Abstract
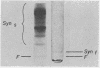
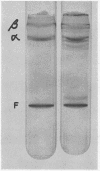
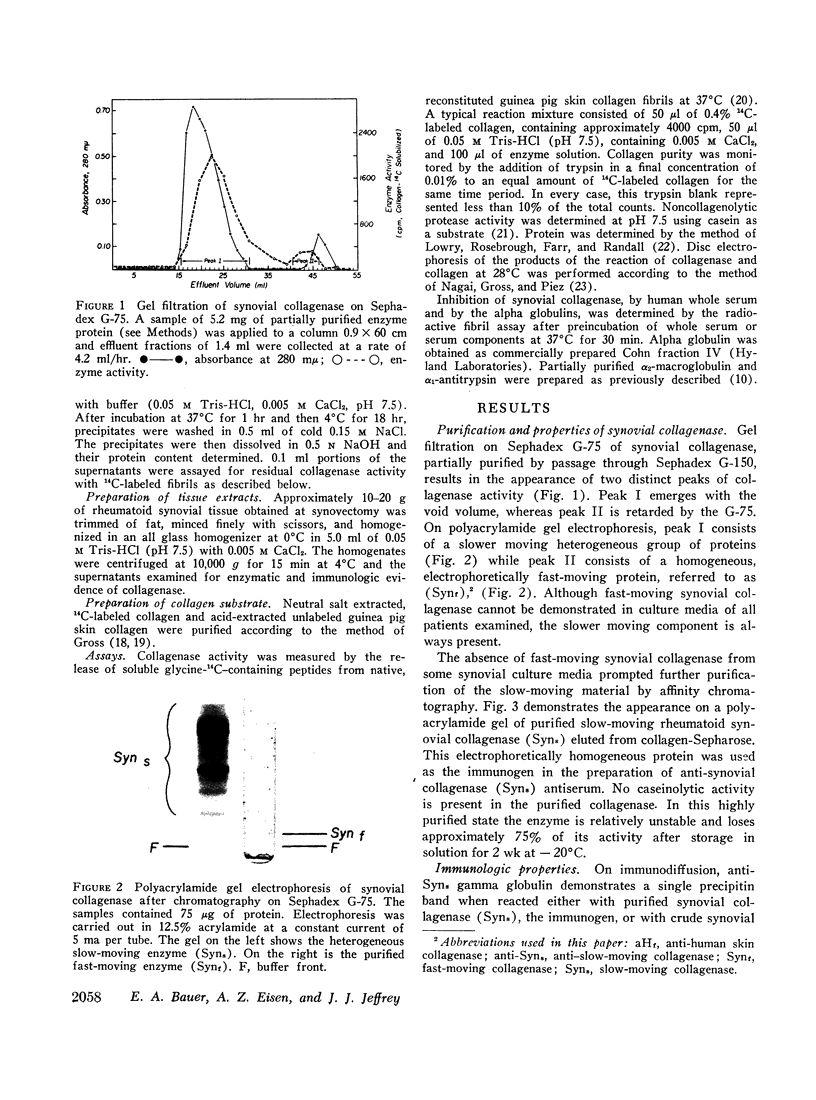
Rheumatoid synovial collagenase obtained from culture medium can be separated by Sephadex gel filtration into two peaks of enzyme activity. These have been designated as fast-moving and slow-moving rheumatoid synovial collagenases on the basis of their electrophoretic mobility on polyacrylamide gels. The slow-moving rheumatoid synovial collagenase has been highly purified by affinity chromatography on collagen conjugated to Sepharose and used to prepare a monospecific anti-synovial collagenase antiserum.
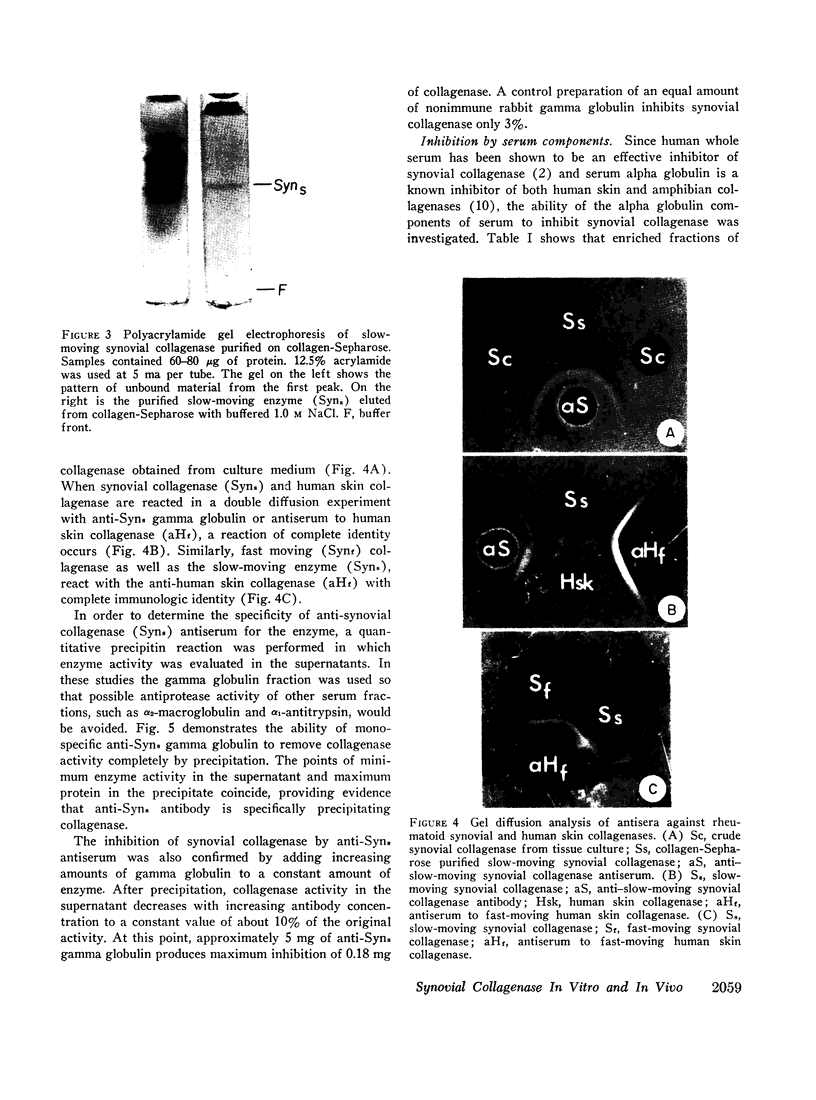
The antiserum against rheumatoid synovial collagenase has permitted the demonstration of immunoreactive collagenase in extracts of rheumatoid synovial tissue that have no detectable enzymatic activity. Collagenase has also been detected immunologically in enzymatically inactive culture medium from the first 24 hr of culture. Recovery of collagenase activity appears to be related to the chromatographic separation of the enzyme from serum antiproteases. The demonstration of collagenase in vivo in rheumatoid synovium adds further support for the concept that the enzyme is present in tissue at levels that are of significance in the pathogenesis of rheumatoid arthritis.
In addition, rheumatoid synovial collagenase and human skin collagenase show complete immunologic identity when reacted with monospecific antiserum prepared against either of these purified enzymes, indicating that organ specificity between these two human collagenases is unlikely.
Full text
PDF

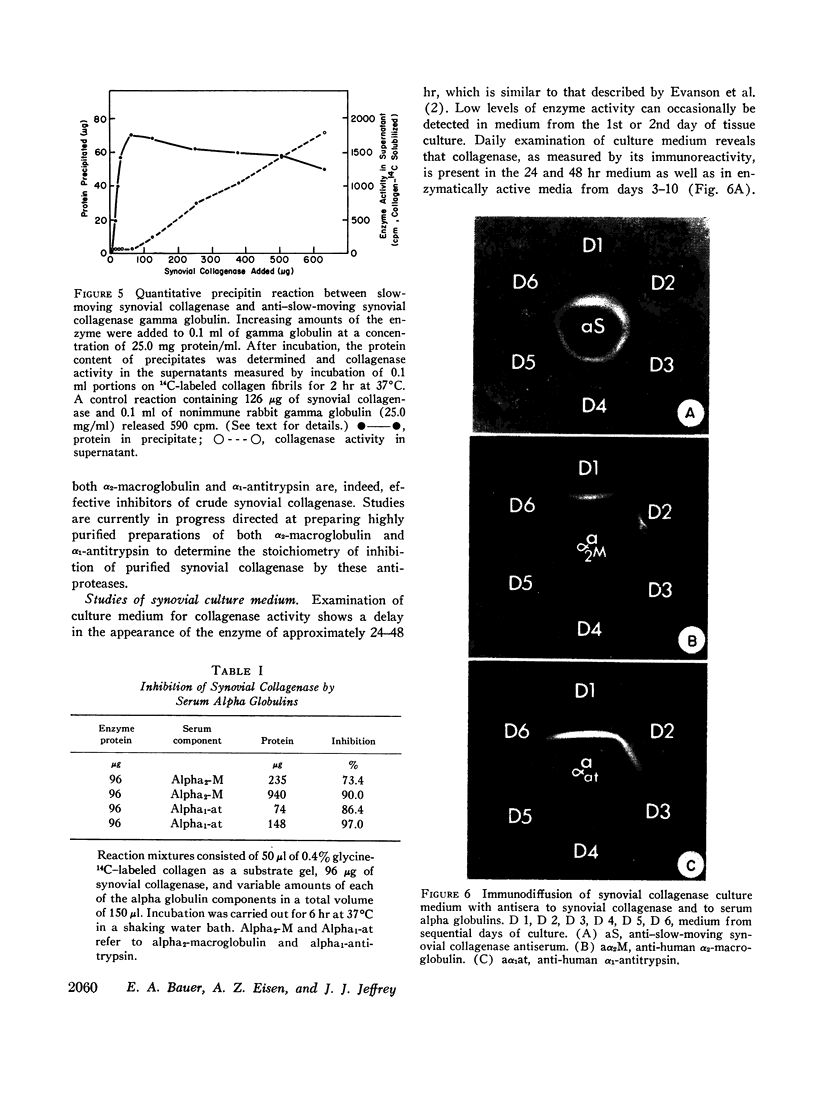
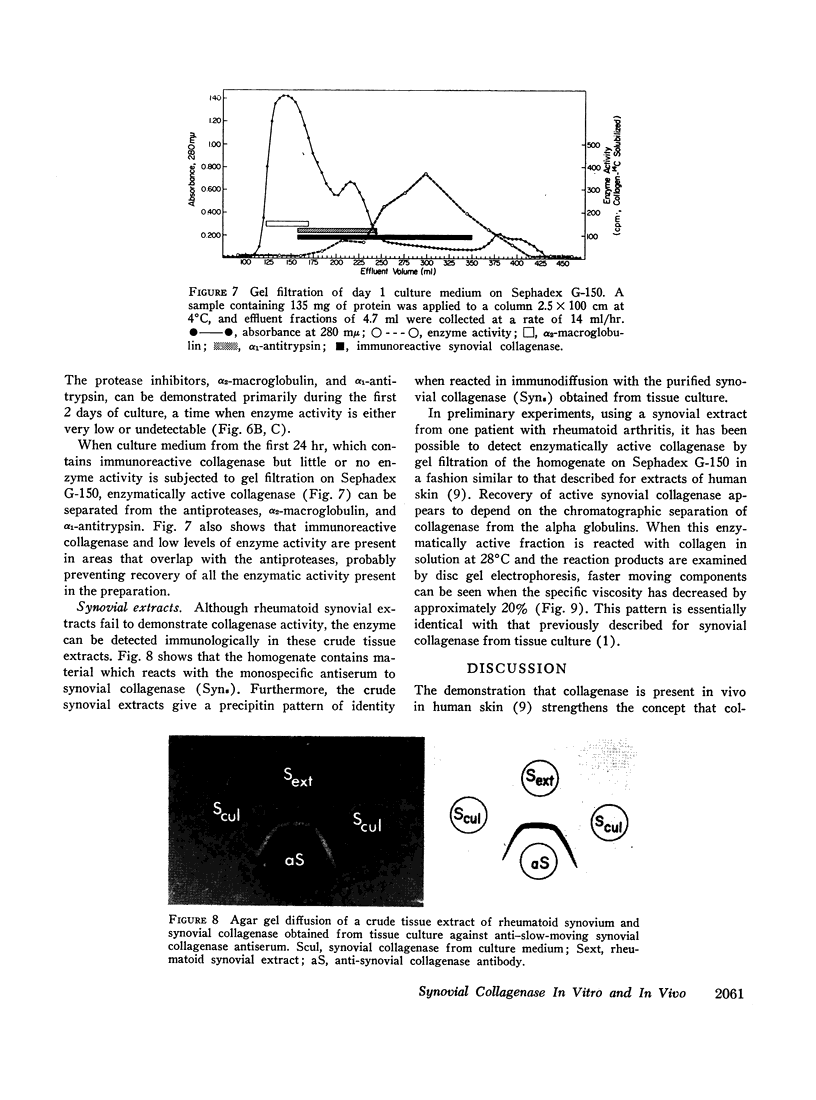
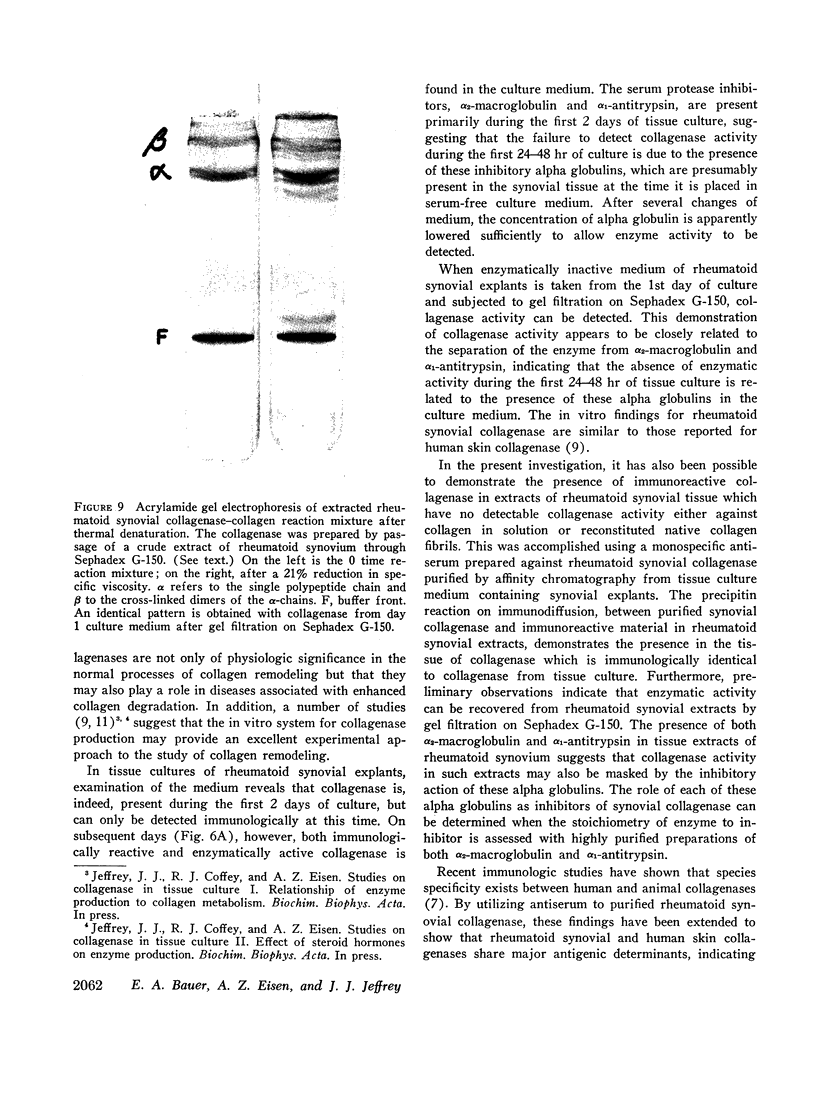


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Eisen A. Z., Jeffrey J. J. Immunologic relationship of a purified human skin collagenase to other human and animal collagenases. Biochim Biophys Acta. 1970 Apr 22;206(1):152–160. doi: 10.1016/0005-2744(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bauer E. A., Jeffrey J. J. Animal and human collagenases. J Invest Dermatol. 1970 Dec;55(6):359–373. doi: 10.1111/1523-1747.ep12260483. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin collagenase. The role of serum alpha-globulins in the control of activity in vivo and in vitro. Proc Natl Acad Sci U S A. 1971 Jan;68(1):248–251. doi: 10.1073/pnas.68.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A. Z., Bloch K. J., Sakai T. Inhibition of human skin collagenase by human serum. J Lab Clin Med. 1970 Feb;75(2):258–263. [PubMed] [Google Scholar]
- Eisen A. Z., Jeffrey J. J., Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968 Mar 25;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Human collagenase: identification and characterization of an enzyme from rheumatoid synovium in culture. Science. 1967 Oct 27;158(3800):499–502. doi: 10.1126/science.158.3800.499. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Studies on collagenase from rheumatoid synovium in tissue culture. J Clin Invest. 1968 Dec;47(12):2639–2651. doi: 10.1172/JCI105947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- GROSS J. Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. J Exp Med. 1958 Feb 1;107(2):247–263. doi: 10.1084/jem.107.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Cohen G. L., Krane S. M. Synovial collagenase: its presence in culture from joint disease of diverse etiology. Arthritis Rheum. 1969 Apr;12(2):92–102. doi: 10.1002/art.1780120206. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NAGAI Y., GROSS J., PIEZ K. A. DISC ELECTROPHORESIS OF COLLAGEN COMPONENTS. Ann N Y Acad Sci. 1964 Dec 28;121:494–500. doi: 10.1111/j.1749-6632.1964.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]