Abstract
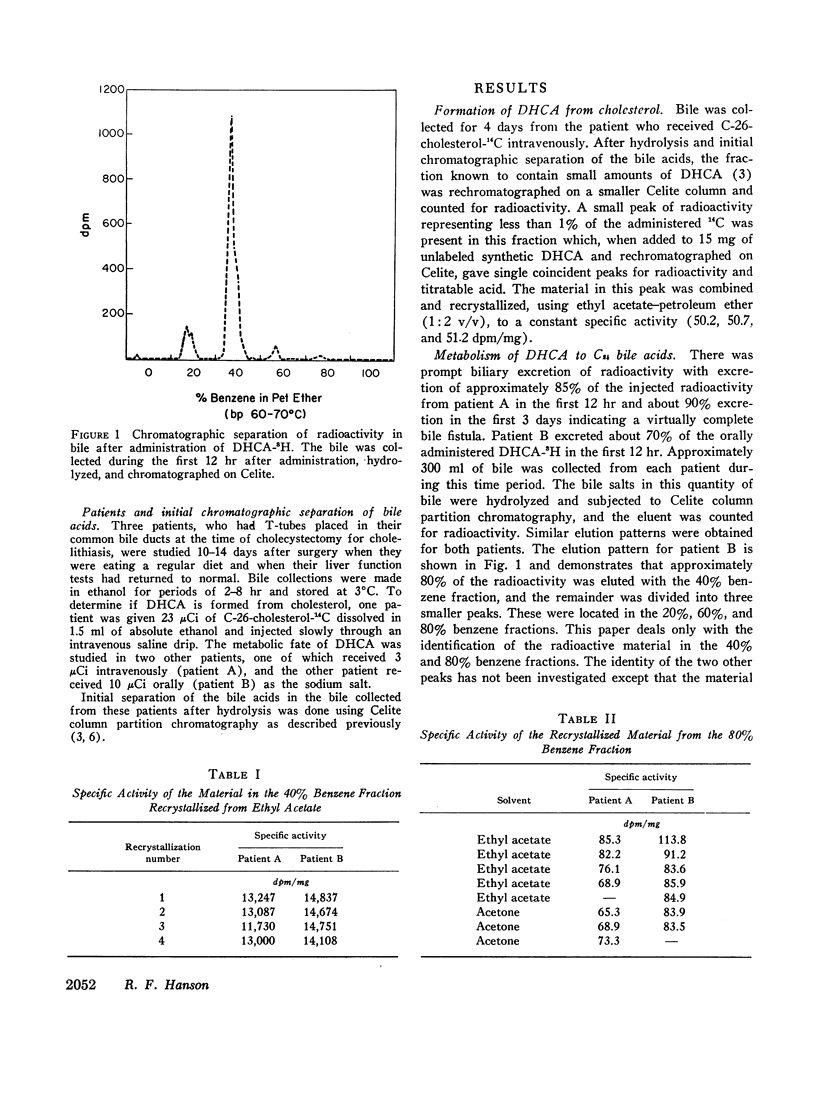
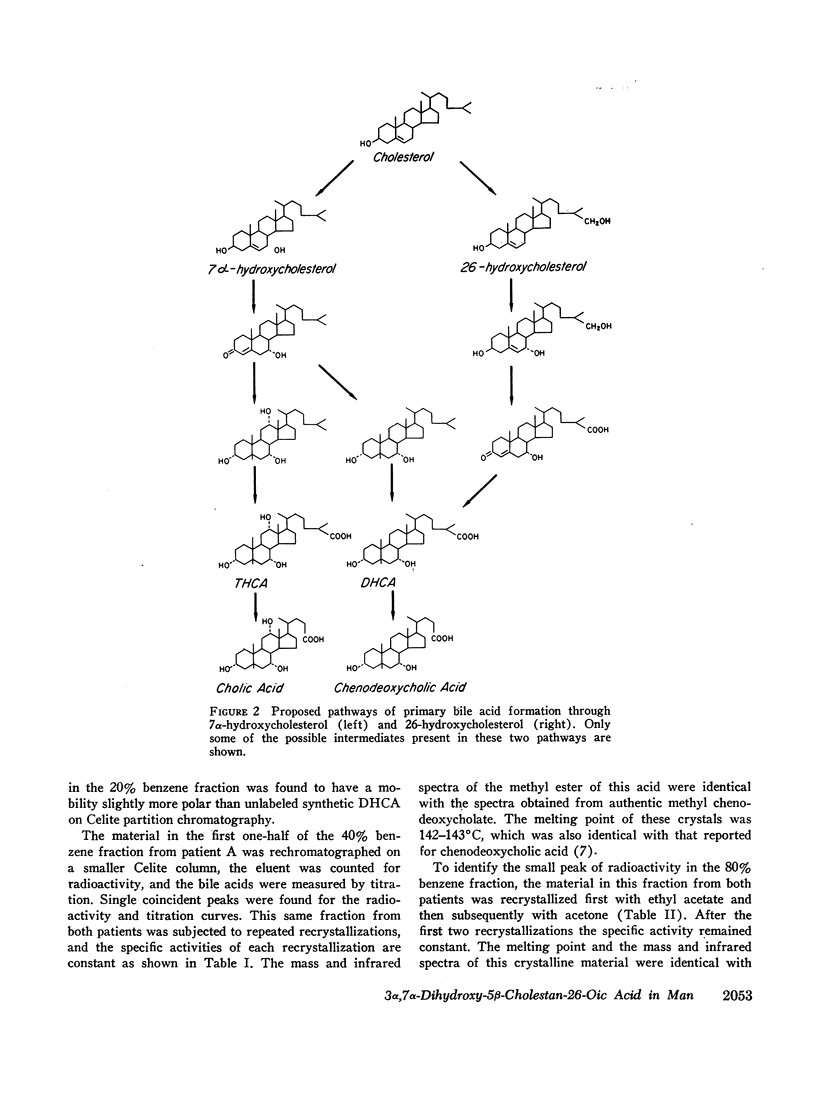
The formation and metabolism of a naturally occurring C27 bile acid, 3α,7α-dihydroxy-5β-cholestan-26-oic acid, was studied in patients with T-tube bile fistulas. C-26-cholesterol-14C was shown to be converted to this C27 bile acid. After synthesis and labeling with tritium, 3α,7α-dihydroxy-5β-cholestan-26-oic acid was efficiently metabolized to chenodeoxycholic acid. After oral and i.v. administration there was conversion of about 80% of the administered amount to chenodeoxycholic acid. A small amount, less than 2% of the administered radioactivity, was converted to cholic acid. The remainder of the radioactivity was excreted in two unidentified peaks of radioactivity.
The results of this study demonstrate that 3α,7α-dihydroxy-5β-cholestan-26-oic acid is a metabolic product of cholesterol and is further metabolized, predominantly to chenodeoxycholic acid and to a minor extent to cholic acid in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRIDGWATER R. J. Partial synthesis of the two 3alpha:7alpha:12alpha-trihydroxycoprostanic acids and of similar bile acids with extended chains. Biochem J. 1956 Dec;64(4):593–599. doi: 10.1042/bj0640593a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY J. B., Jr CONVERSION OF CHOLESTEROL TO TRIHYDROXYCOPROSTANIC ACID AND CHOLIC ACID IN MAN. J Clin Invest. 1964 Jul;43:1443–1448. doi: 10.1172/JCI105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY J. B., Jr The serum trihydroxy-dihydroxy bile acid ratio in liver and biliary tract disease. J Clin Invest. 1958 Nov;37(11):1494–1503. doi: 10.1172/JCI103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. D., Aplin R. T. Mass spectrometric studies on bile acids: the differentiation between chenodeoxycholic acid and deoxycholic acid and the identification of 3alpha, 7alpha-dihydroxy-5beta-cholestanoic acid in alligator bile. Steroids. 1966 Oct;8(4):565–579. doi: 10.1016/0039-128x(66)90051-1. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Williams G. The isolation and identification of 3 alpha, 7 alpha-dihydroxy-5 beta-cholestan-26-oic acid from human bile. Biochem J. 1971 Mar;121(5):863–864. doi: 10.1042/bj1210863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSBACH E. H., ZOMZELY C., KENDALL F. E. Separation of bile acids by column-partition chromatography. Arch Biochem Biophys. 1954 Jan;48(1):95–101. doi: 10.1016/0003-9861(54)90309-4. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Myant N. B. The formation of lithocholic acid, chenodeoxycholic acid and other bile acids from 3 beta-hydroxychol-5-enoic acid in vitro and in vivo. Biochim Biophys Acta. 1967 Oct 2;144(2):430–439. doi: 10.1016/0005-2760(67)90173-7. [DOI] [PubMed] [Google Scholar]
- SULD H. M., STAPLE E., GURIN S. Mechanism of formation of bile acids from cholesterol: oxidation of 5bita-choles-tane-3alpha,7alpha,12alpha-triol and formation of propionic acid from the side chain by rat liver mitochondria. J Biol Chem. 1962 Feb;237:338–344. [PubMed] [Google Scholar]
- Wachtel N., Emerman S., Javitt N. B. Metabolism of cholest-5-ene-3 beta, 26-diol in the rat and hamster. J Biol Chem. 1968 Oct 10;243(19):5207–5212. [PubMed] [Google Scholar]



