Abstract
Background
Patients identified with sepsis in the emergency department often are treated on the basis of the presumption of infection; however, various noninfectious conditions that require specific treatments have clinical presentations very similar to that of sepsis. Our aim was to describe the etiology of illness in patients identified and treated for severe sepsis in the emergency department.
Methods
We conducted a prospective observational study of patients treated with goal-directed resuscitation for severe sepsis in the emergency department. Inclusion criteria were suspected infection, 2 or more criteria for systemic inflammation, and evidence of hypoperfusion. Exclusion criteria were age of <18 years and the need for immediate surgery. Clinical data on eligible patients were prospectively collected for 2 years. Blinded observers used a priori definitions to determine the final cause of hospitalization.
Results
In total, 211 patients were enrolled; 95 (45%) had positive culture results, and 116 (55%) had negative culture results. The overall mortality rate was 19%. Patients with positive culture results were more likely to have indwelling vascular lines (P = .03) be residents of nursing homes (P = .04), and have a shorter time to administration of antibiotics in the emergency department (83 vs 97 min; P = .03). Of patients with negative culture results, 44% had clinical infections, 8% had atypical infections, 32% had noninfectious mimics, and 16% had an illness of indeterminate etiology.
Conclusion
In this study, we found that >50% of patients identified and treated for severe sepsis in the emergency department had negative culture results. Of patients identified with a sepsis syndrome at presentation, 18% had a noninfectious diagnosis that mimicked sepsis, and the clinical characteristics of these patients were similar to those of patients with culture-positive sepsis.
Sepsis is the 10th leading cause of death in the United States, with estimates indicating that ~750,000 patients will be hospitalized with sepsis in the United States annually [1, 2]. The rate of hospitalizations due to severe sepsis doubled during the last decade, and with a present mortality rate of 30%, recent estimates have indicated that the age-adjusted population-based mortality is increasing [2, 3]. All indicators suggest that the incidence of hospitalizations due to sepsis will increase and is projected to be >1 million per year in the near future [2].
The diagnosis of sepsis is made on the basis of the presence of inflammatory response indicators in the setting of suspected or confirmed infection. The sepsis syndromes are a continuum of a disease process that progresses from sepsis (infection with an inflammatory response) to severe sepsis (sepsis with organ dysfunction) to septic shock (sepsis with tissue hypoperfusion). The criteria used by clinicians to define the stages of sepsis were initially developed in 1991 and were revised in 2001 by a group of experts convened by the North American and European intensive care societies [4, 5]. It is important to recognize that the criteria by which sepsis syndrome diagnoses are made rely on the suspicion of infection, and confirmation of infection is often not possible for several days after patient presentation and diagnosis. One of the conclusions from the proceedings of the 2001 conference was to underscore the challenges that both clinicians and researchers still face in making an accurate diagnosis of sepsis in the present day [5].
Recent evidence has suggested that an outcome benefit is associated with early diagnosis and structured resuscitation of severe sepsis and septic shock [6]. As a result, many institutions have reported favorable results associated with the development and implementation of early management protocols that seek to identify and treat patients with severe sepsis during the earliest stages of presentation, such as in the emergency department [7–10]. As the emphasis of sepsis care has moved to the more proximal phases of hospital care, it is possible that many patients are treated for sepsis on the basis of suspicion of infection, raising the possibility of including critically ill patients with alternate etiologies in sepsis-management pathways. Accordingly, the aim of the present study was to describe both the final etiology of illness and hospital outcomes among patients identified and treated with protocolized resuscitation for severe sepsis in the emergency department.
METHODS
Study design and setting
This study was a secondary analysis of a prospectively collected registry of patients treated with an institutional standard-of-care protocol for early quantitative resuscitation of severe sepsis. The registry was compiled from patients identified and treated for severe sepsis in the emergency department; the methodology has been reported elsewhere [7]. The Institutional Review Board and Privacy Board of Carolinas Healthcare System approved this study.
Selection of patients and data collection
Patient enrollment took place from November 2005 through October 2007 in the emergency department of Carolinas Medical Center, an urban, 850-bed, tertiary teaching hospital with >110,000 emergency department visits per year. The emergency department is staffed by emergency medicine residents supervised by board-certified emergency physicians. Eligible patients met the following criteria: (1) suspected infection; (2) two or more systemic inflammatory response syndrome (SIRS) criteria [4]; and (3) hypoperfusion, defined as systolic blood pressure of <90 mm Hg after a 20 cc/kg fluid challenge or as a lactate level of ≥4 mmol/L. We excluded patients who required immediate surgical intervention. In all cases, the emergency department physicians and staff identified the patients, initiated the resuscitation protocol, placed the central venous catheter, and followed the protocol until a bed in the intensive care unit was available for patient transfer. A standing standardized institutional protocol was provided to clinicians as a guide for antimicrobial therapy; however, clinicians were able to deviate from the protocol at their discretion. At the time of patient transfer from the emergency department to the intensive care unit, clinical care was transferred from the emergency department physicians to the admitting physicians.
Data elements collected at enrollment included demographic information, physiological variables, comorbidities, laboratory measurements, suspected source of infection, previous antibiotic use, and severity of illness in the form of the sequential organ failure assessment (SOFA) score [11], and administered treatments.
The criterion standard final diagnosis was established using a predefined, structured method of medical record review that was developed before the study was begun. This plan required a 2-step process in an attempt to ensure maximal accuracy of the assigned diagnoses. In the first step, independent physician observers (A.C.H. and J.M.H.) reviewed hospitalization records and completed case report forms that required categorization of patients into 1 of 2 groups: culture positive or culture negative. The culture-positive group required at least 1 of the following criteria based on consensus criteria [12]: (1) that a bacterial or fungal pathogen be isolated from a normally sterile site by routine clinical culture, including blood, peritoneal or pleural fluid, cerebrospinal fluid, surgical specimen, or synovial fluid; (2) that a bacterial pathogen be isolated from sputum culture derived from forced sputum production, tracheal aspiration, or bronchoaveolar lavage; or (3) a bacterial pathogen isolated from a urine specimen with bacterial growth of ≥1 × 103 colony-forming units/mL. If there was disagreement between the 2 physician observers in the first step, a third observer (A.E.J.), who was blinded to the categorization of the first 2 observers, adjudicated the case.
The second step of the categorization process focused on the patients in the culture-negative group. Because some infections can be supported by clinical and other ancillary diagnostic data or atypical diagnostic modalities (eg, polymerase chain reaction or enzyme-linked immunosorbent serologic assay), a process similar to step 1 (ie, initial review by 2 independent physicians and adjudication by a blinded third observer) was used to further categorize the patients with negative culture results into 1 of 4 groups. Group 1 comprised clinical infections, defined as cases with a clinical history and course suspicious for an primary infectious etiology that was supported by radiological, laboratory (without supportive Gram stain or culture data), or surgical findings in which the patient responded to a full course of antimicrobial therapy and no alternative diagnosis could explain the clinical manifestations (examples include pneumonia or cellulitis). Group 2 comprised atypical infections, defined as cases with a clinical history and course suspicious for a primary infectious etiology and a positive specialty diagnostic confirmation (examples include influenza or tuberculosis). Group 3 comprised noninfectious mimics, which included cases with a clinical history and course that met predefined consensus definitions (examples include adrenal insufficiency or acute myocardial infarction). Group 4 comprised indeterminate cases, defined as those in which an infection source was unclear (such that an infection was possible but not clearly identified according to any of the above-described criteria [eg, gastroenteritis]) or in which a multifactorial etiology was identified (defined as a case in which an infection was not suspected according to the above-described criteria but in which a single alternative etiology could not sufficiently explain the clinical presentation).
Explicit criteria for each potential criterion standard final diagnosis were compiled from widely accepted published definitions or were adapted from specialty textbooks, using a process we have described elsewhere [13]. The explicit criteria were also reviewed by and represented a consensus of 2 board-certified emergency physicians with >5 years of experience, 1 critical care specialist, and 1 infectious disease specialist.
Data analysis
Continuous data are presented as means ± standard deviations or medians and interquartile ranges and when appropriate were compared for statistical differences using the unpaired t or Mann-Whitney U test. Categorical data are reported as proportions rounded to the nearest whole number and where applicable were tested for significance using the χ2 or Fisher exact test. For all statistical tests, P ≤ .05 was considered to indicate significance.
RESULTS
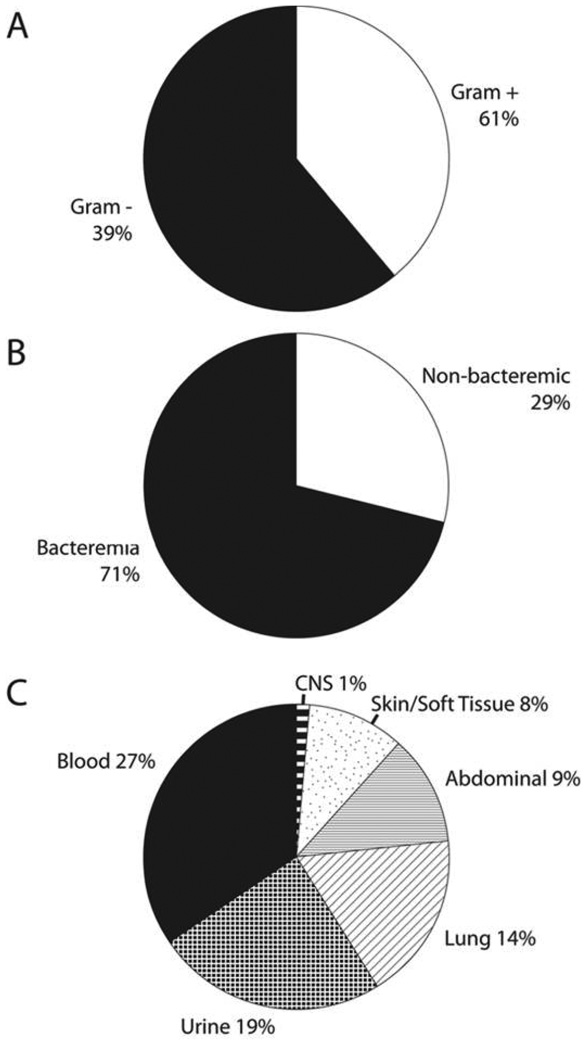
We enrolled 211 patients. Of these patients, 95 (45%) were positive by culture, and 116 (55%) were negative by culture. Figure 1 shows the classification of the culture-positive group according to microbiological appearance and source of infection. Table 1 shows the organisms isolated from blood cultures. Methicillin-resistant Staphylococcus areus accounted for 18% of episodes of bacteremia.
Figure 1.
Classification of the culture-positive group, by Gram stain appearance (A), blood involvement (B), and source of infection (C). CNS, central nervous system.
Table 1.
Organisms Isolated from Blood Cultures
| Organism | No. isolated (n = 67) |
|---|---|
| Bacteroides fragilis | 3 |
| Candida albicans | 1 |
| Enterobacter cloacae | 1 |
| Enterococcus faecalis | 7 |
| Enterococcus cassel | 1 |
| Escherichia coli | 12 |
| Klebsiella pneumoniae | 3 |
| Peptostreptococcus | 1 |
| Proteus mirabilis | 1 |
| Pseudomonas aeruginosa | 3 |
| Serratia marcescens | 1 |
| Staphylococcus aureus (MR) | 12 |
| Staphylococcus aureus (MS) | 8 |
| Staphylococcus epidermidis | 3 |
| Streptococcus pneumoniae | 5 |
| Streptococcus group A | 1 |
| Streptococcus group B | 2 |
| Streptococcus group G | 1 |
| Streptococcus viridans | 1 |
NOTE. MR, methicillin resistant; MS, methicillin susceptible.
Table 2 shows the initial clinical and demographic characteristics of the groups. Patients in the culture-positive group were more likely to have indwelling vascular lines and active malignancy and to be residents of nursing homes. There were no significant differences in severity of illness between the groups according to vital signs, lactate levels, or SOFA scores. Table 3 shows the initial resuscitation interventions. There were no significant differences in the treatments administered between the groups with the exception of a significantly shorter time to antibiotic administration in the culture-positive group (83 vs 97 min).
Table 2.
Initial Clinical and Demographic Information
| Variable | Positive group (n = 95) |
Negative group (n = 116) |
P |
|---|---|---|---|
| Mean age ± SD, years | 59 ± 19 | 55 ± 17 | .09 |
| Race | |||
| White | 47 (50) | 65 (56) | .35 |
| Black | 43 (45) | 43 (37) | .23 |
| Sex | |||
| Male | 52 (55) | 51 (44) | .12 |
| Female | 43 (45) | 65 (56) | .12 |
| Comorbidities | |||
| Diabetes mellitus | 25 (26) | 29 (25) | .83 |
| COPD | 16 (17) | 25 (22) | .40 |
| HIV infection | 10 (11) | 15 (13) | .60 |
| End-stage renal disease | 14 (15) | 14 (12) | .58 |
| Active malignancy | 21 (22) | 13 (11) | .04 |
| Organ transplant | 1 (1) | 3 (3) | .48 |
| Indwelling vascular line | 18 (19) | 10 (9) | .03 |
| Nursing home resident | 24 (25) | 16 (14) | .04 |
| Do not resuscitate | 3 (3) | 2 (2) | .54 |
| Previous antibiotics | 20 (21) | 23 (20) | .86 |
| ED vital signs | |||
| Mean lowest SBP ± SD, mm Hg | 71 ± 15 | 73 ± 17 | .33 |
| Mean highest PR ± SD, beats/min | 122 ± 27 | 118 ± 23 | .27 |
| Mean highest RR ± SD, breaths/min | 31 ± 12 | 30 ± 10 | .39 |
| Median highest temperature (IQR), °C | 38.3 (36.7–39.3) | 38.3 (37–39) | .92 |
| Mean lowest O2 saturation level ± SD, % | 92.9 ± 6.9 | 91.0 ± 7.5 | .07 |
| Median lowest CVP (IQR), mm Hg | 6 (4–9) | 6 (3–9) | .30 |
| Median highest CVP (IQR), mm Hg | 13 (10–15) | 11 (9–16) | .99 |
| Mean lowest ScvO2 level ± SD, % | 65 ± 13 | 67 ± 11 | .20 |
| Mean highest ScvO2 level ± SD, % | 73 ± 13 | 76 ± 13 | .17 |
| Median ED SOFA score (IQR) | 6 (3–11) | 7 (4–8.5) | .97 |
| Mean lactate level ± SD, mmol/L | 4.1 ± 3.7 | 3.7 ± 3.2 | .45 |
| Suspected infection source in ED | |||
| Pulmonary | 33 (35) | 57 (49) | .04 |
| Urinary tract | 41 (43) | 17 (15) | <.001 |
| Intra-abdominal | 16 (17) | 29 (25) | .15 |
| Skin or soft tissue | 12 (13) | 16 (14) | .81 |
| Blood (bacteremia) | 17 (18) | 5 (4) | .002 |
| Unknown | 4 (4) | 12 (10) | .10 |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Dichotomous characteristics were compared using the Fisher exact test; continuous characteristics were compared using the Student t test with the exception of highest temperature, highest and lowest central venous pressure (CVP), and emergency department (ED) sequential organ failure assessment (SOFA) score, which were compared using the Mann-Whitney U test. COPD, chronic obstructive pulmonary disease; IQR, interquartile range; HIV, human immunodeficiency virus; PR, pulse rate; RR, respiratory rate; SBP, systolic blood pressure; ScvO2, central venous oxygen saturation; SD, standard deviation.
Table 3.
Initial Treatments Administered
| Intervention | Positive group (n = 95) |
Negative group (n = 116) |
P |
|---|---|---|---|
| Endotracheal intubation | 26 (27) | 32 (28) | .97 |
| Mean crystalloid volume ± SD, L | 5.7 ± 3.2 | 6.0 ± 3.3 | .91 |
| Vasopressor administration | 68 (72) | 83 (72) | .99 |
| Dobutamine administration | 5 (5) | 4 (3) | .54 |
| PRBC transfusion | 7 (7) | 6 (5) | .52 |
| Other | |||
| Median time to initial antibiotics (IQR), min | 83 (43–133) | 97 (62–179) | .03 |
| Steroid administration | 42 (44) | 53 (46) | .83 |
| Activated protein C | 4 (4) | 1 (1) | .15 |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Dichotomous characteristics were compared using the Fisher exact test. The Student t test was used to compare crystalloid volume. Time to initial antibiotics was compared using the Mann-Whitney U test. IQR, interquartile range; PRBC, packed red blood cell; SD, standard deviation.
Table 4 outlines the categorization of the patients with negative culture results into the predefined groups. Of the 116 patients in the culture-negative group, 60 (52%) had clinical infections confirmed via our predefined criteria (51 patients) or atypical infections (9 patients). Thirty-seven (32%) of the 116 patients in the culture-negative group met the predefined criteria for categorization as noninfectious mimics, comprising 18% (37 of 211) of the entire population identified as having severe sepsis at emergency department presentation. Inflammatory colitis, hypovolemia, medication effects, adrenal insufficiency, acute myocardial infarction, and acute pulmonary embolus were the most common noninfectious diagnoses. The cause of illness was unclear or multifactorial in 19 (16%) of the 116 patients in the culture-negative group.
Table 4.
Categorization of Patients Negative by Culture
| Category, source of illness | No. of cases |
|---|---|
| Group 1: clinical infections | |
| Pneumonia | 38 |
| Skin or soft tissue | 8 |
| Intra-abdominal | 3 |
| Genitourinary | 1 |
| Oropharyngeal | 1 |
| Subtotal | 51 |
| Group 2: atypical infections | |
| Clostridium difficile | 5 |
| Disseminated Cryptococcus | 2 |
| Tuberculosis | 1 |
| Viral encephalitis | 1 |
| Subtotal | 9 |
| Group 3: noninfectious mimics | |
| Inflammatory colitis | 7 |
| Hypovolemia | 5 |
| Medication effect | 5 |
| Adrenal insufficiency | 4 |
| Acute myocardial infarction | 3 |
| Pulmonary embolus | 3 |
| Pancreatitis | 2 |
| Diabetic ketoacidosis | 2 |
| Small bowel obstruction | 2 |
| Anemia | 1 |
| Heart failure | 1 |
| Anaphylaxis | 1 |
| Systemic lupus | 1 |
| Subtotal | 37 |
| Group 4: indeterminate cases | |
| Unclear | 10 |
| Multifactorial | 9 |
| Subtotal | 19 |
| Total | 116 |
Hospital mortality was higher (although not statistically significantly so) in the culture-positive group than in the culture-negative group (25% vs 14%; P = .05). The mortality rate among patients categorized as having any infection (culture-positive group plus groups 1 and 2 of the culture-negative group; n = 155) was significantly higher than that among patients without an identified infection (groups 3 and 4 of the culture-negative group; n = 56) (15% vs 9%; P = .03). A comparison of the classic indicators of infection—the presence of abnormal temperature (<36°C or >38°C) and abnormal peripheral white blood cell count (<4000 or >12,000 cells/µL)—between the culture-negative and culture-positive group revealed no statistically significant differences, and there was no significant differences in these indicators between the patients with any infection and those without an identified infection (described above).
DISCUSSION
This study documents the final etiology of illnesses among subjects with a primary hospital admission diagnosis of severe sepsis. We found that 55% of subjects identified and treated for severe sepsis in the emergency department have negative culture results. Eighteen percent of patients identified as having a sepsis syndrome at presentation had discrete noninfectious diagnoses that mimicked sepsis, some of which may require urgent alternate disease-specific therapy (eg, pulmonary embolism). Furthermore, the classic indicators of infection (abnormal body temperature and abnormal white blood cell count) in the patients with noninfectious etiologies were statistically similar to those in patients with culture-positive sepsis. These data are useful to clinicians when the etiology of illness among patients with presumed sepsis and negative culture results is considered.
Management of patients admitted with presumed sepsis who have negative culture results is a common and complex problem that requires both further diagnostic consideration and difficult decisions regarding antibiotic management. In this study, we found that patients with negative culture results and confirmed infection were the most common subgroup, comprising over half of our culture-negative group. Pneumonia was the most common source, accounting for 55% of the final infectious etiologies in the culture-negative group. Although our study was not designed to test diagnostic or treatment algorithms, clinicians should consider further diagnostic workup for pneumonia in patients with culture-negative sepsis and suspicion of an infectious etiology.
Atypical infections were found in 8% of the patients in our culture-negative group. Fulminate Clostridium difficile colitis is uncommonly recognized at emergency department presentation but was the primary diagnosis in 4% of our patients with negative culture results. Viral, fungal, and atypical bacterial diseases are also represented in the present study; however, since undertaking this project we have also diagnosed parasitic (malaria and pneumocystis) and rickettsial (Rocky Mountain spotted fever) diseases in patients treated in our emergency department sepsis pathway. Knowledge of these potential sources may assist follow-up diagnostic testing before antibiotic de-escalation and highlights consideration of atypical pathogens among patients admitted with sepsis.
Noninfectious diseases comprised the final diagnosis in 18% of the patients in the study and accounted for 32% of the diagnoses in the culture-negative group. These data highlight the difficulties that clinicians face with respect to establishing the diagnosis of sepsis syndromes [5]. SIRS criteria, which are required to establish the diagnosis of sepsis, are nonspecific and are often present in noninfectious disease states [14, 15]. Additionally, to date there is no single diagnostic tests that allows for confident inclusion or exclusion of sepsis as a definitive diagnosis. As a result, the diagnosis of sepsis is made on the basis of the entire clinical picture, including history and physical and diagnostic testing [5]. In our experience, clinicians will occasionally chose to treat patients who have undifferentiated clinical scenarios—particularly critically ill patients—with sepsis-specific therapy (ie, antibiotics) while awaiting the results of further diagnostic testing. Our report serves to underscore this possibility and suggests that clinicians assuming the care of these patients after initial diagnosis and resuscitation, particularly in undifferentiated scenarios, should continue to consider etiologies other than sepsis.
Among the etiologies of illness in the noninfectious category, some required urgent disease-specific therapy—for example, 3 of our patients were given a diagnosis of pulmonary embolism, which would require anticoagulation and possible thrombolysis. However, it should be noted that the resuscitative interventions among patients in the culture-negative group were statistically similar to those in patients in the culture-positive group (Table 3). Furthermore, the patients with no definitively identified infection (groups 3 and 4 of the culture-negative group) had lower hospital mortality than did patients with identified infection (9% vs 15%; P = .03). Taken together, these data suggest that no harm was imparted by resuscitating patients with noninfectious SIRS in our emergency department sepsis resuscitation pathway. We recognize that our sample did not include a large number of patients with noninfectious mimics of sepsis; however, our experience should serve as a solid reminder of the wide differential diagnostic spectrum of patients presenting with SIRS.
Clinicians understand that sepsis is often a challenging diagnosis to establish at bedside. Our report provides data supporting this assertion—namely, that in clinical practice ~1 in 5 patients with suspected sepsis at admission may actually have a noninfectious disease that mimics the presentation of sepsis. However, it is important to recognize that our data was not collected as a part of a rigorous research trial with meticulously scrutinized inclusion criteria; rather, it was a registry of what actually occurs in clinical practice. Thus, our results cannot be extrapolated to a population enrolled in a randomized clinical trial of a sepsis therapeutic agent, which likely would be comprised of a more homogenous population because of the inherit external control of the enrolled population. An important message that is underscored by our data, however, is that sepsis is comprised of a continuum of a syndrome rather than a discrete specific disease. We believe clinical trialists should consider our data when designing efficacy trials of new interventions for sepsis and thus should maintain tight control of entry criteria to ensure homogenous populations.
This report has several limitations that warrant discussion. First, this is a single-center study that was not conducted as a tightly controlled experimental investigation. As such, our results may not be generalizable to other populations. Second, although we used a priori definitions and a 3-reviewer adjudication process to determine outcome, it is possible that diagnostic misclassification occurred. Third, we were unable to establish a firm diagnosis for 19 patients (culture-negative group 4). Finally, had a larger sample been studied it is possible that different incidences of alternate etiologies of disease could have been found.
In conclusion, we found that >50% of subjects identified and treated for severe sepsis in the emergency department have negative culture results. Of patients identified with a sepsis syndrome at presentation, 18% had a noninfectious diagnosis that mimicked sepsis, and the clinical characteristics of these subjects were similar to those of subjects with culture-positive sepsis. Clinicians should carefully consider alternative noninfectious causes of SIRS in patients admitted to the hospital with a sepsis syndrome and persistently negative culture results.
Acknowledgments
Financial support. National Institute of General Medical Sciences, National Institutes of Health (grant GM76652 to A.E.J.).
A.E.J. has received research support from Critical Biologics Corporation and Hutchinson Technology. J.M.H. has received research support from Gilead. A.C.H. has been a speaker for Edwards Lifesciences.
Footnotes
Potential conflicts of interest. All other authors: no conflicts.
References
- 1.Centers for Disease Control and Prevention. Current trends increase in national hospital discharge survey rates for septicemia—United States, 1979–1987. Report no. 39. 1991. [Google Scholar]
- 2.Angus D, Linde-Zwirble W, Lidicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 4.Bone R, Balk R, Cerra F, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: the ACCP/SCCM Consensus Conference Committee—American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 6.Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a metaanalysis. Crit Care Med. 2008;36:2734–2739. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trzeciak S, Dellinger RP, Abata NL, et al. Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest. 2006;129:225–235. doi: 10.1378/chest.129.2.225. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro NI, Howell MD, Talmor D, et al. Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med. 2006;34:1025–1032. doi: 10.1097/01.CCM.0000206104.18647.A8. [DOI] [PubMed] [Google Scholar]
- 10.Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.Calandra T, Cohen J. for the International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The International Sepsis Forum Consensus Conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 13.Jones A, Tayal V, Sullivan D, Kline J. Randomized controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32 doi: 10.1097/01.ccm.0000133017.34137.82. 1703-178. [DOI] [PubMed] [Google Scholar]
- 14.Vincent J-L. Dear SIRS, I’m sorry to say that I don’t like you. Crit Care Med. 1997;25:372–374. doi: 10.1097/00003246-199702000-00029. [DOI] [PubMed] [Google Scholar]
- 15.Alberti C, Brun-Buisson C, Goodman V, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168:77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]