Abstract
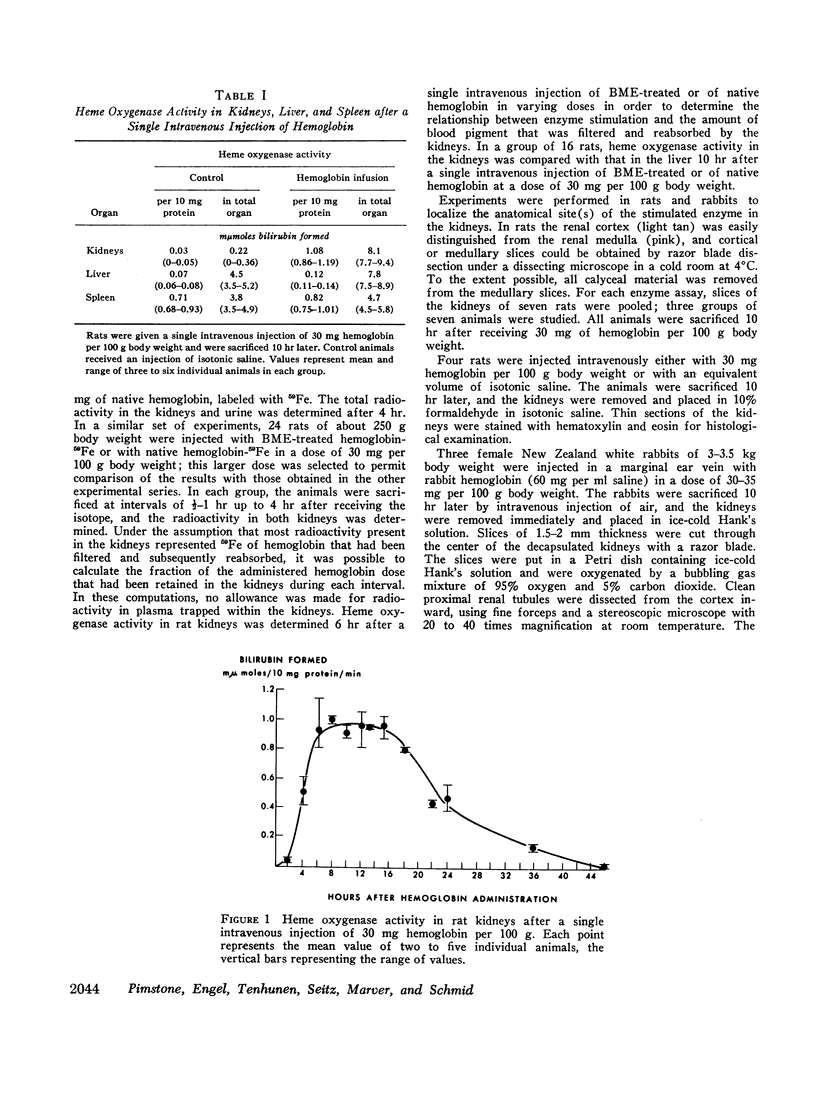
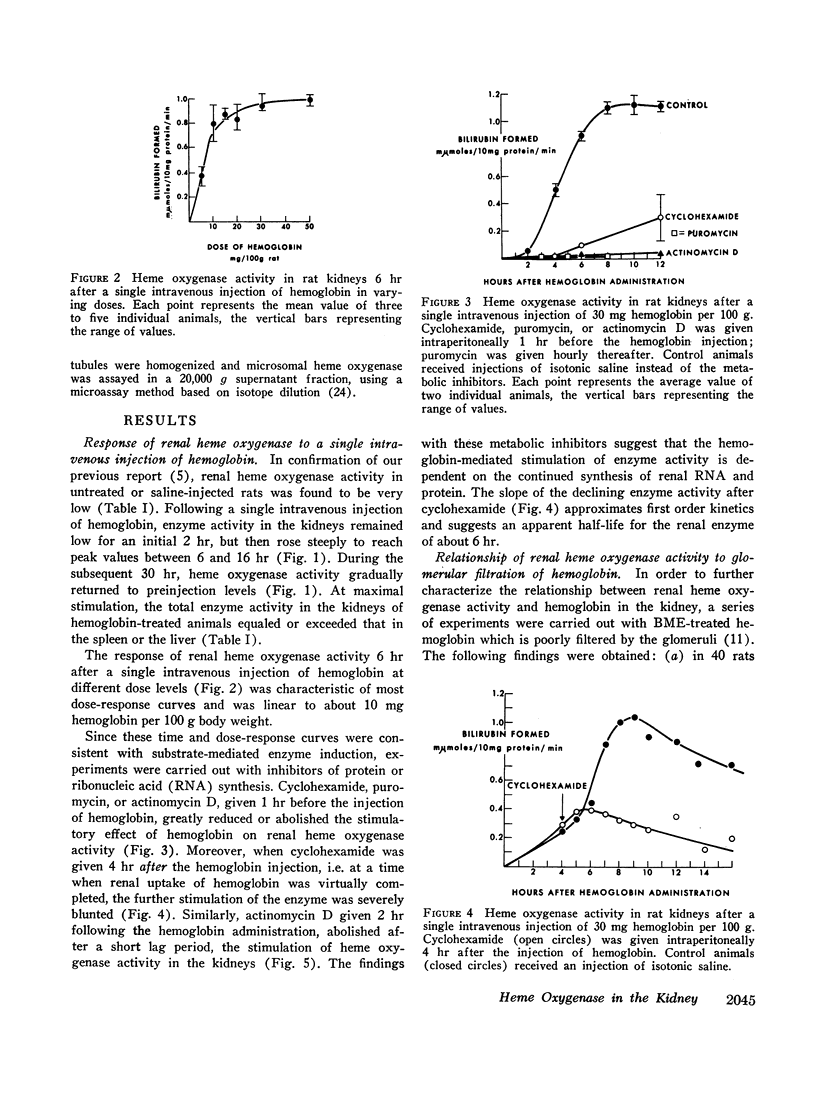
We have recently identified and characterized NADPH-dependent microsomal heme oxygenase as the major enzymatic mechanism for the conversion of hemoglobin-heme to bilirubin-IXα in vivo. Enzyme activity is highest in tissues normally involved in red cell breakdown, that is, spleen, liver, and bone marrow, but it usually is negligible in the kidney. However, renal heme oxygenase activity may be transiently increased 30- to 100-fold following hemoglobinemia that exceeded the plasma haptoglobin-binding capacity and consequently resulted in hemoglobinuria. Maximal stimulation of enzyme activity in rats is reached 6-16 hr following a single intravenous injection of 30 mg of hemoglobin per 100 g body weight; activity returns to basal levels after about 48 hr. At peak level, total enzyme activity in the kidneys exceeds that of the spleen or liver. Cyclohexamide, puromycin, or actinomycin D, given just before, or within a few hours after, a single intravenous injection of hemoglobin minimizes or prevents the rise in renal enzyme activity; this suggests that the increase in enzyme activity is dependent on continued synthesis of ribonucleic acid and protein. The apparent biological half-life of renal heme oxygenase is about 6 hr. These observations indicate that functional adaptation of renal heme oxygenase activity reflects enzyme induction either directly or indirectly by the substrate, hemoglobin.
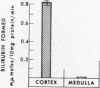
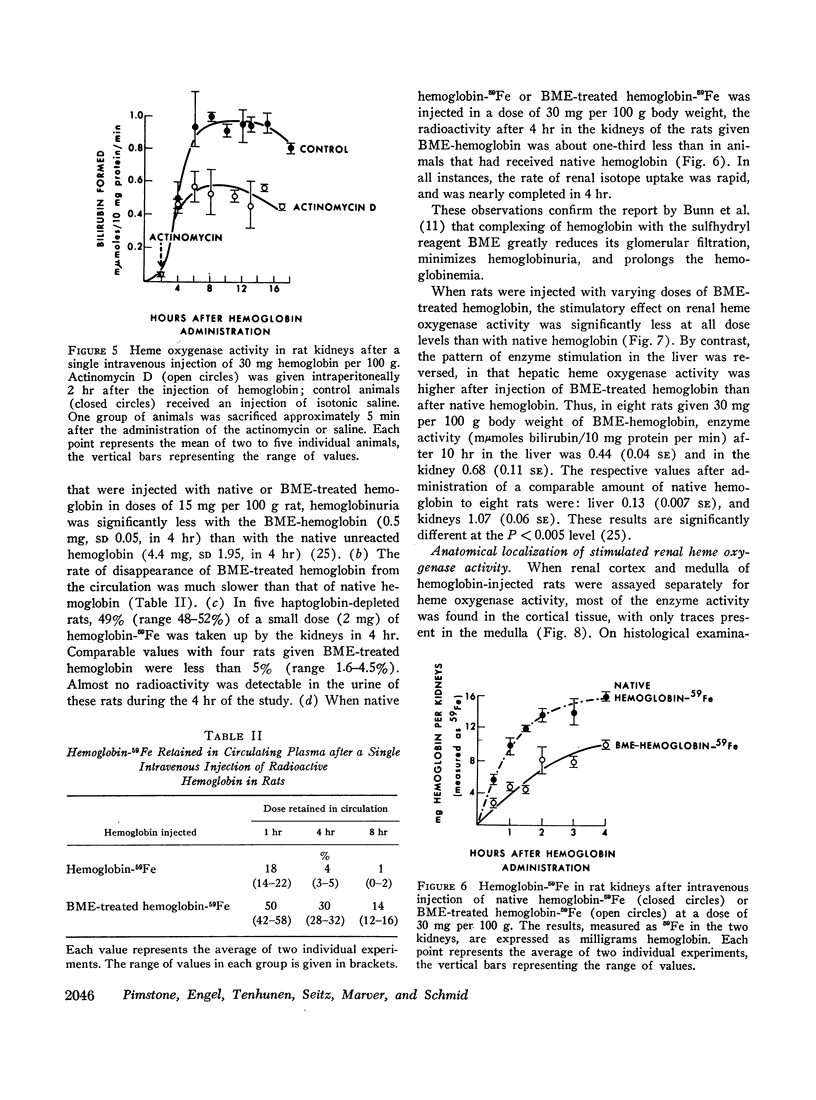
Filtered rather than plasma hemoglobin appears to regulate renal heme oxygenase activity. Thus, stabilization of plasma hemoglobin in its tetrameric form with bis (N-maleimidomethyl) ether, which diminishes its glomerular filtration and retards it plasma clearance, results in reduced enzyme stimulation in the kidney, but enhances its activity in the liver. These findings suggest that the enzyme is localized in the tubular epithelial cells rather than in the glomeruli and is activated by luminal hemoglobin. Direct support for this concept was obtained by the demonstration of heme oxygenase activity in renal tubules isolated from rabbits that had been injected with hemoglobin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awai M., Brown E. B. Examination of the role of xanthine oxidase in iron absorption by the rat. J Lab Clin Med. 1969 Mar;73(3):366–378. [PubMed] [Google Scholar]
- Braun S. R., Weiss F. R., Keller A. I., Ciccone J. R., Preuss H. G. Evaluation of the renal toxicity of heme proteins and their derivatives: a role in the genesis of acute tubule necrosis. J Exp Med. 1970 Mar 1;131(3):443–460. doi: 10.1084/jem.131.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. B., Hwang Y. F., Nicol S., Ternberg J. Absorption of radiation-labeled hemoglobin by dogs. J Lab Clin Med. 1968 Jul;72(1):58–64. [PubMed] [Google Scholar]
- Bunn H. F., Esham W. T., Bull R. W. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969 May 1;129(5):909–923. doi: 10.1084/jem.129.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. The renal handling of hemoglobin. II. Catabolism. J Exp Med. 1969 May 1;129(5):925–934. doi: 10.1084/jem.129.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSBY W. H., DAMESHEK W. The significance of hemoglobinemia and associated hemosiderinuria, with particular reference to various types of hemolytic anemia. J Lab Clin Med. 1951 Dec;38(6):829–841. [PubMed] [Google Scholar]
- CROSBY W. H., FURTH F. W. A modification of the benzidine method for measurement of hemoglobin in plasma and urine. Blood. 1956 Apr;11(4):380–383. [PubMed] [Google Scholar]
- CROSBY W. H., HOUCHIN D. N. Preparing standard solutions of cyanmethemoglobin. Blood. 1957 Dec;12(12):1132–1136. [PubMed] [Google Scholar]
- CROSBY W. H. Paroxysmal nocturnal hemoglobinuria: relation of the clinical manifestations to underlying pathogenic mechanisms. Blood. 1953 Sep;8(9):769–812. [PubMed] [Google Scholar]
- Conrad M. E., Benjamin B. I., Williams H. L., Foy A. L. Human absorption of hemoglobin-iron. Gastroenterology. 1967 Jul;53(1):5–10. [PubMed] [Google Scholar]
- Dawson R. B., Rafal S., Weintraub L. R. Absorption of hemoglobin iron: the role of xanthine oxidase in the intestinal heme-splitting reaction. Blood. 1970 Jan;35(1):94–103. [PubMed] [Google Scholar]
- ERICSSON J. L. ABSORPTION AND DECOMPOSITION OF HOMOLOGOUS HEMOGLOBIN IN RENAL PROXIMAL TUBULAR CELLS. Acta Pathol Microbiol Scand Suppl. 1964;168:SUPPL 168–168:1+. [PubMed] [Google Scholar]
- GABRIELI E. R., HECKERT P., ELLIOTT A. Kinetics of plasma hemoglobin catabolism in the rat. I. Blood clearance of I-131 tagged hemoglobin. Proc Soc Exp Biol Med. 1962 Apr;109:787–791. doi: 10.3181/00379727-109-27336. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., JONES A. R., CASTLE W. B. The destruction of red cells by antibodies in man. I. Observations of the sequestration and lysis of red cells altered by immune mechanisms. J Clin Invest. 1957 Oct;36(10):1428–1459. doi: 10.1172/JCI103542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J. R., Schneeberger E. E. The renal lesion associated with hemoglobinemia. II. Its structural characteristics in the rat. J Exp Med. 1966 Mar 1;123(3):537–545. doi: 10.1084/jem.123.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J. R. The renal lesion associated with hemoglobinemia: a study of the pathogenesis of the excretory defect in the rat. J Clin Invest. 1967 Mar;46(3):378–387. doi: 10.1172/JCI105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene W. R., Jandl J. H. The sites of hemoglobin catabolism. Blood. 1965 Dec;26(6):705–719. [PubMed] [Google Scholar]
- Kuriyama Y., Omura T., Siekevitz P., Palade G. E. Effects of phenobarbital on the synthesis and degradation of the protein components of rat liver microsomal membranes. J Biol Chem. 1969 Apr 25;244(8):2017–2026. [PubMed] [Google Scholar]
- LATHEM W., DAVIS B. B., ZWEIG P. H., DEW R. The demonstration and localization of renal tubular reabosorption of hemoglobin by stop flow analysis. J Clin Invest. 1960 Jun;39:840–845. doi: 10.1172/JCI104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaw S. A., Winchell H. S. Endogenous production of 14CO: A method for calculation of RBC life-span in vivo. Blood. 1970 Nov;36(5):642–656. [PubMed] [Google Scholar]
- MALMENDIER C. L., DEKOSTER J. P., VANDER VEIKEN F., BRAUMAN H., DEMYTTENAERE M., LAMBERT P. Stopflow analysis applied to the excretion of hemoglobin. Am J Physiol. 1960 Aug;199:292–294. doi: 10.1152/ajplegacy.1960.199.2.292. [DOI] [PubMed] [Google Scholar]
- MILLER F. Hemoglobin absorption by the cells of the proximal convoluted tubule in mouse kidney. J Biophys Biochem Cytol. 1960 Dec;8:689–718. doi: 10.1083/jcb.8.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- OSTROW J. D., JANDL J. H., SCHMID R. The formation of bilirubin from hemoglobin in vivo. J Clin Invest. 1962 Aug;41:1628–1637. doi: 10.1172/JCI104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimstone N. R., Tenhunen R., Seitz P. T., Marver H. S., Schmid R. The enzymatic degradation of hemoglobin to bile pigments by macrophages. J Exp Med. 1971 Jun 1;133(6):1264–1281. doi: 10.1084/jem.133.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. H., Owen C. A., Jr, Flock E. V., Schmid R. Bilirubin formation in the liver from nonhemoglobin sources. Experiments with isolated, perfused rat liver. Blood. 1965 Dec;26(6):823–829. [PubMed] [Google Scholar]
- Robinson S. H. The origins of bilirubin. N Engl J Med. 1968 Jul 18;279(3):143–149. doi: 10.1056/NEJM196807182790306. [DOI] [PubMed] [Google Scholar]
- Schmid R., Marver H. S., Hammaker L. Enhanced formation of rapidly labelled bilirubin by phenobarbital: hepatic microsomal cytochromes as a possible source. Biochem Biophys Res Commun. 1966 Aug 12;24(3):319–328. doi: 10.1016/0006-291x(66)90158-6. [DOI] [PubMed] [Google Scholar]
- Simon S. R., Konigsberg W. H. Chemical modification of hemoglobins: a study of conformation restraint by internal bridging. Proc Natl Acad Sci U S A. 1966 Aug;56(2):749–756. doi: 10.1073/pnas.56.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of hemoglobin to bilirubin. Trans Assoc Am Physicians. 1969;82:363–371. [PubMed] [Google Scholar]
- Tenhunen R., Ross M. E., Marver H. S., Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970 Jan 20;9(2):298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- Wheby M. S., Suttle G. E., Ford K. T., 3rd Intestinal absorption of hemoglobin iron. Gastroenterology. 1970 May;58(5):647–654. [PubMed] [Google Scholar]