A mammary epithelial cells | 46 |
| Wnt/β-catenin/TCF | EPHRIN-B | down | Ls174T colon cancer cells | 209 |
Chromosomal locations from the NCBI Human Genome Resources (www.ncbi.nih.gov/projects/genome/guide/human)
Cancer GeneticsWeb (www.cancer-genetics.org)
Atlas of genetics and cytogenetics in oncology and haematology (http://atlasgeneticsoncology.org/index.html).
Chromosomal alterations and changes in mRNA stability also regulate Eph and ephrin expression in cancer cells (Table 1). A number of Eph receptor and ephrin genes are located in chromosomal regions frequently lost in cancer cells. For example, EPHA2, EPHA8 and EPHB2 are clustered at chromosomal region 1p36, which undergoes loss of heterozygosity in many cancers48,49. Some Eph genes, however, are in amplified regions50. Nonsense-mediated mRNA decay and interaction with mRNA-binding proteins can also regulate Eph mRNA stability in cancer cells49,51. These complex mechanisms of regulation parallel the multiplicity of Eph activities in cancer cells.
Expression in the tumor microenvironment
Several Eph receptors and ephrins are upregulated in vascular cells by tumor-derived factors and hypoxia. For example, tumor necrosis factor α (TNFα), vascular endothelial growth factor-A (VEGF-A) and the hypoxia-inducible factor HIF-2α have been shown to upregulate EPHRIN-A1 in cultured endothelial cells52–54. Endothelial EPHRIN-B2 is upregulated by VEGF through the Notch pathway, by cyclic stretch, hypoxic stress and contact with smooth muscle cells, whereas shear stress seems to decrease EPHRIN-B2 expression in endothelial cells but increase it in endothelial precursors by inducing their differentiation55–59. Moreover, EPHRIN-B2 is expressed in pericytes and vascular smooth muscle cells57,60. Expression of EPHA2/EPHRIN-A1 and EPHB4/EPHRIN-B2 in tumor blood vessels has been most extensively characterized, but other Eph receptors and ephrins are also present in the tumor vasculature54–57,61,62. In contrast, little is known about Eph and ephrin expression in other tumor compartments, such as activated fibroblasts and infiltrating immune and inflammatory cells. Nevertheless, Eph-dependent communication between these cells and tumor cells likely plays an important role in tumor homeostasis.
Eph mutations with cancer relevance
Screens of tumor specimens and cell lines have recently identified mutations in the genes encoding all of the Eph receptors, whereas cancer-related ephrin mutations have not been reported so far, perhaps in part because many of the screens have focused on the kinome63–67 (http://www.sanger.ac.uk/genetics/CGP/cosmic). Mutations of at least some Eph receptors are predicted to play a role in cancer pathogenesis. For example, EPHB2 mutations have been identified in human prostate, gastric, colorectal and melanoma tumors40,49,67–69. Some of these mutations may impair kinase function, and some are accompanied by loss of heterozygosity, suggesting a tumor suppressor role for EPHB2 forward signaling. Furthermore, a number of Eph receptors –particularly EPHA3 and EPHA5 – are frequently mutated in lung cancer63,70. The mutations are typically scattered throughout the Eph domains, including the ephrin-binding domain and other extracellular regions67,70. Elucidating the effects of the mutations will provide important insight into the functional roles of the Eph system in cancer.
Tumor Suppression
In many cancer cell lines, Eph receptors appear to be highly expressed but poorly activated by ephrins, as judged by their low level of tyrosine phosphorylation1,29,37,47,71,72. This was one of the first clues that ephrin-dependent Eph forward signaling may be detrimental to tumor progression. Furthermore, recent expression profiling of ApcMin/+ intestinal tumors from wild-type and Ephb4+/− mice has revealed an extensive transcriptional reprogramming that suggests anti-proliferative and anti-invasive activities of EPHB4 in colorectal cancer73.
Eph forward signaling inhibits cell transformation
Forcing Eph receptor activation with soluble Fc fusion proteins of ephrin ligands can inhibit proliferation, survival, migration and invasion of many types of cancer cells in culture as well as tumor growth in several mouse models5,29,42,47,74. Conversely, a dominant negative form of EPHB4 has been shown to promote colorectal cancer proliferation and invasion73. These studies demonstrate that Eph forward signaling pathways can lead to tumor suppression (Fig. 3). Indeed, Eph receptors activated by ephrins acquire the remarkable ability to inhibit oncogenic signaling pathways, such as the HRAS-Erk, PI3 kinase-Akt and Abl-Crk pathways. Interestingly, this may reflect a physiological function of the Eph system in epithelial homeostasis by promoting contact-dependent growth inhibition and decreasing motility and invasiveness. These changes are reminiscent of mesenchymal-to-epithelial transition (Box 1).
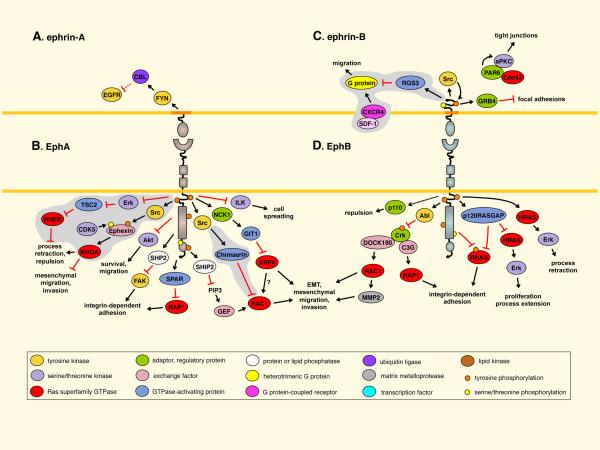
Fig. 3. Tumor suppression through bidirectional signaling.
A. EPHRIN-A5 reverse signaling downregulates epidermal growth factor receptor (EGFR) levels in glioma cells43. B. EphA receptors activate tuberous sclerosis complex 2 (TSC2) in neurons to inactivate RHEB181. EphA activation of RHOA involves Ephexin family exchange factors and other pathways (Fig. 4)5,7. EPHA2 inhibits Akt72,179 and inactivates focal adhesion kinase (FAK) through the SHP2 phosphatase5. EPHA4 inhibits RAP1 through spine-associated RAPGAP (SPAR)1,7. Recruitment of the lipid phosphatase SHIP2 by EPHA2 inhibits RAC1 and EPHA2 internalization182. EPHA4 inhibits RAC1 through Chimaerins1,183. EPHA2-mediated inhibition of ADP-ribosylation factor 6 (ARF6) in epithelial cells inhibits epithelial-to-mesenchymal transition (EMT)170. EphA1 inhibits integrin-linked kinase (ILK)184. C. EPHRIN-B1 disrupts focal adhesions through GRB45. Phosphorylation inhibits EPHRIN-B1 binding to PAR6, allowing PAR6 to bind GTP-bound CDC42 and activate atypical PKC (aPKC)87. Ephrin-Bs also inhibit signaling by the CXCR4 G protein-coupled chemokine receptor5. D. EphB signaling increases expression of the p110 subunit of PI3 kinase91. EphB receptors (and EPHA2) activate Abl, which ultimately inhibits RAP1 and RAC142,47,107. EPHB2 inactivates RRAS through phosphorylation5. EPHB2 (and EPHA2) activates p120RASGAP to inhibit the HRAS and RRAS5,180. Finally, EPHB2 can activate Erk79. Some pathways are assembled from different sources, so the complete pathways are hypothetical. Pathways identified in neurons, and predicted to have tumor suppressing activity, are on gray background. Most other pathways were identified in cultured cells and their significance in cancer also remains to be proven. Dotted lines indicate regulation of expression levels. For more details see references 1,5–7,88,127. CDK5, cyclin-dependent kinase 5; GIT1, G protein-coupled receptor kinase-interacting ARFGAP 1; MMP2, matrix metalloproteinase 2; RAPGEF1, Rap guanine nucleotide exchange factor 1; RGS3, regulator of G-protein signaling 3; SDF-1, stromal cell-derived factor-1.
Silencing of Eph forward signals in cancer cells
Cancer cells appear to use a variety of mechanisms to minimize the tumor suppressor effects of Eph forward signaling. For example, the high EPHA2 or EphB expression and low ephrin expression observed in some cancers result in low bidirectional signaling23,46,47. Furthermore, co-expressed Eph receptors and ephrins often do not interact effectively in cancer cells20. This may be because they engage in lateral interactions that silence their signaling function, as has been shown in neurons and transfected cells5. Alternatively, loss of E- or VE-cadherin impairs endogenous EPHA2-EPHRIN-A1 interaction in malignant breast cancer and melanoma cells, respectively20,75. The two cadherins appear to promote EPHA2-EPHRIN-A1 interaction by stabilizing intercellular contacts and promoting the localization of EPHA2 at cell-cell junctions. Phosphotyrosine phosphatases also negatively regulate Eph receptor forward signaling in some cancer cells76. For example, the low molecular weight phosphotyrosine protein phosphatase (LMW-PTP) has been implicated in cell transformation through its ability to dephosphorylate EPHA2, thus counteracting ephrin-dependent activation77. The receptor-type phosphatases PTPRO and PTPRF, and PTEN in C. elegans, also dephosphorylate Eph receptors78–80. However, it is not known whether this plays a role in cancer. Eph mutations may also contribute to disrupting forward signaling by impairing ephrin binding or kinase activity. For example, the EPHA3 E53K mutation in the MeWo melanoma cell line abrogates ephrin binding66,81 and the EPHB2 G787R mutation found in colorectal cancer impairs kinase activity69. It will also be interesting to investigate whether soluble Eph ectodomains generated by alternative splicing82,83 or proteolysis14–18,24 and proteins containing a major sperm protein (MSP) domain84 (Fig. 1) may decrease Eph signaling in cancer cells by acting as naturally occurring antagonists.
Tumor confinement by surrounding ephrins
The tumor suppressor effects of Eph forward signaling may be active at the tumor periphery if the surrounding tissues express ephrins. In mouse tumor models, ephrins present in normal tissues have been proposed to inhibit expansion and invasiveness of incipient colorectal and skin tumors expressing Eph receptors23,85,86. In addition, recent experiments in the developing zebrafish hindbrain raise the possibility that elevated Eph or ephrin levels may drive segregation of tumor cells from surrounding normal tissues, thereby decreasing invasiveness, not only through repulsive mechanisms but also by promoting adhesiveness between tumor cells22. Eph receptors may further decrease tumor invasiveness by promoting the formation of tight junctions in neighboring epithelial cells through stimulation of ephrin-B reverse signaling87 (see next section). Indeed, recent systems-level studies have implicated complex, asymmetric signaling networks in the sorting of EPHRIN-B1-expressing HEK293 cells from EPHB2-expressing cells88. It is tempting to speculate that Eph receptors may contribute to tumor dormancy through these types of bidirectional signaling mechanisms that restrict tumor expansion. Accordingly, high EPHA5 levels have been detected in various dormant but not fast-growing tumor xenograft models89.
Ephrin reverse signaling in tumor cells
Ephrin reverse signaling in cancer cells may in some cases also contribute to tumor suppression (Fig. 3). In the Xenopus system and HT29 colon cancer cells, EPHRIN-B1 tyrosine phosphorylation (which can be induced by interaction with EphB receptors or by activated growth factor receptors and Src) disrupts binding of the ephrin to the scaffolding protein PAR6, promoting the formation of tight junctions between cells87,90. Similar to its role in neurons, ephrin-B reverse signaling may also inhibit the migratory and invasive effects of the CXCR4 G protein-coupled chemokine receptor in cancer cells5,6. EPHRIN-A5 can downregulate epidermal growth factor receptor (EGFR) levels in glioblastoma cells43.
Tumor promotion
Conversely, forward and/or reverse Eph-ephrin signals can enhance malignant transformation in some cases. There is also increasing evidence that the Eph receptors are capable of unconventional signaling activities that do not depend on activation by ephrin ligands and that support cancer progression. Moreover, it is well established that the Eph system promotes tumor angiogenesis.
Eph forward signaling
In certain cellular contexts, Eph receptors activated by ephrins may have lost the ability to suppress tumorigenicity, and even acquired oncogenic ability. For example, activating mutations may render oncogenic signaling pathways resistant to inhibition by Eph forward signaling. Furthermore, EPHB2 can promote proliferation in mouse intestinal progenitor cells and ApcMin/+ adenomas through Abl-mediated increase in CYCLIN-D1 levels even though it inhibits invasiveness through other pathways91 (Fig. 4). Activation of RHOA downstream of EPHA2 and EPHB4 promotes ameboid-type migration of cancer cells and destabilizes epithelial adherens junctions in various cancer cell lines (Fig. 4), even though RHOA inhibits mesenchymal-type migration92–94 (Fig. 3). EPHA2 forward signaling in malignant melanoma and ovarian cancer cells can also promote vasculogenic mimicry75,95.
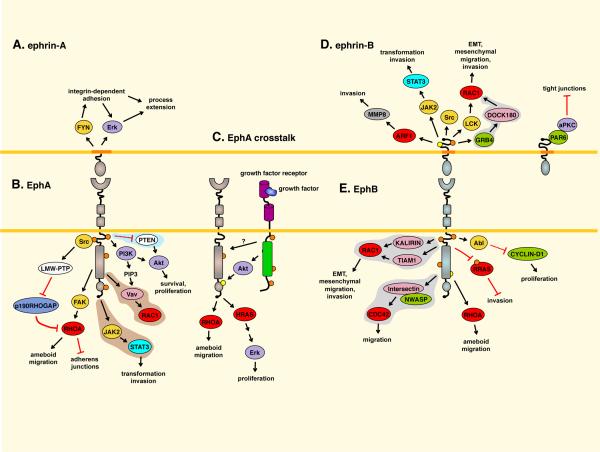
Fig. 4. Eph tumor promoting pathways.
A. EPHRIN-A5 reverse signaling promotes activation of FYN, β1-integrins, and Erk in fibroblasts5. B. Low-molecular-weight phosphotyrosine phosphatase (LMW-PTP) is activated by Src and dephosphorylates and inactivates p190RHOGAP. This increases RHOA activity to destabilize adherens junctions in EPHA2-overexpressing epithelial cells103. EPHA2 (and EPHB2) activate RHOA through focal adhesion kinase (FAK)7,94. EPHA4 activates signal transducer and activator of transcription 3 (STAT3)185. A pathway involving EPHA2, PI3 kinase and Vav family exchange factors for RAC1 operates in endothelial cells27,186. Activation of EPHA2 activates Akt in pancreatic cancer cells138. The C. elegans Eph receptor inhibits PTEN expression80. C. EPHA2-ERBB2 crosstalk activates the HRAS-Erk pathway and RHOA in a mouse mammary tumor model, enhancing tumor growth and in vitro cell proliferation and migration33,105. Akt, activated by ERBB2 or other pathways, phosphorylates EPHA2. D. Ephrin-B reverse signaling affects pathways that promote invasiveness, including matrix metalloproteinase 8 (MMP8) secretion17 and activation of STAT3,118 Src and RAC1113114. In contrast, non-phosphorylated EPHRIN-B1 can bind PAR6 to inhibit atypical protein kinase C (aPKC)87. E. EphB forward signaling activates various RAC1 and CDC42 exchange factors5,7,127, which could promote cancer cell migration and invasion. EphB4 activates RHOA93. EPHB2-mediated RRAS tyrosine phosphorylation increases glioma cell invasiveness96. EPHB2-mediated ABL activation increases CYCLIN-D1 levels91. Pathways identified in neurons, endothelial/muscle cells, or C. elegans that are predicted to have tumor promoting activity are on gray, light-brown or light blue background, respectively. Most other pathways were identified in cultured cells and their significance in cancer remains to be proven. For more details see references 1,5–7,127. ARF1, ADP-ribosylation factor 1; EMT, epithelial-to-mesenchymal transition; JAK2, Janus kinase 2.
RRAS phosphorylation downstream of EPHB2 (Fig. 4) can enhance glioma cell invasiveness, possibly by decreasing cell substrate adhesion96, even though in other cell types Eph forward signals decrease cell adhesion and migration5,47,97. Instead of inhibiting the HRAS-Erk MAP kinase pathway, depending on the circumstances, EPHB2 can sometimes activate it5,79. In turn, activation of the Erk MAP kinase pathway enhances ephrin-dependent activation of overexpressed EPHB2 in cultured cells79. This may result in different EPHB2/MAP kinase feedback loops (Fig. 2B) that could either enhance or diminish cancer cell malignancy. Indeed, activation of an engineered membrane-anchored cytoplasmic domain of fibroblast growth factor receptor 1 (FGFR1) inhibits ephrin-dependent repulsive signaling by overexpressed EPHB2 through a mechanism involving downregulation of the HRAS-Erk pathway, suggesting that FGFR1 activation could neutralize the anti-invasive effects of EPHB2 in cancer cells79. In contrast, overexpressed EPHA4 and FGFR1 associate and potentiate each other's oncogenic activities in cultured glioma and other cell types98,99. It will also be interesting to determine whether Eph receptors can downregulate PTEN levels and perhaps activity in cancer cells, as suggested by recent studies in C. elegans80.
Unconventional Eph receptor activities
Downregulation of EPHA2 or EPHB4 by small interfering RNAs (siRNAs) or antisense oligonucleotides decreases cancer cell malignancy in culture and inhibits tumor growth in a number of mouse cancer models36,37,100–102. Furthermore, EPHA2 overexpression causes oncogenic transformation of mammary epithelial cells in culture as well as in vivo71,103. These experiments demonstrate positive effects of Eph receptors on cancer progression. Given the low levels of Eph forward signaling observed in many cancer cells, these tumor promoting activities are likely to be independent of ephrin stimulation and possibly also of kinase activity. Recent evidence indeed shows that oncogenic signaling pathways can co-opt Eph receptors to increase cancer cell malignancy.
Notable examples of how the altered signaling networks of cancer cells can subvert Eph function involve EPHA2. This receptor has been found to mediate some of the oncogenic activities of EGFR family members, including cancer cell migration in culture and tumor growth and metastasis in a transgenic mouse breast cancer model104,105 (Fig. 4). EPHA2 also appears to be required for Src-dependent invasiveness of colorectal cancer cells in culture90. These effects may be ligand-independent and explained at least in part by the recently discovered crosstalk between EPHA2 and Akt, a serine/threonine kinase frequently activated in cancer cells72 (Figs. 2C and 4). Phosphorylation by Akt of a single serine in EPHA2 appears to promote cancer cell migration and invasion, an effect that interestingly does not require EPHA2 kinase activity and is reversed by EPHRIN-A1 stimulation72. It will be important to investigate the details of the Akt/EPHA2 crosstalk and whether other Eph receptors may contribute to cancer progression through analogous mechanisms. EPHA2 has also been recently shown to promote epithelial proliferation and branching morphogenesis in the developing mouse mammary gland by mediating hepatocyte growth factor (HGF)-dependent inhibition of RHOA activity106, which is in contrast to the RHOA activation induced by EPHA2 overexpression, ephrin stimulation, or crosstalk with the ERBB2 receptor103,105,107. It is not yet known if an ephrin-independent EPHA2/HGF receptor crosstalk may play a role in cancer. Ephrin-independent activities of Eph receptors may also include modulation of the subcellular localization of signaling partners that are constitutively associated with an Eph receptor (Fig. 1) or become associated as a result of Eph phosphorylation by other kinases.
With regard to other receptors, the recently discovered ephrin-independent downregulation of β1-integrin levels and cell substrate adhesion by endogenous EPHB4 may promote migration and invasiveness in some cancer cell types although it is inhibitory in others96,97. Additionally, distinctive signaling activities of Eph intracellular domain fragments generated by metalloprotease and γ-secretase cleavage may promote cancer cell malignancy. For example, the EPHA4 cytoplasmic domain released by γ-secretase can enhance RAC1 activity in cultured cells independently of ephrin stimulation and kinase activity24. Furthermore, EPHRIN-B3 stimulation can block apoptosis caused by caspase-dependent cleavage of overexpressed EPHA4 in cultured cells, which interestingly suggests a role for EPHA4 as a “dependence” receptor108.
Tumor promotion by ephrin signaling in cancer cells
Little is known about the effects of ephrin-A reverse signaling in epithelial cells. One study has shown that ephrin-A1 is highly upregulated in hepatocellular carcinoma and promotes the proliferation and expression of genes associated with proliferation and invasion in human liver cancer cells109. In fibroblasts, EphA-dependent stimulation of EPHRIN-A5 activates the FYN Src family kinase, integrin-mediated adhesion and Erk MAP kinases5,6 (Fig. 4). Accordingly, EPHRIN-A5 overexpression can increase fibroblast growth in soft agar, invasion and morphological transformation110. Ephrin-B reverse signaling also involves Src family kinases, which phosphorylate the ephrin-B cytoplasmic domain thus regulating its interaction with signaling molecules5,6. Src activation has been proposed to require release of the ephrin-B intracellular domain by metalloprotease and γ-secretase cleavage following EphB binding, which decreases Src association with its inhibitory kinase CSK14. Furthermore, homophilic engagement of claudins, which are tight junction proteins, causes Src-mediated EPHRIN-B1 phosphorylation that slows down the formation of epithelial cell junctions and may thus enhance invasiveness111. This is in contrast to the promotion of tight junction formation due to EPHRIN-B1 phosphorylation discussed above. Whether phosphorylation of different tyrosines, different levels of phosphorylation, or the cellular context may lead to positive versus negative effects of ephrin-Bs on intercellular adhesion remains to be determined.
Other recurring themes in ephrin-B reverse signaling are a localization in lipid rafts and RAC1 activation, which can occur through multiple mechanisms and increase cancer cell migration and invasion112–114 (Fig. 4). For example, EPHRIN-B3 is upregulated in invading cells of glioma biopsies and promotes RAC1-dependent invasion of glioma cell lines112 while EPHRIN-B2 is upregulated in invading cells of glioma and melanoma biopsies and its forced overexpression in the cultured cancer cells enhances integrin-mediated attachment, migration and invasion115,116. Furthermore, EPHRIN-B1 reverse signaling has been reported to induce secretion of matrix metalloprotease 8 (MMP8) and promote invasion of glioma, pancreatic, gastric and leukemic cancer cells in vitro and in mouse tumor models17,113,117.
Ephrin-B reverse signaling may also modulate gene transcription in cancer cells. Ephrin-B1 binds and activates STAT3, a transcription factor involved in cancer progression118 (Fig. 4). Furthermore, in neural progenitors EPHRIN-B1 intracellular domain fragments can localize to the nucleus and bind the ZHX2 transcriptional repressor, potentiating its activity, although it is not known whether this regulation also plays a role in cancer25.
Tumor angiogenesis
Blood vessels are critical for tumor growth and represent an important venue for metastatic dissemination. Several Eph receptors and ephrins promote angiogenesis by mediating communication of vascular cells with other vascular cells as well as tumor cells. The latter interactions may occur especially during blood vessel growth and in tumor vessels with discontinuous endothelial lining. Furthermore, they may affect not only the endothelial cells but also, reciprocally, tumor cell behavior119.
Analysis of tumors grown in Epha2 mutant mice or mice treated with inhibitory EphA-Fc fusion proteins suggests that EPHA2 forward signaling promotes tumor angiogenesis27,31,56. In contrast, EPHA2 does not seem to play a major role in developmental angiogenesis, and only recently abnormalities in capillary development that may be due to defective pericyte coverage have been revealed in Epha2-deficient mice120. In vitro and in vivo data also show that EPHA2 forward signaling can increase blood vessel permeability, perhaps in part through phosphorylation of claudins8,121. A major ligand for endothelial EPHA2 is EPHRIN-A1, whose upregulation in endothelial cells and consequent activation of EPHA2 have been reported to play an important role in the angiogenic effects of VEGF-A and TNFα52,53. In tumors, EPHRIN-A1 can be expressed by both endothelial and tumor cells52,122,123. Interestingly, the upregulation of EPHA2 and EPHRIN-A1 observed in pancreatic tumors of mice treated with VEGF inhibitors suggests that EPHA2-dependent angiogenesis may contribute to the development of resistance to anti-VEGF therapies, perhaps by promoting endothelial coverage by pericytes and smooth muscle cells120,124. Curiously EPHRIN-A3, another ephrin ligand for EPHA2, is downregulated in hypoxic endothelial cells in culture by the microRNA miR-210 and appears to inhibit angiogenic responses in hypoxic human umbilical vein endothelial cells62. It will be important in future studies to evaluate the combined activities of all relevant EphA receptors and ephrin-A ligands in the regulation of capillary sprouting, vessel permeability and pericyte coverage, as well as their possible redundancies and opposing functions in tumor blood vessels.
EPHB4 and EPHRIN-B2 also play a role in tumor angiogenesis. During development, they are characteristically expressed in the endothelial cells of veins and arteries, respectively, and enable arterial-venous vessel segregation and vascular remodeling55–57,125. The information available so far highlights the importance of EPHRIN-B2 reverse signaling in tumor angiogenesis, while little is known about the role of EPHB4 forward signaling56,126–128. Reverse signaling by EPHRIN-B2, and possibly other ephrin-Bs, in tumor endothelial cells, pericytes and smooth muscle cells likely depends on interaction with several EphB receptors expressed by vascular and/or tumor cells and has been shown to be important for blood vessel assembly, enlargement and decreased permeability both in cell culture and in vivo57,126,127. EPHRIN-B2 signaling also promotes the interaction between endothelial cells and pericytes or vascular smooth muscle cells60,128, suggesting that upregulation of this ephrin may stabilize the vessels of tumors recurring after anti-VEGF therapy129. EPHRIN-B2 in the tumor endothelium may also play additional roles. For example, it may enhance the recruitment of bone marrow-derived endothelial progenitor cells that could participate in tumor vascularization, through a mechanism involving EPHB4-dependent upregulation of selectin ligands130. It will be interesting to determine whether EPHRIN-B2 may also promote extravasation of EphB-positive metastatic tumor cells through the vascular endothelium, similar to its in vitro effect on monocytes58,131.
Eph proteins as therapeutic targets
Eph receptors and ephrins represent promising new therapeutic targets in cancer. A variety of strategies are under evaluation to interfere with their tumor-promoting effects or enhance their tumor-suppressing effects, although our limited mechanistic understanding of the dichotomous Eph activities represents a challenge in the design of therapeutic agents. Other approaches that do not rely on interfering with Eph function involve using Eph receptor-targeting molecules for the selective delivery of drugs, toxins or imaging agents to tumors, and the use Eph-derived antigenic peptides to stimulate anti-tumor immune responses.
Interfering with Eph/ephrin function
Inhibiting the Eph system may be particularly useful for anti-angiogenic therapies, and possibly to overcome resistance to anti-VEGF therapies27,29,55,124,129. Efforts to identify small molecules that target the Eph kinase domain have begun to yield some high affinity inhibitors132–136 (Table 2). Furthermore, a number of inhibitors designed to target other kinases also inhibit Eph receptors. For example dasatinib, a multi-targeted kinase inhibitor already used in the treatment of chronic myelogenous leukemia and under clinical evaluation to treat solid tumors, potently inhibits EPHA2 and other Eph receptors besides its primary targets Abl and Src34,137,138 (http://clinicaltrials.gov/ct2/results?term=epha2). Interestingly, EPHA2 has also been identified as a biomarker for dasatinib sensitivity of cancer cells34,35. Moreover XL647, an orally bioavailable EGF and VEGF receptor inhibitor being evaluated in clinical trials for lung cancer, also targets EPHB4 (http://clinicaltrials.gov/ct2/results?term=EphB4).
Table 2.
Eph/ephrin targeting molecules
| Molecules | Targets | Activity | Ref. |
|---|---|---|---|
| Kinase inhibitors | |||
| anilinopyrimidine derivatives | EPHB4a | ATP competitors | 133, 211 |
| benzenesulfonamide derivative | EPHB4a | ATP competitor | 132 |
| XL647 (EXEL-7647)b | EPHB4a | ATP competitor | 212 |
| xanthine derivatives | Eph receptors | ATP competitors | 135, 213 |
| LDN-211904 | Eph receptors | ATP competitor | 136 |
| pyrido[2,3-d]pyrimidine PD173955 | Eph receptors | ATP competitor | 214 |
| nilotinib and analogsb | Eph receptors | ATP competitors | 134, 215 |
| dasatiniba | Eph receptors | ATP competitor | 34, 35, 137, 138 |
|
| |||
| Inhibitors of Eph expression | |||
| siRNA | EPHA2 | mRNA downregulation | 101, 102, 139 |
| oligonucleotides | EPHA2 | protein downregulation | 100 |
| siRNA | EPHB4 | mRNA downregulation | 36, 37, 194, 216, 217 |
| oligonucleotides | EPHB4 | Protein downregulation | 36, 194, 216, 217 |
|
| |||
| Inhibitors of Eph-ephrin interaction | |||
| EPHA2 Fc, EPHA3 Fc | EPHRIN-A | Eph competitor | 53, 218–220 |
| sEPHB4 | EPHRIN-B | Eph competitor | 142, 221, 222 |
| KYL and other peptidese | EPHA4 | ephrin competitor | 147, 150, 223 |
| SNEW and other peptides | EPHB2 | ephrin competitor | 145, 149 |
| TNYL-RAW peptide | EPHB4 | ephrin competitor | 145, 148, 224 |
| dimethyl-pyrrole derivatives | EPHA2, EPHA4 | ephrin competitor | 150, 151 |
| mAb 2H9 antagonistic antibody | EPHB2 | ephrin competitor | 143 |
|
| |||
| Activators of Eph forward signaling (also downregulate Eph expression) | |||
| EA1.2 antibody | EPHA2 | Eph activation/degradation, ADCCC? | 100 |
| EA2, B233, 3F2-WT (humanized B233) antibody | EPHA2 | Eph activation/degradation, ADCC? | 152, 155 |
| EA5 antibody | EPHA2 | Eph activation/degradation, reduced Src phosphorylation & VEGF levels, ADCC? | 153 |
| Ab20, 1G9-H7 antibodiesd | EPHA2 | Eph activation/degradation | 156 |
| mAB208 | EPHA2 | Eph degradation & enhanced presentation of peptide antigens on tumor cell surface | 154 |
| YSA, SWL peptides | EPHA2 | ephrin competitor, Eph activation/degradation | 146 |
| dimerized IIIA4 antibody | EPHA3 | Eph activation | 161 |
| EPHRIN-A1 Fc | EphA receptors | Eph activation/degradation | 74 |
| EPHRIN-B2 Fc | EPHB4 | Eph activation/degradation | 127 |
|
| |||
| Cytotoxic molecules | |||
| 1C1 antibody-mc-MMAFf conjugateb | EPHA2 | receptor-mediated internalization & disruption of microtubule dynamics | 157, 158 |
| 3F2-3M antibody (mutated 3F2-WT with enhanced effector function) | EPHA2 | ADCC | 155 |
| bscEphA2xCD3 bispecific single-chain antibody | EPHA2/CD3 | redirection of unstimulated cytotoxic T cells to EphA2-positive tumor cells | 162 |
| YSA-modified adenoviruse | EPHA2 | adenoviral transduction of EphA2-expressing tumor cells | 225 |
| EPHRIN-A1-PE38QQR Pseudomonas exotoxin A conjugate | EphA receptors | EphA-mediated internalization and exotoxin-dependent cell death | 226 |
| EPHRIN-A1-gold-coated nanoshells | EphA receptors | absorption of near infrared light for photothermal ablation of tumor cells | 159 |
| 2H9 antibody-vc-MMAEg conjugate | EPHB2 | receptor-mediated internalization & disruption of microtubule dynamics | 143 |
|
| |||
| Imaging agents | |||
| 64Cu-DOTA-1C1 antibody | EPHA2 | binding, which enables radioimmunoPET | 160 |
| YSA peptide-magnetic nanoparticles | EPHA2 | binding, which enables cell capture | 227, 228 |
| 111Indium-labelled IIIA4 antibody | EPHA3 | binding to low affinity ephrin-binding site, which enables tumor detection | 161 |
Eph receptor selectivity has not been reported
In clinical trials
ADCC, antibody-dependent cell-mediated cytotoxicity
not effective in vivo
tested in vivo in a model of spinal cord injury
mc-MMAF, stable maleimidocaproyl linker-monomethylauristatin F
vc-MMAE, cathepsin B-cleavable valine-citrulline linker-monomethylauristatin E..
Downregulation of EPHA2 or EPHB4 expression with siRNAs or antisense oligonucleotides has been shown to inhibit malignant cell behavior in culture and tumor growth in vivo36,37,100–102 (Table 2). For example, delivery of EPHA2 siRNA to tumors using neutral liposomes inhibits tumor growth and metastasis in mouse models of ovarian cancer, particularly when combined with delivery of siRNA silencing focal adhesion kinase (FAK) or with paclitaxel chemotherapy102,139. Eph receptor levels and function might also be reduced in vivo, as they are in vitro, by drugs that target the chaperone protein HSP90140,141, although other proteins will also be concomitantly downregulated.
Another strategy that shows promise for cancer anti-angiogenic therapy is to inhibit Eph-ephrin interactions. A variety of molecules can be used for this purpose (Table 2). The dimeric EPHA2 ectodomain fused to Fc (which inhibits EPHA forward signaling but promotes reverse signaling) and the monomeric soluble EPHB4 ectodomain (which inhibits both forward and reverse signaling) can both reduce tumor growth in mouse cancer models, at least in part by inhibiting tumor angiogenesis27,31,57,142. Antagonistic antibodies143,144 and peptides that inhibit ephrin binding to individual Eph receptors or subsets of receptors145–147 could be useful for inhibiting Eph-ephrin interactions and bidirectional signaling with greater selectivity than the promiscuous Eph ectodomains. At least two of these peptides bind to the high-affinity ephrin-binding channel of their target receptor148,149. This Eph channel also appears suitable for targeting with chemical compounds, and two isomeric small molecules that preferentially inhibit ephrin binding to EPHA2 and EPHA4, albeit with low affinity, have been identified150,151. Structural characterization of additional small molecules and peptides in complex with Eph receptors may reveal general rules enabling the rational design of chemical compounds capable of selectively targeting Eph receptors with high affinity.
Intriguingly, ephrin ligands and agonistic antibodies have also been successfully used to inhibit tumor progression in mouse cancer models despite being activators rather than inhibitors of Eph-ephrin signaling (Table 2). These agonists have been proposed to act by stimulating Eph forward signaling pathways with tumor suppressor activity and/or receptor degradation in the cancer cells47,152–154. Antibody-dependent cell-mediated cytotoxicity may also contribute to the anti-cancer effects of some of the antibodies155, perhaps explaining the discrepancies in the effectiveness of different EPHA2 antibodies with similar agonistic properties155,156. Eph agonistic antibodies may also be useful in combination with chemotherapy33,153.
Eph-targeting agents likely act through a combination of multiple effects on cancer cells and the tumor microenvironment, which may explain the efficacy of agents with opposite mechanisms of action. For example, EPHA2 agonists would be expected to enhance tumor suppressor signaling pathways and receptor degradation in the cancer cells but promote tumor angiogenesis31. On the other hand, some Eph kinase inhibitors with anti-angiogenic activity might also block possible Eph tumor suppressor activities. Such inhibitors may therefore be particularly effective for the treatment of tumors where Eph forward signaling pathways with tumor suppressor activity are not activated. EPHB4 agonists that also antagonize ephrin binding may be particularly beneficial by both enhancing EPHB4-dependent tumor suppression in cancer cells and inhibiting EPHRIN-B2-dependent angiogenesis47,127. Ultimately, how a tumor will respond to a particular Eph-targeted strategy will likely depend on the tumor type, stage and microenvironment. Selecting optimal strategies to interfere with Eph function for cancer therapy will therefore require a better understanding of Eph signaling mechanisms in the different cellular compartments of tumors. Eph-dependent oncogenic signaling networks may also represent suitable therapeutic targets. Newly developed targeting molecules, in particular those with selectivity for individual Eph receptors or ephrins, in turn represent useful research tools to further our knowledge of Eph cancer biology.
Targeted delivery of drugs/toxins and imaging agents
Because of their elevated expression in many tumors compared to normal tissues, Eph receptors also represent attractive targets for the delivery of drugs or toxins and imaging agents to cancer tissue. Several chemotherapeutic drugs and toxins conjugated to Eph antibodies or an ephrin, which cause receptor-mediated drug internalization, appear promising in initial studies (Table 2). EPHA2- or EPHB2-targeting antibodies coupled to derivatives of the peptide drug auristatin, which disrupts microtubule dynamics, inhibit the growth of several cancers in rodent models143,157,158. Another potential application is the targeted delivery of gold-coated nanoshells conjugated to ephrins for photothermal destruction of Eph-positive cancer cells159. Importantly, systemic toxic effects have not been apparent so far and the EPHA2 antibody coupled to an auristatin derivative is under clinical evaluation (http://clinicaltrials.gov/ct2/show/NCT00924235). Notably, targeting Eph surfaces that are preferentially exposed on tumor cells, which may include the ephrin-binding channel, could further improve the therapeutic index152.
Antibodies, ephrins and peptides can also be used to deliver imaging agents for diagnostic purposes. EPHA2 is a particularly attractive target for this application given its widespread expression in both cancer cells and tumor vasculature and low expression in most adult tissues27,56,160. Promising results have been obtained in animal models by using an EPHA2 antibody labeled with 64Cu through the chelating agent 1,4,7,10-tetraazacyclododecane N,N′,N″,N″′-tetraacetic acid (DOTA) for radioimmunoPET imaging and an EPHA3 antibody coupled to 111Indium for gamma camera imaging160,161.
Immunotherapy
In addition to the immune cell-mediated cytotoxicity that can be elicited by Eph-targeted antibodies, a bispecific single-chain antibody that simultaneously binds both EPHA2 and the T-cell receptor/CD3 complex causes T-cell-mediated destruction of EPHA2-positive tumor cells in vitro and decreases tumor growth in vivo162 (Table 2). Eph receptors that are preferentially expressed in tumors compared to normal tissues are also attractive targets for cancer vaccines. EPHA2, EPHA3 and an EPHB6 isoform have been identified as sources of tumor-associated peptide antigens that are recognized by cancer-specific cytotoxic T-cells163–166. Interestingly, agonists and drugs that stimulate Eph receptor degradation may inhibit tumor growth at least in part by enhancing the presentation of Eph-derived peptides that can be recognized by effector T-cells141,154. Vaccination with Eph-derived epitopes also shows promise as a strategy to elicit tumor rejection167,168.
Perspectives
Accumulating evidence implicates deregulation of the Eph cell communication system in cancer pathogenesis. The Eph receptors are emerging as master regulators capable of either potentiating the activities of oncogenic signaling networks or repressing them, depending on ephrin stimulation and other contextual factors. Remarkably, Eph receptors and ephrins can switch between contrasting activities by using bidirectional signaling as well as other signaling modalities to influence cancer cell behavior. We still know relatively little about how the Eph system regulates tumorigenesis at the molecular level, but clearly there is extensive cell context dependency for many Eph pathways. For example, many of the differences observed in Eph/ephrin signaling outcomes may relate to differences in spatial and temporal coordination of imput signals and relays, and thus vary between cell types and in vitro versus in vivo environments169. An important step forward will be to understand in detail the Eph activities beyond bidirectional signaling and the crosstalk with oncogenic pathways. Furthermore, systems-level studies will be instrumental for: providing a comprehensive overview of the effects of Eph bidirectional and unconventional signaling mechanisms in cancer and stromal cells; comparing the signaling activities of different Eph/ephrin family members; examining the consequences of changes in Eph/ephrin expression, for example to compare the effects of Eph receptor downregulation by agonists and by transcriptional silencing; and elucidating the effects of cancer-relevant Eph/ephrin mutations. Indeed, a recent proteomic analysis combined with siRNA screening and data-driven network modeling has provided a wealth of tantalizing new information on the asymmetric bidirectional signaling networks initiated by ephrin-B1 and EphB2 at sites of cell-cell contact88. Another area of great interest is how the Eph system influences the metastatic process, including tissue invasion, dissemination through the vascular system, possible reversal of epithelial-to-mesenchymal transition at distant sites, and dormancy of Eph-expressing micrometastases seeded in ephrin-rich tissues.
To advance our understanding of Eph cancer biology, it will also be important to examine the effects of Eph or ephrin loss, increased expression, and cancer relevant mutations in genetically engineered mouse models that mimic the progression of human cancers. Such in vivo models are key for studying the Eph system, given its penchant for regulating communication between different cell types, which is difficult to accurately recapitulate in vitro. The mouse models will also be useful for preclinical evaluation of new Eph-based therapies.
Eph and ephrin expression promises to be a powerful predictor of prognosis and perhaps drug sensitivity. For example, increased EPHA2 expression can confer sensitivity to dasatinib but resistance to the ERBB2-targeting antibody trastuzumab33–35. Therefore, there is a need for a comprehensive assessment of Eph and ephrin protein expression in large cohorts of human tumors in correlation with stages of malignancy and clinical outcome. Carefully validated antibodies and quantitative proteomics approaches are needed to ensure the reliability of such studies. Understanding the complexities of the Eph system will contribute to clarify the mechanisms of cancer development, progression and metastasis as well as aid development of novel anti-cancer therapies.
At a glance.
The Eph receptors are the largest family of receptor tyrosine kinases. They bind GPI-linked and transmembrane ephrin ligands, generating bidirectional signals at sites of cell-cell contact.
Eph receptors and/or ephrins are widely expressed in cancer cells and tumor stroma, but they can be downregulated at advanced cancer stages. Often Eph receptor and ephrin levels are discordantly regulated. Not only changes in expression levels, but also Eph receptor mutations likely play a role in cancer pathogenesis.
In many cellular contexts, Eph bidirectional signaling promotes an epithelial phenotype and suppresses cancer cell-substrate adhesion, migration, invasion and growth. Consistent with this, Eph receptor signaling appears to be low in many cancer cells due to imbalance of Eph/ephrin expression or inability of receptor and ligand to interact effectively.
Eph receptors and ephrins can also promote cancer progression through poorly understood mechanisms that do not involve reciprocal association but rather depend on crosstalk with oncogenic signaling pathways. In addition, Eph bidirectional signals promote tumor angiogenesis.
Eph receptors and ephrins represent promising new therapeutic targets in cancer, and many Eph-based approaches show promise for prognosis and therapy.
Box 1. The Eph system can promote an epithelial phenotype.
Forward signaling by EPHA2 and several EphB receptors in epithelial and cancer cells can induce morphological changes reminiscent of mesenchymal-to-epithelial transition. For example, stimulation of EPHA2 forward signaling with EPHRIN-A1-Fc in sparse MDCK epithelial cells enhances maturation of cell-cell junctions and cell compaction170,171 (see figure) In a positive feedback loop, E-CADHERIN can promote EPHA2 expression and surface localization in epithelial and cancer cells that have reached high density, thereby prolonging EPHA2 interaction with co-expressed EPHRIN-A1 and forward signaling19,20,170 (Fig. 2D). Stimulation of EPHB2 forward signaling with EPHRIN-B1-Fc can also couple increased intercellular adhesion with cell contraction and apico-basal polarization in colorectal cancer cells by promoting the membrane localization of E-CADHERIN86. Interestingly, the consequences are dramatically different in colorectal cancer cells expressing EPHB2 but lacking E-CADHERIN, where EPHRIN-B1-Fc stimulation causes cell contraction and separation instead of promoting cell-cell adhesion86. Stable transfection of EPHB3 in HT29 colon cancer cells, which endogenously express ephrin-Bs and E-CADHERIN, also causes changes consistent with mesenchymal-to-epithelial transition42. Furthermore, moderate ephrin-B expression and phosphorylation can promote the integrity of adherens and tight junctions in Xenopus and HT29 cells87. Conversely, EPHB4-EPHRIN-B2 antagonists have been shown to disturb intercellular junctions in MCF-10A mammary epithelial cells47. Thus, interplay with E-CADHERIN can convert Eph repulsive signals into signals that promote cell-cell adhesion. It is not known whether a similar interplay may occur with N-CADHERIN, which often replaces E-CADHERIN in malignant cancer cells that have undergone epithelial-to-mesenchymal transition. Studies in normal tissues suggest that Eph receptors can promote N-CADHERIN-dependent adhesion. For example, EPHA4 forward signaling is critical for the N-CADHERIN-dependent mesenchymal-to-epithelial transition that occurs at the borders of developing zebrafish somites172. Interestingly, EPHA2 mutations in humans and EPHA2 or EPHRIN-A5 loss in mice disrupt the N-CADHERIN-dependent intercellular junctions in the lens epithelium, causing cataracts173,174.
Acknowledgments
The author thanks members of her laboratory for helpful comments on the manuscript. Work in the author's laboratory is supported by grants from the National Institutes of Health, the Department of Defense, the Tobacco-Related Disease Research Program, and Sanford Children's Health.
Glossary
- Basal phenotype
Phenotype of highly aggressive breast and prostate cancers with gene expression profiles similar to basal cells. Basal-type breast cancers are typically negative for estrogen, progesterone and HER2 receptors. Basal-type prostate cancers have high expression of cytokeratin 5 and low expression of the androgen receptor and prostate specific antigen.
- Nonsense-mediated mRNA decay
The process by which mRNA molecules carrying premature stop codons are degraded by a regulated pathway, thereby limiting the synthesis of abnormal proteins.
- Cyclic stretch
Periodic stretch (or strain) to which vascular endothelial cells are subjected as a result of the rhythmic changes in vessel diameter caused by pulsatile blood flow.
- Shear stress
The physical force exerted on endothelial cells as a result of blood flow.
- Pericytes
Mesenchymal cell precursors to vascular smooth muscle that associate with endothelial cells during angiogenesis and provide support to small capillaries.
- ApcMin/+
Mouse that carries the Min (multiple intestinal neoplasia) point mutation in one Apc allele and spontaneously develops intestinal adenomas. It is a commonly used model for human familial adenomatous polyposis and for human sporadic colorectal cancer.
- Mesenchymal-to-epithelial transition
The conversion of non-polarized and motile mesenchymal cells into polarized epithelial cells. Typically associated with increased E-CADHERIN levels and with low cancer cell invasion and metastasis. It is the reverse of the better known epithelial-to-mesenchymal transition.
- Ameboid-type migration
Motility frequently exhibited by cancer cells and leukocytes and characterized by high speeds, lack of stable polarity and a relatively amorphous cell shape. Does not require stable integrin-dependent adhesion for traction but depends on RHOA to increase actomyosin contractility and allow invasion in the absence of extracellular proteolysis.
- Mesenchymal-type migration
Movement of cells with elongated morphology and a front-back polarity, where traction is generated through integrin-dependent adhesion. Requires extracellular proteolysis for cell invasion and is thought to depend on RAC1.
- Vasculogenic mimicry
The formation by the tumor cells of blood vessel-like channels that contribute to tumor blood perfusion.
- “Dependence” receptors
Structurally unrelated receptors that can induce cell death by apoptosis when unoccupied by ligand, thus creating cellular dependence on their ligands. In the presence of ligand, these receptors mediate survival, differentiation or migration.
- Neutral liposomes
Small vesicles made of neutral phospholipids (such as DOPC, 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine), which can be filled with siRNA for efficient in vivo intracellular delivery to tumor tissue.
- RadioimmunoPET imaging
Positron emission tomography (PET) imaging using a radioactively labeled antibody. It allows non-invasive in vivo visualization of a tissue of interest, such as tumor tissue, that expresses the antigen as well as quantification of antigen levels.
- Gamma camera imaging
Imaging with a camera that detects radioisotopes emitting gamma radiation. It is also known as scintigraphy and allows non-invasive in vivo visualization of radioisotopes coupled, for example, to an antibody that targets tumor tissue.
- Epithelial-to-mesenchymal transition
A complex process in which genetic and epigenetic events lead to epithelial cells acquiring a mesenchymal architecture concomitant with increased cell motility. Typically associated with the loss of E-CADHERIN expression, disruption of cell-cell junctions, and cancer cell invasion and metastasis.
- Apico-basal polarization
Epithelial cells are polarized, with an apical membrane that faces the external environment or a lumen and is opposite the basolateral membrane, which functions in cell–cell interactions and contacts the basement membrane.
References
- 1.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- 3.Maru Y, Hirai H, Takaku F. Overexpression confers an oncogenic potential upon the eph gene. Oncogene. 1990;5:445–447. [PubMed] [Google Scholar]
- 4.Bartley TD, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- 5.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 6.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- 8.Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2009;41:762–770. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alford SC, Bazowski J, Lorimer H, Elowe S, Howard PL. Tissue transglutaminase clusters soluble A-type ephrins into functionally active high molecular weight oligomers. Exp Cell Res. 2007;313:4170–4179. doi: 10.1016/j.yexcr.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Wykosky J, et al. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–7273. doi: 10.1038/onc.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu C, Park S. The EphA8 Receptor Regulates Integrin Activity through p110{gamma} Phosphatidylinositol-3 Kinase in a Tyrosine Kinase Activity-Independent Manner. Mol. Cell. Biol. 2001;21:4579–4597. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuoka H, Obama H, Kelly ML, Matsui T, Nakamoto M. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J Biol Chem. 2005;280:29355–29363. doi: 10.1074/jbc.M500010200. [DOI] [PubMed] [Google Scholar]
- 13.Miao H, et al. Inhibition of Integrin-mediated Cell Adhesion but Not Directional Cell Migration Requires Catalytic Activity of EphB3 Receptor Tyrosine Kinase. J Biol Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- 14.Georgakopoulos A, et al. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. Embo J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 16.Litterst C, et al. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J Biol Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka M, Sasaki K, Kamata R, Sakai R. The C-terminus of ephrin-B1 regulates metalloproteinase secretion and invasion of cancer cells. J Cell Sci. 2007;120:2179–2189. doi: 10.1242/jcs.008607. [DOI] [PubMed] [Google Scholar]
- 18.Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J Cell Sci. 2000;113:1793–1802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
- 20.Zantek ND, et al. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth & Differentiation. 1999;10:629–638. [PubMed] [Google Scholar]
- 21.Yumoto N, et al. Meltrin beta/ADAM19 interacting with EphA4 in developing neural cells participates in formation of the neuromuscular junction. PLoS ONE. 2008;3:e3322. doi: 10.1371/journal.pone.0003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemp HA, Cooke JE, Moens CB. EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev Biol. 2009;327:313–326. doi: 10.1016/j.ydbio.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batlle E, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]; This is an important article demonstrating that Eph receptors can be upregulated during early stages of cancer progression and subsequently silenced to circumvent their tumor suppressor activity. This bimodal regulation may explain the contradictory reports of both increased and decreased Eph expression in cancer versus normal tissues.
- 24.Inoue E, et al. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, et al. ZHX2 Interacts with Ephrin-B and regulates neural progenitor maintenance in the developing cerebral cortex. J Neurosci. 2009;29:7404–7412. doi: 10.1523/JNEUROSCI.5841-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15:419–433. doi: 10.1016/j.cytogfr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- 28.Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9:1179–1187. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 29.Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- 30.Castano J, Davalos V, Schwartz S, Jr., Arango D. EPH receptors in cancer. Histol Histopathol. 2008;23:1011–1023. doi: 10.14670/HH-23.1011. [DOI] [PubMed] [Google Scholar]
- 31.Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–1806. doi: 10.1158/1541-7786.MCR-08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarron JK, Stringer BW, Day BW, Boyd AW. Ephrin expression and function in cancer. Future Oncol. 2010;6:165–176. doi: 10.2217/fon.09.146. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang G, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 2010;70:299–308. doi: 10.1158/0008-5472.CAN-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang F, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 35.Wang XD, et al. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: rationale for patient selection and efficacy monitoring. Genome Biol. 2007;8:R255. doi: 10.1186/gb-2007-8-11-r255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar SR, et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer. 2007;96:1083–1091. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a provocative study showing an opposite transcriptional regulation of the EPHB2 and EPHB4 receptors during human colorectal cancer progression, despite both receptors being under the transcriptional control of β-catenin and TCF.
- 37.Kumar SR, et al. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–3745. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- 38.Hafner C, Becker B, Landthaler M, Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod Pathol. 2006;19:1369–1377. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- 39.Herath NI, Doecke J, Spanevello MD, Leggett BA, Boyd AW. Epigenetic silencing of EphA1 expression in colorectal cancer is correlated with poor survival. Br J Cancer. 2009;100:1095–1102. doi: 10.1038/sj.bjc.6604970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alazzouzi H, et al. Mechanisms of inactivation of the receptor tyrosine kinase EPHB2 in colorectal tumors. Cancer Res. 2005;65:10170–10173. doi: 10.1158/0008-5472.CAN-05-2580. [DOI] [PubMed] [Google Scholar]
- 41.Davalos V, et al. EPHB4 and survival of colorectal cancer patients. Cancer Res. 2006;66:8943–8948. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- 42.Chiu ST, et al. Over-expression of EphB3 enhances cell-cell contacts and suppresses tumor growth in HT-29 human colon cancer cells. Carcinogenesis. 2009;30:1475–1486. doi: 10.1093/carcin/bgp133. [DOI] [PubMed] [Google Scholar]
- 43.Li JJ, Liu DP, Liu GT, Xie D. EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Oncogene. 2009;28:1759–1768. doi: 10.1038/onc.2009.15. [DOI] [PubMed] [Google Scholar]
- 44.Muller-Tidow C, et al. Identification of metastasis-associated receptor tyrosine kinases in non-small cell lung cancer. Cancer Res. 2005;65:1778–1782. doi: 10.1158/0008-5472.CAN-04-3388. [DOI] [PubMed] [Google Scholar]
- 45.Fu T, et al. c-Rel is a transcriptional repressor of EPHB2 in colorectal cancer. J Pathol. 2009;219:103–113. doi: 10.1002/path.2590. [DOI] [PubMed] [Google Scholar]
- 46.Macrae M, et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]; This study identifies the Abl-Crk pathway as a critical mediator of EPHB4-dependent tumor suppression. References 42 and 107 further characterize the involvement of Abl and/or Crk downstream of other Eph receptors.
- 48.Sulman EP, et al. ECK, a human EPH-related gene, maps to 1p36.1, a common region of alteration in human cancers. Genomics. 1997;40:371–374. doi: 10.1006/geno.1996.4569. [DOI] [PubMed] [Google Scholar]
- 49.Huusko P, et al. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer. Nat Genet. 2004;36:979–983. doi: 10.1038/ng1408. [DOI] [PubMed] [Google Scholar]
- 50.Kang JU, Koo SH, Kwon KC, Park JW, Kim JM. Identification of novel candidate target genes, including EPHB3, MASP1 and SST at 3q26.2–q29 in squamous cell carcinoma of the lung. BMC Cancer. 2009;9:237. doi: 10.1186/1471-2407-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter J, et al. Comparative 3'UTR analysis allows identification of regulatory clusters that drive Eph/ephrin expression in cancer cell lines. PLoS ONE. 2008;3:e2780. doi: 10.1371/journal.pone.0002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 53.Cheng N, et al. Blockade of EphA Receptor Tyrosine Kinase Activation Inhibits Vascular Endothelial Cell Growth Factor-Induced Angiogenesis. Mol Cancer Res. 2002;1:2–11. [PubMed] [Google Scholar]
- 54.Yamashita T, et al. Hypoxia-inducible transcription factor-2alpha in endothelial cells regulates tumor neovascularization through activation of ephrin A1. J Biol Chem. 2008;283:18926–18936. doi: 10.1074/jbc.M709133200. [DOI] [PubMed] [Google Scholar]
- 55.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 56.Pasquale EB. In: Modern concepts in angiogenesis. Simons M, Rubanyi G, editors. Imperial College Press; London: 2007. pp. 27–66. [Google Scholar]
- 57.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–151. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Korff T, Braun J, Pfaff D, Augustin HG, Hecker M. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood. 2008;112:73–81. doi: 10.1182/blood-2007-12-128835. [DOI] [PubMed] [Google Scholar]
- 59.Obi S, et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 2009;106:203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 60.Foo SS, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]; In this study, conditional deletion of EPHRIN-B2 in pericytes and vascular smooth muscle cells demonstrates a critical role of EPHRIN-B2 in the association of these cells with small diameter blood vessels and, therefore, in vessel integrity. This work extends previous studies implicating endothelial EPHRIN-B2 in vascular development.
- 61.Hafner C, et al. Differential gene expression of Eph receptors and Ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 62.Fasanaro P, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies H, et al. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005;65:7591–7595. doi: 10.1158/0008-5472.CAN-05-1855. [DOI] [PubMed] [Google Scholar]; This screen for somatic mutations in cancer specimens and cell lines, and those reported in references 64, 65, and 70, identified mutations of many genes in each sample examined. This suggests that mutations involving distinctive constellations of many genes, rather than mutations involving only a few genes as was previously believed, contribute to the malignant transformation of normal epithelial cells into cancer cells.
- 64.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 65.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruhe JE, et al. Genetic alterations in the tyrosine kinase transcriptome of human cancer cell lines. Cancer Res. 2007;67:11368–11376. doi: 10.1158/0008-5472.CAN-07-2703. [DOI] [PubMed] [Google Scholar]
- 67.Prickett TD, et al. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davalos V, et al. High EPHB2 mutation rate in gastric but not endometrial tumors with microsatellite instability. Oncogene. 2007;26:308–311. doi: 10.1038/sj.onc.1209780. [DOI] [PubMed] [Google Scholar]
- 69.Zogopoulos G, et al. Germline EPHB2 receptor variants in familial colorectal cancer. PLoS One. 2008;3:e2885. doi: 10.1371/journal.pone.0002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding L, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 72.Miao H, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article identifies a mechanism that converts the EPHA2 receptor from a tumor suppressor (when activated by its ligand EPHRIN-A1) to a tumor promoter (when phosphorylated by the serine/threonine kinase Akt). In a negative feedback loop, EPHA2 inhibits Akt when activated by EPHRIN-A1.
- 73.Dopeso H, et al. The receptor tyrosine kinase EPHB4 has tumor suppressor activities in intestinal tumorigenesis. Cancer Res. 2009;69:7430–7438. doi: 10.1158/0008-5472.CAN-09-0706. [DOI] [PubMed] [Google Scholar]
- 74.Noblitt LW, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 75.Hess AR, et al. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- 76.Wimmer-Kleikamp SH, et al. Elevated protein tyrosine phosphatase activity provokes Eph/ephrin-facilitated adhesion of pre-B leukemia cells. Blood. 2008;112:721–732. doi: 10.1182/blood-2007-11-121681. [DOI] [PubMed] [Google Scholar]
- 77.Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 Kinase by the Low Molecular Weight Tyrosine Phosphatase Induces Transformation. J. Biol. Chem. 2002;277:39274–39279. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 78.Shintani T, et al. Eph receptors are negatively controlled by protein tyrosine phosphatase receptor type O. Nat Neurosci. 2006;9:761–769. doi: 10.1038/nn1697. [DOI] [PubMed] [Google Scholar]
- 79.Poliakov A, Cotrina ML, Pasini A, Wilkinson DG. Regulation of EphB2 activation and cell repulsion by feedback control of the MAPK pathway. J Cell Biol. 2008;183:933–947. doi: 10.1083/jcb.200807151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brisbin S, et al. A role for C. elegans Eph RTK signaling in PTEN regulation. Dev Cell. 2009;17:459–469. doi: 10.1016/j.devcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 81.Smith FM, et al. Dissecting the EphA3/Ephrin-A5 interactions using a novel functional mutagenesis screen. J Biol Chem. 2004;279:9522–9531. doi: 10.1074/jbc.M309326200. [DOI] [PubMed] [Google Scholar]
- 82.Zisch AH, Pasquale EB. The Eph family: a multitude of receptors that mediate cell recognition signals. Cell & Tissue Research. 1997;290:217–226. doi: 10.1007/s004410050926. [DOI] [PubMed] [Google Scholar]
- 83.Jin P, et al. Novel splice variants derived from the receptor tyrosine kinase superfamily are potential therapeutics for rheumatoid arthritis. Arthritis Res Ther. 2008;10:R73. doi: 10.1186/ar2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsuda H, et al. The amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted, and acts as a ligand for Eph receptors. Cell. 2008;133:963–977. doi: 10.1016/j.cell.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo H, et al. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 86.Cortina C, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]; This article, together with reference 23, demonstrates that repulsive interactions between Eph receptors expressed in tumor tissue and ephrin ligands expressed in the surrounding normal tissue can have powerful tumor suppressor effects by restricting tumor invasion and expansion.
- 87.Lee HS, Nishanian TG, Mood K, Bong YS, Daar IO. EphrinB1 controls cell-cell junctions through the Par polarity complex. Nat Cell Biol. 2008;10:979–986. doi: 10.1038/ncb1758. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that ephrin-B phosphorylation, due to reverse signaling or interplay with growth factor receptors, regulates the integrity of epithelial cell-cell junctions.
- 88.Jorgensen C, et al. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]; This study using integrative network biology approaches is the first to analyze overall signaling networks that are modulated in cells expressing EPHB2 and EPHRIN-B1 that come in contact with each other. The results show that the bidirectional networks that regulate segregation of the two cell populations are asymmetric and sensitive to stimulating conditions, and regulate multiple cellular processes to achieve the repulsive outcome. This work opens the way for similar studies to investigate endogenous Eph receptors and ephrins.
- 89.Almog N, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 90.Leroy C, et al. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–2286. doi: 10.1158/0008-5472.CAN-08-2354. [DOI] [PubMed] [Google Scholar]
- 91.Genander M, et al. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 93.Yang NY, Pasquale EB, Owen LB, Ethell IM. The EphB4 Receptor-tyrosine Kinase Promotes the Migration of Melanoma Cells through Rho-mediated Actin Cytoskeleton Reorganization. J Biol Chem. 2006;281:32574–32586. doi: 10.1074/jbc.M604338200. [DOI] [PubMed] [Google Scholar]
- 94.Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P. EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res. 2009;69:2072–2081. doi: 10.1158/0008-5472.CAN-08-1845. [DOI] [PubMed] [Google Scholar]
- 95.Wang JY, et al. Functional significance of VEGF-a in human ovarian carcinoma: role in vasculogenic mimicry. Cancer Biol Ther. 2008;7:758–766. doi: 10.4161/cbt.7.5.5765. [DOI] [PubMed] [Google Scholar]
- 96.Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem J. 2009;422:433–442. doi: 10.1042/BJ20090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yokote H, et al. Trans-activation of EphA4 and FGF receptors mediated by direct interactions between their cytoplasmic domains. Proc Natl Acad Sci U S A. 2005;102:18866–18871. doi: 10.1073/pnas.0509741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fukai J, et al. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7:2768–2778. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- 100.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody Targeting of the EphA2 Tyrosine Kinase Inhibits Malignant Cell Behavior. Cancer Res. 2002;62:2840–2847. [PubMed] [Google Scholar]
- 101.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 102.Landen CN, Jr., et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]; This article reports the optimization of liposomes for efficient siRNA delivery to tumors and uses this technology to demonstrate that downregulation of EPHA2 in ovarian cancer xenografts enhances the therapeutic effects of the drug paclitaxel.
- 103.Fang WB, et al. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–368. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- 104.Larsen AB, et al. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol Cancer Res. 2007;5:283–293. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- 105.Brantley-Sieders DM, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses transgenic mouse models of mammary tumorigenesis to show that crosstalk with EPHA2 enhances the tumorigenic effects of the ERBB2 receptor but not of polyomavirus middle T antigen.
- 106.Vaught D, Chen J, Brantley-Sieders DM. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell. 2009;20:2572–2581. doi: 10.1091/mbc.E08-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol. 2008;183:711–723. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Furne C, et al. EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim Biophys Acta. 2009;1793:231–238. doi: 10.1016/j.bbamcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iida H, et al. Ephrin-A1 expression contributes to the malignant characteristics of {alpha}-fetoprotein producing hepatocellular carcinoma. Gut. 2005;54:843–851. doi: 10.1136/gut.2004.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Campbell TN, Attwell S, Arcellana-Panlilio M, Robbins SM. Ephrin A5 expression promotes invasion and transformation of murine fibroblasts. Biochem Biophys Res Commun. 2006;350:623–628. doi: 10.1016/j.bbrc.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka M, Kamata R, Sakai R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell-cell contact formation. Embo J. 2005;24:3700–3711. doi: 10.1038/sj.emboj.7600831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakada M, Drake KL, Nakada S, Niska JA, Berens ME. Ephrin-B3 Ligand Promotes Glioma Invasion through Activation of Rac1. Cancer Res. 2006;66:8492–8500. doi: 10.1158/0008-5472.CAN-05-4211. [DOI] [PubMed] [Google Scholar]
- 113.Jiang G, et al. In human leukemia cells ephrin-B-induced invasive activity is supported by Lck and is associated with reassembling of lipid raft signaling complexes. Mol Cancer Res. 2008;6:291–305. doi: 10.1158/1541-7786.MCR-07-0047. [DOI] [PubMed] [Google Scholar]
- 114.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meyer S, et al. Ephrin-B2 overexpression enhances integrin-mediated ECM-attachment and migration of B16 melanoma cells. Int J Oncol. 2005;27:1197–1206. [PubMed] [Google Scholar]
- 116.Nakada M, et al. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int J Cancer. 2010;126:1155–1165. doi: 10.1002/ijc.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tanaka M, Kamata R, Takigahira M, Yanagihara K, Sakai R. Phosphorylation of ephrin-B1 regulates dissemination of gastric scirrhous carcinoma. Am J Pathol. 2007;171:68–78. doi: 10.2353/ajpath.2007.070033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bong YS, et al. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc Natl Acad Sci U S A. 2007;104:17305–17310. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- 120.Okazaki T, et al. Capillary defects and exaggerated inflammatory response in the airways of EphA2-deficient mice. Am J Pathol. 2009;174:2388–2399. doi: 10.2353/ajpath.2009.080949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L431–439. doi: 10.1152/ajplung.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ogawa K, et al. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 123.Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–10324. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 124.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Herbert SP, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Erber R, et al. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. Embo J. 2006;25:628–641. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Noren NK, Lu M, Freeman AL, Koolpe M, Pasquale EB. Interplay between EphB4 on tumor cells and vascular ephrin-B2 regulates tumor growth. Proc Natl Acad Sci U S A. 2004;101:5583–5588. doi: 10.1073/pnas.0401381101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salvucci O, et al. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang J, et al. Vascular remodeling marks tumors that recur during chronic suppression of angiogenesis. Mol Cancer Res. 2004;2:36–42. [PubMed] [Google Scholar]
- 130.Foubert P, et al. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pfaff D, et al. Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J Cell Sci. 2008;121:3842–3850. doi: 10.1242/jcs.030627. [DOI] [PubMed] [Google Scholar]
- 132.Miyazaki Y, et al. Design and effective synthesis of novel templates, 3,7-diphenyl-4-aminothieno and furo-[3,2-c]pyridines as protein kinase inhibitors and in vitro evaluation targeting angiogenetic kinases. Bioorg Med Chem Lett. 2007;17:250–254. doi: 10.1016/j.bmcl.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 133.Bardelle C, et al. Inhibitors of the tyrosine kinase EphB4. Part 2: structure-based discovery and optimisation of 3,5-bis substituted anilinopyrimidines. Bioorg Med Chem Lett. 2008;18:5717–5721. doi: 10.1016/j.bmcl.2008.09.087. [DOI] [PubMed] [Google Scholar]
- 134.Choi Y, et al. Discovery and structural analysis of Eph receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009;19:4467–4470. doi: 10.1016/j.bmcl.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lafleur K, Huang D, Zhou T, Caflisch A, Nevado C. Structure-based optimization of potent and selective inhibitors of the tyrosine kinase erythropoietin producing human hepatocellular carcinoma receptor B4 (EphB4) J Med Chem. 2009;52:6433–6446. doi: 10.1021/jm9009444. [DOI] [PubMed] [Google Scholar]
- 136.Qiao L, et al. Structure-activity relationship study of EphB3 receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009;19:6122–6126. doi: 10.1016/j.bmcl.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]; Screen of a panel of 317 kinases (representing approximately half of the predicted human kinome) to determine the selectivity of 38 kinase inhibitors. Most inhibitors had previously been screened only against a limited subset of kinases and had therefore a poorly characterized selectivity.
- 138.Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074–1082. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shahzad MM, et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther. 2009;8:1027–1034. doi: 10.4161/cbt.8.11.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Annamalai B, Liu X, Gopal U, Isaacs JS. Hsp90 is an essential regulator of EphA2 receptor stability and signaling: implications for cancer cell migration and metastasis. Mol Cancer Res. 2009;7:1021–1032. doi: 10.1158/1541-7786.MCR-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawabe M, et al. Heat shock protein 90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin enhances EphA2+ tumor cell recognition by specific CD8+ T cells. Cancer Res. 2009;69:6995–7003. doi: 10.1158/0008-5472.CAN-08-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scehnet JS, et al. The role of Ephs, Ephrins, and growth factors in Kaposi sarcoma and implications of EphrinB2 blockade. Blood. 2009;113:254–263. doi: 10.1182/blood-2008-02-140020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mao W, et al. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004;64:781–788. doi: 10.1158/0008-5472.can-03-1047. [DOI] [PubMed] [Google Scholar]
- 144.Xu Z, Jin H, Qian Q. Humanized anti-EphB4 antibodies for the treatment of carcinomas and vasculogenesis-related diseases. Expert Opin Ther Pat. 2009;19:1035–1037. doi: 10.1517/13543770902835525. [DOI] [PubMed] [Google Scholar]
- 145.Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem. 2005;280:17301–17311. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]; This study reports the identification by phage display of peptides that inhibit ephrin binding to several EphB receptors. Some of these peptides selectively target an individual EphB receptor, unlike the promiscuous ephrin-B ligands, and an optimized peptide inhibits ephrin binding to EPHB4 at low nanomolar concentrations.
- 146.Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974–46979. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- 147.Murai KK, et al. Targeting the EphA4 receptor in the nervous system with biologically active peptides. Mol Cell Neurosci. 2003;24:1000–1011. doi: 10.1016/j.mcn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 148.Chrencik JE, et al. Structure and thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321–330. doi: 10.1016/j.str.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 149.Chrencik JE, et al. Three-dimensional Structure of the EphB2 Receptor in Complex with an Antagonistic Peptide Reveals a Novel Mode of Inhibition. J Biol Chem. 2007;282:36505–36513. doi: 10.1074/jbc.M706340200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Noberini R, et al. Small Molecules Can Selectively Inhibit Ephrin Binding to the EphA4 and EphA2 Receptors. J Biol Chem. 2008;283:29461–29472. doi: 10.1074/jbc.M804103200. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report identifying small molecules that target Eph receptors and inhibit ephrin binding and biological effects.
- 151.Qin H, Shi J, Noberini R, Pasquale EB, Song J. Crystal Structure and NMR Binding Reveal That Two Small Molecule Antagonists Target the High Affinity Ephrin-binding Channel of the EphA4 Receptor. J Biol Chem. 2008;283:29473–29484. doi: 10.1074/jbc.M804114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coffman KT, et al. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63:7907–7912. [PubMed] [Google Scholar]
- 153.Landen CN, Jr., et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 154.Wesa AK, et al. Enhancement in specific CD8+ T cell recognition of EphA2+ tumors in vitro and in vivo after treatment with ligand agonists. J Immunol. 2008;181:7721–7727. doi: 10.4049/jimmunol.181.11.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bruckheimer EM, et al. Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors. Neoplasia. 2009;11:509–517. doi: 10.1593/neo.81578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kiewlich D, et al. Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth. Neoplasia. 2006;8:18–30. doi: 10.1593/neo.05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jackson D, et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 2008;68:9367–9374. doi: 10.1158/0008-5472.CAN-08-1933. [DOI] [PubMed] [Google Scholar]; This study shows that an antibody-drug conjugate that targets EPHA2 inhibits tumor growth in rodent cancer models without any evident toxic effects.
- 158.Lee JW, et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2009;101:1193–1205. doi: 10.1093/jnci/djp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine. 2008;3:351–358. [PMC free article] [PubMed] [Google Scholar]
- 160.Cai W, et al. Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur J Nucl Med Mol Imaging. 2007;34:2024–2036. doi: 10.1007/s00259-007-0503-5. [DOI] [PubMed] [Google Scholar]
- 161.Vearing C, et al. Concurrent binding of anti-EphA3 antibody and ephrin-A5 amplifies EphA3 signaling and downstream responses: potential as EphA3-specific tumor-targeting reagents. Cancer Res. 2005;65:6745–6754. doi: 10.1158/0008-5472.CAN-05-0758. [DOI] [PubMed] [Google Scholar]
- 162.Hammond SA, et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer Res. 2007;67:3927–3935. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- 163.Chiari R, et al. Identification of a tumor-specific shared antigen derived from an Eph receptor and presented to CD4 T cells on HLA class II molecules. Cancer Res. 2000;60:4855–4863. [PubMed] [Google Scholar]
- 164.Tatsumi T, et al. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–4489. [PubMed] [Google Scholar]
- 165.Alves PM, et al. EphA2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res. 2003;63:8476–8480. [PubMed] [Google Scholar]
- 166.Jin M, et al. Erythropoietin-producing hepatocyte B6 variant-derived peptides with the ability to induce glioma-reactive cytotoxic T lymphocytes in human leukocyte antigen-A2+ glioma patients. Cancer Sci. 2008;99:1656–1662. doi: 10.1111/j.1349-7006.2008.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hatano M, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamaguchi S, et al. Immunotherapy of murine colon cancer using receptor tyrosine kinase EphA2-derived peptide-pulsed dendritic cell vaccines. Cancer. 2007;110:1469–1477. doi: 10.1002/cncr.22958. [DOI] [PubMed] [Google Scholar]
- 169.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they're apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol Biol Cell. 2009;20:1949–1959. doi: 10.1091/mbc.E08-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Miao H, et al. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J Cell Biol. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barrios A, et al. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 173.Cooper MA, et al. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc Natl Acad Sci U S A. 2008;105:16620–16625. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jun G, et al. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 2009;5:e1000584. doi: 10.1371/journal.pgen.1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 176.Kalo MS, Pasquale EB. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry. 1999;38:14396–14408. doi: 10.1021/bi991628t. [DOI] [PubMed] [Google Scholar]
- 177.Lim YS, et al. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Miao H, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 179.Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–2940. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- 180.Dail M, Richter M, Godement P, Pasquale EB. Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J Cell Sci. 2006;119:1244–1254. doi: 10.1242/jcs.02842. [DOI] [PubMed] [Google Scholar]
- 181.Nie D, et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. doi: 10.1038/nn.2477. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhuang G, Hunter S, Hwang Y, Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J Biol Chem. 2007;282:2683–2694. doi: 10.1074/jbc.M608509200. [DOI] [PubMed] [Google Scholar]
- 183.Takeuchi S, Yamaki N, Iwasato T, Negishi M, Katoh H. Beta2-chimaerin binds to EphA receptors and regulates cell migration. FEBS Lett. 2009;583:1237–1242. doi: 10.1016/j.febslet.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 184.Yamazaki T, et al. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci. 2009;122:243–255. doi: 10.1242/jcs.036467. [DOI] [PubMed] [Google Scholar]
- 185.Lai KO, et al. Identification of the Jak/Stat proteins as novel downstream targets of EphA4 signaling in muscle: implications in the regulation of acetylcholinesterase expression. J Biol Chem. 2004;279:13383–13392. doi: 10.1074/jbc.M313356200. [DOI] [PubMed] [Google Scholar]
- 186.Hunter SG, et al. Essential Role of Vav Family Guanine Nucleotide Exchange Factors in EphA Receptor-Mediated Angiogenesis. Mol Cell Biol. 2006;26:4830–4842. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Frohling S, Dohner H. Chromosomal abnormalities in cancer. N Engl J Med. 2008;359:722–734. doi: 10.1056/NEJMra0803109. [DOI] [PubMed] [Google Scholar]
- 188.Oba SM, et al. Genomic structure and loss of heterozygosity of EPHB2 in colorectal cancer. Cancer Letters. 2001;164:97–104. doi: 10.1016/s0304-3835(00)00716-3. [DOI] [PubMed] [Google Scholar]
- 189.Laiho P, et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene. 2007;26:312–320. doi: 10.1038/sj.onc.1209778. [DOI] [PubMed] [Google Scholar]
- 190.Ikegaki N, et al. Molecular characterization and chromosomal localization of DRT (EPHT3): a developmentally regulated human protein-tyrosine kinase gene of the EPH family. Hum Mol Genet. 1995;4:2033–2045. doi: 10.1093/hmg/4.11.2033. [DOI] [PubMed] [Google Scholar]
- 191.Narayan G, et al. Genetic analysis identifies putative tumor suppressor sites at 2q35–q36.1 and 2q36.3–q37.1 involved in cervical cancer progression. Oncogene. 2003;22:3489–3499. doi: 10.1038/sj.onc.1206432. [DOI] [PubMed] [Google Scholar]
- 192.Kasahara K, et al. Detection of genetic alterations in advanced prostate cancer by comparative genomic hybridization. Cancer Genet Cytogenet. 2002;137:59–63. doi: 10.1016/s0165-4608(02)00552-6. [DOI] [PubMed] [Google Scholar]
- 193.Sinha UK, et al. The association between elevated EphB4 expression, smoking status, and advanced-stage disease in patients with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1053–1059. doi: 10.1001/archotol.132.10.1053. [DOI] [PubMed] [Google Scholar]
- 194.Xia G, et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 195.Yang TL, et al. High-resolution 19p13.2–13.3 allelotyping of breast carcinomas demonstrates frequent loss of heterozygosity. Genes Chromosomes Cancer. 2004;41:250–256. doi: 10.1002/gcc.20080. [DOI] [PubMed] [Google Scholar]
- 196.Dottori M, Down M, Huttmann A, Fitzpatrick DR, Boyd AW. Cloning and characterization of EphA3 (Hek) gene promoter: DNA methylation regulates expression in hematopoietic tumor cells. Blood. 1999;94:2477–2486. [PubMed] [Google Scholar]
- 197.Guan M, Xu C, Zhang F, Ye C. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int J Cancer. 2009;124:88–94. doi: 10.1002/ijc.23890. [DOI] [PubMed] [Google Scholar]
- 198.Wang J, et al. Differential expression of EphA7 receptor tyrosine kinase in gastric carcinoma. Hum Pathol. 2007;38:1649–1656. doi: 10.1016/j.humpath.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 199.Wang J, et al. Downregulation of EphA7 by hypermethylation in colorectal cancer. Oncogene. 2005;24:5637–5647. doi: 10.1038/sj.onc.1208720. [DOI] [PubMed] [Google Scholar]
- 200.Dawson DW, et al. Global DNA methylation profiling reveals silencing of a secreted form of Epha7 in mouse and human germinal center B-cell lymphomas. Oncogene. 2007;26:4243–4252. doi: 10.1038/sj.onc.1210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nosho K, et al. Genetic and epigenetic profiling in early colorectal tumors and prediction of invasive potential in pT1 (early invasive) colorectal cancers. Carcinogenesis. 2007;28:1364–1370. doi: 10.1093/carcin/bgl246. [DOI] [PubMed] [Google Scholar]
- 202.Fox BP, Kandpal RP. Transcriptional silencing of EphB6 receptor tyrosine kinase in invasive breast carcinoma cells and detection of methylated promoter by methylation specific PCR. Biochem Biophys Res Commun. 2006;340:268–276. doi: 10.1016/j.bbrc.2005.11.174. [DOI] [PubMed] [Google Scholar]
- 203.Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008;582:2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 204.Dohn M, Jiang JY, Chen XB. Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20:6503–6515. doi: 10.1038/sj.onc.1204816. [DOI] [PubMed] [Google Scholar]
- 205.Jin YJ, et al. A novel mechanism for p53 to regulate its target gene ECK in signaling apoptosis. Mol Cancer Res. 2006;4:769–778. doi: 10.1158/1541-7786.MCR-06-0178. [DOI] [PubMed] [Google Scholar]
- 206.Yu J, et al. Identification and classification of p53-regulated genes. Proc Natl Acad Sci U S A. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.van Doorn R, et al. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 208.Ting MC, et al. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development. 2009;136:855–864. doi: 10.1242/dev.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Batlle E, et al. beta-Catenin and TCF Mediate Cell Positioning in the Intestinal Epithelium by Controlling the Expression of EphB/EphrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 210.Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrinB2 during mammary gland morphogenesis. J Cell Sci. 1998;111:2741–2751. doi: 10.1242/jcs.111.18.2741. [DOI] [PubMed] [Google Scholar]
- 211.Bardelle C, et al. Inhibitors of the tyrosine kinase EphB4. Part 1: Structure-based design and optimization of a series of 2,4-bis-anilinopyrimidines. Bioorg Med Chem Lett. 2008;18:2776–2780. doi: 10.1016/j.bmcl.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 212.Gendreau SB, et al. Inhibition of the T790M gatekeeper mutant of the epidermal growth factor receptor by EXEL-7647. Clin Cancer Res. 2007;13:3713–3723. doi: 10.1158/1078-0432.CCR-06-2590. [DOI] [PubMed] [Google Scholar]
- 213.Kolb P, Kipouros CB, Huang D, Caflisch A. Structure-based tailoring of compound libraries for high-throughput screening: discovery of novel EphB4 kinase inhibitors. Proteins. 2008;73:11–18. doi: 10.1002/prot.22028. [DOI] [PubMed] [Google Scholar]
- 214.Caligiuri M, et al. MASPIT: three-hybrid trap for quantitative proteome fingerprinting of small molecule-protein interactions in mammalian cells. Chem Biol. 2006;13:711–722. doi: 10.1016/j.chembiol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 215.Melnick JS, et al. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc Natl Acad Sci U S A. 2006;103:3153–3158. doi: 10.1073/pnas.0511292103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kumar SR, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xia G, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25:769–780. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 218.Dobrzanski P, et al. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–919. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- 219.Brantley DM, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 220.Cheng N, et al. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445–456. doi: 10.1016/s1476-5586(03)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Martiny-Baron G, et al. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia. 2004;6:248–257. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kertesz N, et al. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107:2330–2338. doi: 10.1182/blood-2005-04-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fabes J, Anderson P, Brennan C, Bolsover S. Regeneration-enhancing effects of EphA4 blocking peptide following corticospinal tract injury in adult rat spinal cord. Eur J Neurosci. 2007;26:2496–2505. doi: 10.1111/j.1460-9568.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 224.Salvucci O, et al. EphrinB reverse signaling contributes to endothelial and mural cell assembly into vascular structures. Blood. 2009 doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.van Geer MA, et al. Ephrin A2 receptor targeting does not increase adenoviral pancreatic cancer transduction in vivo. World J Gastroenterol. 2009;15:2754–2762. doi: 10.3748/wjg.15.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–3218. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 227.Scarberry KE, Dickerson EB, McDonald JF, Zhang ZJ. Magnetic nanoparticle-peptide conjugates for in vitro and in vivo targeting and extraction of cancer cells. J Am Chem Soc. 2008;130:10258–10262. doi: 10.1021/ja801969b. [DOI] [PubMed] [Google Scholar]
- 228.Scarberry KE, Dickerson EB, Zhang ZJ, Benigno BB, McDonald JF. Selective removal of ovarian cancer cells from human ascites fluid using magnetic nanoparticles. Nanomedicine. doi: 10.1016/j.nano.2009.11.003. in press. [DOI] [PubMed] [Google Scholar]