Abstract
Background
Treatment of relapsed hematological malignancies after an allogeneic peripheral blood stem cell transplant (SCT) is challenging. Donor lymphocyte infusion (DLI) from the stem cell donor is an attractive clinical option to salvage this group of patients.
Methods
We reviewed the important studies looking at donor lymphocyte infusion as a therapy for the treatment of hematological disorders that are either refractory to or have relapsed after allogeneic SCT.
Results
The response to DLI is dependent upon type of disease, dose of infused lymphocytes, and the development of graft versus host disease (GvHD). The best response rates are seen in patients with chronic myeloid leukemia (CML) followed by patients with lymphomas, multiple myeloma and acute leukemias, respectively. The responses in patients with CML are durable whereas durable responses in other diseases are rare.
Conclusions
Given the development of new drugs to treat some hematological diseases, DLI has taken a backseat. New modalities to target the infused cells to the tumor and new approaches to reduce GvHD that will augment the graft versus leukemia/lymphoma (GvL) effect and decrease the injury to normal host tissues need to be developed. Understanding the factors and mechanisms that differentiate the GvL effect from GvHD will help in the development of newer treatment modalities.
Keywords: Donor lymphocyte infusion, Donor lymphocyte transfusion, immunotherapy, graft-vs-leukemia, graft-vs-host disease: hematopoietic stem cell transplantation, T-cells
Introduction
We celebrated the 50th anniversary of bone marrow transplantation (BMT)/peripheral blood stem cell transplantation (PBSCT) about a year ago [1]. Over the last 50 years, the field of stem cell transplantation has grown rapidly and has become the treatment of choice for many hematological malignancies and non- malignant conditions such as immunodeficiency disorders, hemoglobinopathies, genetic disorders, and aplastic anemia. The anti-leukemic effect of allogeneic BMT is further exemplified in the decrease of relapse rates observed in patients transplanted with resistant or relapsed hematological malignancies.
As the clinical “art” of BMT developed, investigators realized that depleting T cells from bone marrow or peripheral blood stem cell grafts would decrease the chances of severe GvHD [2-8]. However, it was soon realized that T cell depletion of the product had major disadvantages. There was a much higher incidence of graft failure and disease relapse [9-12] and infectious complications such as bacteria, viral, fungal, and other opportunistic infections. While confronting this clinical dilemma, it was theorized that DLI may lead to a GvL effect and help patients combat their underlying disease [13, 14]. In one study, donor T cells reactive to recipient minor histocompatibility antigens (mHA) inhibited the growth of leukemic colonies and prevented the development of acute myeloid leukemia derived from human cells in immunologically susceptible mice [15]. Donor T cells can also target aberrantly expressed proteins (e.g., proteinase 3 in myeloid leukemias) and can inhibit leukemic, but not normal, colony formation [16]. Theoretically, the donor T cells can recognize the foreign antigens on the recipient leukemic cells and eliminate them.
In the era before DLI, the patients who developed graft failure or disease relapse only had the options of either going through a second transplant or to succumb to their disease. The first report of DLI leading to remission of disease following disease relapse after BMT was published in 1990 by Kolb and his colleagues [17]. This report was followed by reports of other attempts at controlling relapsed disease with the use of this new clinical strategy [17-22]. Although DLI was originally described in patients with relapsed chronic myeloid leukemia, it was then utilized for other hematological malignancies like multiple myeloma, acute leukemias (both acute myeloid and acute lymphoid leukemia) and lymphomas [23-27].
The initial data was in patients who had relapsed chronic myeloid leukemia after allogeneic bone marrow transplantation [17, 18, 28-31]. During the first few years, DLI was performed in patients who had received stem cell grafts from a matched family member, but subsequently there were reports of DLI being effective in patients who had received matched unrelated grafts.
In this review, we will present the current data related to the use of DLI as a viable option for various relapsed diseases after allogeneic peripheral or bone marrow stem cell transplantation.
Pathophysiology
The first evidence that GvHD had a beneficial effect on decreasing the incidence of leukemic relapse with a survival advantage was reported by Weiden et al [32, 33]. In addition, there were key clinical observations that supported the GVL effect mediated by donor T cells [34-40]: 1) patients who received syngeneic stem cell transplants relapsed more frequently than those who received allogeneic stem cell transplant; 2) a rapid decrease or elimination of post-grafting prophylaxis or treatment immunosuppression would often lead to remissions; and 3) higher numbers of relapses were seen in patients who received T cell depleted grafts compared to patients who received unmanipulated grafts.
Kolb et al published a report in 1990 wherein they infused donor lymphocytes into patients who had relapsed after allogeneic stem cell transplant for chronic myeloid leukemia and showed that cytogenetic remission could be induced [17]. This led to the development of DLI as a viable option for treatment of relapsed disease after allogeneic SCT.
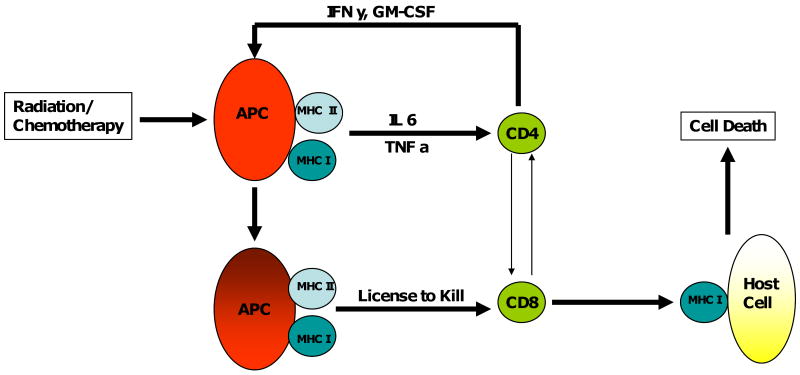
In GvHD, the antigen presenting cells (APC) of the host, which have been activated by the preparative regimen of chemotherapy and/or radiation present minor histocompatibility (mHA) and activate the donor helper and cytotoxic T-cells. These cells are co-activated by stimulatory cytokines which leads to a vicious cycle of activation, killing, and release of more stimulatory cytokines (Figure 1).
Figure 1.
Radiation/chemotherapy leads to damage to host cells, the antigen presenting cells of the host become activated and become highly proficient at presenting the antigens to the donor CD4/CD8 cells, which are able to costimulate and become effector cells which lead to tissue damage/anti-disease activity. The tissue damage leads to the release of cytokines which further accentuate the vicious cycle.
Miller et al showed that patients who receive a lymphodepleting conditioning regimen in their therapy for recurrent leukemia prior to DLI developed significantly more GvHD [41]. This finding emphasizes the role of conditioning regimen as stimulant of GvHD. This could be explained either by more space for donor lymphocytes to expand in vivo or the activation of antigen presenting function of host cells. Patients who receive a T cell depleted graft have a higher risk of GvHD after DLI, as compared to patients who receive an unmanipulated graft, supporting the preclinical model that shows that regulatory T cells from the donor can down-regulate the ability of host APC to decrease GVHD and help in the development of tolerance [42].
Graft versus leukemia (GvL) effect is thought to be maintained by host APC that stimulate the donor T cells by presenting target antigens expressed on the specific hematopoietic cells. Some of the candidate mHA on hematopoietic cells are HA-1, HA-2, HB-1 and BCL2A1 [13, 43].
Chronic Myeloid Leukemia
In the era before tyrosine kinase inhibitors like imatinib revolutionized the treatment of CML [44-46], patients with CML were treated with interferon alpha and/or hematopoietic SCT. Patients who relapsed after hematopoietic SCT could be retreated with interferon alpha or could undergo a second transplant. DLI was found to be a viable option for the treatment of CML relapse after SCT. Various investigators over the years described patients who were managed with DLI after relapse with relatively good outcomes after the initial report by Kolb et al [17]. Most of the early data regarding CML and DLI was reported in patients who relapsed after receiving the graft from matched siblings, but by around 2000, data on patients who received grafts from matched unrelated donors was also reported (Table 1) [17, 42, 47-50]. The best response was seen in patients who had molecular and/or cytogenetic relapse. Among patients with hematological relapse, patients who were in chronic phase did better than accelerated/blastic phase. Patients with only molecular and cytogenetic relapses almost always went into remission with DLI, while patients with chronic phase hematologic relapse went into remission about 75% of the time. Those in accelerated or blastic phase were less responsive to DLI with responses ranging from 12.5 to 33%.
Table 1. Chronic Myeloid Leukemia Studies.
| Authors | Number of patients | Graft Source RD/UD | Median time to relapse from SCT* | Other treatments | Mean number of DLI | DLI dose Median T cells / Kg (range) | CR rates after DLI | Survival |
|---|---|---|---|---|---|---|---|---|
| Kolb et al, 1990 [17] | 3 | 3/0 | 23- 39 months | IFN alpha | 4 | (4.4 - 7.4) ×108 | 100 %CR | NA |
| Kolb et al, 1995 [42] | 84 | 77/7 | 24 months | 9 pts with transformed CML | NA | 3 (0.25 -12.3 ) × 108 | 82 % cytogenetic relapse, 78% hematological relapse, and 12.5 % transformed phase | 67% at 2 yrs |
| Collins et al, 1997 [47] | 56 | 50/6 | NA | NA | 3 | (1.0 - 8.2) × 108 | 100% cytogenetic relapse, 73.5% hematologic relapse, 33 % transformed phase | 60% at 2 yrs |
| Porter et al, 2000 [48] | 25 | 0/25 | 9 months | 2 received SCT for blast phase | 1 in 67%, 2 in 21% and >3 in 12% | 0.85 ×108 | 46% | 53% and 12% at 2 yrs for early and late phase CML |
| Dazzi et al, 2000 [49] | 66 | 35/31 | 12 months | NA | I in 34 patients, rest had >1 | 1.5 (0.4 – 5.3) × 108 | 68% | 91% and 48% at 2 yrs in responders and non responders |
| Shiobara et al, 2000 [50] | 23 | NA | 8 months | 5 pts received IFN alpha | I pt received 8 infusions | 3.0 × 108 | 91 % in chronic phase€ and 27 % in acute phase£ | 82 % at 2 yrs in chronic phase 16% at 2 yrs in acute phase |
Abbreviations RD=related donor, UD=unrelated donor, CML= chronic myeloid leukemia, NA=Information not available, CR= complete remission, DLI = Donor lymphocyte infusion, SCT=Stem cell transplant, IFN= Interferon
=Chronic phase included molecular, cytogenetic and stable phase relapses,
= Acute phase included accelerated and blastic phase
The duration of responses to DLI in patients with relapsed CML after SCT is longstanding. The first patient who received the DLI remains in remission about 20 years after the procedure [51]. The GvL effect of the DLI is not immediate and may not be appreciable for as long as 1 yr after the DLI. On average, some form of a clinical response is apparent in 2-3 months.
Schapp et al attempted to identify patients who would benefit from DLI preemptively in patients who had received grafts from HLA-matched siblings and did not develop acute or chronic GvHD. They found that patients who received DLI had a better 3 year disease free survival of 77% versus 45% in patients who did not receive DLI. Furthermore, the relapse rate was lower in patients who received DLI when compared to the control group (18% versus 44% at 3 years) [25].
The difference in response rates in molecular relapse versus relapse in accelerated phase emphasizes the point that DLI is best suited for treating slowly relapsing leukemia. This principle is underscored by the lack of responses to DLI in diseases with high proliferation indices like the acute leukemias. The use of DLI to boost anti-leukemic/anti-lymphoma effects may be clinically useful in patients who fail to develop GvHD. The key questions that remain are: 1) how many T cells should be infused in the dose of DLI for prophylaxis or treatment; 2) how many doses should be given; 3) how frequently should they be given to maintain a GvL effect; and 4) when should infusions be stopped.
Recently, the standard of care of CML has been transformed with a number of clinically effective and successful tyrosine kinase inhibitors (TKI) such as imatinib, dasatinib and nilotinib; hematopoietic SCT has taken a backseat for CML. However, the TKIs do not cure the disease and have to be taken life-long to keep the disease under control. Moreover, the patients who develop resistance to the development of the T315I mutation or are unable to tolerate the side effects of the FDA-approved TKI drugs end up undergoing a SCT for the management of their disease.
The technology to detect the relapse of CML has steadily improved. With the incorporation of polymerase chain reaction for detecting molecular relapses, even small increases in the number of cells positive for BCR-ABL can detected earlier. Although the number of SCT for CML has gone down in the past decade, there still are some patients who need it. In this subgroup of patients, DLI can be an important tool in keeping their disease in check especially when used together with sensitive cytogenetic and molecular methods. The use of DLI for patients with only cytogenetic/molecular relapse continues to give excellent remission rates.
Acute Myeloid Leukemia
Donor lymphocyte infusion for patients with relapsed AML following hematopoietic SCT is not very effective in getting patients into remission. Overall remission rates range between 15-42%, with a 2 year overall survival of approximately 15-20% (Table-2) [42, 47, 48, 52, 53]. Parenthetically, it is important to understand that a second SCT in such patients results in only 10-35% long term survival with treatment related mortality in the range of 50 % [54-56].
Table 2. Acute Myeloid Leukemia Studies.
| Authors | Number of patients | Graft Source RD/UD | Median time to relapse from SCT | Other treatments | Mean number of DLI | DLI dose Median T cells / Kg (range) | Disease state after DLI | Survival |
|---|---|---|---|---|---|---|---|---|
| Kolb et al, 1995 [42] | 23 | 21/2 | 8 months | 8 pts got chemo | NA | 2.4 (0.25 – 12.3) × 108 | 29% CR | 15% at 2 yrs |
| Collins et al, 1997 [47] | 46 | 44/2 | NA | 7 pts got chemo and were not evaluated | 3 | (1.0 - 8.2) × 108 | 15.4% CR for pts who did not get Chemo | 17% at 2 yrs |
| Porter et al, 2000 [48] | 23 | 0/23 | 9 months | 4 pts were in remission prior to DLI | 1 in 67%, 2 in 21% and >3 in 12% | 1.34 × 108 | 42% for the remaining 19 pts | 21% at 1.4 yrs |
| Shiobara et al, 2000 [50] | 21 | NA | 4 months | None | 1 | 2.3 × 108 | 38% CR | 7 % at 2 yrs |
| Choi et al, 2004 [52] | 16 | 15/1 | N/A (2.4 – 23.7 months) | All pts got pre-DLI chemo | NA | 4.5 × 108 | 63 % CR | 31 % at 2 yrs |
| Schmid et al, 2007 [53] | 399 DLI=171, No DLI =228 | 128/43 | 5.5 months | 124 pts got pre DLI chemotherapy | 1 in 61%, 2 in 26% and >3 in 13% | 2.8 × 108 | 35 % CR | 20% at 3 yrs |
Abbreviations: RD=related donor, UD=unrelated donor, NA=Information not available, CR= complete remission, DLI = Donor lymphocyte infusion, SCT=Stem cell transplant
Schmid et al in 2007 [53] published data on a large group of patients, who received DLI for relapse after allogeneic SCT for AML. They showed an overall survival of 20% at 2 years, but when the authors looked at patients who had favorable cytogenetics and who went into remission with chemotherapy induction therapy, survival was surprisingly far better (56% at 2 years). They also noted that female patients who did not go into remission with induction therapy and had less than 35% blasts at the time of relapse had better survival (21% at 2 yrs). If these patients were removed from the analysis, the rest of the patients fared rather poorly with 9% survival at 2 yrs.
As Intuition might predict, lower initial disease burdens, reductions in the tumor burden with chemotherapy prior to DLI and favorable cytogenetics appear to be beneficial for patients with relapsed leukemia after SCT.
Overall, AML that relapses after allogeneic SCT is a hard disease to treat. The ability of DLI to induce a remission is approximately 15-20%. In the absence of systemic chemotherapy, DLI by itself is not effective. The most likely reason for the relative ineffectiveness of DLI can be explained by the tumor burden in AML and the rapid rate of cell division. The tumor burden needs to be reduced with systemic chemotherapy and DLI can be an adjunct to improve survival, while possibly awaiting a second transplant in candidates who do qualify for one. In other patients, DLI may be another tool in the immunologic arsenal to delay inevitable AML relapse. There are a small number of patients who become long term survivors after DLI and further investigation is needed to determine the subset patients who may benefit from DLI in relapsed AML after an allogeneic SCT.
Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL), which is of B-cell or pre B-cell origin, responds even more poorly to DLI than the myeloid leukemias (CML and AML). Although some patients may go into a remission with chemotherapy and DLI, most remissions are short in duration. There is an occasional exception; Slavin et al have reported a child with ALL who went into remission after DLI and is in remission 15 years after the DLI.[57]
For patients with ALL, who relapse after allogeneic SCT, reinduction chemotherapy can induce remissions in about 40-60% of the patients. Most of the patients who go into remission eventually relapse and die of their disease with 3 year disease free survival rates in the range of 10 % [58-61]. A second allogeneic SCT provides a 5 year overall survival of around 15 -20% in this patient population, with a treatment related mortality of around 50% [55, 62, 63]. The treatment mortally is attributed mainly to veno-occlusive disease, acute GvHD, infections, interstitial pneumonia, and failure to engraft. However, only a small fraction (∼15%) of the patients who relapse after allogeneic SCT will be candidates for a second transplant due to a decline in performance status and failure of remission induction after salvage chemotherapy [56].
The results from various trials reporting remission rates and overall survival for patients with ALL who relapse after SCT and receive DLI are summarized in Table 3 [42, 47, 48, 50, 64, 65]. Kolb et al showed in 1995 that DLI was not beneficial for patients who did not go into remission after salvage chemotherapy [42]. It is clearly evident from the data presented by Collins et al [65] that achieving remission is a critical surrogate marker that predicts overall survival. The best overall survival was seen in the study by Choi et al [64] and the worse by Shiobara et al [50]. The critical difference in the 2 groups of patients was the number of patients who got pre DLI chemotherapy (100% vs. 0 %).
Table 3. Acute Lymphoblastic Leukemia Studies.
| Authors | Number of patients | Graft Source RD/UD | Median time to relapse from SCT | Other treatments | Mean number of DLI | DLI dose Median T cells / Kg (range) | Disease state after DLI | Survival |
|---|---|---|---|---|---|---|---|---|
| Kolb et al, 1995 [42] | 22 | 21/1 | 8 months | 17 pts got chemo | NA | 2.9 ( 0.3 – 11) × 108 | 9 pts in CR after chemo 0% CR after DLI | 12% at 1 yr |
| Collins et al, 1997 [47] | 15 | 13/2 | NA | 4 pts got chemo | 2 | (1.0 - 8.2) × 108 | 18.2% CR (2/11) | 18% at 1.5 yr |
| Porter et al, 2000 [48] | 7 | 0/7 | 9 months | 3 in CR prior to DLI | 1 in 67%, 2 in 21% and >3 in 12% | 0.9 × 108 | 2/4 not in CR after chemo went into CR | 25% at 3 yrs (1/4 pts) |
| Collins et al, 2000 [64] | 44 | 34/10 | NA | 28 got chemo | 36 pts had 1 8 pts had 2 | (0.01 – 8.8) × 108 | 5/16 who did not get any chemo went into CR | 17% at 2 yrs for all pts(44) 12.5 % at 2 yrs for pts who only got DLI(26) |
| Shiobara et al, 2000 [50] | 23 | NA | 4 months | None | 1 | 2.1 × 108 | 25 % CR | 5 % at 2 yrs |
| Choi et al, 2005 [63] | 10 | 8/2 | 5.5 – 83.6 months | All 10 got chemo | NA | (2.9-7.0) × 108 | 70 % in CR | 20% at 2 yrs |
Abbreviations: RD=related donor, UD=unrelated donor, NA=Information not available, CR= complete remission, DLI = Donor lymphocyte infusion, SCT=Stem cell transplant
It is well documented that the clinically evident GVL effect of DLI requires weeks to months to become apparent [30, 66]. Unlike CML, ALL is a rapidly proliferating disease and without a significant reduction in leukemia burden, DLI alone is unable to control the disease. The poor response of relapsed ALL to DLI is thought to be mediated by diverse immune escape mechanisms of the leukemia cells [67-72].
Overall management of ALL which has relapsed after allogeneic SCT is challenging. Salvage chemotherapy is essential if the patient is to be given DLI since the best overall survival can be achieved with a combination of chemotherapy and DLI. Although it is unclear whether DLI adds anything to salvage chemotherapy, there are some long term survivors who had received both chemotherapy and DLI.
Lymphomas
There is data to suggest that there is a graft versus lymphoma effect following allogeneic SCT as shown by Ratanatharathorn et al and Jones et al [73, 74]. The data to suggest that DLI is effective in inducing a remission for relapsed lymphoma after allogeneic SCT is limited with small number of patients reported.
The studies in which patients got DLI in different lymphomas for relapse after allogeneic SCT are summarized in Table 4[75-80]. These studies show that highest response rates were seen in the indolent lymphomas, while the more aggressive lymphomas had a lower response rates. In the study by Morris et al [79], 3 out of 9 patients with high grade NHL, 1 of out 2 patients with mantle cell lymphoma, and 6 out of 10 patients (60%) with low grade NHL responded to DLI. This finding further supports the clinical hypothesis that low tumor burdens and slower growing malignancies are essential conditions for DLI to be effective.
Table 4. Lymphoma studies.
| Authors | Number of patients | Graft Source RD/UD | Median time to relapse from SCT | Other treatments | Mean number of DLI | DLI dose Median T cells / Kg (range) | Disease state after DLI | Survival |
|---|---|---|---|---|---|---|---|---|
| Mandigers et al,‡ 2003 [79] | 7 (5 FL, 2 SLL) | 7/0 | 25 months | NA | 4 got chemo | (0.1 – 1.0) × 108 | 85 % response (4/7 CR) | 71% at 2 yrs |
| Morris et al, 2004 [78] | 21 (HG-NHL 9, MCL 2, LG-NHL 10) | 19/2 | NA | NA | 3 | (0.01 – 0.1) × 108 | 42 % CR | NA for DLI pts |
| Anderlini et al, 2004 [74] | 9 HL | 7/2 | 7 months | none | 4 pts got more than 1 | 0.77 × 108 | 44% CR | NA |
| Peggs et al,‡ 2007 [77] | 14 HL | NA | NA | 8 got chemo | NA | (0.01 – 1) × 108 | 57% CR | 35 % at 2 yrs |
| Bloor et al,‡ 2008 [76] | 17 (FL 6, CLL3, MCL 3, tFL 5) 11 had Mixed Chimerism | 14/3 | NA | 7 got chemo | NA | (0.01 – 1) × 108 | 76 % CR | Overall survival 88% (including 11 pts who had mixed chimerism) |
| Bishop et al, 2008 [75] | 5 DLBCL | NA | Relapse/refractory Disease at day +100 | 4 pts got chemo | 2 pts got 2 DLI | (0.01 – 1) × 108 | 60% CR (2/3 had previous chemo) | All 3 alive at 6 yrs |
Abbreviations :RD=related donor, UD=unrelated donor, NA=Information not available, DLBCL=Diffuse large B cell lymphoma, FL= Follicular Lymphoma, tFL=Transformed follicular lymphoma, CLL= Chronic lymphocytic leukemia, HL= Hodgkin's' lymphoma, NHL=Non hodgkin lymphoma HG-NHL= High grade non hodgkin lymphoma, MCL= mantle cell lymphoma, LG-NHL= Low grade non hodgkin lymphoma, SLL= Small lymphocytic lymphoma, CR= complete remission, DLI = Donor lymphocyte infusion, SCT=Stem cell transplant
Studies used an escalating dose of DLI
The role of allogeneic SCT in lymphomas remains controversial as an upfront treatment. Bierman et al reviewed the role of DLI in autologus and allogeneic transplant in patients for low grade lymphoma in the Center for International Blood and Marrow Transplant Research (CIBMTR) and European Group for Blood and Marrow Transplant (EBMT) registries [81]. Patients with low grade lymphoma had a significantly lower relapse rate following allogeneic SCT while patients with high grade lymphomas had no significant reduction in their relapse rates. A detailed discussion is beyond the scope of this review.
Overall, DLI is an attractive option for the patients who have relapsed lymphoma after an allogeneic SCT especially in patients with low grade lymphomas. Significant responses can be attained in more aggressive lymphomas, but the addition of chemotherapy appears to be a prerequisite for success.
Multiple Myeloma
Multiple myeloma is the most common indication for SCT, albeit most of these are autologus SCT rather than allogeneic SCT. A retrospective analysis from EBMT, showed no advantage of allogeneic graft over an autologus graft [82]. The study showed that the auto SCT patients had better median survival than allogeneic SCT (34 vs 18 months). However, in patients alive at 1 year post allogeneic SCT there was a trend for better long-term survival and significantly better progression-free survival as compared with auto SCT. The treatment related mortality from an allogeneic SCT has been reported to be range between 25 and 56% [83, 84]. The myeloma community is divided into 2 camps with regard to the benefit of allogeneic SCT over autologus SCT and a detailed discussion is beyond the scope of this review.
The various studies reporting the role of DLI in relapsed multiple myeloma are summarized in Table 5 [23, 24, 47, 85, 86]. Lokhorst et al [23] used DLI in 13 patients who had relapsed multiple myeloma after an allogeneic SCT. The authors showed a response rate of about 62 % with approximately half of the responders attaining a complete response. Although long term survival data was not provided, there were some long term survivors up to 38 months after the DLI. Salama et al [24] showed a response rate of 36% with DLI and 48% of the patients were alive at 1 year after DLI. This emphasizes the point that some non-responding patients can be long term survivors. The reasons for this observation are unclear, but could be related to non-responding patients receiving more chemotherapy or having slower progression of their disease. Overall, multiple myeloma is a disease that has up to a 50% chance of responding to DLI.
Table 5. Multiple Myeloma Studies.
| Authors | Number of patients | Graft Source RD/UD | Median time to relapse from SCT | Other treatments | Mean number of DLI | DLI dose Median T cells / Kg (range) | Disease state after DLI | Survival |
|---|---|---|---|---|---|---|---|---|
| Collins et al, 1997 [47] | 5 | 5/0 | NA | 1 PR, 4 refractory disease | 2 | (1.0 - 8.2) × 108 | 50% CR | 40% at 2 yrs |
| Lokhorst et al, 1997 [23] | 13 | 9/0 | 5-87 months | 6 PR, 7 refractory disease | NA | (0.01 – 3.3) × 108 | 62 % response, 31 % CR | 5-38 months |
| Lokhorst et al, 2000 [84] | 27 | NA | 30 months | 13 pts got chemo | NA | (0.01 – 5) × 108 | 52 % response, 30 % CR | 40% at 5 yrs (70% for responders) |
| Salama et al, 2000 [24] | 25 | 24/1 | 57.5 months | 4 pts got chemo | 9 pts got 2 | (0.02 – 5.55) × 108 | 36 % response, 28% CR | 48 % at 1 yr |
| van de Donk et al, 2006 [85] | 63 | 46/17 | Included persistent and relapsed dis | None | 7 got more than 1 | (0.01 – 3) × 108 | 38 % response | 50% at 2 yrs (62 %for responders) |
Abbreviations RD=related donor, UD=unrelated donor, CML= chronic myeloid leukemia, NA=Information not available, CR= complete remission, DLI = Donor lymphocyte infusion, SCT=Stem cell transplant
The main reason for the difference in the response of the 2 studies mentioned above is the number of patients who received chemotherapy in some form prior to the DLI. This again emphasizes the point that DLI is active against diseases when the tumor burden is low. A number of investigators have studied prophylactic DLI in patients who receive allogeneic SCT [87-89]. With this approach, investigators have reported complete remission rates ranging from 43 to 85 %.
A major confounding factor for the high response rates seen with DLI for multiple myeloma is that steroids are used for treating GvHD and steroids have a known anti- myeloma effect which could potentially enhance response rates in patients who receive DLI.
Currently, most patients with multiple myeloma undergo an autologus SCT and not an allogeneic SCT. The overall role of transplantation in this disease continues to be under investigation with the advent of highly active drugs in myeloma like lenalidomide and bortezomib.
It is relatively uncommon for patients with multiple myeloma to be candidates for allogeneic SCT. Patients, who relapse after allogeneic SCT, can avail of some active drugs like lenalidomide or bortezomib. In this setting, the role of DLI is unclear, but can be considered as an option to provide some chance of clinical response. The chance of a clinical response may be increased by treating patients with chemotherapy prior to the DLI. However, it is unclear whether DLI will add to the durability of response once the patients are in a chemotherapy induced remission.
Cell Dose
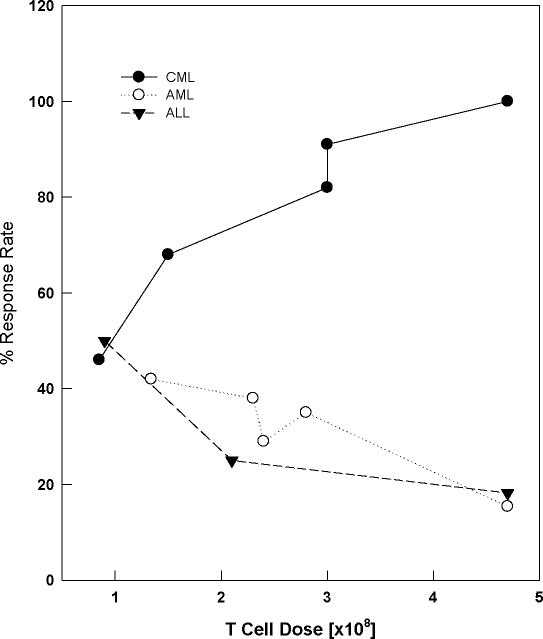
The cell dose used in the various studies reviewed in this article ranged from 0.01 to 8.8 × 108 T cells/kg. On closer examination with specific attention to the cell doses used for DLI and response rates in CML (Figure 2), it is evident that a cell dose of less than 1 × 108 T cells /kg is suboptimal. A cell dose above 4.5 ×108 T cells /kg does not seem to offer better response rates and may possibly adds to the complications and be detrimental. The relationship between cell dose and response rates in AML (Figure 2) shows that increasing the cell dose beyond 1.5 × 108 T cells/kg does not add to the response rate. Only the study by Choi and colleagues [52] seemed to show a better response rate with higher dose of T cells (not included in graph), but all patients in this study received chemotherapy for cytoreduction prior to chemotherapy. When evaluating the data from ALL studies (Figure 2), higher response rates were seen in studies using 1- 2 × 108 T cells /kg, while studies using higher cell dose had lower response rates. One possible explanation for lower response rates seen with higher T cell dose could be due to the infusion of higher absolute numbers of T-regs, which in turn would lead to decreased GvL. Another explanation could be the fact that higher cell doses lead to more GvHD which would offset the benefit from DLI. Almost all of the studies looking at patients with lymphoma (Table 4) used DLI at the dose of 0.01 – 1.0 × 108 T cells/kg and higher response rates were seen in studies utilizing an escalating dose regimen. In patients with multiple myeloma, there does not seem to be a well-defined relationship between cell dose and response rates with DLI cell doses ranging from 0.01 to 8.2 ×108 T cells/kg (Table 5). The limitation of the cell dose data reviewed here are median cell doses and individual doses and responses are not available.
Figure 2.
Graph showing the dose of T cells given during DLI and the response rates seen in CML, AML and ALL.
Complications
The following sections discuss the complications seen in patients who undergo DLI.
GvHD
GvHD develops in about 50-60 % of the patients receiving DLI [90, 91]. There does not seem to be any correlation between the type of disease for which DLI was given and the development of GvHD. The development of GvHD not only predicts a response to DLI but predicts a longer disease free survival [92]. The only exceptions to this fact are the patients who were treated with DLI for chronic phase CML due to relapse after allogeneic SCT. The risk of developing GvHD was also dependent on the dose of DLI, with almost 50% or more of the patients developing GvHD when the dose of DLI was greater than 1 × 108 T cells/kg while less than 10% developing GvHD when the dose of infused cells is < 107 T cells/kg
Interestingly, the there was low mortality associated with GvHD and most of the mortality was from disease relapse or refractory disease rather than GvHD. One mechanism that may be responsible for the low mortality from GvHD after DLI is the absence of conditioning regimens prior to DLI which may potentiate the severity of GvHD as discussed earlier.
APLASIA
Aplasia following DLI has been reported in 20 - 40% of the patients, with overall mortality of about 5%. The patients who develop DLI are at risk for death due to infections or bleeding. The main factor that predicts the development of aplasia is the extent of residual host hematopoiesis [93]. The mechanism(s) responsible for the aplasia are not known. Aplasia was seen to develop most commonly in patients who received DLI for hematological relapse of chronic phase of CML and lower in patients with acute leukemias. Most of the patients will eventually have spontaneous recovery of counts and require supportive care during the interim.
Discussion
T cell depleted stem cell graft followed after engraftment with DLI maybe the trend of the future. This is supported by the fact that the development of GvHD is less after T cell depletion. The main factors critical for the development of more effective DLI that require further study are approaches that: 1) decrease the tumor burden prior to DLI; 2) target the infused lymphocytes specifically to the tumor antigens; 3) augment the immunogenicity of the tumor cells using biologic response modifiers; 4) promote clonal expansion of infused tumor specific lymphocytes in the host; 5) control the activity of the infused donor lymphocytes against normal host cells; 6) enhance the role of antigen presenting cells such as monocytes and B cells in the GVL effect; 7) help to understand the T cell dose response relationships between inducing remissions and not causing GvHD; and 8) explain the role of the innate immune system (NK) cells.
A number of strategies have been attempted to address the points raised above. In the data summarized in this review, only patients with chronic phase of CML benefited from DLI alone whereas all others derived benefit from DLI after receiving some cytoreductive chemotherapy prior to the DLI.
There are ongoing attempts by investigators to target lymphocytes to the specific tumor antigens with bispecific antibodies [94], T cells transduced with chimeric antibody receptors [95] or T cells armed with bispecific antibodies [96-98]. These strategies target circulating effector cells to tumor. The approach needs further development with selection of appropriate antigens for specific types of cancer as well as avoiding dose limiting toxicities due to cytokine storm when bispecific antibodies are infused alone [99].
It is postulated that the T cells require two signals to be activated, the first signal is derived by MHC molecule and the second signal is a stimulatory signal from the antigen presenting cells. The tumor cells may escape by either inadequate expression of the MHC or by inability of the antigen presenting cells to provide the co-stimulatory signal [100]. Better understanding of the mechanism by which the tumor cells prevent the activation of the infused T cells is needed; this may provide an opportunity to develop a therapeutic approach in which the tolerance for antigens on tumor cells may be broken to induce clinically effective immune responses.
Investigators so far have not been able to identify specific antigens present on tumors that can be safely used to vaccinate the DLI donors [101-104]. If specific tumor antigens are isolated, they may be useful in vaccinating the donor prior to collection of T cells which may lead to better targeting of the tumor cells.
There is evidence to suggest that host T cells play a regulatory role in the expansion of the infused donor T cells. The depletion of host T cells helps the donor T cells to expand after being infused into the recipient [101]. The combination of chemotherapy to optimize the effect of DLI may be an important approach to explore in patients resistant to DLI since the mechanism(s) of responsible for its effectiveness in the presence of chemotherapy is not known. Aside from the cytoreduction provided by chemotherapy, the infused T cells in the DLI may be much more effective in providing a anti-leukemia effect if the chemotherapy provides lymphodepletion to provide immunologic space for T cells to undergo homeostatic expansion. The use of DLI and IL-2 was shown by Slavin et al to induce remission in patients who failed to achieve remission with DLI alone [22].
An innovative approach to control GvHD is to insert the herpes simplex thymidine kinase gene (HSV-TK) suicide gene into T lymphocytes into donor lymphocytes. Bonini et al [105] inserted the HSV-TK into donor lymphocytes. They subsequently infused these cells into patients requiring DLI for relapsed disease after allogeneic transplant. Once GVHD developed in these patients the authors were able to control the GVHD with ganciclovir which eliminated the HSV-TK transduced T cells [105, 106]. There is ongoing research into the insertion of various suicide genes into the infused donor T cells to have a way to modulate or eliminate the activity of these cells [107].
One approach to decrease GvHD following DLI could be selectively depleting CD8+ cells from DLI product prior to infusion of the cells. This approach is based on the fact that depletion of CD8+ T cells from the graft led to reduced risk of GvHD without any change in disease free survival [108]. This approach has not been tested using DLI.
Another possible approach is the use of reduced intensity conditioning (RIC) regimens followed by planned prophylactic DLI. The strategy is to use RIC to provide immunosuppression that enables the donor stem cells to engraft. Then DLI can be given to provide a GvL effect after a less toxic conditioning regimen followed by HSCT. The lower toxicities associated with this approach makes it a viable option for patients who are older or have significant co-morbidities. Schmid et al investigated the role of RIC with prophylactic DLI in patients with AML/MDS. Out of 75 patients in the study, 12 were eligible to receive prophylactic DLI and of those 10 were long term disease-free survivors [109]. Furthermore, Levenga et al, using RIC followed by SCT and pre-emptive DLI in patients with multiple myeloma, showed only 29 % transplant related mortality. Thirteen of 24 patients received prophylactic DLI, Ten patients were in CR after DLI, but 5 patients were in CR before DLI and 5 had VGPR prior to DLI. Again, these data emphasize the role of DLI as being effective in low tumor burden state. 69% of these 13 patients were long term survivors [110].
Conclusion
Donor lymphocyte infusion is an important therapeutic weapon in the arsenal of hematologists treating patients who have relapsed or have refractory disease after allogeneic SCT. The response rates are best in CML, followed by lymphomas, multiple myeloma and acute leukemias, respectively. Other than CML, clinical responses are most effective when chemotherapy induction is used to reduce the tumor burden and/ or provide lymphodepletion for the T cells in the DLI product to undergo homeostatic expansion.
With the advent of newer TKI-targeted therapies and data showing that allogeneic SCT may be not be superior to an autotransplant in some hematological disorders, the role of allogeneic SCT is being revisited for some of the hematologic malignancies. Despite these considerations, allogeneic SCT will continue to be the mainstay of refractory diseases or diseases with a high risk of relapse. With better understanding of the immune reconstitution and the transplantation immunobiology in the allogeneic and autologous transplantation, transplant physicians continue to try to achieve lower transplant related mortality for allogeneic transplant while maintaining a GvL effect. It would be ideal to decrease relapse rates in hematologic malignancies without increasing deaths due to GvHD or infectious complications. In other words, achieve a allogeneic anti-GvL effect with transplant related toxicities equivalent to an “autologous” SCT.
Future directions are focused on enhancing the anti-tumor activity of the infused donor T cells against the tumor cells while decreasing the toxicities related to GvHD from DLI. Future strategies may involve: 1) infusion of selected T cell(s) subsets or other monuclear cells; 2) infusion of T cells with chimeric targeting receptors; 3) modification of the host environment with new immunosuppressive agents or other targeting agents prior to DLI; 4) chemotherapy to provide tumor reduction and/ or lymphodepletion prior to DLI; and 5) provide a DLI or T cell product prophylactically to avert relapse. In the era of targeted therapy, this polyclonal strategy of DLI for the treatment of unfortunate patients with relapsed or resistant disease after an allogeneic SCT remains a cornerstone of therapeutic options to achieve remission and long term survival.
Acknowledgments
L.G.L is supported in part by R01 CA092344 from the NIH, Translational Grants #60660-5 LLS and #6092-09 LLS from the Leukemia & Lymphoma Society, Susan G. Komen Foundation Grant #BCTR0707125, and startup funding from the Barbara Ann Karmanos Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Abhinav Deol, Email: deola@karmanos.org.
Lawrence G. Lum, Email: luml@karmanos.org.
References
- 1.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957 Sep 12;257(11):491–6. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 2.Reinherz EL, Geha R, Rappeport JM, Wilson M, Penta AC, Hussey RE, et al. Reconstitution after transplantation with T-lymphocyte-depleted HLA haplotype-mismatched bone marrow for severe combined immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6047–51. doi: 10.1073/pnas.79.19.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spruce WE, McMillan R, Miller W, Fox R, Carson D, Schwartz DB, et al. Transplantation of T-lymphocyte-depleted bone marrow between HLA-mismatched individuals. Transplantation. 1983 Oct;36(4):369–72. doi: 10.1097/00007890-198310000-00004. [DOI] [PubMed] [Google Scholar]
- 4.de Witte T, Raymakers R, Plas A, Koekman E, Wessels H, Haanen C. Bone marrow repopulation capacity after transplantation of lymphocyte-depleted allogeneic bone marrow using counterflow centrifugation. Transplantation. 1984 Feb;37(2):151–5. doi: 10.1097/00007890-198402000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Champlin RE, Mitsuyasu RT, Gale RP. Transplantation of T lymphocyte depleted bone marrow to prevent graft-versus-host disease: its implications for fetal liver transplantation. Prog Clin Biol Res. 1985;193:315–25. [PubMed] [Google Scholar]
- 6.Trigg ME, Sondel PM, Billing R, Finlay JL, Peterson A, Hong R, et al. Mismatched bone marrow transplantation in children with hematologic malignancy using T lymphocyte depleted bone marrow. J Biol Response Mod. 1985 Dec;4(6):602–12. [PubMed] [Google Scholar]
- 7.Lowenberg B, Wagemaker G, van Bekkum DW, Sizoo W, Sintnicolaas K, Hendriks WD, et al. Graft-versus-host disease following transplantation of ‘one log’ versus ‘two log’ T-lymphocyte-depleted bone marrow from HLA-identical donors. Bone Marrow Transplant. 1986 Dec;1(2):133–40. [PubMed] [Google Scholar]
- 8.Patterson J, Prentice HG, Brenner MK, Gilmore M, Janossy G, Ivory K, et al. Graft rejection following HLA matched T-lymphocyte depleted bone marrow transplantation. Br J Haematol. 1986 Jun;63(2):221–30. doi: 10.1111/j.1365-2141.1986.tb05544.x. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner N, Lawler M, O'Riordan J, De'Arce M, McCann SR. Donor chimaerism is a strong indicator of disease free survival following bone marrow transplantation for chronic myeloid leukaemia. Leukemia. 1997 Apr;11(Suppl 3):512–5. [PubMed] [Google Scholar]
- 10.Apperley JF, Jones L, Hale G, Waldmann H, Hows J, Rombos Y, et al. Bone marrow transplantation for patients with chronic myeloid leukaemia: T-cell depletion with Campath-1 reduces the incidence of graft-versus-host disease but may increase the risk of leukaemic relapse. Bone Marrow Transplant. 1986 May;1(1):53–66. [PubMed] [Google Scholar]
- 11.Bortin MM, Horowitz MM, Rimm AA. Increasing utilization of allogeneic bone marrow transplantation. Results of the 1988-1990 survey. Ann Intern Med. 1992 Mar 15;116(6):505–12. doi: 10.7326/0003-4819-116-6-505. [DOI] [PubMed] [Google Scholar]
- 12.Leclair B, Fregeau CJ, Aye MT, Fourney RM. DNA typing for bone marrow engraftment follow-up after allogeneic transplant: a comparative study of current technologies. Bone Marrow Transplant. 1995 Jul;16(1):43–55. [PubMed] [Google Scholar]
- 13.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004 May;4(5):371–80. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 14.Peggs KS, Thomson K, Hart DP, Geary J, Morris EC, Yong K, et al. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood. 2004 Feb15 15;103(4):1548–56. doi: 10.1182/blood-2003-05-1513. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet D, Warren EH, Greenberg PD, Dick JE, Riddell SR. CD8(+) minor histocompatibility antigen-specific cytotoxic T lymphocyte clones eliminate human acute myeloid leukemia stem cells. Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8639–44. doi: 10.1073/pnas.96.15.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997 Oct 1;90(7):2529–34. [PubMed] [Google Scholar]
- 17.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990 Dec 15;76(12):2462–5. [PubMed] [Google Scholar]
- 18.Cullis JO, Jiang YZ, Schwarer AP, Hughes TP, Barrett AJ, Goldman JM. Donor leukocyte infusions for chronic myeloid leukemia in relapse after allogeneic bone marrow transplantation. Blood. 1992 Mar 1;79(5):1379–81. [PubMed] [Google Scholar]
- 19.Ferster A, Bujan W, Mouraux T, Devalck C, Heimann P, Sariban E. Complete remission following donor leukocyte infusion in ALL relapsing after haploidentical bone marrow transplantation. Bone Marrow Transplant. 1994 Aug;14(2):331–2. [PubMed] [Google Scholar]
- 20.Pati AR, Godder K, Lamb L, Gee A, Henslee-Downey PJ. Immunotherapy with donor leukocyte infusions for patients with relapsed acute myeloid leukemia following partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 1995 Jun;15(6):979–81. [PubMed] [Google Scholar]
- 21.Sica S, Di Mario A, Salutari P, Rutella S, Chiusolo P, Rumi C, et al. Chemotherapy and recombinant human granulocyte colony-stimulating factor primed donor leukocyte infusion for treatment of relapse after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995 Sep;16(3):483–5. [PubMed] [Google Scholar]
- 22.Slavin S, Naparstek E, Nagler A, Ackerstein A, Samuel S, Kapelushnik J, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996 Mar 15;87(6):2195–204. [PubMed] [Google Scholar]
- 23.Lokhorst HM, Schattenberg A, Cornelissen JJ, Thomas LL, Verdonck LF. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood. 1997 Nov 15;90(10):4206–11. [PubMed] [Google Scholar]
- 24.Salama M, Nevill T, Marcellus D, Parker P, Johnson M, Kirk A, et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant. 2000 Dec;26(11):1179–84. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- 25.Schaap N, Schattenberg A, Bar B, Preijers F, van de Wiel van Kemenade E, de Witte T. Induction of graft-versus-leukemia to prevent relapse after partially lymphocyte-depleted allogeneic bone marrow transplantation by pre-emptive donor leukocyte infusions. Leukemia. 2001 Sep;15(9):1339–46. doi: 10.1038/sj.leu.2402203. [DOI] [PubMed] [Google Scholar]
- 26.van der Griend R, Verdonck LF, Petersen EJ, Veenhuizen P, Bloem AC, Lokhorst HM. Donor leukocyte infusions inducing remissions repeatedly in a patient with recurrent multiple myeloma after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999 Jan;23(2):195–7. doi: 10.1038/sj.bmt.1701546. [DOI] [PubMed] [Google Scholar]
- 27.Verdonck LF, Petersen EJ, Lokhorst HM, Nieuwenhuis HK, Dekker AW, Tilanus MG, et al. Donor leukocyte infusions for recurrent hematologic malignancies after allogeneic bone marrow transplantation: impact of infused and residual donor T cells. Bone Marrow Transplant. 1998 Dec;22(11):1057–63. doi: 10.1038/sj.bmt.1701496. [DOI] [PubMed] [Google Scholar]
- 28.Drobyski WR, Roth MS, Thibodeau SN, Gottschall JL. Molecular remission occurring after donor leukocyte infusions for the treatment of relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992 Sep;10(3):301–4. [PubMed] [Google Scholar]
- 29.Bar BM, Schattenberg A, Mensink EJ, Geurts Van Kessel A, Smetsers TF, Knops GH, et al. Donor leukocyte infusions for chronic myeloid leukemia relapsed after allogeneic bone marrow transplantation. J Clin Oncol. 1993 Mar;11(3):513–9. doi: 10.1200/JCO.1993.11.3.513. [DOI] [PubMed] [Google Scholar]
- 30.Drobyski WR, Keever CA, Roth MS, Koethe S, Hanson G, McFadden P, et al. Salvage immunotherapy using donor leukocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. 1993 Oct 15;82(8):2310–8. [PubMed] [Google Scholar]
- 31.Leber B, Walker IR, Rodriguez A, McBride JA, Carter R, Brain MC. Reinduction of remission of chronic myeloid leukemia by donor leukocyte transfusion following relapse after bone marrow transplantation: recovery complicated by initial pancytopenia and late dermatomyositis. Bone Marrow Transplant. 1993 Oct;12(4):405–7. [PubMed] [Google Scholar]
- 32.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979 May 10;300(19):1068–73. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 33.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981 Jun 18;304(25):1529–33. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 34.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990 Feb 1;75(3):555–62. [PubMed] [Google Scholar]
- 35.Odom LF, August CS, Githens JH, Humbert JR, Morse H, Peakman D, et al. Remission of relapsed leukaemia during a graft-versus-host reaction. A “graft-versus-leukaemia reaction” in man? Lancet. 1978 Sep 9;2(8089):537–40. doi: 10.1016/s0140-6736(78)92879-9. [DOI] [PubMed] [Google Scholar]
- 36.Higano CS, Brixey M, Bryant EM, Durnam DM, Doney K, Sullivan KM, et al. Durable complete remission of acute nonlymphocytic leukemia associated with discontinuation of immunosuppression following relapse after allogeneic bone marrow transplantation. A case report of a probable graft-versus-leukemia effect. Transplantation. 1990 Jul;50(1):175–7. [PubMed] [Google Scholar]
- 37.Collins RH, Jr, Rogers ZR, Bennett M, Kumar V, Nikein A, Fay JW. Hematologic relapse of chronic myelogenous leukemia following allogeneic bone marrow transplantation: apparent graft-versus-leukemia effect following abrupt discontinuation of immunosuppression. Bone Marrow Transplant. 1992 Oct;10(4):391–5. [PubMed] [Google Scholar]
- 38.Goldman JM, Gale RP, Horowitz MM, Biggs JC, Champlin RE, Gluckman E, et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann Intern Med. 1988 Jun;108(6):806–14. doi: 10.7326/0003-4819-108-6-806. [DOI] [PubMed] [Google Scholar]
- 39.Apperley JF, Mauro FR, Goldman JM, Gregory W, Arthur CK, Hows J, et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988 Jun;69(2):239–45. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 40.Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991 Oct 15;78(8):2120–30. [PubMed] [Google Scholar]
- 41.Miller JS, Weisdorf DJ, Burns LJ, Slungaard A, Wagner JE, Verneris MR, et al. Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood. 2007 Oct 1;110(7):2761–3. doi: 10.1182/blood-2007-05-090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995 Sep 1;86(5):2041–50. [PubMed] [Google Scholar]
- 43.Dolstra H, Fredrix H, Maas F, Coulie PG, Brasseur F, Mensink E, et al. A human minor histocompatibility antigen specific for B cell acute lymphoblastic leukemia. J Exp Med. 1999 Jan 18;189(2):301–8. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002 Mar 15;99(6):1928–37. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 45.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002 May 15;99(10):3530–9. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003 Mar 13;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 47.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997 Feb;15(2):433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 48.Porter DL, Collins RH, Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000 Feb 15;95(4):1214–21. [PubMed] [Google Scholar]
- 49.Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000 Oct 15;96(8):2712–6. [PubMed] [Google Scholar]
- 50.Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transplant. 2000 Oct;26(7):769–74. doi: 10.1038/sj.bmt.1702596. [DOI] [PubMed] [Google Scholar]
- 51.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008 Dec 1;112(12):4371–83. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 52.Choi SJ, Lee JH, Kim S, Seol M, Lee YS, Lee JS, et al. Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullar relapse. Leukemia. 2004 Nov;18(11):1789–97. doi: 10.1038/sj.leu.2403523. [DOI] [PubMed] [Google Scholar]
- 53.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007 Nov 1;25(31):4938–45. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 54.Mrsic M, Horowitz MM, Atkinson K, Biggs JC, Champlin RE, Ehninger G, et al. Second HLA-identical sibling transplants for leukemia recurrence. Bone Marrow Transplant. 1992 Apr;9(4):269–75. [PubMed] [Google Scholar]
- 55.Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W, et al. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993 Feb;11(2):304–13. doi: 10.1200/JCO.1993.11.2.304. [DOI] [PubMed] [Google Scholar]
- 56.Mehta J, Powles R, Treleaven J, Horton C, Meller S, Pinkerton CR, et al. Outcome of acute leukemia relapsing after bone marrow transplantation: utility of second transplants and adoptive immunotherapy. Bone Marrow Transplant. 1997 Apr;19(7):709–19. doi: 10.1038/sj.bmt.1700720. [DOI] [PubMed] [Google Scholar]
- 57.Slavin S, Nagler A, Shapira MY, Aker M, Gabriel C, Or R. Treatment of leukemia by alloreactive lymphocytes and nonmyeloablative stem cell transplantation. J Clin Immunol. 2002 Mar;22(2):64–9. doi: 10.1023/a:1014423617596. [DOI] [PubMed] [Google Scholar]
- 58.Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989 Jan;7(1):50–7. doi: 10.1200/JCO.1989.7.1.50. [DOI] [PubMed] [Google Scholar]
- 59.Frassoni F, Barrett AJ, Granena A, Ernst P, Garthon G, Kolb HJ, et al. Relapse after allogeneic bone marrow transplantation for acute leukaemia: a survey by the E.B.M.T. of 117 cases. Br J Haematol. 1988 Nov;70(3):317–20. doi: 10.1111/j.1365-2141.1988.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 60.Bostrom B, Woods WG, Nesbit ME, Krivit W, Kersey J, Weisdorf D, et al. Successful reinduction of patients with acute lymphoblastic leukemia who relapse following bone marrow transplantation. J Clin Oncol. 1987 Mar;5(3):376–81. doi: 10.1200/JCO.1987.5.3.376. [DOI] [PubMed] [Google Scholar]
- 61.Barrett AJ, Joshi R, Tew C. How should acute lymphoblastic leukaemia relapsing after bone-marrow transplantation be treated? Lancet. 1985 May 25;1(8439):1188–91. doi: 10.1016/s0140-6736(85)92865-x. [DOI] [PubMed] [Google Scholar]
- 62.Barrett AJ, Locatelli F, Treleaven JG, Gratwohl A, Szydlo R, Zwaan FE. Second transplants for leukaemic relapse after bone marrow transplantation: high early mortality but favourable effect of chronic GVHD on continued remission. A report by the EBMT Leukaemia Working Party. Br J Haematol. 1991 Dec;79(4):567–74. doi: 10.1111/j.1365-2141.1991.tb08083.x. [DOI] [PubMed] [Google Scholar]
- 63.Bosi A, Laszlo D, Labopin M, Reffeirs J, Michallet M, Gluckman E, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2001 Aug 15;19(16):3675–84. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 64.Choi SJ, Lee JH, Kim S, Lee YS, Seol M, Ryu SG, et al. Treatment of relapsed acute lymphoblastic leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a prospective study. Bone Marrow Transplant. 2005 Jul;36(2):163–9. doi: 10.1038/sj.bmt.1705024. [DOI] [PubMed] [Google Scholar]
- 65.Collins RH, Jr, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000 Sep;26(5):511–6. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 66.Dazzi F, Szydlo RM, Craddock C, Cross NC, Kaeda J, Chase A, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood. 2000 Jan 1;95(1):67–71. [PubMed] [Google Scholar]
- 67.Brouwer RE, van der Heiden P, Schreuder GM, Mulder A, Datema G, Anholts JD, et al. Loss or downregulation of HLA class I expression at the allelic level in acute leukemia is infrequent but functionally relevant, and can be restored by interferon. Hum Immunol. 2002 Mar;63(3):200–10. doi: 10.1016/s0198-8859(01)00381-0. [DOI] [PubMed] [Google Scholar]
- 68.Ossendorp F, Eggers M, Neisig A, Ruppert T, Groettrup M, Sijts A, et al. A single residue exchange within a viral CTL epitope alters proteasome-mediated degradation resulting in lack of antigen presentation. Immunity. 1996 Aug;5(2):115–24. doi: 10.1016/s1074-7613(00)80488-4. [DOI] [PubMed] [Google Scholar]
- 69.Hirano N, Takahashi T, Ohtake S, Hirashima K, Emi N, Saito K, et al. Expression of costimulatory molecules in human leukemias. Leukemia. 1996 Jul;10(7):1168–76. [PubMed] [Google Scholar]
- 70.Cardoso AA, Schultze JL, Boussiotis VA, Freeman GJ, Seamon MJ, Laszlo S, et al. Pre-B acute lymphoblastic leukemia cells may induce T-cell anergy to alloantigen. Blood. 1996 Jul 1;88(1):41–8. [PubMed] [Google Scholar]
- 71.Bergmann L, Schui DK, Brieger J, Weidmann E, Mitrou PS, Hoelzer D. The inhibition of lymphokine-activated killer cells in acute myeloblastic leukemia is mediated by transforming growth factor-beta 1. Exp Hematol. 1995 Dec;23(14):1574–80. [PubMed] [Google Scholar]
- 72.Buzyn A, Petit F, Ostankovitch M, Figueiredo S, Varet B, Guillet JG, et al. Membrane-bound Fas (Apo-1/CD95) ligand on leukemic cells: A mechanism of tumor immune escape in leukemia patients. Blood. 1999 Nov 1;94(9):3135–40. [PubMed] [Google Scholar]
- 73.Ratanatharathorn V, Uberti J, Karanes C, Abella E, Lum LG, Momin F, et al. Prospective comparative trial of autologous versus allogeneic bone marrow transplantation in patients with non-Hodgkin's lymphoma. Blood. 1994 Aug 15;84(4):1050–5. [PubMed] [Google Scholar]
- 74.Jones RJ, Ambinder RF, Piantadosi S, Santos GW. Evidence of a graft-versus-lymphoma effect associated with allogeneic bone marrow transplantation. Blood. 1991 Feb 1;77(3):649–53. [PubMed] [Google Scholar]
- 75.Anderlini P, Acholonu SA, Okoroji GJ, Andersson BS, Couriel DR, De Lima MJ, et al. Donor leukocyte infusions in relapsed Hodgkin's lymphoma following allogeneic stem cell transplantation: CD3+ cell dose, GVHD and disease response. Bone Marrow Transplant. 2004 Sep;34(6):511–4. doi: 10.1038/sj.bmt.1704621. [DOI] [PubMed] [Google Scholar]
- 76.Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol. 2008 Nov;19(11):1935–40. doi: 10.1093/annonc/mdn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bloor AJ, Thomson K, Chowdhry N, Verfuerth S, Ings SJ, Chakraverty R, et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008 Jan;14(1):50–8. doi: 10.1016/j.bbmt.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Peggs KS, Sureda A, Qian W, Caballero D, Hunter A, Urbano-Ispizua A, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007 Oct;139(1):70–80. doi: 10.1111/j.1365-2141.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 79.Morris E, Thomson K, Craddock C, Mahendra P, Milligan D, Cook G, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004 Dec 15;104(13):3865–71. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 80.Mandigers CM, Verdonck LF, Meijerink JP, Dekker AW, Schattenberg AV, Raemaekers JM. Graft-versus-lymphoma effect of donor lymphocyte infusion in indolent lymphomas relapsed after allogeneic stem cell transplantation. Bone Marrow Transplant. 2003 Dec;32(12):1159–63. doi: 10.1038/sj.bmt.1704290. [DOI] [PubMed] [Google Scholar]
- 81.Bierman PJ, Sweetenham JW, Loberiza FR, Jr, Taghipour G, Lazarus HM, Rizzo JD, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin's lymphoma: a comparison with allogeneic and autologous transplantation--The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003 Oct 15;21(20):3744–53. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 82.Bjorkstrand BB, Ljungman P, Svensson H, Hermans J, Alegre A, Apperley J, et al. Allogeneic bone marrow transplantation versus autologous stem cell transplantation in multiple myeloma: a retrospective case-matched study from the European Group for Blood and Marrow Transplantation. Blood. 1996 Dec 15;88(12):4711–8. [PubMed] [Google Scholar]
- 83.Gahrton G, Tura S, Ljungman P, Blade J, Brandt L, Cavo M, et al. Prognostic factors in allogeneic bone marrow transplantation for multiple myeloma. J Clin Oncol. 1995 Jun;13(6):1312–22. doi: 10.1200/JCO.1995.13.6.1312. [DOI] [PubMed] [Google Scholar]
- 84.Bensinger WI, Buckner CD, Anasetti C, Clift R, Storb R, Barnett T, et al. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996 Oct 1;88(7):2787–93. [PubMed] [Google Scholar]
- 85.Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000 Aug;18(16):3031–7. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 86.van de Donk NW, Kroger N, Hegenbart U, Corradini P, San Miguel JF, Goldschmidt H, et al. Prognostic factors for donor lymphocyte infusions following non-myeloablative allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2006 Jun;37(12):1135–41. doi: 10.1038/sj.bmt.1705393. [DOI] [PubMed] [Google Scholar]
- 87.Alyea E, Weller E, Schlossman R, Canning C, Webb I, Doss D, et al. T-cell--depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 2001 Aug 15;98(4):934–9. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]
- 88.Badros A, Barlogie B, Morris C, Desikan R, Martin SR, Munshi N, et al. High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood. 2001 May 1;97(9):2574–9. doi: 10.1182/blood.v97.9.2574. [DOI] [PubMed] [Google Scholar]
- 89.Peggs KS, Banerjee L, Thomson K, Mackinnon S. Post transplant lymphoproliferative disorders following reduced intensity conditioning with in vivo T cell depletion. Bone Marrow Transplant. 2003 Apr;31(8):725–6. doi: 10.1038/sj.bmt.1703893. author reply 7. [DOI] [PubMed] [Google Scholar]
- 90.Marks DI, Lush R, Cavenagh J, Milligan DW, Schey S, Parker A, et al. The toxicity and efficacy of donor lymphocyte infusions given after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2002 Nov 1;100(9):3108–14. doi: 10.1182/blood-2002-02-0506. [DOI] [PubMed] [Google Scholar]
- 91.Peggs KS, Mackinnon S, Linch DC. The role of allogeneic transplantation in non-Hodgkin's lymphoma. Br J Haematol. 2005 Jan;128(2):153–68. doi: 10.1111/j.1365-2141.2004.05251.x. [DOI] [PubMed] [Google Scholar]
- 92.Carlens S, Remberger M, Aschan J, Ringden O. The role of disease stage in the response to donor lymphocyte infusions as treatment for leukemic relapse. Biol Blood Marrow Transplant. 2001;7(1):31–8. doi: 10.1053/bbmt.2001.v7.pm11215696. [DOI] [PubMed] [Google Scholar]
- 93.Keil F, Haas OA, Fritsch G, Kalhs P, Lechner K, Mannhalter C, et al. Donor leukocyte infusion for leukemic relapse after allogeneic marrow transplantation: lack of residual donor hematopoiesis predicts aplasia. Blood. 1997 May 1;89(9):3113–7. [PubMed] [Google Scholar]
- 94.Ball ED, Guyre PM, Mills L, Fisher J, Dinces NB, Fanger MW. Initial trial of bispecific antibody-mediated immunotherapy of CD15-bearing tumors: cytotoxicity of human tumor cells using a bispecific antibody comprised of anti-CD15 (MoAb PM81) and anti-CD64/Fc gamma RI (MoAb 32) J Hematother. 1992 Spring;1(1):85–94. doi: 10.1089/scd.1.1992.1.85. [DOI] [PubMed] [Google Scholar]
- 95.Willemsen RA, Debets R, Chames P, Bolhuis RL. Genetic engineering of T cell specificity for immunotherapy of cancer. Hum Immunol. 2003 Jan;64(1):56–68. doi: 10.1016/s0198-8859(02)00730-9. [DOI] [PubMed] [Google Scholar]
- 96.Gall JM, Davol PA, Grabert RC, Deaver M, Lum LG. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol. 2005 Apr;33(4):452–9. doi: 10.1016/j.exphem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Lum LG, Davol PA. Retargeting T cells and immune effector cells with bispecific antibodies. Cancer Chemother Biol Response Modif. 2005;22:273–91. doi: 10.1016/s0921-4410(04)22013-0. [DOI] [PubMed] [Google Scholar]
- 98.Buhmann R, Simoes B, Stanglmaier M, Yang T, Faltin M, Bund D, et al. Immunotherapy of recurrent B-cell malignancies after allo-SCT with Bi20 (FBTA05), a trifunctional anti-CD3 × anti-CD20 antibody and donor lymphocyte infusion. Bone Marrow Transplant. 2009 Mar;43(5):383–97. doi: 10.1038/bmt.2008.323. [DOI] [PubMed] [Google Scholar]
- 99.Sebastian M, Kiewe P, Schuette W, Brust D, Peschel C, Schneller F, et al. Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti-EpCAM × Anti-CD3): results of a phase 1/2 study. J Immunother. 2009 Feb-Mar;32(2):195–202. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]
- 100.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975 Feb;53(1):27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 101.Anderson LD, Jr, Petropoulos D, Everse LA, Mullen CA. Enhancement of graft-versus-tumor activity and graft-versus-host disease by pretransplant immunization of allogeneic bone marrow donors with a recipient-derived tumor cell vaccine. Cancer Res. 1999 Apr 1;59(7):1525–30. [PubMed] [Google Scholar]
- 102.Kwak LW, Pennington R, Longo DL. Active immunization of murine allogeneic bone marrow transplant donors with B-cell tumor-derived idiotype: a strategy for enhancing the specific antitumor effect of marrow grafts. Blood. 1996 Apr 1;87(7):3053–60. [PubMed] [Google Scholar]
- 103.Kwak LW, Taub DD, Duffey PL, Bensinger WI, Bryant EM, Reynolds CW, et al. Transfer of myeloma idiotype-specific immunity from an actively immunised marrow donor. Lancet. 1995 Apr 22;345(8956):1016–20. doi: 10.1016/s0140-6736(95)90757-2. [DOI] [PubMed] [Google Scholar]
- 104.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996 Jan;2(1):52–8. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 105.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997 Jun 13;276(5319):1719–24. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 106.Verzeletti S, Bonini C, Marktel S, Nobili N, Ciceri F, Traversari C, et al. Herpes simplex virus thymidine kinase gene transfer for controlled graft-versus-host disease and graft-versus-leukemia: clinical follow-up and improved new vectors. Hum Gene Ther. 1998 Oct 10;9(15):2243–51. doi: 10.1089/hum.1998.9.15-2243. [DOI] [PubMed] [Google Scholar]
- 107.Thomis DC, Marktel S, Bonini C, Traversari C, Gilman M, Bordignon C, et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001 Mar 1;97(5):1249–57. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 108.Nimer SD, Giorgi J, Gajewski JL, Ku N, Schiller GJ, Lee K, et al. Selective depletion of CD8+ cells for prevention of graft-versus-host disease after bone marrow transplantation. A randomized controlled trial. Transplantation. 1994 Jan;57(1):82–7. doi: 10.1097/00007890-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 109.Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005 Aug 20;23(24):5675–87. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 110.Levenga H, Levison-Keating S, Schattenberg AV, Dolstra H, Schaap N, Raymakers RA. Multiple myeloma patients receiving pre-emptive donor lymphocyte infusion after partial T-cell-depleted allogeneic stem cell transplantation show a long progression-free survival. Bone Marrow Transplant. 2007 Aug;40(4):355–9. doi: 10.1038/sj.bmt.1705742. [DOI] [PubMed] [Google Scholar]