Abstract
Purpose:
To investigate multifocal visual evoked potentials (mfVEP) of the amblyopic and fellow eye in amblyopia due to anisometropia.
Methods:
We recorded mfVEP in both eyes of 15 anisometropic amblyopic patients and 15 normal control subjects. The responses from the central 7.0° arc of the visual field were measured, and changes in latency and amplitude were compared between the amblyopic, fellow, and normal control eyes.
Results:
There was a significant difference in the latency and amplitude of mfVEP between the amblyopic and fellow eyes. The responses in the central region of the visual field (rings 1 and 2) had a longer latency and smaller amplitude in the amblyopic eye. In contrast, there was no difference in mfVEP latency or amplitude between the fellow eye and normal control eyes.
Conclusion:
These results suggest that mfVEP may be used as an alternative objective method for diagnosis and monitoring of anisometropic amblyopia.
Keywords: amblyopia, anisometropia, multifocal visual evoked potentials
Introduction
Amblyopia is a developmental loss of visual sensitivity caused by experience of discordant binocular images early in life, and refers to a decrease in best corrected visual acuity of an eye with no organic pathology.1 Despite many interesting theories and neurophysiologic investigations in animal models, it is not clearly understood how and where in the visual system the visual connections that result in amblyopia are altered.2–4 Amblyopia is primarily a cortical phenomenon, caused by unequal competitive input from the two eyes into area 17 of the primary visual cortex. However, additional structural and functional abnormalities have also been observed in the lateral geniculate body of animals and humans.1,2,5,6
A number of studies have indicated that the conventional visual evoked responses in these cases are abnormal.3,5,6 These abnormalities in visual evoked potentials (VEP) are related to loss of high spatial frequency contrast sensitivity, and can be marked in anisometropic amblyopic patients. It has also been shown that the decrease in visual acuity for amblyopic eyes is greater in the fovea than at the periphery of the visual field, and the contrast sensitivity for a fixed spatial frequency across the visual field of amblyopic patients shows a greater depression in the fovea than peripherally.7,8 Recently, multifocal VEP (mfVEP) have been used widely to investigate pathologic changes or functional variations in the visual system.9–11 Using this technique, numerous locations in the visual fields can be stimulated simultaneously, and individual responses from each of them can be extracted.
In this study, we measured the mfVEP across the foveal and parafoveal area in each eye of anisometropic amblyopic patients and compared the differences between the amblyopic eye, fellow eye, and normal control eyes.
Materials and methods
Fifteen patients aged 6–10 years (mean 7.66 ± standard deviation [SD] 1.44) with amblyopia due to anisometropia were examined in the Department of Ophthalmology at the University of Athens. In all of the amblyopic eyes examined, the cornea and lens were clear, and no retinal or optic nerve diseases which might influence mfVEP values were observed. Also, there was no nystagmus or latent nystagmus. Amblyopia was diagnosed on the basis of a clear history after the age of 5 years and on orthoptic examination revealing foveal fixation without strabismus or microtropia. Refractive errors were corrected before testing. Age, refraction, and best corrected visual acuity of the subjects are shown in Table 1. In the normal control subjects, the age was 10–15 years, the spherical or astigmatic error was less than 1.0 diopter, and best corrected visual acuity was 6/6. The research followed the tenets of the Declaration of Helsinki and informed consent was obtained from the parents of the patients after the nature of the study was explained.
Table 1.
Clinical data for studied subjects
| Case | Age (years) |
Refraction |
Visual acuity (Snellen card) |
||
|---|---|---|---|---|---|
| Amblyopic eye | Fellow eye | Amblyopic eye | Fellow eye | ||
| 1 | 6 | sph +6.5 | sph +1.0 | 6/18 | 6/7.5 |
| 2 | 28 | sph +6.0 | normal | 6/9 | 6/6 |
| 3 | 25 | sph +6.0 | normal | 6/12 | 6/6 |
| 4 | 30 | sph +5.5 | sph +1.0 | 6/12 | 6/6 |
| 5 | 32 | sph +6.0 | sph +1.5 | 6/7.5 | 6/6 |
| 6 | 5 | sph +4.5 | normal | 6/9 | 6/6 |
| 7 | 16 | sph +3.5, cyl +2.5 | cyl +0.75 | 6/12 | 6/6 |
| 8 | 25 | sph +3.0, cyl +3.0 | cyl +1.0 | 6/60 | 6/6 |
| 9 | 12 | sph −9.0 | sph −0.75 | 6/60 | 6/6 |
| 10 | 21 | sph +6.5 | normal | 6/18 | 6/6 |
| 11 | 19 | sph +4.0, cyl −3.0 | cyl +1.0 | 6/60 | 6/6 |
| 12 | 28 | sph +3.0, cyl +2.5 | cyl +0.5 | 6/12 | 6/6 |
| 13 | 12 | sph +6.5 | sph +1.0 | 6/60 | 6/6 |
| 14 | 13 | sph +6.0, cyl +2.0 | sph +1.0 | 6/24 | 6/6 |
| 15 | 21 | sph +5.5 | normal | 6/15 | 6/6 |
Recording of multifocal visual evoked potentials
We used theVERIS system 4.2 (Visual Evoked Response Imaging System 4.2, Electro-Diagnostic Imaging, San Francisco, CA) to record the mfVEP. The stimulus array consisted of 60 sectors, each with 16 checks, comprising 8 black and 8 white. The stimulus array was scaled and displayed on a monochrome monitor driven at 75 Hz. The luminance of the white checks was 200 cd/m2 and for the black checks was 3 cd/m2, producing a contrast of 97%. The background luminance of the screen was 100 cd/m2. The diameter of the first stimulus ring was 0.5–3.0° of arc, 3.0–7.0° for the second stimulus ring, and 7.0–12.0° for the third stimulus ring. To obtain mfVEP, the signals were fed into an amplifier and band-pass filtered at 3–100 Hz. The gain of the amplifier was × 100,000.
For signal derivation, the active electrode was placed 2 cm above the inion and the reference electrode was placed 2 cm below the inion. A ground electrode was attached to the center of the forehead. The fellow eye was closed and the total recording time was 8 minutes.
Subjects viewed with appropriate refractive correction and were instructed to maintain fixation at the center of the stimulus marked with an “X”. The mfVEP waveforms were divided into five groups, from the center to the periphery, according to their different eccentricities. Because the intersubject and intrasubject variance of traces of the outermost rings was very large, only data from rings 1 and 2 were analyzed.
Statistical analysis
Continuous data were tested using the Kolmogorov and Smirnov method to determine whether they followed a Gaussian distribution. The data sampled from the Gaussian distribution were compared using the unpaired t-test. Categoric data were tabulated and compared using the Chi-square test. A P value less than 0.05 was considered to indicate statistical significance.
Results
All patients were diagnosed with hypermetropia or hypermetropic astigmatism, except for one case (Case 9) who suffered from myopia (Table 1). The fellow eye was hypermetropic or emmetropic. The anisometropia was higher than 4 diopters between the amblyopic and fellow eye. Also, there was no significant difference in visual acuity between the fellow and normal control eyes.
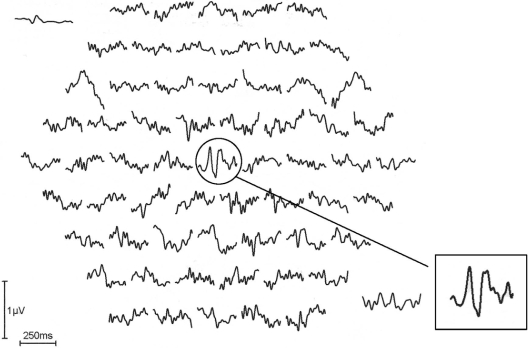
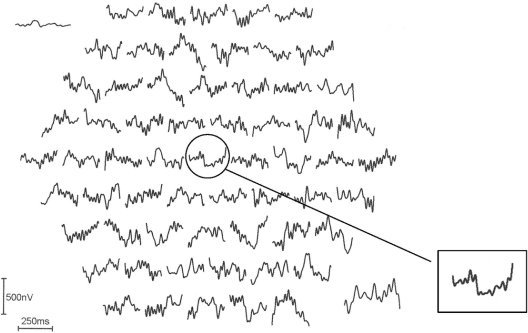
Figure 1 shows a monocular pattern reversal mfVEP recorded from the fellow eye of an amblyopic patient (Case 9 in Table 1). The inset shows a typical normal single foveal response. Figure 2 shows a monocular pattern reversal mfVEP recorded from the amblyopic eye of the same patient. The amplitude of the center stimulus hexagon (ring 1) is attenuated compared with the trace of the fellow eye and traces adjacent to it.
Figure 1.
Pattern reversal multifocal visual evoked potentials recorded from the fellow left eye of Case 10 in Table 1. The inset shows a normal foveal trace without attenuation and normal latency.
Figure 2.
The multifocal visual evoked potentials recorded from the amblyopic right eye of the same subject in Figure 1. The inset shows the pathologic foveal trace with decreased amplitude and increased latency.
Table 2 shows the amplitude and latency in ring 1 and 2 of the mfVEP in the amblyopic, fellow, and normal control eyes.
Table 2.
Values of multifocal visual evoked potentials for rings 1 and 2 in amblyopic, fellow, and normal control eyes
| Variables |
Eye |
Pvalue* | Pvalue** | ||
|---|---|---|---|---|---|
|
Amblyopic |
Fellow |
Normal |
|||
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Amplitude | |||||
| Ring 1 | 17.53 ± 7.85 | 50.93 ± 8.06 | 54.20 ± 5.93 | <0.0001 | 0.216 |
| Ring 2 | 6.97 ± 1.95 | 8.47 ± 1.71 | 9.47 ± 1.70 | 0.033 | 0.119 |
| Latency | |||||
| Ring 1 | 127.12 ± 7.99 | 101.67 ± 12.47 | 101.79 ± 4.98 | <0.0001 | 0.971 |
| Ring 2 | 98.75 ± 3.07 | 97.61 ± 3.09 | 97.79 ± 2.94 | 0.317 | 0.867 |
Notes:
P value derived from t-test for the comparison between amblyopic and fellow eye;
P value derived from t-test for the comparison between fellow and normal eye.
Table 2 shows that the mean amplitude of mfVEP from the foveal area (ring 1) in the amblyopic eye was 17.5 μV/deg2 (SD ± 7.5), and the mean latency was 127.1 msec (SD ± 7.7). In the parafoveal area (ring 2), the mean amplitude was 6.9 μV/deg2 (SD ± 1.88) and the mean latency was 98.7 msec (SD ± 2.96). In the fellow eye, the mean amplitude of mfVEP in the foveal area (ring 1) was 50.75 μV/deg2 (SD ± 7.78) and the mean latency was 101.66 msec (SD ± 12.04). In the parafoveal area (ring 2), the mean amplitude was 8.4 μV/deg2 and the mean latency was 96.6 msec (SD ± 1.64).
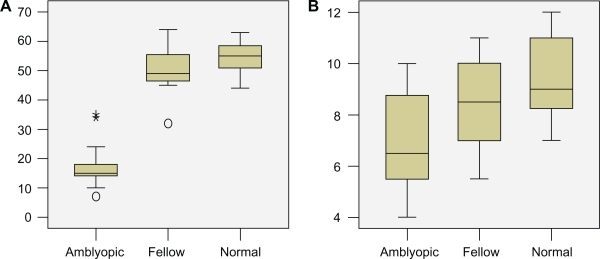
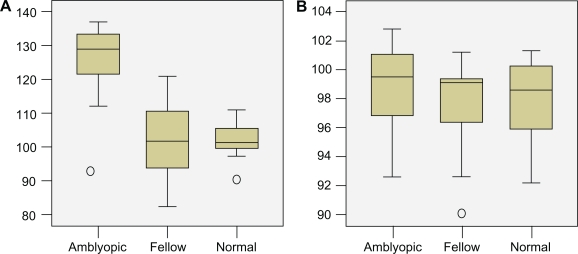
The Kolmogorov and Smirnov method assumed that the data for both groups followed Gaussian distributions. The statistical analysis performed by using the unpaired t-test demonstrated that the retinal response density of ring 1 was significantly lower in the amblyopic eye (P < 0.0001). Conversely, the decrease in the parafoveal area (ring 2) was not statistically significant (P = 0.0333, Figure 3). The mean latency in the amblyopic eye was significantly higher in ring 1 than in the fellow eye (P < 0.0001). Conversely, the difference between the amblyopic and fellow eye in ring 2 was lower but not significantly so (P = 0.3175, Figure 4). Finally, there was no statistical difference in the values of multifocal electroretinography between the fellow and normal control eyes.
Figure 3.
Box plots of data showing the difference of mean amplitude of multifocal visual evoked potentials in ring 1 (A) and ring 2 (B) in the amblyopic, fellow, and normal control eyes.
Figure 4.
Box plots of data showing the difference of mean latency of multifocal visual evoked potentials in ring 1 (A) and ring 2 (B) in the amblyopic, fellow, and normal control eyes.
Discussion
In our study, the results from the mfVEP recordings concur with data showing impairment of vision in amblyopia due to anisometropia. We also demonstrated that the multifocal responses to a 7° stimulation in amblyopic eyes were severely depressed within the foveal and parafoveal areas compared with the fellow eyes. These differences in amplitude, as well as in latency, were statistically significant, especially within ring 1, which represents the foveal area. In contrast, there was no significant difference in response amplitude and latency between the fellow and normal control eyes.
Many previous studies have demonstrated that the latency and amplitude of pattern VEP, obtained by using large stimulating fields, are abnormal in amblyopia.12–14 However, these data do not show how the increase of latency and attenuation of amplitude vary with eccentricity in amblyopia. Our results show that the increase in latency and decrease of P1-N2 amplitude is more marked at the foveal region and is diminished in the parafoveal area. This is consistent with the results of Levi et al8 and Yu et al11 who demonstrated that visual acuity was more severely impaired in the foveal area than in the periphery of amblyopic eyes. A possible explanation of this phenomenon is that the center of the visual field in the normal eye has keen visual acuity and its development demands are an accurately focused image, whereas the periphery of the visual field has poorer visual acuity and requires a less accurate focus of the image.11 However, recent studies suggest that the observed mfVEP deficits of amblyopic eyes may be largely attributed to their unsteady fixation.15
Some studies have focused attention on the anatomic location of the VEP abnormalities. Shan et al16 suggested that anisometropic amblyopia is primarily associated with an abnormal parvocellular visual system rather than function of the magnocellular visual system, which is why the dorsal layers of the lateral geniculate body are the most abnormal.17 Parvocellular pathways tend to reflect visual function of the fovea, and account for the relatively greater defects observed in central than peripheral visual function in amblyopic individuals.18 This may explain why mfVEP are more attenuated in the central region of the visual field, with less of an effect at the periphery.13,16
In summary, we suggest that assessment of mfVEP may enable objective and quantitative identification of depression of visual function in anisometropic amblyopia and that this may help to provide a more accurate diagnosis in ophthalmic practice.
Footnotes
Disclosure
The authors report no conflict on interest in this work.
References
- 1.von Noorden GK. Binocular Vision and Ocular Motility. 6th ed. Saint Louis, MO: Mosby; 2002. [Google Scholar]
- 2.Arden GB, Barnard WM, Mushin AS. Visually evoked responses in amblyopia. Br J Ophthalmol. 1974;58:183–192. doi: 10.1136/bjo.58.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiorpes L. Visual processing in amblyopia: Animal studies. Strabismus. 2006;14:3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- 4.Hoyt CS, Nickel BL, Nilson FA. Ophthalmological examination of the infant. Developmental aspects. Surv Ophthalmol. 1982;26:177–189. doi: 10.1016/0039-6257(82)90078-9. [DOI] [PubMed] [Google Scholar]
- 5.Sokol S. Abnormal evoked potentials in amblyopia. Br J Ophthalmol. 1983;67:310–314. doi: 10.1136/bjo.67.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ET, Bass SJ, Sherman J. Flash visual evoked potential (VEP) in amblyopia and optic nerve disease. Optom Vis Sci. 1995;72:612–618. doi: 10.1097/00006324-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Kirschen DG, Flom MC. Visual acuity at different retinal loci of eccentrically fixating functional amblyopia. Am J Optom Physiol Opt. 1978;55:144–150. doi: 10.1097/00006324-197803000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Levi DM, Klein SA, Aitsebaomo AP. Detection and discrimination of the direction of the motion in central and peripheral vision of normal and amblyopic observers. Vision Res. 1984;24:789–800. doi: 10.1016/0042-6989(84)90150-0. [DOI] [PubMed] [Google Scholar]
- 9.Hood DC, Odel JG, Winn BJ. The multifocal visual evoked potential. J Neuroophthalmol. 2003;23:270–289. doi: 10.1097/00041327-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Zhao K. Multifocal VEP difference between early- and late-onset strabismus amblyopia. Doc Ophthalmol. 2005;110:173–180. doi: 10.1007/s10633-005-4312-5. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Brown B, Edwards MH. Investigation of multifocal visual evoked potentials in anisometropic and esotropic amblyopes. Invest Ophthalmol Vis Sci. 1998;39:2033–2040. [PubMed] [Google Scholar]
- 12.Sokol S. Pattern visual evoked potentials. Their use in pediatric ophthalmology. In: Sokol S, editor. Electrophysiology and Psychophysics: Their Use in Ophthalmic Diagnosis. Boston, MA: Little, Brown & Co; 1980. [Google Scholar]
- 13.Wagner P, Nilsson BY. Visual evoked responses to pattern-reversal stimulation in patients with amblyopia and/or defective binocular functions. Acta Ophthalmol. 1978;56:617–627. doi: 10.1111/j.1755-3768.1978.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner P, Persson HE. Visual evoked responses to pattern-reversal stimulation in childhood amblyopia. Acta Ophthalmol. 1980;58:697–706. doi: 10.1111/j.1755-3768.1980.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Stevenson SS, Cheng H, et al. Effects of fixation instability on multifocal VEP (mfVEP) responses in amblyopes. J Vis. 2008;16:1–14. doi: 10.1167/8.3.16. [DOI] [PubMed] [Google Scholar]
- 16.Shan Y, Moster ML, Roemer RA, et al. Abnormal function of the parvocellular visual cells in amblyopia. J Pediatr Ophthalmol Strabismus. 2000;37:73–78. doi: 10.3928/0191-3913-20000301-05. [DOI] [PubMed] [Google Scholar]
- 17.Maguire GW, Smith EL, Harwerth RS, Crawford ML. Optically induced anisometropia in kittens. Invest Ophthalmol Vis Sci. 1982;23:263–264. [PubMed] [Google Scholar]
- 18.Donahue SP. The relationship between anisometropia, patient age and the development of amblyopia. Trans Am Ophthalmol Soc. 2005;103:313–316. [PMC free article] [PubMed] [Google Scholar]