Abstract
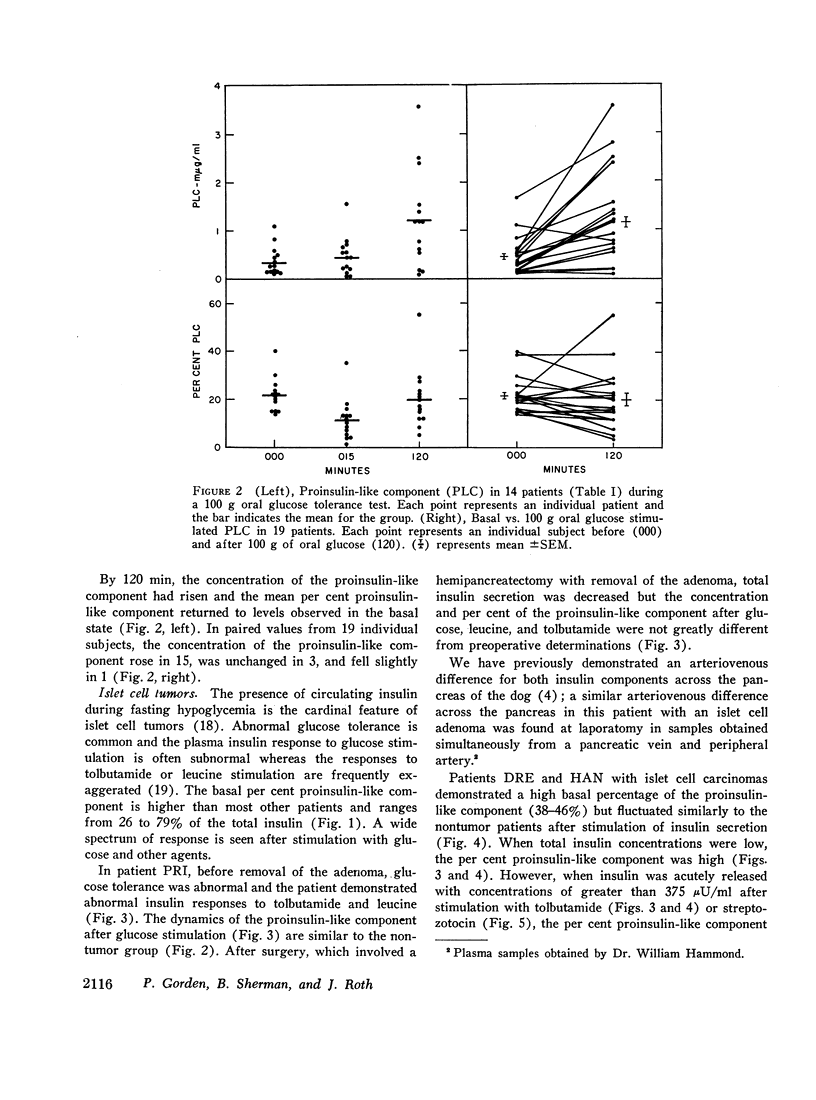
The proinsulin-like component comprised approximately 20% of total circulating basal immunoreactive insulin in 15 patients without islet cell tumors. 15 min after oral glucose, the concentration of the proinsulin-like component was unchanged and its percentage of the total immunoreactive insulin decreased with the acute release of the insulin component. By 2 hr after oral glucose, the concentration of the proinsulin-like component increased and the insulin component concentration decreased so that the percentage of the proinsulin-like component was essentially the same as in the basal state.
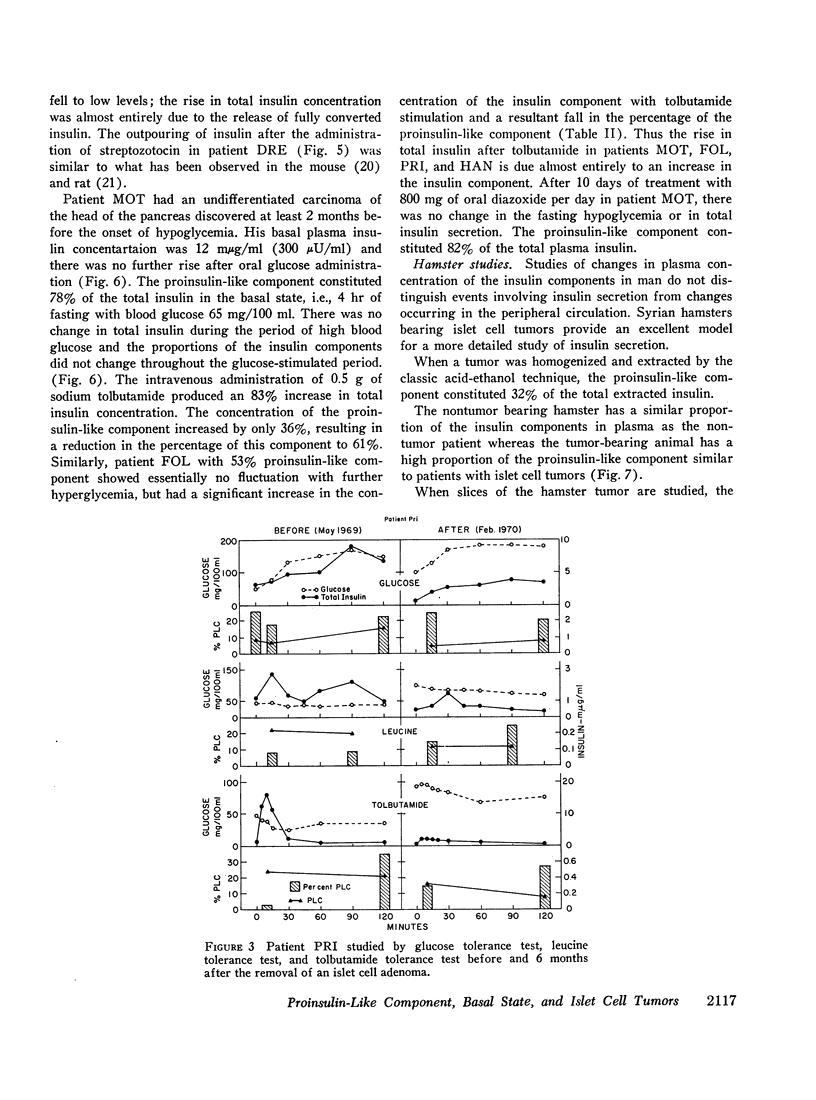
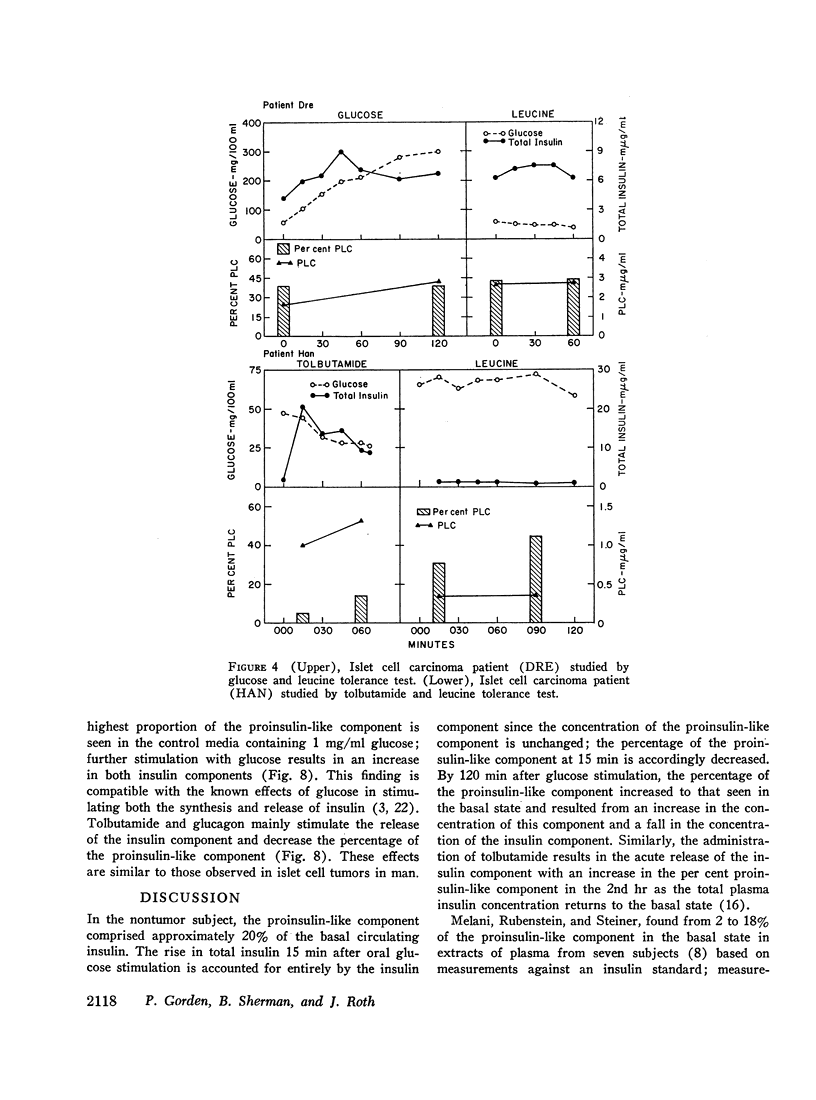
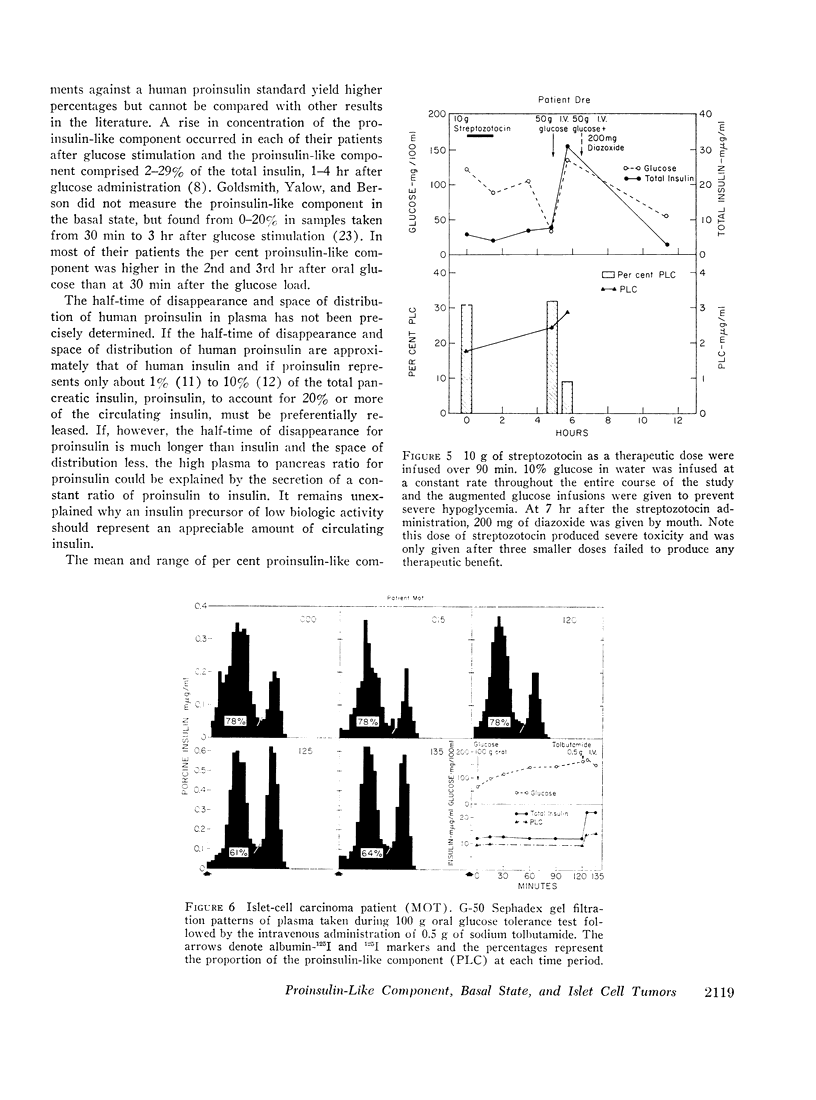
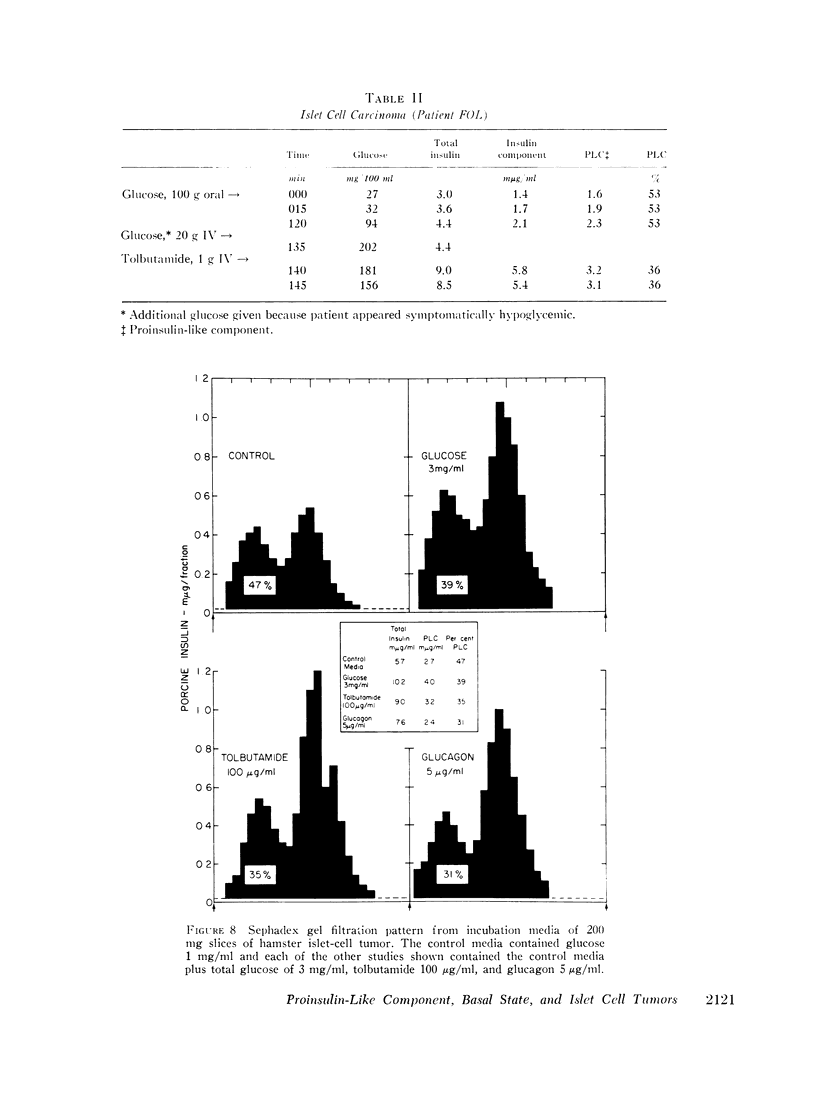
In five patients with islet cell tumors and fasting hypoglycemia, basal proinsulin-like component ranged from 26 to 79% of the total immunoreactive insulin. While basal proinsulin-like component was higher in the islet cell tumor patients, the fluctuations after stimulation were qualitatively similar to the nontumor patients. Acute stimulation with glucose, tolbutamide, leucine, and streptozotocin mainly released the insulin component resulting in a fall in the per cent proinsulin-like component with a subsequent increase in percentage of this component as the total insulin concentration returns towards basal levels. Three islet-cell tumor patients with less than 46% proinsulin-like component had favorable therapeutic responses to diazoxide whereas one patient with over 80% proinsulin-like component was completely refractory.
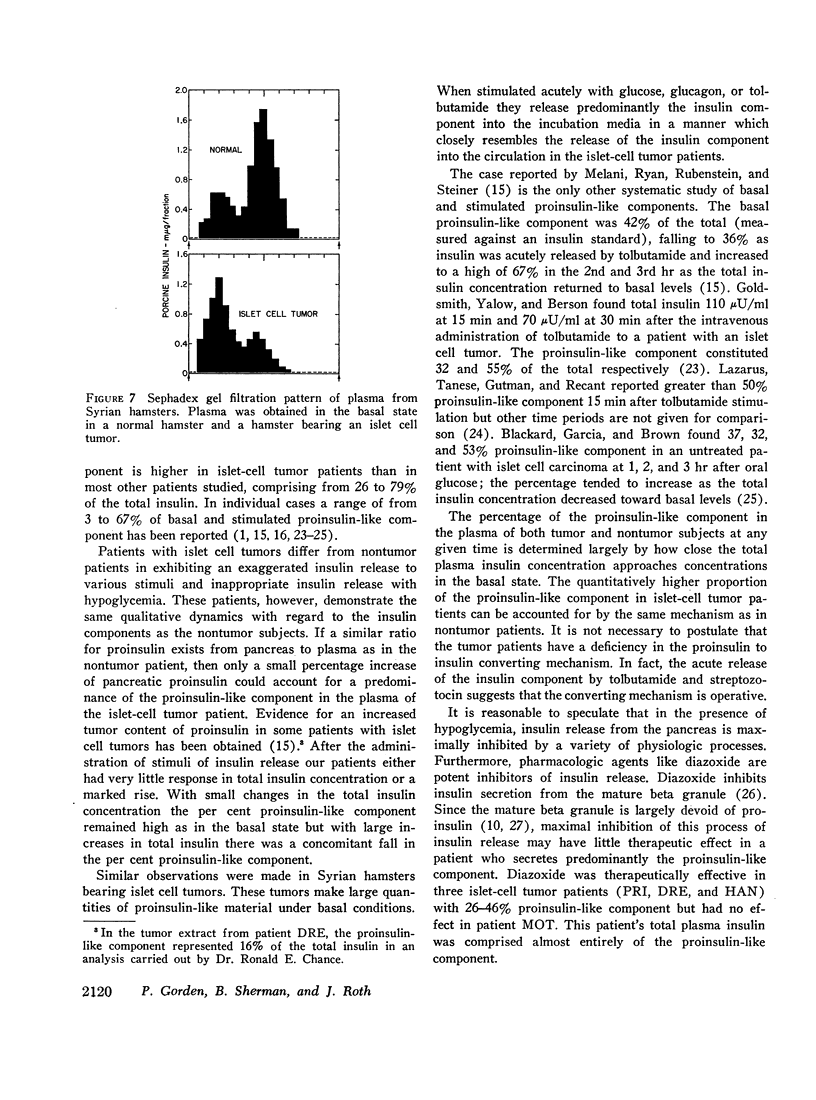
Syrian hamsters bearing islet cell tumors provided an excellent model for islet cell tumors in man. These animals have a high proportion of a proinsulin-like component in plasma; stimulation of tumor slices in vitro with tolbutamide and glucagon releases mainly the insulin component similar to the observations in man.
These studies suggest that the mechanisms regulating the release of the proinsulin-like and of the insulin components are different.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackard W. G., Garcia A. R., Brown C. L., Jr Effect of streptozotocin on qualitative aspects of plasma insulin in a patient with a malignant islet cell tumor. J Clin Endocrinol Metab. 1970 Aug;31(2):215–219. doi: 10.1210/jcem-31-2-215. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Creutzfeldt C., Frerichs H., Perings E., Sicklinger K. The morphological substrate of the inhibition of insulin secretion by diazoxide. Horm Metab Res. 1969 Mar;1(2):53–64. doi: 10.1055/s-0028-1095173. [DOI] [PubMed] [Google Scholar]
- FLOYD J. C., Jr, FAJANS S. S., KNOPF R. F., CONN J. W. PLASMA INSULIN IN ORGANIC HYPERINSULINISM: COMPARATIVE EFFECTS OF TOLBUTAMIDE, LEUCINE AND GLUCOSE. J Clin Endocrinol Metab. 1964 Aug;24:747–760. doi: 10.1210/jcem-24-8-747. [DOI] [PubMed] [Google Scholar]
- Goldsmith S. J., Yalow R. S., Berson S. A. Significance of human plasma insulin Sephadex fractions. Diabetes. 1969 Dec;18(12):834–839. doi: 10.2337/diab.18.12.834. [DOI] [PubMed] [Google Scholar]
- Gorden P., Roth J. Circulating insulins. "Big" and "little". Arch Intern Med. 1969 Mar;123(3):237–247. [PubMed] [Google Scholar]
- Gorden P., Roth J. Plasma insulin: fluctuations in the "big" insulin component in man after glucose and other stimuli. J Clin Invest. 1969 Dec;48(12):2225–2234. doi: 10.1172/JCI106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. The secretion of newly synthesized insulin in vitro. Biochem J. 1967 Mar;102(3):922–927. doi: 10.1042/bj1020922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Orci L., Pictet R., Gonet A. E., Renold A. E. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967 Oct;126(1):201–205. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- Lazarus N. R., Tanese T., Gutman R., Recant L. Synthesis and release of proinsulin and insulin by human insulinoma tissue. J Clin Endocrinol Metab. 1970 Mar;30(3):273–281. doi: 10.1210/jcem-30-3-273. [DOI] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Oyer P. E., Steiner D. F. Identification of proinsulin and C-peptide in human serum by a specific immunoassay. Proc Natl Acad Sci U S A. 1970 Sep;67(1):148–155. doi: 10.1073/pnas.67.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F., Ryan W. G., Rubenstein A. H., Steiner D. F. Proinsulin secretion by a pancreatic beta-cell adenoma. Proinsulin and C-peptide secretion. N Engl J Med. 1970 Oct 1;283(14):713–719. doi: 10.1056/NEJM197010012831401. [DOI] [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Cho S., Steiner D. F. Evidence for proinsulin in human urine and serum. Lancet. 1968 Jun 22;1(7556):1353–1355. doi: 10.1016/s0140-6736(68)92040-0. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Bates R. W. Plasma glucose levels in normal and adrenalectomized mice treated with streptozotocin and nicotinamide. Diabetes. 1968 Dec;17(12):760–765. doi: 10.2337/diab.17.12.760. [DOI] [PubMed] [Google Scholar]
- Sherman B. M., Gorden P., Roth J., Freychet P. Circulating insulin: th proinsulin-like properties of "big" insulin in patients withou islet cell tumors. J Clin Invest. 1971 Apr;50(4):849–858. doi: 10.1172/JCI106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson R. L., Steffes M. W., Lindall A. W. Subcellular localization of proinsulin to insulin conversion in isolated rat islets. Endocrinology. 1970 Jan;86(1):88–96. doi: 10.1210/endo-86-1-88. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. DYNAMICS OF INSULIN SECRETION IN HYPOGLYCEMIA. Diabetes. 1965 Jun;14:341–349. doi: 10.2337/diab.14.6.341. [DOI] [PubMed] [Google Scholar]



