Abstract
BACKGROUND
Aging is associated with reduced endothelium-dependent dilation (EDD) and increased risk for cardiovascular disease (cVD), but the mechanisms are incompletely understood. clinically elevated plasma low-density lipoprotein cholesterol (LDL-C) is associated with impaired EDD. The purpose of this study was to determine whether circulating LDL-C within the “normal” range modulates EDD in healthy older adults and whether young age or habitual aerobic exercise protects against this adverse effect.
METHODS
In 83 healthy men with optimal/near optimal LDL-C (<130 mg/dl) or borderline high LDL-C (130–159 mg/dl), EDD (brachial artery flow-mediated dilation, FMD), and endothelium-independent dilation (sublingual glyceryl trinitrate, GTN) were assessed.
RESULTS
FMD was 35% lower in older nonexercising men with borderline high LDL-C vs. optimal/near optimal LDL-C (3.1 ± 0.5 vs. 4.8 ± 0.4%Δ, P < 0.05), whereas the GTN response did not differ (P = 0.86). In contrast, FMD was similar between groups of young nonexercising men and between groups of older exercising men differing in LDL-C (P = 0.89–0.95). FMD was inversely related to LDL-C among the older nonexercising men (r = −0.43, P < 0.001), whereas there was no relation in the other groups (P > 0.05).
CONCLUSIONS
Borderline high plasma LDL-C is associated with impaired EDD in older sedentary men, but not in young sedentary or older exercising men. Thus, modest elevations in plasma LDL-C within the normal range may contribute to the increased risk of cVD in sedentary older men by exacerbating vascular endothelial dysfunction, whereas resistance to this adverse influence may help explain the enhanced endothelial function and reduced CVD risk associated with young age and regular aerobic exercise.
Adult aging is associated with a marked increase in the risk of cardiovascular disease (CVD).1 This is thought to be attributable, in part, to the development of vascular endothelial dysfunction, as indicated by a reduction in endothelium- dependent dilation (EDD).2,3 As such, identifying the mechanisms that contribute to decreases in EDD with aging has important clinical implications for the prevention of age-associated CVD.
Plasma low-density lipoprotein cholesterol (LDL-C) is a major risk factor for CVD.4,5 Patients with hypercholesterolemia (i.e., LDL-C >160 mg/dl) demonstrate impaired EDD,6 and reducing LDL-C with lipid-lowering agents improves EDD in these patients.7,8 However, it is unknown whether LDL-C within the clinically “normal” range (i.e., LDL-C <160 mg/dl) modulates EDD in healthy older adults.
If LDL-C influences EDD in older adults, two related issues would seem important to address. One would be to determine whether this association also is observed in young adults. It is possible that older age somehow renders individuals more susceptible to the potentially adverse effects of LDL-C on the vascular endothelium. Another would be to determine whether lifestyle or other factors might protect against the negative influence of LDL-C in older adults. In this context, habitual aerobic exercise can exert a favorable effect on vascular endothelial function in older adults9–14 and, thus, could provide a protective effect against LDL-C.
Accordingly, the goal of the present study was to gain insight into these issues. We first tested the hypothesis that conduit artery EDD, measured by brachial artery flow-mediated dilation (FMD), but not endothelium-independent dilation (glyceryl trinitrate (GTN)-induced dilation), is influenced by differences in LDL-C within the normal range in older nonexercising adults. To do so, we compared groups of healthy older nonexercising men with optimal/near optimal LDL-C (<130 mg/dl) or borderline high LDL-C (130–159 mg/dl) based on National Cholesterol Education Program/Adult Treatment Panel III (NCEP/ATP III) classification15 that were carefully matched for other conventional CVD risk factors. We then tested the hypothesis that young age would have a protective effect as shown by similar EDD in groups of healthy young nonexercising men with either optimal/near optimal or borderline high LDL-C. Finally, we tested the hypothesis that habitual exercise protects EDD from the adverse effects of LDL-C in older adults, as indicated by no difference in EDD between groups of older healthy men with optimal/near optimal or borderline high LDL-C who regularly performed aerobic exercise.
METHODS
Subjects
A total of 83 healthy men were studied: 33 young (aged 18–31 years) and 50 older (aged 60–79 years). During the previous 2 years the young men and a portion of the older men had performed no regular exercise (nonexercising men), whereas the other portion of the older men performed >3 sessions/week of vigorous aerobic-endurance exercise (exercising men). All of the men had LDL-C <160 mg/dl and were normotensive (resting blood pressure <140/90 mm Hg), non-smokers, nondiabetic (fasting blood glucose <126 mg/dl), nonobese (body mass index <30 kg/m2), and free of clinical diseases as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG (older men only). No subjects had the metabolic syndrome according to NCEP/ATP III criteria.15 Subjects were not taking medications and had not taken antioxidants (e.g., vitamins C and E) within 6 weeks of the study. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Procedures
All measurements were performed at the University of Colorado at Boulder General Clinical Research Center after a 12-h fast and 24-h abstention from alcohol and physical activity.
Subject characteristics
Waist and hip circumferences and body mass index were measured by anthropometry.16 Percent body fat was measured by dual-energy X-ray absorptiometry (DXA-GE; Lunar, Madison, WI; software version 5.60.003). Arterial blood pressure and resting heart rate were measured over the brachial artery during supine rest using a semiautomated device (Dinamap Pro 100, GE Healthcare, Waukesha, WI). Maximal oxygen consumption was measured during incremental treadmill exercise using open-circuit spirometry as previously described.14 Fasting plasma metabolic factors were determined by the General Clinical Research Center core laboratory using standard assays. Framingham coronary heart disease risk score was calculated for each subject.17
Brachial artery FMD and GTN-induced dilation
Duplex ultra-sonography was used to assess brachial artery FMD and GTN-induced dilation in the supine position, as previously described by our laboratory.12,13 Briefly, an ultrasound probe was clamped 3–6 cm proximal to the antecubital crease. After obtaining a baseline image, reactive hyperemia was produced by inflation of a blood pressure cuff placed on the upper forearm distal to the antecubital fossa for 5 min at 250 mm Hg followed by a rapid deflation. GTN-induced dilation was assessed by measurement of brachial artery dilation in response to sublingual GTN (0.4 mg). Because group differences in FMD can result from differences in dilatory stimulus (i.e., shear stress),18 we measured baseline and peak mean blood velocities as previously described.12 Because blood viscosity was not measured, shear rate was calculated instead of shear stress using the formula: shear rate = V/D, where V and D represent velocity and baseline diameter, respectively. The percent change in diameter was used as the primary expression of brachial FMD based on recent findings and recommendations.19
Statistics
Statistical analyses were performed with SPSS (version 16.0; Chicago, IL). Differences in subject characteristics among peer groups were assessed by t-tests for independent sample comparisons with a statistical significance for the analyses set at P < 0.05. Group differences in main outcomes were determined by one-way analysis of variance. In the case of significant F values, post hoc analyses were performed using a Bonferroni corrected P value of 0.008 (i.e., six comparisons) to identify significant differences among mean values. Bivariate relations were determined using Pearson product–moment correlations. Values are mean ± s.e.
RESULTS
Plasma LDL-C concentrations
Values for plasma LDL-C concentrations are presented in Tables 1–3 and illustrated in Figure 1. The plasma LDL-C concentrations of the groups were consistent with the study design and experimental approach.
Table 1.
Subject characteristics for older nonexercising men
| Optimal/near optimal LDL-C | Borderline high LDL-C | |
|---|---|---|
| N | 15 | 12 |
| Age, year | 66 ± 1 | 65 ± 1 |
| Body mass, kg | 80 ± 3 | 82 ± 3 |
| Body mass index, kg/m2 | 26 ± 1 | 26 ± 1 |
| Body fat, % | 25.7 ± 2.0 | 27.0 ± 1.7 |
| Waist:hip ratio | 0.95 ± 0.01 | 0.96 ± 0.01 |
| VO2max, ml/kg/min | 29.9 ± 1.2 | 29.6 ± 1.1 |
| Systolic blood pressure, mm Hg | 124 ± 3 | 119 ± 4 |
| Diastolic blood pressure, mm Hg | 74 ± 2 | 76 ± 2 |
| Total cholesterol, mg/dl | 178 ± 4 | 217 ± 3* |
| LDL-C, mg/dl | 105 ± 3 | 142 ± 2* |
| HDL-C, mg/dl | 48 ± 3 | 48 ± 2 |
| Triglycerides, mg/dl | 127 ± 16 | 140 ± 19 |
| Cholesterol/HDL-C ratio | 3.94 ± 0.26 | 4.65 ± 0.23* |
| Fasting blood glucose, mg/dl | 94 ± 3 | 101 ± 2* |
| Framingham risk score, % | 9.1 ± 0.7 | 11.9 ± 0.7* |
| Base diameter, mm | 4.36 ± 0.13 | 4.46 ± 0.16 |
| FMD, mm change | 0.21 ± 0.02 | 0.13 ± 0.02* |
| GTN, mm change | 0.98 ± 0.06 | 0.95 ± 0.06 |
| SR, % change | 528 ± 56 | 536 ± 71 |
| SR, 1/s change | 85 ± 4 | 78 ± 7 |
Data are mean ± s.e.
FMD, flow-mediated dilation; GTN, glyceryl trinitrate-induced dilation; HDL-C, high density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SR, shear rate; VO2max, maximal oxygen consumption.
P < 0.05 vs. older nonexercising men with optimal/near optimal LDL-C.
Table 3.
Subject characteristics for older exercising men
| Optimal/near optimal LDL-C | Borderline high LDL-C | |
|---|---|---|
| N | 16 | 7 |
| Age, year | 66 ± 1 | 64 ± 2 |
| Body mass, kg | 70 ± 2 | 77 ± 4* |
| Body mass index, kg/m2 | 23 ± 1 | 24 ± 1 |
| Body fat, % | 16.3 ± 1.1 | 21.9 ± 2.8* |
| Waist:hip ratio | 0.87 ± 0.01 | 0.90 ± 0.02 |
| VO2max, ml/kg/min | 42.8 ± 1.3 | 39.5 ± 1.6 |
| Systolic blood pressure, mm Hg | 123 ± 3 | 120 ± 4 |
| Diastolic blood pressure, mm Hg | 72 ± 2 | 74 ± 3 |
| Total cholesterol, mg/dl | 186 ± 6 | 224 ± 8* |
| LDL-C, mg/dl | 104 ± 4 | 138 ± 4* |
| HDL-C, mg/dl | 64 ± 4 | 64 ± 9 |
| Triglycerides, mg/dl | 90 ± 13 | 111 ± 26 |
| Cholesterol/HDL-C ratio | 3.06 ± 0.23 | 3.92 ± 0.56 |
| Fasting blood glucose, mg/dl | 94 ± 3 | 99 ± 4 |
| Framingham risk score, % | 8.4 ± 0.9 | 10.6 ± 1.3 |
| Base diameter, mm | 3.97 ± 0.11 | 4.08 ± 0.12 |
| FMD, mm change | 0.24 ± 0.02 | 0.25 ± 0.02 |
| GTN, mm change | 0.84 ± 0.06 | 1.00 ± 0.06 |
| SR, % change | 395 ± 76 | 364 ± 92 |
| SR, 1/s change | 79 ± 9 | 76 ± 6 |
Data are mean ± s.e.
FMD, flow-mediated dilation; GTN, glyceryl trinitrate-induced dilation; HDL-C, high density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SR, shear rate; VO2max, maximal oxygen consumption.
P < 0.05 vs. older exercising men with optimal/near optimal LDL-C.
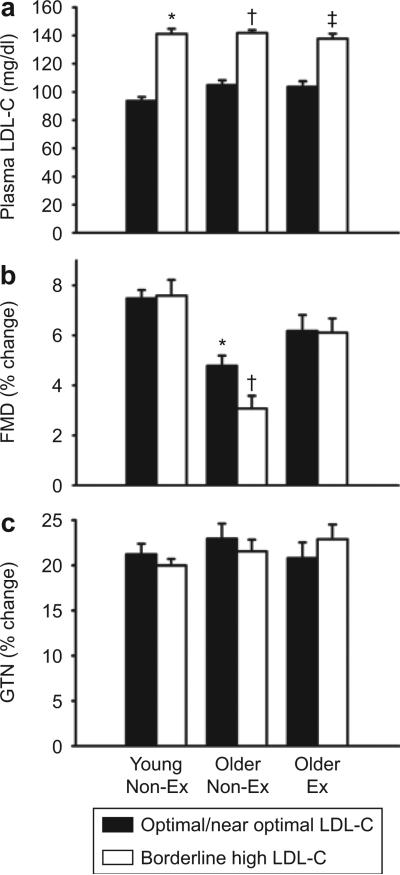
Figure 1.
Plasma low-density lipoprotein cholesterol (LDL-C) concentrations, flow-mediated dilation and endothelium-independent dilation: (a) LDL-C, (b) endothelium-dependent dilation (brachial artery flow-mediated dilation, FMD); and (c) endothelium-independent dilation (brachial artery response to sublingual glyceryl trinitrate, GTN) in young nonexercising (young non-ex), older nonexercising (older non-ex) and older exercising (older ex) men with optimal/near optimal or borderline high LDL-C. Values are mean ± s.e. *P < 0.05 vs. young nonexercising men with optimal/near optimal LDL-C, †P < 0.05 vs. older nonexercising men with optimal/near optimal LDL-C, ††P < 0.05 vs. older exercising men with optimal/near optimal LDL-C.
Other subject characteristics
Among the older nonexercising men, the optimal/near optimal and borderline high LDL-C groups differed (P < 0.05) in total cholesterol, cholesterol/ HDL-C ratio, fasting blood glucose, and Framingham risk score (Table 1). Among the young nonexercising men, the two groups differed in total cholesterol, body mass index, percent body fat, waist:hip ratio, plasma triglycerides, cholesterol/ HDL-C ratio, and Framingham risk score (Table 2). Among the older exercising men, the two groups differed in total cholesterol, body mass, and percent body fat (Table 3).
Table 2.
Subject characteristics for young nonexercising men
| Optimal/near optimal LDL-C | Borderline high LDL-C | |
|---|---|---|
| N | 26 | 7 |
| Age, year | 23 ± 1 | 24 ± 1 |
| Body mass, kg | 78 ± 3 | 84 ± 3 |
| Body mass index, kg/m2 | 24 ± 1 | 26 ± 1* |
| Body fat, % | 18.1 ± 1.5 | 24.6 ± 3.1* |
| Waist:hip ratio | 0.84 ± 0.01 | 0.88 ± 0.02* |
| VO2max, ml/kg/min | 46.8 ± 0.8 | 44.4 ± 3.2 |
| Systolic blood pressure, mm Hg | 116 ± 2 | 112 ± 3 |
| Diastolic blood pressure, mm Hg | 61 ± 1 | 63 ± 4 |
| Total cholesterol, mg/dl | 156 ± 3 | 214 ± 5* |
| LDL-C, mg/dl | 94 ± 3 | 141 ± 4* |
| HDL-C, mg/dl | 45 ± 2 | 45 ± 4 |
| Triglycerides, mg/dl | 90 ± 5 | 141 ± 15* |
| Cholesterol/HDL-C ratio | 3.60 ± 0.13 | 4.95 ± 0.36* |
| Fasting blood glucose, mg/dl | 87 ± 1 | 89 ± 2 |
| Framingham risk score, % | 1.5 ± 0.1 | 2.9 ± 0.3* |
| Base diameter, mm | 4.08 ± 0.07 | 4.20 ± 0.17 |
| FMD, mm change | 0.31 ± 0.01 | 0.32 ± 0.02 |
| GTN, mm change | 0.89 ± 0.05 | 0.84 ± 0.02 |
| SR, % change | 355 ± 40 | 331 ± 48 |
| SR, 1/s change | 83 ± 5 | 78 ± 8 |
Data are mean ± s.e.
FMD, flow-mediated dilation; GTN, glyceryl trinitrate-induced dilation; HDL-C, high density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SR, shear rate; VO2max, maximal oxygen consumption.
P < 0.05 vs. young nonexercising men with optimal/near optimal LDL-C.
Baseline brachial artery diameter was not different among groups (Tables 1–3). There were no differences in absolute or percent changes in shear rate among the peer groups during reactive hyperemia (Tables 1–3).
Effects of age and habitual exercise on EDD
Among nonexercising men with optimal/near optimal LDL-C, percent change in brachial FMD was ~35% lower in the older compared with the young group (Figure 1). Among older men with optimal/near optimal LDL-C, brachial FMD was 30% greater in the exercising than in the nonexercising group (Figure 1). The absolute changes in diameter with FMD showed the same group differences as percent changes (Tables 1–3). Neither percent nor absolute GTN-induced dilation differed between groups (Figure 1 and Tables 1–3). Thus, in men with optimal/near optimal LDL-C, sedentary aging was associated with reduced brachial FMD, whereas habitual endurance exercise training was associated with enhanced brachial FMD among older men. Neither age nor habitual exercise status influenced brachial artery GTN-induced dilation, indicating selective effects on EDD.
Effects of plasma LDL-C on EDD
Among older nonexercising men, the percent change in brachial FMD was 35% lower in the group with borderline high compared with optimal/near optimal LDL-C, whereas GTN-induced dilation did not differ (Figure 1). The difference in brachial artery FMD between groups of older nonexercising men was not related to the small but significant difference in fasting blood glucose concentrations (r = −0.27, P = 0.17).
In contrast to the older nonexercising men, in older exercising and young nonexercising men there were no differences in percent brachial FMD between groups with optimal/near optimal compared with borderline high LDL-C, nor did GTN-induced dilation differ (Figure 1). These trends were similar when absolute changes in FMD and GTN-induced dilation were compared (Tables 1–3).
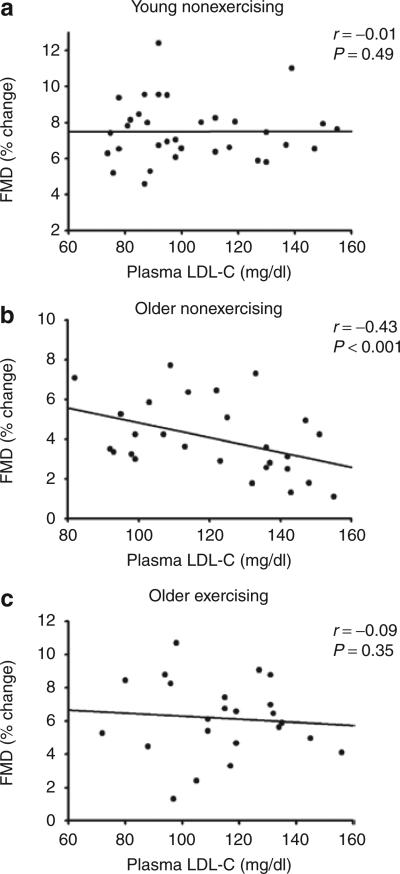
Consistent with these group comparisons, among the older nonexercising men the percent change in brachial FMD was inversely related to plasma LDL-C concentration, whereas among young nonexercising men and the older exercising men brachial FMD was not significantly related to plasma LDL-C concentration (Figure 2). Brachial GTN-induced dilation was not related to plasma LDL-C concentrations within any of the groups (all P > 0.05).
Figure 2.
Relation between plasma LDL-C and brachial FMD. Relations between plasma LDL-C concentrations and brachial FMD in (a) young nonexercising men, (b) older nonexercising men, and (c) older exercising men. For each panel, men with optimal/near optimal and borderline high plasma LDL-C concentrations are pooled.
DISCUSSION
The results of this study provide new insight into the influence of “normal” (defined by current NCEP/ATP III classification) circulating LDL-C on EDD with aging in healthy humans. We have shown previously that brachial artery FMD is lower in older men compared with young healthy sedentary men.12,13,20 However, there is significant variability in FMD among the older men, with function ranging from normal or near normal to substantially impaired. The mechanisms contributing to impaired EDD with aging and the factors responsible for this interindividual variability are incompletely understood. One possible influencing factor is LDL-C. Patients with hypercholesterolemia exhibit impaired EDD, which is improved after treatment with lipid-lowering agents.6–8 In the present study, we found that brachial FMD was ~35% lower in healthy older nonexercising men with borderline high LDL-C compared with those with optimal/near optimal LDL-C based on NCEP/ATP III guidelines.15 Plasma LDL-C was the only risk factor that differed between groups and correlated with brachial FMD. Together, our findings show that even high normal levels of LDL-C are associated with impaired EDD in older sedentary healthy men, and that differences in LDL-C may contribute to interindividual differences in brachial FMD among these men.
In contrast to these older nonexercising men, brachial FMD was not different in young men with optimal/near optimal vs. borderline high LDL-C, and FMD was not related to LDL-C. This suggests that LDL-C within the normal range does not influence brachial FMD in young healthy men without risk factors for CVD. Our observations are unique in that a recent study found that brachial FMD was modestly, but significantly, greater in young (33–39 years) Spanish military men with optimal/near optimal LDL-C compared to men with less favorable LDL-C.21 However, some of the soldiers smoked, had hypertension, and had high LDL-C (up to 232 mg/dl). An earlier study found that EDD, assessed by the increase in leg blood flow in response to intra-femoral artery infusion of methacholine chloride, was smaller in young men and women with LDL-C in the normal range (25th to 75th age group percentile, 100–155 mg/dl) vs. those with lower LDL-C (<25th age group percentile, 47–144 mg/dl).22 However, the inclusion of smokers in this sample could explain at least some of the endothelial dysfunction found in this young cohort. The results also could be explained by differences in the measurements of EDD used, as brachial artery FMD and pharmacological stimulation of EDD generally do not correlate.23,24 Thus, LDL-C can be associated with EDD in young adults with risk factors for CVD and, perhaps, with EDD of resistance vessels in the leg, although the latter has not been shown to be a predictor of future cardiovascular events. The results of our study demonstrate that LDL-C does not modulate brachial artery FMD in young men without CVD risk factors.
We12,13 and others10,11 have shown that baseline brachial artery FMD is greater in older men who exercise regularly compared with their sedentary peers. In the present study, we found that in contrast to older men who do not exercise, brachial FMD does not differ among groups of older exercising men with optimal/near optimal vs. borderline high LDL-C. This suggests that, like young men, older men who habitually perform aerobic exercise may be “protected” from the potential adverse effects of LDL-C. This apparent protection in the young and exercising older groups was observed despite the fact that some other risk factors typically associated with impaired EDD were greater (or tended to be greater) in the men with borderline high LDL-C.
We can only speculate on the mechanisms underlying these age- and habitual exercise-specific influences of LDL-C on brachial FMD. We previously demonstrated that supraphysiological infusion of vitamin C, a potent antioxidant, improves brachial FMD in older sedentary, but not in young sedentary or older exercise-trained healthy men.12 These findings are consistent with the idea that oxidative stress tonically suppresses brachial FMD in older sedentary men, but not in young sedentary or older exercising men. As such, it seems reasonable that the presence vs. absence of oxidative stress may be at least one mechanism involved in the selective effects of LDL-C on brachial FMD in our older sedentary men. Indeed, hypercholesterolemia is associated with increased superoxide production in isolated vessels,25 primarily as the result of increased synthesis by the endothelium.26 Sources of vascular superoxide production with elevated LDL-C include NADPH oxidase,27 xanthine oxidase,28 and uncoupled endothelial nitric oxide synthase.29 Recently, vascular inflammation, a process tightly linked to oxidative stress, was shown to contribute to vascular endothelial dysfunction in older rats.30 Systemic and vascular endothelial markers of inflammation increase with age in some sedentary adults31,32 and habitual exercise is thought to exert an anti-inflammatory influence.33 Thus, it is possible that vascular inflammation plays a role in the age- and habitual exercise-specific relations between LDL-C and brachial FMD observed in the present study.
Our findings have potentially important clinical implications. Arterial aging, including impaired vascular endothelial function, is an important factor in the development of age- associated CVD.1 Brachial artery FMD predicts future cardiovascular events,34,35 thus, impaired brachial FMD precedes the development of clinical symptoms and disease. We have shown previously that brachial FMD decreases and LDL-C increases moderately on average with aging in healthy adults.12,13 The present results indicate that even elevations in LDL-C within the normal range adversely affect brachial FMD in older healthy nonexercising men, a group at increased risk of CVD.1 Current NCEP/ATP III guidelines indicate interventions should be considered when LDL-C is >160 mg/dl in adults with 0–1 risk factors.15 Our findings suggest that lifestyle or pharmacological treatment may need to be considered for older men with LDL-C between 130 and 159 mg/dl. Our results also suggest that in contrast to older nonexercising men, young age and regular aerobic exercise may protect against the negative influence of high normal LDL-C on brachial FMD. As is the case with statins, habitual aerobic exercise may have pleiotropic effects on EDD. Regular exercise and/or high aerobic fitness are associated with reduced risk of CVD36–38 and protecting the vascular endothelium from the adverse effects of LDL-C may be a previously unrecognized mechanism for this reduced CVD risk. Our findings in this regard also reinforce current recommendations for regular exercise in the prevention of CVD.
This study has several limitations that are important to note. First, we did not have data on sufficient numbers of healthy women with borderline high LDL-C to include females in the analysis. It is possible that, for example, women who are premenopausal or in the early postmenopausal period also may be protected against high normal LDL-C. This is an important question to address in future studies. Second, our subject groups for young nonexercising men and older exercising men with borderline high LDL-C are relatively small as a result of the difficulty in finding such subjects. Indeed, we calculate that, ideally, 11 subjects per group would be necessary to have the proper power to detect differences in our outcome measures between men with optimal/near optimal vs. borderline high LDL-C. However, in the young and older exercising men the mean FMD values are similar or greater in the borderline high compared with the optimal/near optimal LDL-C groups. Thus, it is unlikely that adding more subjects to the young sedentary and older exercising group comparisons would change our results. Third, our results are limited to brachial artery FMD. Although hypercholesterolemia is associated with impaired EDD in forearm resistance vessels,6 another common measure of EDD and predictor of future CV events,39 the effect of borderline high LDL-C on these vessels is unknown. Fourth, we were able to assess the peak shear rate, but not the shear rate area under the curve.40 However, non-normalized expressions of FMD, not FMD corrected for shear rate, predict cardiovascular events,34,35 and recent findings indicate that the percent change in FMD is the most reliable and risk factor-sensitive expression of the response.19 Fifth, the standard dose of GTN used in the present study18,35 produces an approximately fourfold greater dilation than FMD. It is possible that group differences may exist in GTN-induced dilation at lower doses. Finally, we are unable to provide any insight into the mechanisms by which borderline elevations in LDL-C selectively modulate brachial artery FMD with aging and habitual exercise. Future studies should explore the potential roles of oxidative stress and inflammation.
In conclusion, the present findings show for the first time that high normal LDL-C is negatively associated with brachial artery FMD in older sedentary healthy men. Our results also provide novel evidence that young age and habitual aerobic exercise protect against the adverse effects of borderline high LDL-C on brachial FMD. Increased vascular endothelial vulnerability to moderate elevations in LDL-C may be an important mechanism by which the risk of CVD is increased with aging in sedentary men, whereas protection against this effect may be a novel mechanism by which regular aerobic exercise reduces CVD risk in this group. As such, our results provide additional support for an important role of aerobic exercise in the prevention of vascular endothelial dysfunction with aging.
Acknowledgments
We thank Brooke Lawson for technical assistance. This work was supported by National Institutes of Health awards AG006537, AG013038, AG022241, AG000279, AG031617, and RR00051 and American Heart Association 0715735Z.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 4.The Lipid Research Clinics Coronary Primary Prevention Trial results II The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 5.The Lipid Research Clinics Coronary Primary Prevention Trial results I Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 6.Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 8.O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- 9.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 10.Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol. 1999;87:2136–2142. doi: 10.1152/jappl.1999.87.6.2136. [DOI] [PubMed] [Google Scholar]
- 11.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88:761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- 12.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 15.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 16.Van Pelt RE, Davy KP, Stevenson ET, Wilson TM, Jones PP, Desouza CA, Seals DR. Smaller differences in total and regional adiposity with age in women who regularly perform endurance exercise. Am J Physiol. 1998;275:E626–E634. doi: 10.1152/ajpendo.1998.275.4.E626. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 19.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 21.Laclaustra M, Frangi AF, Frangi AG, Casasnovas JA, Cia P. Association of endothelial function and vascular data with LDL-c and HDL-c in a homogeneous population of middle-aged, healthy military men: evidence for a critical role of optimal lipid levels. Int J Cardiol. 2008;125:376–382. doi: 10.1016/j.ijcard.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg HO, Bayazeed B, Hook G, Johnson A, Cronin J, Baron AD. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation. 1997;96:3287–3293. doi: 10.1161/01.cir.96.10.3287. [DOI] [PubMed] [Google Scholar]
- 23.Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–2375. doi: 10.1161/01.ATV.0000184769.22061.da. [DOI] [PubMed] [Google Scholar]
- 24.Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol. 2001;88:1067–1069. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Benna S, Hamilton CA, McClure JD, Rogers PN, Berg GA, Ford I, Delles C, Dominiczak AF. Low-density lipoprotein cholesterol determines oxidative stress and endothelial dysfunction in saphenous veins from patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:218–223. doi: 10.1161/01.ATV.0000193626.22269.45. [DOI] [PubMed] [Google Scholar]
- 26.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donnell RW, Johnson DK, Ziegler LM, DiMattina AJ, Stone RI, Holland JA. Endothelial NADPH oxidase: mechanism of activation by low-density lipoprotein. Endothelium. 2003;10:291–297. doi: 10.1080/10623320390272280. [DOI] [PubMed] [Google Scholar]
- 28.White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stepp DW, Ou J, Ackerman AW, Welak S, Klick D, Pritchard KA., Jr Native LDL and minimally oxidized LDL differentially regulate superoxide anion in vascular endothelium in situ. Am J Physiol Heart Circ Physiol. 2002;283:H750–H759. doi: 10.1152/ajpheart.00029.2002. [DOI] [PubMed] [Google Scholar]
- 30.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–398. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 34.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 35.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 37.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 38.Powell KE, Thompson PD, Caspersen CJ, Kendrick JS. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health. 1987;8:253–287. doi: 10.1146/annurev.pu.08.050187.001345. [DOI] [PubMed] [Google Scholar]
- 39.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 40.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]