Abstract
Src family kinases (SFK) are critical for initiating and regulating the response of mast cells activated by engagement of the high-affinity IgE receptor, FcεRI. Lyn is the predominant SFK in mast cells and has been ascribed both positive and negative roles in regulating mast cell activation. We analyzed the mast cell phenotype of WeeB, a recently described mouse mutant that expresses a Lyn protein with profoundly reduced catalytic activity. Surprisingly, we found that this residual activity is sufficient for wild-type levels of cytokine production and degranulation in bone marrow-derived mast cells after low-intensity stimulation with anti-IgE. High-intensity stimulation of lyn−/− bone marrow-derived mast cells with highly multivalent Ag resulted in enhanced cytokine production as previously reported, and WeeB cells displayed an intermediate phenotype. Under this latter condition, SFK inhibition using PP2 increased cytokine production in wild-type and WeeB but not lyn−/− cells, resulting in substantially higher levels in the PP2-treated WeeB than in lyn−/− cells. Restoration of wild-type and WeeB lyn alleles in lyn−/− cells generated activation phenotypes similar to those in nontransduced wild-type and WeeB cells, respectively, whereas a kinase-dead allele resulted in a phenotype similar to that of empty-vector–transduced cells. These data indicate that inhibition of Lyn and/or SFK activity can result in higher levels of mast cell activation than simple deletion of lyn and that only near-complete inhibition of Lyn can impair its positive regulatory functions. Furthermore, the data suggest that both positive and negative regulatory functions of Lyn are predominantly carried out by its catalytic activity and not an adaptor function.
Mast cells are effector cells of the innate immune system that respond to bacterial and parasitic infections and are important mediators of IgE-dependent allergic and other inflammatory reactions (1). They secrete a variety of pre-formed and newly synthesized mediators of the inflammatory response, such as histamine, serotonin, leukotrienes, cytokines, and chemokines. FcεRI, the high-affinity IgE receptor expressed on the mast cell surface, is a tetrameric complex consisting of an IgE-binding α subunit, a signal-modulating β subunit, and two copies of the signal-generating FcRγ subunit, which associates with a number of immunoreceptors (2). Aggregation of FcεRI induced by IgE-specific multivalent Ag or by other means, such as anti-IgE cross-linking, results in activation of β subunit-associated Lyn, a Src family tyrosine kinase (SFK). Lyn phosphorylates the ITAMs in the cytoplasmic domains of the β- and γ-chains, which leads to the recruitment of additional Lyn to the β-chain, the tyrosine kinase Syk to the γ-chains, and other signaling and scaffolding molecules to the aggregated receptor complex (3–5).
Lyn is the predominant SFK in mast cells and is estimated to be at least 20-fold more abundant in mast cells than two other SFKs, Fyn and Hck, that have been demonstrated to play a role in FcεRI-dependent signaling (6, 7). Despite its predominance, Lyn-deficient bone marrow-derived mast cells (BMMCs) still respond to FcεRI engagement, with hyperresponsiveness observed under some conditions and hyporesponsiveness observed in others (6, 8–11). Consistent with the observation of Lyn-independent mast cell activation, Fyn- and Hck-deficient BMMCs have reduced biological activity in most functional assays, indicating that these two SFKs contribute substantially toward the signals required for mast cell activation (i.e., positive regulation). For example, Fyn has been shown to mediate phosphorylation of the adaptor Gab2 and subsequently activate PI3K and Ca2+-independent, microtubule-dependent translocation of granules (6, 12). Gab2 phosphorylation and microtubule formation are also reduced in Hck-deficient BMMCs, and Hck further appears to negatively regulate Lyn activity, potentially via a mechanism involving the C-terminal Src kinase-interacting protein Cbp/PAG (7).
In vivo and in vitro studies using Lyn-deficient mice and BMMCs derived from these mice, respectively, have begun to unravel a surprisingly complex regulatory structure in which Lyn plays both positive and negative roles in regulating mast cell activation. Mast cell responses induced via low-intensity stimulation (e.g., anti-IgE cross-linking or monomeric IgE binding) are severely curtailed in lyn−/− BMMCs, highlighting the activating role of Lyn and suggesting that with these “weak” stimuli the residual SFK activity supplied by Fyn and Hck is insufficient to compensate for the loss of Lyn. High-intensity stimulation of lyn−/− BMMCs, via highly multivalent Ag-induced aggregation of receptor-bound IgE, results in enhanced mast cell activation, indicating a dampening role for Lyn under these conditions (10). Low- and high-intensity stimuli are operationally defined by the initial velocity of receptor internalization and are directly correlated with the degree of phosphorylation of proximal signaling molecules (13). The overall effects of Fyn and Hck on mast cell activation appear to be somewhat more straightforward: The absence of either protein generally results in decreased mast cell activity (6, 7). This may in part reflect their substantially lower expression in mast cells and/or lower level of activation in response to FcεRI activation signals; if high-intensity signals are required for negative regulatory events to occur, then these kinases may not generate sufficiently intense signals under most scenarios for this to take place.
The kinase domain of the SFKs is composed of N- and C-terminal lobes flanking an ATP- and substrate-binding cleft. The G loop of the N lobe is important for catalysis by acting as a flexible clamp that covers and anchors the nontransferable ATP α/β phosphates, orienting the ATP for optimal γ-phosphate transfer. WeeB, a mouse strain possessing a lyn mutation that results in the expression of a protein with strongly reduced kinase activity, has recently been described (14). The mutant kinase LynWeeB is the result of an E260G mutation in the G loop of the N lobe and has undetectable kinase activity when immunoprecipitated from BCR-stimulated B cells. Baculovirus-expressed and purified LynWeeB has ≤17% residual catalytic activity, with reduced ATP (~7-fold) and substrate (~5-fold) binding. B cell development and B cell receptor signaling are impaired in these mice but to a lesser extent than observed in lyn−/− mice, and WeeB mice ultimately develop autoimmune glomerulonephritis, although at a much later age than lyn−/− mice (14–17). These data suggested that the positive and negative regulatory impairments observed in Lyn-deficient mast cells under the appropriate conditions might be more moderate in WeeB mast cells, thus confirming the results of our previous study, which indicated that the intensity of FcεRI signaling can determine whether Lyn positively or negatively regulates mast cell activation (10). Moreover, we reasoned that the dramatic reduction of Lyn kinase activity in WeeB cells should provide a more realistic model of the effects of Lyn-specific inhibition on mast cell biology than models in which the protein itself is either knocked out or knocked down. Therefore, the in vitro and in vivo mast cell phenotypes of WeeB mice were analyzed.
Materials and Methods
Abs and other reagents
The anti-DNP IgE mAb [1H]DNP-ε-206 has been previously described (18). DNP21-BSA was purchased from Biosearch Technologies (Novato, CA). Anti-IgE mAb B1E3 was provided by D.H. Conrad (Virginia Commonwealth University, Richmond, VA). Commercial sources of other Abs were as follows: anti-phosphotyrosine mAb 4G10, anti-linker for activation of T cells (LAT), and anti-SHIP1 from Millipore (Bedford, MA); anti-ERK from Invitrogen (Carlsbad, CA); anti-Lyn, anti-Fyn, anti-Hck, anti-phospholipase C (PLC)-γ2, anti-Akt1, anti-p38, anti-JNK1/2, anti-IκB, and anti-β-actin from Santa Cruz Biotechnology (Santa Cruz, CA). All of the other antibodies were from Cell Signaling Technology (Beverly, MA).
Cell culture and FcεRI stimulation
WeeB (14) and lyn−/− (15) mice used were extensively backcrossed onto a C57BL/6 genetic background. Bone marrow cells from wild-type (wt) and mutant mice were cultured in IL-3–containing medium for 4–6 wk to generate >95% pure (c-Kit+ FcεRI+) BMMCs. Stimulation of BMMCs with Ag or anti-IgE was performed as previously described (10). Cells were sensitized for 24 h with 0.5 μg/ml [1H]DNP-ε-206 IgE and washed twice with Tyrode’s buffer or media before stimulation with the indicated concentration of Ag (DNP21-BSA) or the anti-IgE mAb B1E3.
Retroviral transduction
Retroviral transduction of lyn−/− mast cells was performed as previously described (19). Briefly, pMX-puro plasmids harboring wt, WeeB, or kinase-dead (KD) lyn cDNAs were transfected into Plat-E packaging cells (20) to generate recombinant retroviruses. BMMCs in culture medium containing IL-3 and stem cell factor (SCF) were then infected with retrovirus-containing supernatants. Mass populations of puromycin-resistant cells were used for subsequent experiments.
Measurement of histamine and cytokines
Histamine secreted from BMMCs was measured as previously described(18). Supernatants from FcεRI-stimulated cells were measured by ELISA for IL-6 (BD Biosciences, San Jose, CA) and IL-13 (R&D Systems, Minneapolis, MN). SFK inhibition via PP2 (EMD Biosciences, San Diego, CA) was carried out by preincubating cells with the drug or vehicle (DMSO) for 15 min prior to stimulation. Competition with monomeric lysine-DNP was carried out similarly.
Migration assays
Chemotaxis of IgE-sensitized BMMCs toward DNP21-BSA or anti-IgE was performed in fibronectin-coated Transwell chambers (Corning, Corning, NY) as previously described (21). Briefly, 1 × 106 cells were loaded into the upper chambers of wells in a volume of 200 μl, and migration into the indicated concentration of chemoattractant-containing lower chamber was measured after 6 h using a hemocytometer.
Immunoblotting, immunoprecipitations, and in vitro kinase assays
Cells were lysed in 1% NP-40-containing lysis buffer (20 mM Tris-HCl [pH 8.0], 0.15 M NaCl, and 1 mM EDTA) containing protease and phosphatase inhibitors and subjected either to direct immunoblotting or to immunoprecipitation as previously described (22). For kinase assays, immune complexes were washed and incubated in kinase buffer (50 mM HEPES (pH 7.4), 10 μM MgCl2, 10 μM MnCl2, and 0.1 μM ATP) in the presence of 10 μCi [γ-32P]ATP. Reaction products were analyzed by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and exposed to x-ray film.
Ca2+ flux
IgE-sensitized BMMCs were loaded with Indo 1-AM (Calbiochem, San Diego, CA) and stimulated with Ag or anti-IgE at the indicated concentrations as previously described (22), except that the fluorescence ratio was continuously monitored using a BD-LSR II flow cytometer (BD Biosciences).
Passive cutaneous anaphylaxis (PCA)
Mice 10–12 wk of age were sensitized by the administration of 25 ng anti-trinitrophenyl (TNP) IgE or PBS (10 μl) in each ear. Twenty-four hours later, mice were challenged i.v. with 100 μg of TNP26-BSA in 200 μl PBS containing 1% Evans blue. Mice were sacrificed after 45 min, and the ears were removed for subsequent analysis. Evans blue extravasation was quantified by measuring the absorbance at OD620 after overnight incubation of ear tissue in 300 μl formamide at 63°C. Blood samples were collected retro-orbitally, and plasma IgE levels were measured by ELISA (BD Pharmingen, San Diego, CA). For mast cell enumeration, Ag stimulation of mice was performed in the absence of Evans blue, and ears were fixed in 10% formalin and paraffinized. Sections were then stained with 1% toluidine blue, and mast cells were counted in at least three 200× fields. Numbers were then normalized to a 400× (high-power) field. Degranulated mast cells were distinguished by morphology. For mast cell reconstitution into C57BL/6-KitW-sh/W-sh mice, 2 × 106 BMMCs were injected intradermally into mouse ears. After 6 wk, PCA experiments were performed as described above, with the exception that 100 ng anti-TNP IgE was used.
Statistical analysis
Statistical significance was determined using an unpaired two-tailed Student t test except that one-way ANOVA was used for analysis of PCA data (Fig. 7). Results were expressed as mean ± SD unless otherwise stated; p < 0.05 was considered significant.
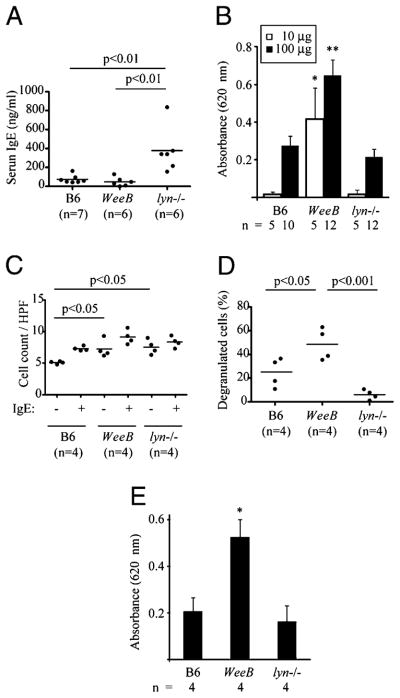
FIGURE 7.
The immediate-phase PCA response in WeeB mice is stronger than the response in wt or lyn−/− mice. A, IgE serum levels of 10-to 12-wk-old mice were measured by ELISA. B, PCA reactions were measured as described in the Materials and Methods. Results are expressed as the mean ± SEM. C, Dermal mast cell numbers in IgE-sensitized and PBS (vehicle)-treated ears after Ag challenge were obtained by staining with toluidine blue. Cells with metachromatically stained granules were counted as mast cells. D, The percentage of degranulated mast cells was determined morphologically. Degranulated mast cells were not observed in PBS-treated ears. E, PCA reactions in C57BL/6-KitW-sh/W-sh mice were performed as in B. *p <0.05; **p <0.001. Error bars represent SEM. Significance was determined by ANOVA.
Results
Growth properties of WeeB mast cells are intermediate to those of wt and lyn−/− mast cells
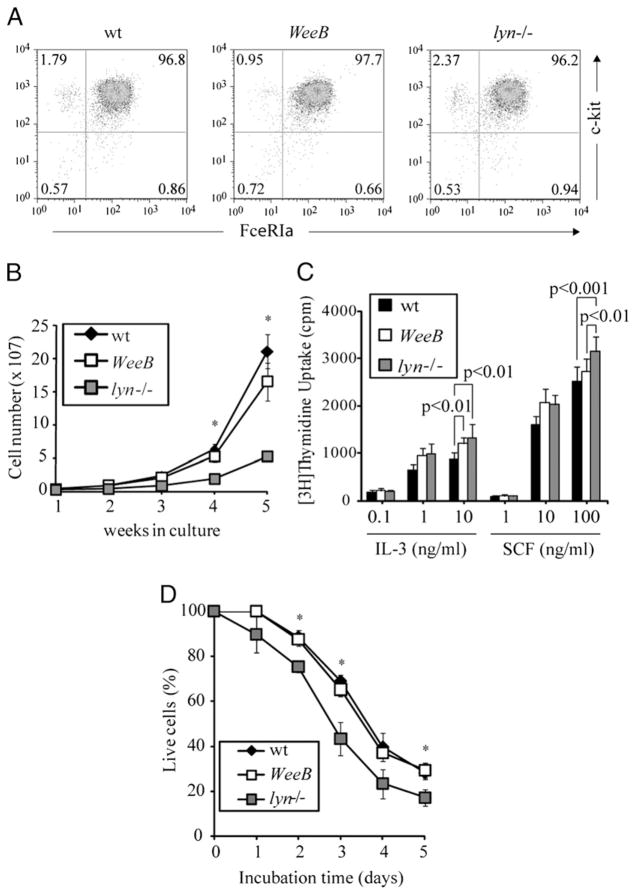
To understand the biological effects of reduced Lyn kinase activity on mast cells, we generated BMMCs from WeeB mice and compared their growth properties with those derived from lyn−/− and wt (C57BL/6) mice. After 4 wk in the presence of IL-3, all three cultures were >95% pure populations of mast cells as defined by surface expression of the FcεRI and c-Kit receptors, with no significant differences in receptor levels detectable between the three genotypes (Fig. 1A). Total cell number increased much more slowly in cultures grown from lyn−/− bone marrow cells than in wt or WeeB cultures. In highly pure populations of BMMCs grown for 4–5 wk, lyn−/− cells proliferated more rapidly than wt cells in the presence of either IL-3 or SCF, as measured by thymidine uptake experiments. WeeB BMMCs proliferated at an intermediate rate (Fig. 1B, 1C). Removal of IL-3 from BMMC cultures results in apoptosis, and this process was accelerated in lyn−/− but not WeeB cells (Fig. 1D). These observations indicate that reduced Lyn kinase activity impacts the in vitro growth properties of mast cells to a lesser degree than the absence of Lyn.
FIGURE 1.
WeeB mast cells have intermediate growth properties. Bone marrow cells were harvested from wt, WeeB, and lyn−/− mice and grown in media containing IL-3 for 4–6 wk. A, Surface expression of FcεRI and c-Kit on 4-wk-old BMMCs derived from B6, WeeB, and lyn−/− mice was assayed by flow cytometry. B, Total cell number was determined during weekly media changes to track the growth of bone marrow cells cultured in IL-3-containing medium. Data represent means and SD. *p<0.05 versus lyn−/− cells. C, BMMCs >4-wk-old were grown in the indicated concentrations of IL-3 or SCF in the presence of [3H]thymidine for 5 h to measure cell proliferation. D, Apoptosis after IL-3 withdrawal was measured by Annexin V and 7-amino-actinomycin D staining using flow cytometry. The percentage of double-negative cells is plotted as a function of incubation time. *p <0.001 versus lyn−/− cells. Representative results from at least three independent experiments are presented.
Reduced Lyn kinase activity does not impair cytokine production or histamine release in WeeB mast cells after low-intensity stimulation
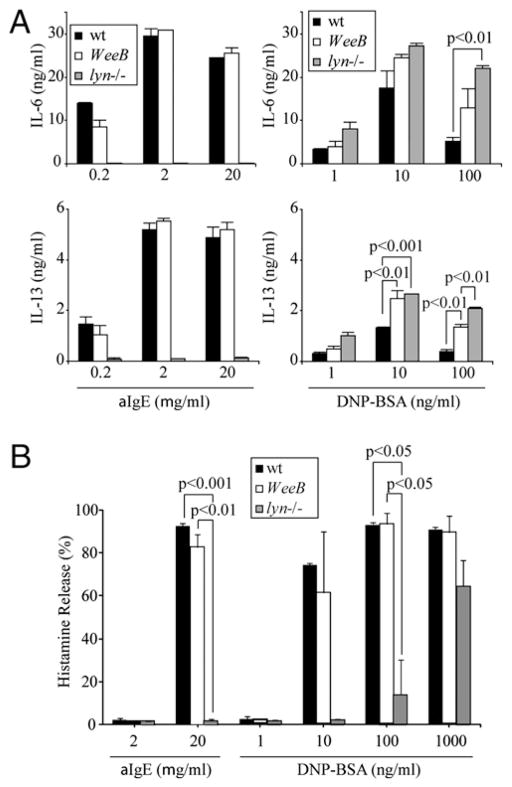
Both increased and decreased activation have been reported in lyn−/− mast cells and other cell types (6, 8–11). Engagement of IgE-FcεRI complexes with high-intensity stimuli, such as moderate to high concentrations of highly multivalent Ag, strongly activates the catalytic activity of Lyn and results in a high degree of phosphorylation and/or activation of its direct and indirect downstream targets. For the most part, these pathways are marginally activated in lyn−/− BMMCs, yet various mast cell effector functions are enhanced, indicating a net negative regulatory role for Lyn under these conditions (10). Cross-linking of IgE-FcεRI complexes with a low-intensity stimulus, such as anti-IgE, results in weak activation of Lyn, but this weak activity appears to be indispensable for generating mast cell effector functions, because low-intensity stimulation of lyn−/− mast cells results in minimal levels of degranulation and cytokine production. We hypothesized that diminished Lyn kinase activity would result in mast cells less hyporesponsive than lyn−/− cells after anti-IgE cross-linking but also less hyperresponsive than lyn−/− cells after multivalent Ag stimulation. To test this hypothesis, wt, WeeB, and lyn−/− BMMCs were sensitized with IgE and then stimulated with increasing concentrations of anti-IgE (0.2–20 μg/ml B1E3) or Ag (1–100 ng/ml DNP21-BSA). Cells were measured for cytokine production and degranulation, two hallmarks of mast cell activation. Cytokine production in Ag-stimulated WeeB cells was elevated as compared with that in wt cells but lower than that in lyn−/− cells, consistent with this hypothesis. In contrast, WeeB cells stimulated with anti-IgE had levels of IL-6 and IL-13 production that were comparable to those from wt cells, whereas cytokine production in lyn−/− cells was negligible (Fig. 2A), as reported previously (10). Both anti-IgE and Ag stimuli induced near-complete histamine release at high and equivalent levels in wt and WeeB cells, whereas lyn−/− cells displayed sharply reduced or undetectable levels of histamine release under most conditions (Fig. 2B). The increasing histamine release in lyn−/− cells with increasing multivalent Ag concentrations indicated that even with a high-intensity stimulus the SFK activity generated in these cells was insufficient to induce degranulation efficiently. These data indicate that the dramatically reduced kinase activity of LynWeeB is sufficient for unimpaired positive regulation of FcεRI-induced mast cell activation.
FIGURE 2.
WeeB BMMCs display wt levels of degranulation and cytokine production in response to low-intensity stimulation. BMMCs were sensitized with anti-DNP IgE 206 overnight, washed, and then stimulated with the indicated concentrations of anti-IgE (B1E3) or Ag (DNP21-BSA) for 16 h for cytokine production (A) or 45 min for histamine release (B). Results shown are representative of at least three independent experiments.
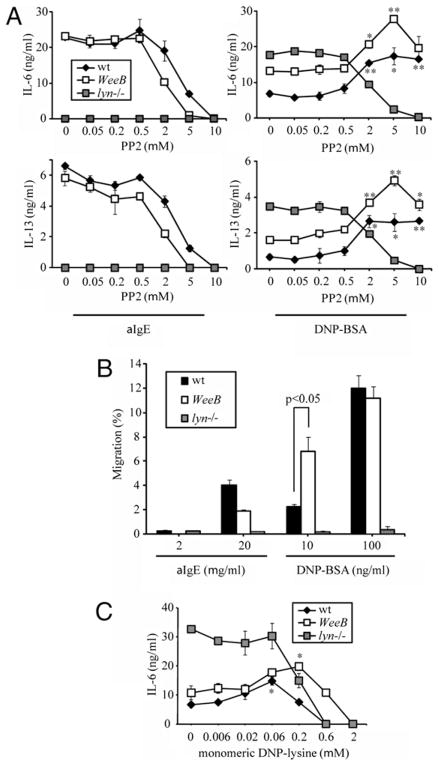
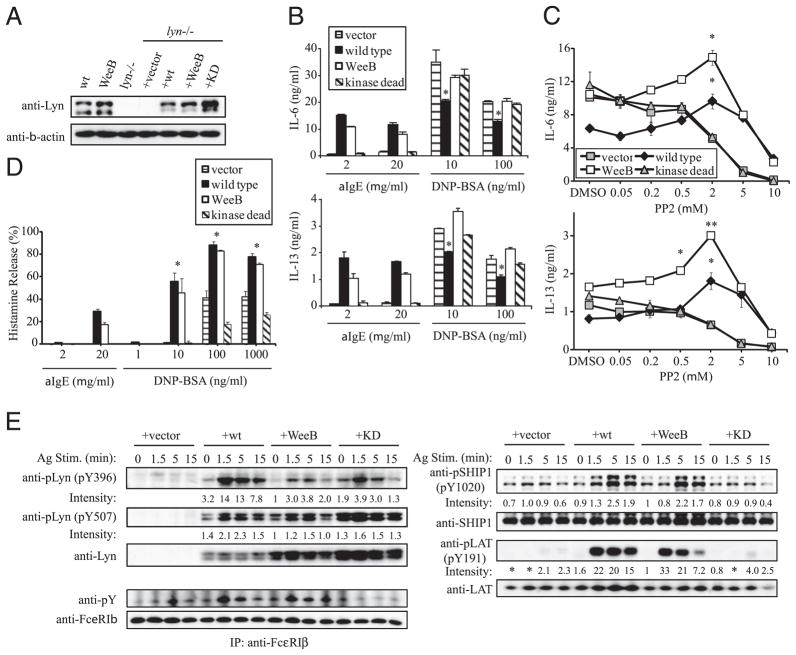
Further reduction of SFK activity in WeeB mast cells increases cytokine production beyond the already elevated levels seen in lyn−/− cells
The finding that the minor kinase activity associated with LynWeeB could result in wt levels of cytokine production and degranulation after anti-IgE stimulation was unexpected. This suggested that the positive regulatory aspects of Lyn reside in a surprisingly small portion of its total kinase capacity. Alternatively, if this residual kinase activity is so low as to be biologically irrelevant, then the activating role of Lyn might reside in its ability to interact with other proteins in a manner independent of its kinase activity (i.e., as an adaptor). To address the former possibility, IgE-sensitized cells were pretreated with increasing concentrations of the SFK inhibitor PP2, stimulated with either anti-IgE or Ag, and assayed for cytokine production (Fig. 3A). Both wt and WeeB cells pre-treated with 2–10 μM PP2 and subsequently stimulated with multivalent Ag (100 ng/ml DNP21-BSA) displayed increased levels of IL-6 production. IL-6 production in PP2-treated WeeB cells was even higher than that observed in lyn−/− mast cells, which themselves display enhanced cytokine production in the absence of SFK inhibition. IL-6 production from lyn−/− cells did not increase with PP2 treatment, nor was a PP2-dependent increase in IL-6 production observed in anti-IgE-stimulated wt or WeeB cells, suggesting the loss of negative regulation in lyn−/− cells under all of the conditions tested and its absence in all of the genotypes stimulated with anti-IgE. A similar response to increasing concentrations of PP2 was observed for IL-13 production (Fig 3A, lower panels). Overall, these data demonstrate that with high-intensity Ag stimulation even WeeB mast cells retain residual net negative regulatory capacity that can be overcome with SFK inhibition. Because PP2-inhibited Ag-stimulated WeeB cells produce higher levels of IL-6 and IL-13 than untreated lyn−/− cells, it seems likely that the hyperresponsive phenotype seen with Lyn deficiency only partially reflects the loss of negative regulation, partly offset by the loss of some positive regulation. Similar to our observation that some conditions resulting in weak Lyn/SFK activation (e.g., PP2 pretreatment) can lead to higher levels of cytokine production in WeeB than in wt or Lyn-deficient BMMCs, we found that moderate levels of multivalent Ag (10 ng/ml DNP21-BSA) induced more chemotaxis in WeeB cells than in the other two genotypes (Fig. 3B). Anti-IgE was very inefficient at inducing migration of even wt BMMCs, and a partial defect in WeeB cells was observed. This suggests that if the threshold for a biological effect to occur is high enough, then even positive regulation is impaired in this genotype. These data indicate that low levels of Lyn or SFK activity lead to a hyperresponsive mast cell phenotype that is more extreme than the lyn−/− mast cell phenotype might have suggested. Some concentrations of multivalent Ag led to higher levels of migration in WeeB cells than in wt or lyn−/− cells, but WeeB cells always produced intermediate amounts of cytokines unless PP2 was included. This observation likely reflects differences in the requirements of cytokine production and migration for a particular level of SFK activity to generate various levels of positive and negative signals (i.e., different biological effects may require more or less stimulus to occur). Nevertheless, it might be expected that stimulation conditions should exist whereby WeeB cells produce more cytokines than either wt or lyn−/− cells, without the need for pharmacological treatment. Although both PP2 and decreased multivalent Ag concentration result in decreased SFK activation, they clearly do not result in equivalent effects on cytokine production. Because anti-IgE cross-linking results in very low levels of SFK activation and overall signal transduction as compared with multivalent Ag stimulation, it is reasonable to assume that low stimulus valence also leads to reduced SFK activity levels. Therefore, we tested the effects of reduced valence on cytokine production. The effective valence of our multivalent Ag stimulus was reduced by titrating with increasing amounts of a monovalent competitor, lysine-DNP (Fig. 3C). Lysine-DNP binds to mast cell surface-bound IgE, reducing the number of IgE-FcεRI complexes available for subsequent clustering and effectively reducing the number of complexes that a given DNP21-BSA molecule will cluster. Although IL-6 levels in lyn−/− cells were particularly high in this experiment (an outlier but not entirely unexpected), the results were quite similar to those seen with PP2 titration (compare Fig. 3A with Fig. 3C). WeeB and wt cells displayed increased IL-6 production with decreased effective stimulus valence, whereas lyn−/− cells did not, and WeeB cells produced more IL-6 than either of the other two genotypes under some conditions, similar to the migration results. This result is consistent with the idea that low Lyn/SFK activity is associated with high biological activity and also with the hypothesis that the manner in which the cellular level of Lyn/SFK activity is achieved (a combination of concentration and valence) is also important.
FIGURE 3.
Treatment with the SFK inhibitor PP2 results in increased levels of IL-6 and IL-13 production in Ag-stimulated B6 and WeeB but not lyn−/− BMMCs. A, Cells were sensitized with anti-DNP IgE 206 overnight, pretreated with the indicated concentrations of PP2 or vehicle (DMSO) for 15 min, and then stimulated for 16 h with either 100 ng/ml DNP21-BSA or 2 μg/ml anti-IgE. Error bars represent SD. Results shown are representative of three independent experiments. B, Migration assays were performed in Transwell chambers using the indicated concentrations of DNP21-BSA or anti-IgE B1E3 in the lower wells. C, Lysine-DNP pretreatments with the indicated concentrations of monomer (in media) were performed as in A. *p <0.05; **p <0.01 versus the same genotype in the absence of PP2 or lysine-DNP.
The weak kinase activity of LynWeeB is sufficient for near-normal phosphorylation levels of Lyn substrates and wt levels of downstream phosphorylation events
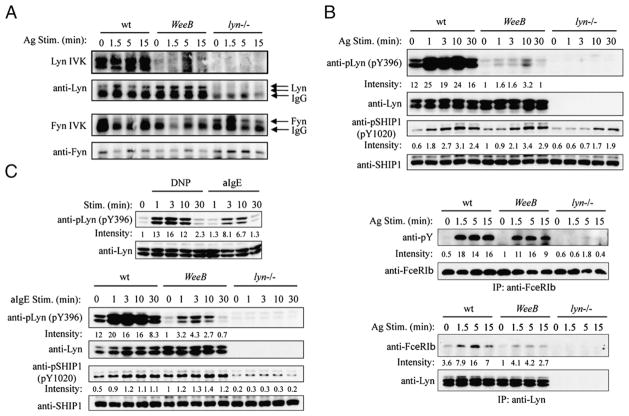
In vitro kinase (IVK) assays from WeeB B cell immunoprecipitates had undetectable levels of kinase activity, whereas assays using purified preparations of baculovirus-expressed proteins indicated that a small amount of kinase activity is still associated with LynWeeB (14). We repeated the IVK experiment in mast cells and were able to detect residual kinase activity associated with LynWeeB (Fig. 4A). Activation loop Tyr396 phosphorylation of Lyn was also greatly reduced in multivalent Ag-stimulated mast cells (Fig. 4B). Nevertheless, the FcεRIβ subunit and the lipid phosphatase SHIP1, shown to be direct Lyn phosphorylation targets (10, 23–26), were only moderately hypophosphorylated or normally phosphorylated, respectively, in WeeB cells. Recruitment of Lyn to the β-chain was reduced, but to a far less extent than activation loop phosphorylation might have suggested (Fig. 4B). Similar data were obtained with anti-IgE-stimulated cells, although the level of phosphorylation was reduced significantly overall (Fig. 4C and data not shown). Fyn activity was increased at 1.5 min of Ag stimulation in lyn−/− BMMCs as expected (6) but was normal in WeeB cells (Fig. 4A). Further downstream, we found no consistent differences between Ag-stimulated wt and WeeB mast cells with respect to the phosphorylation levels of important signaling molecules, including LAT, PLC-γ2, Akt1, and IKK, or the MAPK proteins p38, Erk1/2, and JNK1/2 (Fig. 5A, 5B). Calcium flux was essentially normal in WeeB cells, although, interestingly, there was a short delay compared with wt cells that grew less pronounced with increasing Ag concentration (Fig. 5C). A clear difference in calcium flux was present between wt and WeeB cells stimulated with anti-IgE. Consistent with this observation, PLC-γ2 phosphorylation was decreased in WeeB cells stimulated with anti-IgE, although other signaling molecules had either normal or only slightly reduced levels of phosphorylation (Fig. 5D and data not shown). The small calcium flux measured in anti-IgE-stimulated WeeB cells is apparently sufficient for wt levels of degranulation, just as the barely detectable calcium flux measured in lyn−/− cells treated with the highest tested concentration of multivalent Ag (1000 ng/ml) is sufficient for near-wt levels of degranulation. These results differ from those obtained in B cells isolated from WeeB mice, which generally had intermediate levels of phosphorylation compared with those in wt-and lyn−/−-derived B cells. This may reflect either the biology of different cell types or differences in the reagents used to study and activate different receptor systems in vitro. One possibility is that in vitro BCR stimulation of B cells may be analogous to only a very low level of anti-IgE cross-linking of the FcεRI receptor in mast cells, which could explain why we were able to detect the residual Lyn kinase activity of WeeB BMMCs in IVK assays using lysates derived from multivalent Ag-stimulated cells.
FIGURE 4.
Lyn activity is dramatically reduced in WeeB BMMCs, but phosphorylation levels of Lyn targets are only moderately impaired or normal. A, In vitro kinase assays for Lyn and Fyn were carried out after immunoprecipitation from cell lysates using the appropriate antisera. Equal loading was subsequently confirmed by immunoblotting using either the same blot or a duplicate blot derived from a portion of the immunoprecipitates. B, BMMCs were sensitized with IgE overnight and stimulated with 100 ng/ml DNP21-BSA for the indicated periods. Cell lysates were analyzed by SDS-PAGE followed by immunoblotting with the indicated antibodies. Immunoprecipitates of the FcεRIβ subunit and of Lyn were analyzed by immunoblotting with anti-phosphotyrosine (4G10) and anti-FcεRIβ, respectively, followed by reprobing of the blots with anti-FcεRIβ and anti-Lyn, respectively. C, Analysis of phospho-Lyn and phospho-SHIP1 after stimulation with 20 μg/ml anti-IgE (bottom panel) as described in A. Immunoblotting results are representative of three independent experiments, and kinase assays are representative of two independent experiments.
FIGURE 5.
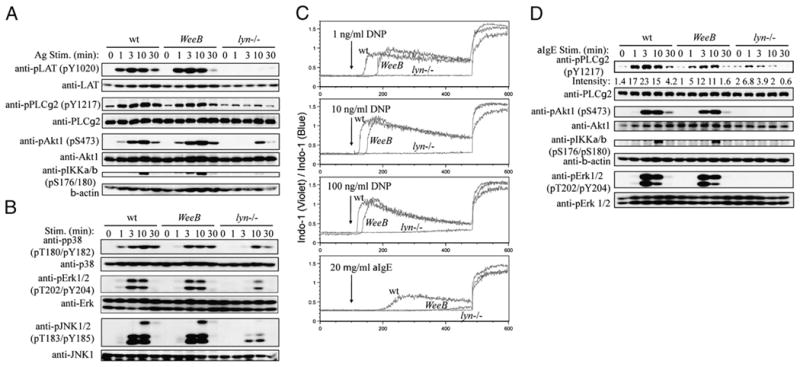
Downstream FcεRI signal transduction is largely intact in WeeB BMMCs. BMMCs were sensitized with IgE overnight and stimulated with 100 ng/ml DNP21-BSA for the indicated periods. A, Membrane proximal phosphorylation events were analyzed by immunoblotting using antisera to phospho-LAT (Tyr191), phospho-PLC-γ2 (Tyr1217), and phospho-Akt1 (Ser473). Blots were subsequently stripped and reprobed using antibodies to LAT, PLC-γ2, and Akt1. B, MAPK phosphorylation events were analyzed as in A using the indicated antibodies. C, Ca2+ flux was measured using BMMCs loaded with Indo 1-AM and stimulated with the indicated concentrations of Ag, followed by the addition of 1 μg/ml ionomycin. D, A panel of phosphospecific antisera were used to probe lysates derived from BMMCs stimulated with 20 μg/ml anti-IgE. In cases where phospho-specific antisera were not effectively stripped from membranes, duplicate samples from the same lysate were run on separate gels. Results are representative of three independent experiments.
LynWeeB mast cells are phenotypically distinct from mast cells expressing a KD Lyn allele
One explanation for the observation that positive regulation is unimpaired in WeeB mast cells could be an unknown adaptor function for Lyn. SFK inhibition enhances IL-6 and IL-13 production in WeeB but not lyn−/− mast cells, suggesting that the residual kinase activity of Lyn is still quite relevant to regulating mast cell activation. However, it is also possible that the presence of the Lyn structure in WeeB cells potentiates the ability of other SFKs implicated in FcεRI-induced signal transduction, and this potentiation is likely to be a PP2-inhibitable event, because it is unlikely that positive regulation is possible without any SFK activity whatsoever. To address this possibility, we transduced lyn−/− bone marrow cells with different LynA-expressing retro-viruses, including the KD K275R mutant (27). Re-expression of Lyn by this method resulted in mast cells expressing LynA at approximately endogenous levels, with the caveat that the K275R mutant (KD) was moderately overexpressed, and all of the genotypes lacked LynB (Fig. 6A). The difference between LynA and LynB, products of alternative mRNA splicing, is the presence and absence, respectively, of an exon coding for 21 residues (28) of unknown significance. IL-6 production in Ag-stimulated empty vector, LynWeeB, and LynKD retrovirally transduced mast cells was similar but was moderately lower in Lynwt-transduced cells, consistent with the restoration of some of the negative regulatory capacity of Lyn (Fig. 6B). Appreciable levels of IL-6 were detected only in Lynwt and LynWeeB cells stimulated with anti-IgE. Similar results were observed with IL-13, although the level of this cytokine was also moderately increased in Ag-stimulated LynWeeB cells. Cells were then pretreated with PP2 (Fig. 6C) and stimulated with Ag as described above. IL-6 and IL-13 production from vector-transduced, Lynwt, and LynWeeB cells was consistent with the PP2 inhibition experiment in Fig. 3. LynKD cells did not display elevated levels of IL-6 or IL-13 with PP2 pretreatment, in contrast with LynWeeB cells, and had similar properties to vector-transduced cells. Histamine release followed a similar pattern, with Lynwt and LynWeeB cells falling into one group and vector-transduced and LynKD cells falling into a second group (Fig. 6D). Overall, LynKD cells exhibited almost exactly the same profiles of cytokine production and phosphorylation of signaling molecules as empty-vector–transduced cells (Fig. 6), suggesting that both positive and negative regulatory functions of Lyn are executed mainly by its catalytic activity. Notably, anti-IgE was far less effective at inducing either cytokine production or degranulation in Lynwt- and LynWeeB-transduced cells than in wt or WeeB BMMCs for reasons that are unclear. Moreover, unlike wt versus WeeB BMMCs, LynWeeB-transduced cells consistently had moderately lower cytokine production and histamine levels than Lynwt cells, which may indicate that in this system even the positive regulatory aspect of Lyn activity is not fully activated by anti-IgE, a low-intensity stimulus. Consistent with this explanation, activation loop phosphorylation of even the re-expressed wt Lyn protein was extremely low, again for reasons that are unclear, as was phosphorylation of its direct and indirect targets, as compared with untransduced wt BMMCs (exposure times were >50-fold longer than those shown in Figs. 4 and 5, data not shown), although the relative comparisons between the four genotypes were as predicted (Fig. 6E).
FIGURE 6.
lyn−/− BMMCs retrovirally reconstituted with LynWeeB are phenotypically distinct from cells reconstituted with KD Lyn. A, Lysates derived from lyn−/− BMMCs transduced with the indicated LynA constructs were analyzed by immunoblotting for expression of Lyn and, as a loading control, β-actin. B-D, Transduced BMMCs were analyzed for cytokine production (B), cytokine production (C) in cells stimulated with 100 ng/ml DNP21-BSA in the presence of increasing concentrations of the SFK inhibitor PP2 as in Fig. 3, and histamine release (D) as described in Fig. 2. Asterisks represent a comparison of wt or WeeB-transduced cells with both vector- and KD-transduced cells. E, Transduced BMMCs were stimulated with 100 ng/ml DNP21-BSA for the indicated periods, and cell lysates were analyzed by immunoblotting or immunoprecipitation followed by immunoblotting using the previously indicated phospho-specific antibodies. Blots were subsequently stripped and reprobed with nonphosphospecific antisera; alternatively, a duplicate blot from the same lysates was probed. *p <0.05; **p <0.01 as indicated in Figs. 2 and 3. Results are representative of two independent experiments.
Anaphylaxis is enhanced in WeeB mice
In vivo studies regarding IgE-dependent mast cell degranulation in lyn−/− mice have been difficult to interpret. Initial findings that the mast cell-dependent immediate-phase PCA reaction (16) is negligible in lyn−/− mice have been tempered by findings that this may be due to dramatically increased FcεRI receptor occupancy resulting from dramatically elevated IgE levels (29). Subsequent experiments using a passive systemic anaphylaxis model determined that histamine release in lyn−/− mice is moderately increased as compared with wt mice, but only in young lyn−/− mice, presumably because FcεRI receptor occupancy has not yet increased to the point where the IgE sensitization step becomes problematic. These studies were carried out on a 129/Sv or mixed genetic background, and it has been shown that even wt 129/Sv mice have much higher serum IgE levels than their wt C57BL/6 counterparts (11). We found that serum IgE levels were indeed elevated in 10- to 12-wk-old lyn−/− mice maintained on a C57BL/6 background [but far lower than those reported (29) for 7-wk-old lyn−/− mice on a 129/Sv X C57BL/6 mixed lineage background], but not in WeeB mice (Fig. 7A). We therefore measured the immediate-phase PCA reaction of 10- to 12-wk-old wt, WeeB, and lyn−/− mice, all on a C57BL/6 background. Interestingly, similar levels of Evans blue extravasation were measured in wt and lyn−/− mice after i.v. Ag injection, but dramatically higher levels were found in WeeB mice (Fig. 7B). We observed a moderate increase in dermal mast cell numbers in WeeB and lyn−/− mice (Fig. 7C) and a substantial increase in the number of degranulated mast cells in WeeB mice (Fig. 7D). Dermal reconstitution of C57BL/6-KitW-sh/W-sh mice with BMMCs from wt, WeeB, and lyn−/− genotypes generated similar PCA data (Fig. 7E), ruling out the possibility that the relatively moderate increase in serum IgE levels found in lyn−/− mice on a C57BL/6 background might still partially suppress the PCA reaction due to increased receptor occupancy. This result provides clear in vivo evidence that specific pharmacological reduction of Lyn kinase activity is likely to lead to a more severe mast cell-dependent anaphylactic response.
Discussion
The current study clearly demonstrates that Lyn switches from a net positive to a net negative regulator of mast cell function(s) when a very low level of its kinase activity is induced or, in the case of LynWeeB, available (Fig. 8). Previous studies have shown that the absence of Lyn leads to increased biological effects for some mast cell functions and decreased biological effects for others, and the data have not been fully consistent among numerous studies. Our previous data partially clarified these observations by demonstrating that the suppressive role of Lyn is associated with high-intensity FcεRI stimulation and the activating role of Lyn is associated with low-intensity stimulation (10). However, it was unanticipated that virtually all of the kinase capacity of Lyn would function as a net negative regulator. That LynKD and empty-vector–transduced lyn−/− cells were phenotypically similar to each other, but phenotypically distinct from LynWeeB-transduced cells nevertheless suggests that this minor catalytic activity, and not an unknown adaptor function, is the main driver of the positive regulatory functions of Lyn.
FIGURE 8.
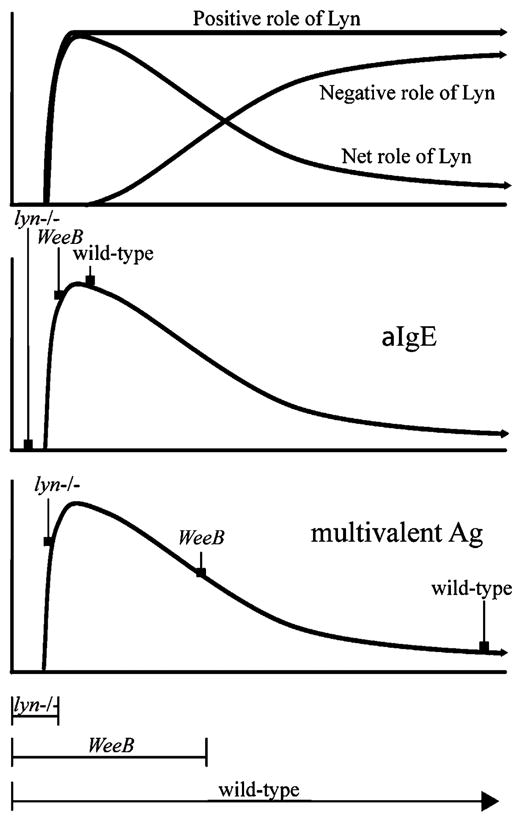
Model for the effects of diminished or absent Lyn kinase activity on mast cell regulation. Top, Hypothetical curves are shown for positive and negative regulatory functions of Lyn. Middle, Low-intensity stimulation of FcεRI (upper panel) leads to weak activation of SFKs. This is insufficient to generate an appreciable biological response (y-axis) in Lyn-deficient cells. Lyn is the predominant SFK in mast cells, and the minor activity associated with the LynWeeB allele, combined with other SFKs, is sufficient for a maximal response in many cases. This suggests a sharply ascending peak as the kinase activity generated by a given stimulus increases. Bottom, The majority of SFK capacity, used during high-intensity stimulation of FcεRI, leads to increasing negative regulation in wt and WeeB cells; biological activity in lyn−/− cells still remains on the ascending region of the curve under these conditions. This simplified model does not take into account potential differences in function for the different SFKs present in mast cells. Note that the reduction in total SFK capacity for lyn−/− and WeeB mast cells from wt levels is far greater than that depicted.
Although the defect in kinase activity for the LynWeeB allele is severe, the reduction in phosphorylation levels of its presumed direct targets in mast cells was more modest. Moreover, determination of the phosphorylation level of the indirect targets of Lyn, at various points in the FcεRI signaling cascade, was not convincingly different in wt and WeeB BMMCs across a range of conditions (Figs. 4 and 5 and data not shown). The calcium flux data for multivalent Ag-stimulated cells (Fig. 5C) suggests that the major effect of reduced Lyn kinase activity in BMMCs is to reduce the rate of signal transduction but not its intensity. Calcium flux in anti-IgE-stimulated WeeB cells was substantially lower than that in wt cells, with only minimal effects on various other signal transduction pathways and at best moderate effects on most biological assays. However, this suggests that with even weaker stimulation of mast cells a wider difference in the intensity of signaling-related phosphorylation events might be observed between WeeB and wt cells. In practice, it was difficult to consistently detect phosphorylation of signaling proteins even in wt BMMCs treated with low concentrations of anti-IgE by Western blot. Nevertheless, the calcium data may indicate that the apparent discrepancy between the intermediate signaling data seen in WeeB B cells stimulated at the BCR and the wt levels of signaling in WeeB mast cells stimulated at FcεRI simply reflects a dramatically stronger activation of Lyn/SFK and the subsequent signaling response in mast cells under these conditions. Recently, mice containing a KD allele of Lyn have been reported and have similar properties to WeeB mice, including delayed autoimmunity (30). It remains to be seen whether mast cells from these mice would have a different phenotype than WeeB mast cells and whether multivalent Ag-stimulated mast cells from these mice would also possess a detectable residual kinase activity.
Correlating the intensity of signal transduction in the various genotypes with the intensity of the different biological outcomes is not straightforward, because the phosphorylation levels of both negative and positive regulators downstream of Lyn/SFK shift in tandem. For example, activation of SHIP1, an important negative regulator of mast cell signaling (31), and activation of Akt1, a positive regulator of mast cell activation (32), both occur to a much higher degree in wt and WeeB BMMCs than in lyn−/− cells. This is the case for both high- and low-intensity FcεRI stimulation, and yet Lyn is a net negative regulator with respect to cytokine production in the former case and a net positive regulator in the latter. Previous studies have shown that the duration of phosphorylation events may be prolonged in lyn−/− BMMCs, which can potentially explain the increased cytokine production (8). In contrast, degranulation typically occurs within minutes; therefore, we speculate that low but prolonged levels of signaling events could lead to increased cytokine production in WeeB and, much more dramatically, lyn−/− cells, over many hours, but only a defect would be observed in degranulation. The defect is not measurable in WeeB cells but is much more easily observed in the more severely SFK-defective lyn−/− cells.
The current study indicates that for a given biological readout there is a point at which the Lyn/SFK activity switches from net positive regulation to net negative regulation (Fig. 8), which the availability of the WeeB mouse has made more easily definable. The differential effects of the SFK inhibitor PP2 and monomeric lysine-DNP on cytokine production in WeeB and lyn−/− BMMCs, and in the respective transduced lyn−/− cells, were striking, and to the best of our knowledge, results of a similar nature have not been reported. With high-intensity stimulation (i.e., a high concentration of multivalent Ag), even LynWeeB can generate net inhibitory signals in mast cells, indicating that perhaps as little as a few percent of the total Lyn and/or SFK capacity of mast cells available to the FcεRI receptor is sufficient for a maximal mast cell response. Even though the contribution of Fyn and Hck toward the total SFK capacity of mast cells is quite small, if these proteins function largely as positive regulators, then their impact on biological outcomes might be much larger than expected. This would seem to be the case, because most (but not all) biological readouts are reduced in fyn−/− and hck−/− mast cells (7, 33). As we and others have previously noted, the phenotypes of lyn−/− cells and mice have been reported as hyperresponsive, hyporesponsive, or equal to the response of wt cells and mice, depending upon the intensity of the stimuli and probably depending upon the conditions used by each laboratory. Furthermore, degranulation is increased in Lyn-deficient BMMCs derived from 129/Sv mice or in mixed genetic lineages but decreased in those derived from C57BL/6 mice, whereas cytokine production is increased in lyn−/− BMMCs derived from both strains, a result that is at least partially dependent upon the expression level of Fyn (11). Our characterization of WeeB BMMCs, combined with inhibitor experiments, clearly demonstrates that a range of diverse biological outcomes likely centers around a point where SFK activity in the cell, compared with its total FcεRI-induced capacity, is very low. One byproduct of this is that lyn−/− BMMCs, in which most SFK activity has been removed, might be expected to be particularly sensitive to differences in experimental procedures and reagents across laboratories, strain variations, etc., as the literature demonstrates is likely the case.
The in vivo susceptibility of lyn−/− mast cells to degranulation has been tested in several studies, taking into account different variables such as the age and strain of the mice. At least in 129/Sv mice prior to the development of diminished FcεRI receptor availability, Lyn deficiency appears to result in a loss of some negative regulation, because there is a moderate increase in histamine levels in lyn−/− mice tested using a passive systemic anaphylaxis model, as compared with wt mice (29). The current study suggests that specific inhibition of Lyn might be more counterproductive than even the lyn−/− mice suggest. Overall, our in vitro and in vivo results indicate that complete or near-complete inhibition of Lyn activity alone (see Fig. 8) would be insufficient to overcome the residual positive activation of the other SFK in mast cells for some functions, would be sufficient for others, and would be clearly counterproductive in a third set. Even pan-SFK inhibition, which has been demonstrated to be therapeutically beneficial in some cases, would appear to be counterproductive in this model.
In conclusion, our results indicate that the catalytic activity of Lyn is critical for both positive and negative regulation of mast cell activation and that a surprisingly small portion of the capacity of Lyn to become activated during FcεRI stimulation is sufficient to achieve wt biological effects. These data do not support the idea that Lyn may have a function independent of its kinase activity, although they do not necessarily rule one out, but do suggest that the sensitivity of Lyn as a rheostat of mast cell activity is most acute at a small range of very weak stimuli.
Acknowledgments
We thank Dr. Dan Conrad for providing anti-IgE mAb and James Faeder for a critical reading of the manuscript. This is publication 1,146 from the La Jolla Institute for Allergy and Immunology.
This study was supported by a grant from the National Institutes of Health AI50209 (to T.K.) and a T32 Training grant (to M.P.).
Abbreviations in this paper
- BMMC
bone marrow-derived mast cell
- IVK
in vitro kinase
- KD
kinase-dead
- LAT
linker for activation of T cells
- PCA
passive cutaneous anaphylaxis
- PLC
phospholipase C
- SCF
stem cell factor
- SFK
Src family tyrosine kinase
- TNP
trinitrophenyl
- wt
wild-type
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 2.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 3.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor FcεRI. Nature. 1999;402(6760 Suppl):B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 4.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 5.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 6.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 7.Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, et al. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 9.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- 10.Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, Constant S, Bower MA, Ryan JJ, Rivera J. Genetic variation influences FcεRI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- 12.Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitaura J, Xiao W, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Kawakami T. Early divergence of Fcε receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J Immunol. 2004;173:4317–4323. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 14.Barouch-Bentov R, Che J, Lee CC, Yang Y, Herman A, Jia Y, Velentza A, Watson J, Sternberg L, Kim S, et al. A conserved salt bridge in the G loop of multiple protein kinases is important for catalysis and for in vivo Lyn function. Mol Cell. 2009;33:43–52. doi: 10.1016/j.molcel.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 16.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 17.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 18.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcεRI. Proc Natl Acad Sci USA. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakami Y, Kitaura J, Yao L, McHenry RW, Kawakami Y, Newton AC, Kang S, Kato RM, Leitges M, Rawlings DJ, et al. A Ras activation pathway dependent on Syk phosphorylation of protein kinase C. Proc Natl Acad Sci USA. 2003;100:9470–9475. doi: 10.1073/pnas.1633695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- 21.Kitaura J, Kinoshita T, Matsumoto M, Chung S, Kawakami Y, Leitges M, Wu D, Lowell CA, Kawakami T. IgE- and IgE+Ag-mediated mast cell migration in an autocrine/paracrine fashion. Blood. 2005;105:3222–3229. doi: 10.1182/blood-2004-11-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimoto H, Lee SW, Hong H, Potter KG, Maeda-Yamamoto M, Kinoshita T, Kawakami Y, Mittler RS, Kwon BS, Ware CF, et al. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood. 2005;106:4241–4248. doi: 10.1182/blood-2005-04-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pribluda VS, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5′-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem. 2003;278:38628–38636. doi: 10.1074/jbc.M305021200. [DOI] [PubMed] [Google Scholar]
- 25.Phee H, Jacob A, Coggeshall KM. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J Biol Chem. 2000;275:19090–19097. doi: 10.1074/jbc.M001093200. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, et al. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 27.Kamps MP, Sefton BM. Neither arginine nor histidine can carry out the function of lysine-295 in the ATP-binding site of p60src. Mol Cell Biol. 1986;6:751–757. doi: 10.1128/mcb.6.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi TL, Bolen JB, Ihle JN. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991;11:2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhagen AM, Wallace ME, Goradia A, Jones SA, Croom HA, Metcalf D, Collinge JE, Maxwell MJ, Hibbs ML, Alexander WS, et al. A kinase-dead allele of Lyn attenuates autoimmune disease normally associated with Lyn deficiency. J Immunol. 2009;182:2020–2029. doi: 10.4049/jimmunol.0803127. [DOI] [PubMed] [Google Scholar]
- 31.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The Src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitaura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. J Exp Med. 2000;192:729–740. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcεRI-dependent gene expression and defective ei-cosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J Immunol. 2005;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]