Abstract
SAG (Sensitive to Apoptosis Gene) (also known as RBX2 or ROC2) is a dual function protein with antioxidant activity while acting alone or E3 ligase activity when complexed with other components of SCF (Skp1-cullins-F box proteins) E3 ubiquitin ligases. SAG acts as a survival protein to inhibit apoptosis induced by a variety of stresses. Our recent work showed that SAG siRNA silencing sensitized cancer cells to radiation but the mechanism responsible remains elusive. Here we report that complete elimination of Sag expression via a gene trapping strategy significantly sensitized mouse embryonic stem cells to radiation with a SER (sensitizing enhancement rate) of 1.5–1.6. Radiosensitization was associated with increased steady-state levels of intracellular ROS (including superoxide) 24 h following irradiation as well as enhancement of radiation-induced apoptosis. Furthermore Sag elimination abrogated IκBα degradation leading to inhibition of NFκB activation. Further detailed analysis revealed that I IκBα is a direct substrate of SAG-SCFβTrCP E3 ubiquitin ligase. Taken together, these results support the hypothesis that Sag elimination via gene disruption sensitizes ES cells to radiation-induced cell killing by mechanisms that involve increased steady-state levels of ROS and decreased activation of NFκB.
Keywords: Apoptosis, NFκB, protein ubiquitination, Radiosensitization, ROS, SAG E3 ligase
Introduction
SAG, also known as RBX2 (RING box protein 2), ROC2 (Regulator of cullins-2), or RNF7 (RING finger protein 7), was originally cloned in our laboratory as a redox inducible antioxidant protein and was later characterized as the second member of ROC/RBX RING component of SCF (Skp1, cullins, F-box proteins) E3 ubiquitin ligases [1–3]. SAG belongs to an evolutionarily conserved gene family and is mapped onto chromosome 3q22-24 with three splice variants and two family pseudogenes in humans [1, 4]. Previous work has revealed that when tested in in vitro, using purified protein, SAG scavenges H2O2 at the expense of self oligomerization [3]; has thiol-linked peroxidase activity [5]; and inhibits peroxynitrite-induced DNA damage [6]. In addition when complexed with other components of SCF E3 ubiquitin ligase, SAG has E3 ubiquitin ligase activity [7, 8]. In cell culture systems, SAG, when over-expressed, inhibits apoptosis induced by disruptions in cellular redox homeostasis [1, 9], nitric oxide [10], ischemia/reoxygenation [11], neurotoxins and 1-methyl-4-phenylpyridinium (MPP) [12], heat-shock [13] and UV-irradiation [14]. SAG over-expression also promotes S-phase entry and cell growth under serum starved conditions [15] and inhibits TPA-induced neoplastic transformation [16]. Silencing SAG expression by anti-sense or siRNA inhibits tumor cell growth [17], enhances apoptosis induced by etoposide and TRAIL [18], enhances TPA-induced neoplastic transformation [16], and sensitizes radioresistant cancer cells to radiation [19]. These biological effects of SAG have been suggested to be mediated by antioxidant activity [1, 10] as well as through its E3 ubiquitin ligase activity by promoting the degradation of p27, c-Jun, pro-caspase-3, IκBα, HIF-1α, and Noxa [15, 16, 18–21].
The antioxidant activity of SAG was mainly demonstrated previously in vitro using purified SAG protein [3, 5] and has been implicated in SAG over expression studies in cultured cells [1, 10, 11] and in mouse brain tissues [22]. A Tat-SAG fusion protein was also recently reported to reduce oxidative stress and brain ischemic insults [23]. None of these previous studies have used SAG knockdown or knockout cells to directly measure alterations in steady-state levels of ROS following exposure to agents that induce oxidative damage in living cells such as ionizing radiation. Furthermore IκBα, a known substrate for RBX1/ROC1-SCF [24, 25], which is subject to enhanced radiation-induced degradation leading to induction of MnSOD [26–28] was recently implicated as a substrate of SAG/RBX2/ROC2-SCF E3 ligase [21], but direct biochemical evidence supporting this relationship is lacking.
In this report we have used Sag gene disruption in mouse embryonic stem cells to show that Sag elimination sensitizes ES cells to radiation-induced cell killing and apoptosis by mechanisms that appear to involve increased steady-state levels of intracellular ROS and decreased activation of NFκB.
Materials and Methods
Generation and maintenance of Sag−/− ES Cell Lines
Blastocysts were isolated from Sag+/− females mated with Sag+/− males. Blastocysts were placed in culture on irradiated mouse embryonic feeder cells in high glucose DMEM (Invitrogen, Carlsbad, CA) supplemented with 15% fetal bovine serum (Harlan, Indianopolis, IN), 0.1 mM β-mercaptoethanol (Sigma, St. Louis, MO), 50 IU/ml penicillin, 50 µg/ml streptomycin, 103u/ ml ESGRO (Chemicon, Temecula, CA) and 12.5 µM PD 98059 (Sigma). Inner cell mass outgrowths were trypsinized and passaged sequentially until ES cell lines were established in 35 mm cell culture dishes. Subsequent culture continued in the absence of antibiotics and PD098059. ES cells were passaged on gelatin coated dishes twice to eliminate feeder cells prior to genotyping as described [29].
Radiation exposure and clonogenic Assay
Cells were seeded in 6-well plates at three different cell densities in duplicate. The next day, cells were exposed to different doses of radiation (Philips RT250, Kimtron Medical), followed by incubation at 37 °C for 7 to 9 days. Survival curves were fitted using the linear-quadratic equation, and the mean inactivation dose was calculated according to the method of Fertil and colleagues [30]. Irradiations were performed in the Experimental Irradiation Core of the University of Michigan Comprehensive Cancer Center.
DNA fragmentation analysis
The cells were seeded in 100mm dishes at 2.5×106. The next day the cells were left untreated or exposed to 10 Gy radiation. Both detached and attached cells were harvested 24 hrs later by scraping, pelleted, and lysed in 600 µl of lysis buffer (5mM Tris-HCl, pH 8, 20 mM EDTA and 0.5% Triton X-100). The DNA was isolated using Phenol:Choroform:Isoamine (Fisher) extraction and ethanol precipitation, and subjected to electrophoresis in a 1.8% agarose gel [31].
Western blotting analysis
ES cells were harvested, lysed in a Titron X-100 lysis buffer and subjected to Western blotting analysis, as described [1], using various antibodies as follows. SAG monoclonal antibody was raised against the RING domain (AA44-113), IκBα, c-Jun, β-catenin, E2F1, and Cul-1 (Santa Cruz Biotechnology, CA), Bcl-xL (Cell Signaling, MA), β-Actin, and FLAG (Sigma, MO), HA (Roche, IN), and p53 (EMD Chemicals, NJ). MnSOD antibody was described previously [32] and cIAP-1 antibody was a gift from Dr. John Silke at La Trobe University, Australia.
Caspase activation assay
The activity of caspase-3 was analyzed using a fluorogenic caspase assay with Ac-DEVA-AFC (Biomol) as a substrate [33]. The results are expressed as fold change compared to control.
Measurement of intracellular superoxide levels using dihydroethidium (DHE) oxidation
Sub-confluent ES cells were left untreated or exposed to 10 Gy radiation. Twenty-four hrs later, cells were washed once with PBS and exposed to 5 µM DHE (in 0.1% DMSO) (Molecular Probes, Eugene, Oregon) in PBS containing 5 mM pyruvate at 37°C for 40 min to measure DHE oxidation. After labeling, cells were trypsinized and resuspended in PBS containing 5mM pyruvate and kept on ice. Samples were analyzed using a FACScan flowcytometer (Becton Dickinson Immunocytometry System, INC., Mountain View, CA) (excitation 488 nm, emission 585 nm band-pass filter). The mean fluorescence intensity (MFI) of 10,000 cells was analyzed in each sample in triplicate and corrected for autofluorescence from unlabeled cells. The MFI data was normalized to control levels as fold increase. To confirm that superoxide was responsible for increases in MFI noted in irradiated cells labeled with DHE, the cells were treated 2 h prior to labeling with 100 U/ml PEG–CuZnSOD (Sigma Chemical Co., St. Louis, MO, USA) or PEG alone (18 µM). The differences in DHE oxidation between PEG–SOD and PEG-alone treated cells were calculated and plotted as fold increase from the controls.
Measurement of intracellular prooxidants (presumably hydroperoxides)
Steady–state levels of pro-oxidants (presumably hydroperoxides) were estimated using the oxidation-sensitive CDCFH2 [5- (and 6-)carboxy-2′,7′-dichlorodihydrofluorescein diacetate] (10 µg/ml) and oxidation-insensitive CDCF [5- (and 6-)carboxy-2′,7′- dichlorofluorescein diacetate] (10 µg/ml) fluorescent dyes (dissolved in 0.1% DMSO) that were obtained from Molecular Probes. Sub-confluent ES cells were left untreated or exposed to 10 Gy radiation. Twenty-four hrs later, the cells were washed once with PBS and then labeled with CDCFH2 or CDCF for 15 mins at 37°C in PBS. After the labeling, the cells were trypsinized and resuspended in PBS and kept on ice. Samples were then analyzed using a FACScan flowcytometer. The MFI of 10,000 cells were analyzed in each sample in triplicate and corrected for autofluorescence from unlabeled cells. The MFI data was normalized to control levels and expressed as fold increase.
Luciferase reporter assay
A luciferase reporter driven by NFκB consensus sequence (pNifty plasmid, Invivogen, CA), along with Renilla construct were electroporated into ES cells. Briefly, cells were harvested from plates by 0.05% trypsin, and suspended at a concentration of 107 cells/ml in ES culture medium. Transfected DNA (20 µg) was then mixed with 0.8 ml of ES cells in a steriled electroporation cuvette. Electroporation was carried out using a BioRad electroporater at room temperature (250 µF and 0.3 Kev for 4 seconds). Cells after electroporation were seeded into a 96-well plate and left untreated or treated with 10 Gy radiation or TNFα (25 ng/ml, BD biospheres), followed by luciferase activity assay (Promega) at indicated time points afterward. The results are presented as the fold activation after normalization with Renilla.
DNA transfection and immunoprecipitation
Cells were transfected with a variety of plasmids in 293 cells and subjected to immunoprecipitation 48 hrs later as described [18] with FLAG-beads (Sigma) to pull-down SAG as well as other SCF components. The immunoprecipitates were washed four times with lysis buffer. One portion was assessed by Western blotting with various antibodies and the other portion was used for in vitro ubiquitination assay.
In vitro ubiquitination of Iκ Bα
The assay was performed as recently described [34]. Briefly, IKKβ-phosphorylated 32P-GST-2TK-Iκ Bα (1–54), was prepared using a two-step procedure. GST-2TK-Iκ Bα (1–54) was first phosphorylated by IKKβ. The IKKβ-phosphorylated GST-2TK-Iκ Bα was adsorbed to glutathione beads, and the bound IKKβ-phosphorylated GST-2TK-Iκ Bα was then 32P labeled by cyclic AMP kinase. Following washing of the beads, the bound 32P-labeled substrate was eluted and used for in vitro ubiquitination assay as follows. SAG-SCFβTrCP2 complex, prepared by immunoprecipitation with FLAG-beads was incubated in a mixture that contained 50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 2 mM NaF, 10 nM okadaic acid, 2 mM ATP, 0.6 mM DTT, 0.1 mg/ml BSA, 32P-GST-2TK-IκBα (20 nM), Ub (80 µM), E1 (10 nM), with or without E2 (Cdc34, 1µM; UbcH5c, 0.06µM) . The reaction mix was incubated at 37°C for 60 min on a thermomixer (1,300 rpm; Eppendorf). The reaction products were visualized by autoradiography after separation by 4 to 20% SDS-PAGE [34].
Statistical analysis
The paired Student t test was used for statistical analysis, using SAS software.
Results
Targeted disruption of Sag sensitized mouse ES cells to radiation
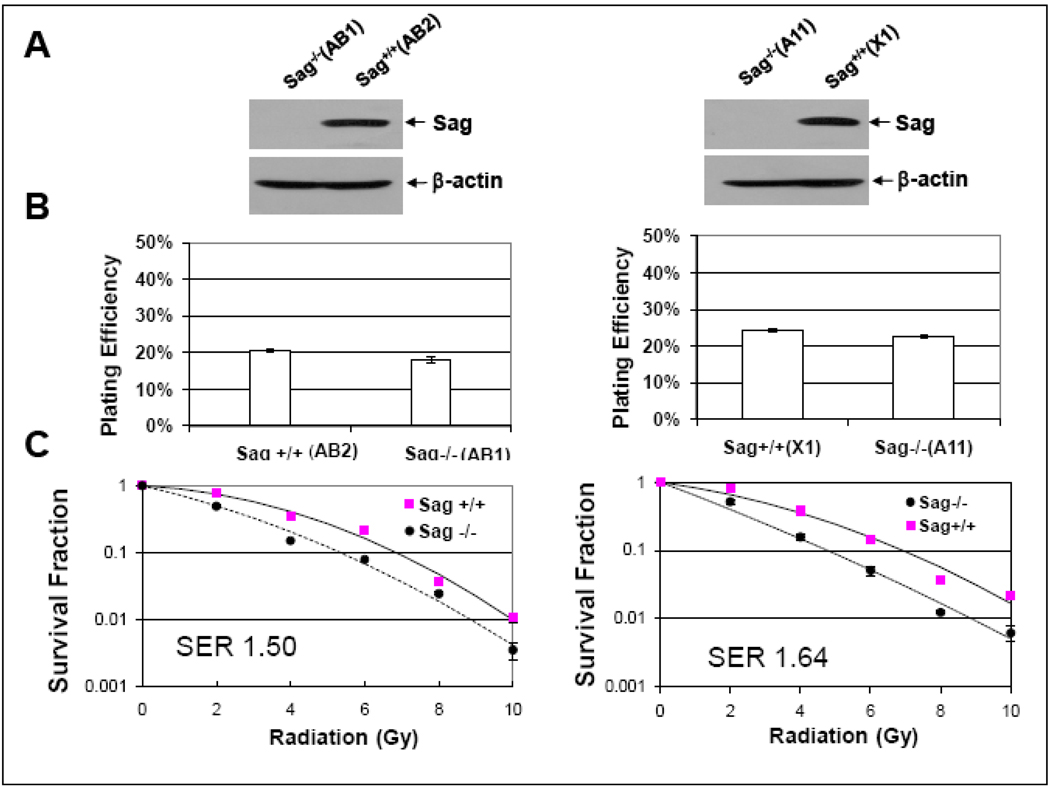
To investigate the role of Sag in radiosensitivity, we generated mouse ES cells from blastocysts derived from intercrossing of Sag gene-trapped heterozygous mice (Sag+/−) (manuscript submitted elsewhere). Two independent pairs of mouse ES cell clones were obtained, in which Sag disruption via gene-trapping completely eliminated Sag protein expression (clones AB1 and A11, compared to wild type AB2 and X1) (Fig. 1A). Two pairs of ES cells were subjected to standard clonogenic assay. Sag disruption had no effect on clonogenic growth as reflected by plating efficiency (Fig. 1B), but significantly sensitized ES cells to radiation with a SER (Sensitizing Enhancement Ratio) of 1.5–1.6 (Fig. 1C).
Figure 1. Sag disruption has no effect on clonogenic growth, but sensitizes mouse ES cells to radiation.
ES cells with genotypes of Sag+/+ and Sag−/− were derived from outgrowth of blastocysts, isolated from intercrossing of Sag+/− mice (manuscript submitted elsewhere), as described [54] and subjected to Western blotting analysis (A), and standard clonogenic assay in the absence (B) or presence (C) of radiation exposure. The colonies with more than 50 cells were counted after 9 days growth in culture. Plating efficiency was calculated by dividing number of colonies formed by number of cells seeded, whereas the surviving fraction was determined by the proportion of seeded cells following irradiation to form colonies relative to untreated cells. SER was calculated as the ratio of the mean inactivation dose under Sag+/+ ES control cells divided by the mean inactivation dose under Sag−/− ES cells. Shown is mean ± SEM (n=3).
Targeted disruption of Sag enhanced radiation-induced apoptosis
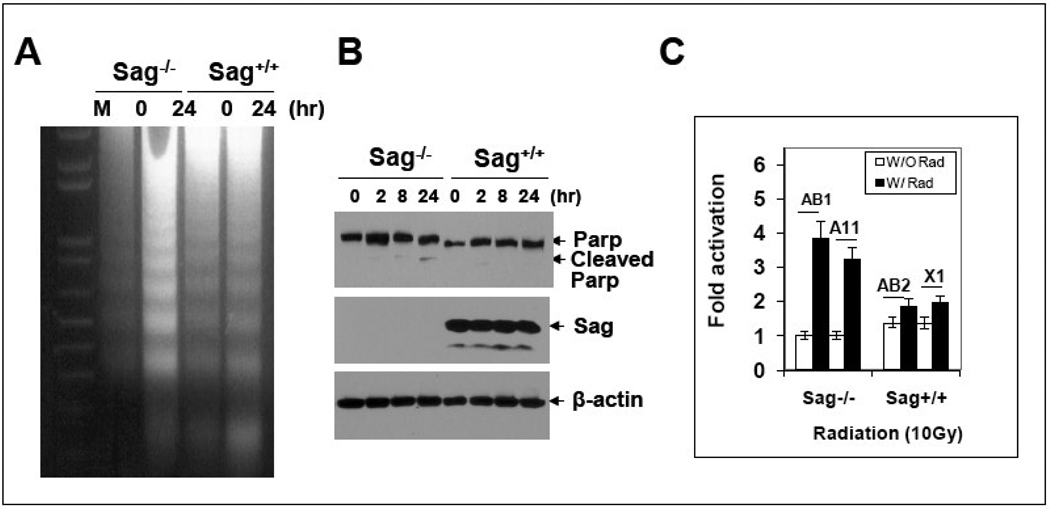
We next determined, using one pair of ES clones, if radiosensitization by Sag disruption was associated with apoptosis induction, since SAG was previously implicated as an anti-apoptotic protein [1, 2]. As shown in Figure 2, Sag disruption indeed enhanced radiation-induced apoptosis as demonstrated by enhanced DNA fragmentation (A), appearance of cleaved PARP (B) and increased caspase-3 activity (C).
Figure 2. Sag disruption sensitizes ES cells to radiation-induced apoptosis.
ES cells with genotype of Sag+/+ and Sag−/− were left untreated or exposed to 10 Gy radiation, followed by DNA fragmentation assay 24 hrs later (A), Western blotting for PARP cleavage at indicated time points (B), or ELISA assay for caspase-3 activity, 24 hrs post radiation (C).
Targeted disruption of Sag increased ROS generation
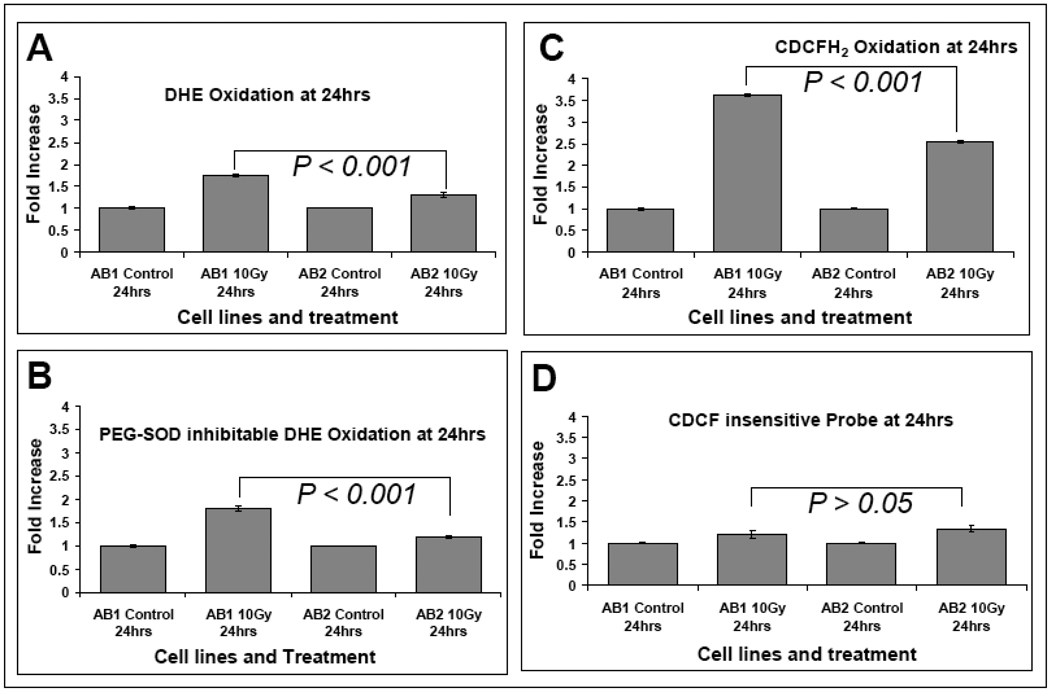
Radiolysis of water caused by ionizing radiation produces ROS which can cause oxidative damage to critical bimolecules leading to persistent increases in steady-state levels of intracellular ROS which are believed to contribute to the biological effects of radiation [35–38]. Since Sag possesses antioxidant activity, likely due to its enriched proportion of cysteine residues (greater than 10% of amino acid composition) [3], we reasoned that loss of Sag would lead to increased steady-state levels of ROS after radiation exposure, which could contribute to increased sensitivity of Sag−/− ES cells to radiation. Using the superoxide-sensitive dye DHE (dihydroethidium), we found that basal levels of DHE oxidation were similar between Sag+/+ (AB2) and Sag−/− ES (AB1) cells. Twenty-four hours following exposure to 10 Gy ionizing radiation, the steady-state levels of DHE oxidation increased in both clones, but a significant 40% greater increase was seen in AB1 cells than that in AB2 cells (Fig. 3A), suggesting Sag disruption lead to increased steady-state levels of superoxide. To confirm the DHE oxidation was truly derived from superoxide anion, we included PEG-SOD (polyethylene glycol conjugated copper-zinc superoxide dismutase) in the assay and found that all increased DHE oxidation was inhibitable by PEG-SOD, supporting the conclusion that the observed increases in DHE oxidiation were caused by superoxide anions (Fig. 3B). We then used the peroxidesensitive dye CDCFH2 to determine steady-state levels of pro-oxidants in cells exposed to radiation and again found significantly higher levels of probe oxidation in Sag−/− AB1 cells upon radiation when compared to Sag+/+ AB2 cells (Fig. 3C). Using the oxidation insensitive analog of CDCFH2 (CDCF) as a control for changes in probe uptake, ester cleavage, and efflux, we found no significant difference between two lines when exposed to radiation. (Fig. 3D). Taken together, these results clearly support the conclusion that disruption of Sag expression and function leads to increased steady-state levels of ROS in cells following exposure to ionizing radiation.
Figure 3. Sag disruption increases ROS levels following exposure to radiation.
ES cells with the Sag+/+ (AB2) and Sag−/− (AB1) genotype were left untreated or exposed to 10 Gy radiation (Cs137 at 0.75 Gy/min). Cells were labeled with DHE as described in the Methods 24 following radiation (panels A & B, 5 µM for 40 min) or CDCFH2/CDCF (10 µg/mL for 15 mins) (panels C & D). The oxidation insensitive probe (CDCF) was used to control for potential changes in probe uptake, ester cleavage, or efflux to ensure that changes in fluorescence seen with CDCFH2 could be attributed to changes in probe oxidation. For measurement of PEG-SOD inhibitable DHE oxidation (panel B), PEG-CuZnSOD (100 U/ml) was given 2 hours before and during DHE labeling. Ten thousand cells from each sample were then analyzed for Mean Fluorescence Intensity (MFI) by flow cytometry. PEG-SOD inhibitable DHE oxidation was obtained by calculating the difference between DHE oxidation in the presence or absence of PEG-SOD. Each data point represents the mean ± SEM from two independent experiments, each run in triplicate.
Targeted disruption of Sag caused IκBα accumulation
SAG is the RING component of SCF E3 ubiquitin ligase required for its ligase activity and SAG disruption would be expected to cause the accumulation of its substrates. We focused on a set of proteins that are known substrates of SAG-SCF E3 ubiquitin ligase involved in cell proliferation and apoptosis, including IκBα, β-catenin, c-Jun, and E2F1 [39, 40]. We found that among all tested proteins, IκBα was the only protein that accumulated in Sag−/− AB1 cells (Figure 4A). We therefore focused our study on SAG regulation of IκBα and its biochemical and biological significance.
Figure 4. Sag disruption causes IκBα accumulation and blocks NF-κB activation.
Subconfluent ES cells with genotype of Sag+/+ (AB2) and Sag−/− (AB1) was subjected to Western blotting analysis (A) or exposed to 10 Gy radiation, followed by Western blotting analysis at indicated time points (B). A NFκB luciferase reporter construct (pNifty plasmid, Invivogene) [55] was transiently transfected, along with Renilla construct, into AB1 or AB2 ES cells via electroporation. Cells were seeded into 96-well plates and cultured for 24 hr, and left untreated or treated with TNFα (10 ng/ml) (C), or exposed to 10 Gy radiation (D) for 8 hr and 24 hr, respectively, followed by luciferase assay. The results, from three independent transfections with six replicates per treatment, are presented as relative luciferase units after normalization with transfection efficiency with Renilla. The paired Student t test was used for statistical analysis. (*, p<0.05; **p < 0.01). Subconfluent cells were left untreated or exposed to 10 Gy radiation for 8 or 24 hrs, followed by Western blotting analysis using indicated antibodies (E).
It is well-established that ionizing radiation causes activation of NF-κB [41] through degradation of IκB [26, 27], whereas activated NF-κB induces an adaptive response to confer cell resistance to ionizing radiation [42]. We therefore determined the levels of IκBα after exposure to radiation for various time points. As shown in Figure 4B, the IκBα levels were much higher in Sag−/− AB1 cells than Sag+/+ AB2 cells in all time points tested. The lack of complete elimination of IκBα in Sag+/+ cells upon radiation is due to the fact that IκBα is a transcriptional target of NFκB, which is likely induced upon NFκB activation [43].
We next determined if NFκB transcription activity is consequently inhibited (due to IκBα accumulation) in Sag−/− ES cells at both basal and induced levels after exposure to TNFα (another known NFκB activator) or radiation, using a luciferase reporter-based transcriptional activation assay. Indeed, NFκB transcriptional activity, as reflected by luciferase units, was ~2-fold lower at the basal level and 2- to 3-fold lower after TNFα treatment (Fig. 4C) or radiation exposure (Fig. 4D) in Sag−/− cells than that in Sag+/+ cells. The difference is statistically significant. We further determined expression of endogenous NFκB target genes to verify the effect of Sag on the transcription function of NFκB. As shown in Figure 4E, expression of three known NFκB target anti-apoptotic proteins, Bcl-xL, MnSod, and cIap-1 [43] were all induced in response to ionizing radiation in Sag+/+, but not in Sag−/− ES cells. Taken together, our results clearly demonstrated that SAG disruption causes the accumulation of IκBα that blocks NFκB activation upon exposure to radiation.
IκBα is a direct substrate of SAG-SCFβ-TrCP E3 ligase
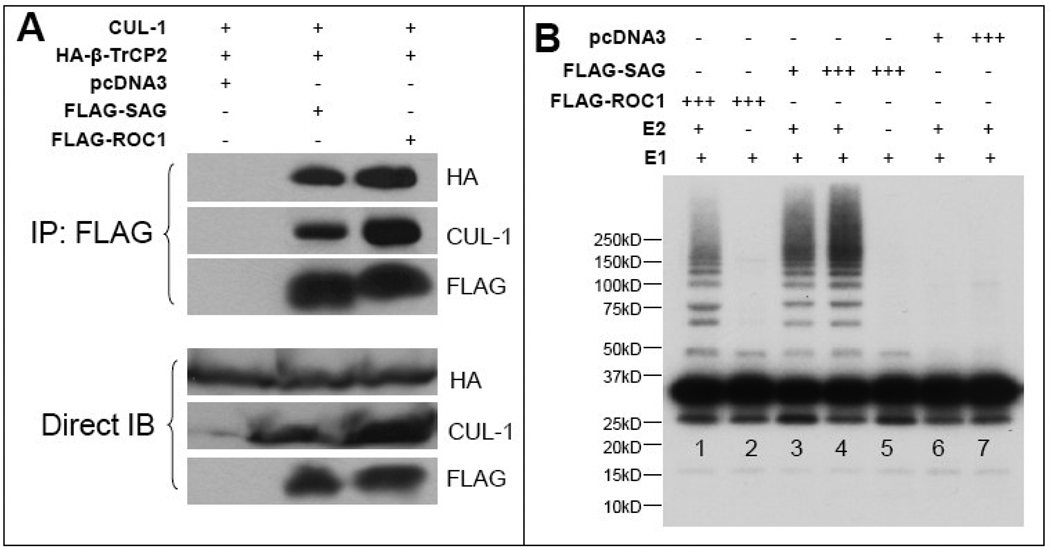
Previous study has shown that IκBα is a direct substrate of RBX1/ROC1-SCFβ-TrCP E3 ligase [24, 25]. Our data presented here, along with our previous observation [21], suggest that IκBα could also be a direct substrate of SAG-SCFβ-TrCP E3 ubiquitin ligases. We therefore went on to demonstrate this using a well-established in vitro E3 ubiquitination assay. SAG-Cul-1/β-TrCP2 (an F-box protein that recognizes IκBα) [24], along with positive control ROC1-Cul-1/β-TrCP2, were transfected into 293 cells. The complex was immunoprecipitated using FLAG-beads (Fig. 5A) and used as the source of SCF E3 ligase in an in vitro biochemical assay. As shown in Figure 5B, the SAG-SCF complex was able to promote IκBα ubiquitination in vitro (Fig. 5B, lanes 3 & 4), as effective as the ROC1-containing complex (lane 1). No ligase activity was detected when E2 was omitted from the reaction (lane 5), nor in the vector control samples even in the presence of E2 (lanes 6 & 7). These data clearly indicated that IκB is a direct target of SAG-SCFβ-TrCP E3 ligases.
Figure 5. SAG-SCFβ-TrCP E3 ligase promotes in vitro IκB ubiquitination.
The 293 cells were co-transfected with FLAG-SAG or FLAG-ROC1 as a positive control [24], HA-β-TrCP2, untagged cullin-1. SAG-SCF or ROC1-SCF complex was pulled down by FLAG-beads and expression of all three components (SAG/ROC1, Cul-1, β-TrCP2) is shown (A). The complex was then tested for its ligase activity in an in vitro ubiquitination assay using in vitro phosphoylated IκB as a substrate, as described in the Materials and Methods, followed by SDS-PAGE and autoradiography. In reactions shown in lanes 3 and 6, 200µl of extracts were used for immuno-affinity purification of the E3 complex. Extracts (600µl) were used in lanes 1, 2, 4, 5 and 7.
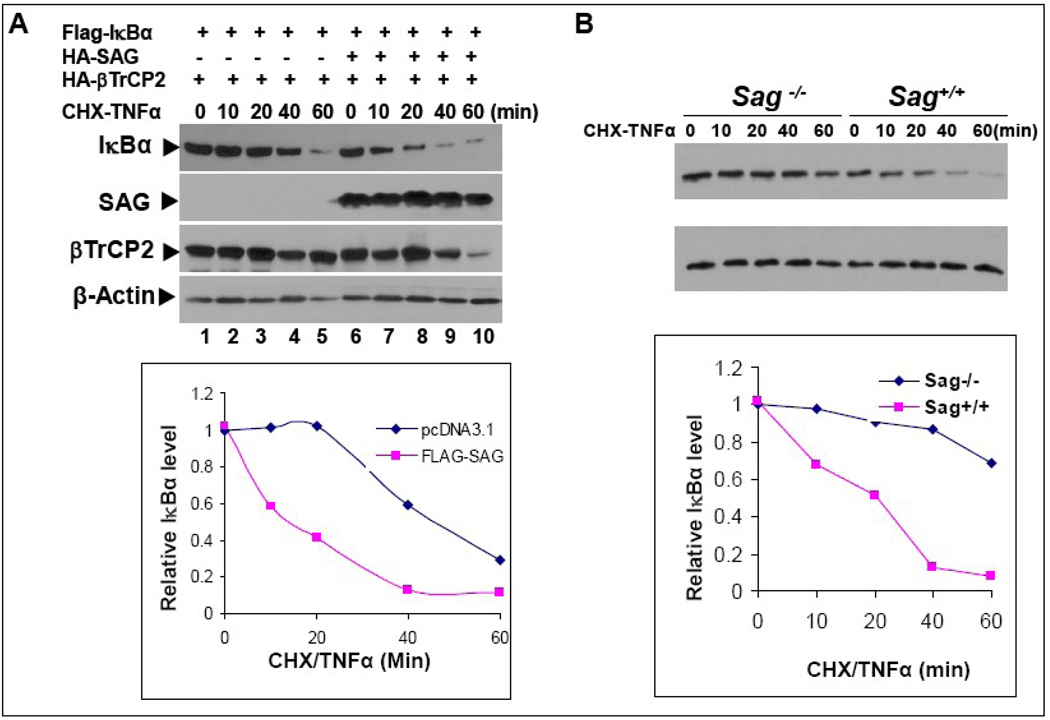
Sag modulates the protein half-life of IκBα
Finally, we determined if modulation of cellular SAG levels would alter the protein half-life of IκBα. As shown in Figure 6A, upon TNFα stimulation, the half-life of the recombinant FLAG-IκBα was ~40 min when cotransfected with β-TrCP2. The half-life value for FLAG-IκBα was significantly longer than that observed with the endogenous protein (with a half-life typically at 5–10min) [44]. This finding suggests that SCF activity was limiting even in the presence of over-expressed β-TrCP2. Importantly, inclusion of SAG in the cotransfection shortened the half-life to ~15 min. Thus, over-expression of SAG shortens the protein half-life of exogenously expressed IκBα. In a reciprocal experiment, we used mouse ES cells to determine the effect of Sag disruption on the protein half-life of endogenous IκBα after TNFα stimulation, which initiates its degradation. As shown in Figure 6B, the half-life of endogeous IκBα was ~20 min in wild type ES cells, which was significantly extended to more than 60 min when Sag was disrupted. Taken together, these results clearly demonstrated that IκBα is a direct substrate of SAG-SCFβ-TrCP E3 ubiquitin ligase and support the conclusion that IκBα accumulates upon Sag disruption preventing NFκB activation and rendering cells more susceptible to radiation-induced apoptosis, which contributes to the increased radiosensitivity seen in Sag−/− ES cells.
Figure 6. Sag regulates IκBα protein half-life upon TNFα stimulation.
(A) SAG overexpression shortens the protein half-life of IκBα: FLAG-IκBα was co-transfected with HA-β-TrCP2 with or without HA-SAG in H1299 cells. Cells were treated simultaneously with TNFα (25 ng/ml) to trigger IκBα degradation and CHX (100 µg/ml) to block new protein synthesis for indicated time periods and subjected to Western blotting analysis. (B) Sag disruption extends the protein half-life of IκBα: AB-1 and AB-2 ES cells were exposed simultaneously to TNFα (25 ng/ml) and CHX (100 µg/ml). Cells were harvested at indicated time point and subjected to Western blotting analysis. Densitometry quantification was performed by ImageJ with β-actin as the loading control.
DISCUSSION
We have recently shown that siRNA silencing of SAG induced moderate radiosensitization of two radioresistant human cancer lines. Relatively weak radiosensitizing activity upon SAG silencing was likely due to only partial reduction of SAG levels. Here, using a genetic approach that disrupted Sag gene, we achieved a complete elimination of Sag expression in mouse embryonic stem cells. As a consequence of Sag elimination, ES cells become much more sensitive to radiation with a SER of 1.6. We also showed that radiosensitization upon Sag elimination was associated with enhanced apoptosis induced by radiation, which is attributable to abrogation of both antioxidant and E3 ligase activities of Sag.
Antioxidant proteins can be classified into two major categories; the first includes primary antioxidant enzymes, such as superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase. These enzymes effectively catalyze the scavenging of ROS generated during respiratory processes and/or after exposure to external stimuli [36]. The second category is thiol antioxidant proteins with typical examples of metallothionein [45] and thioredoxin [46], which usually contain a high percentage of cysteine residues in their amino acid composition. Their antioxidant activity is to use thiol groups in the cysteine residues to buffer the ROS, to chelate the metals, to quench the radicals, and to regulate redox reactions [47]. Because ROS scavenging by these antioxidant proteins does not involve catalytic enzyme reaction, the efficiency in elimination of ROS is relatively lower than that of antioxidant enzymes. SAG, with 10.6% cysteine residues in its composition, belongs to the second category, which scavenges ROS at the expense of the formation of intra- and inter-molecule disulfide bonds as well as by chelating zinc or copper ion [2, 3]. This feature may explain why in Sag−/− ES cells, ROS accumulation following radiation was significantly higher than that in wild type ES cells. Another interesting finding is that increased steady-state levels of superoxide occur in Sag−/− ES cells following radiation. We found that DHE oxidation can be blocked by PEG-SOD, confirming that superoxide is the source of DHE oxidation. This finding is consistent with our previous observation in a mouse brain ischemia model in which SAG-induced superoxide scavenging reduced the infarction size [22]. We also found an increased level of CDCFH2 oxidation in Sag−/− ES cells as compared to wild type ES cells upon radiation, suggesting involvement of other peroxides derived from superoxide which could also be contributing to increased radiosensitivity. Taken together, our study using a gene knockout approach clearly demonstrates that Sag may play a role in modulating ROS levels in living cells, the lack of which contributes to radiosensitization.
Another important finding of this study is that radiosensitization upon Sag elimination is associated with IκBα accumulation that prevents NFκB activation and that IκBα is a direct substrate of SAG-SCFβTrCP E3 ubiquitin ligase. In human and mouse, there are only two family members of RING component of SCF E3 ligase, namely RBX1/ROC1 and RBX2/ROC2/SAG [1, 48–52]. Both RBX1 and RBX2/SAG bind to six members of cullin family (Cul 1–3, Cul4A, B and Cul-5) [50] and have in vitro E3 ubiquitin ligase activity when complexed with cullin-1 [8]. The known differences between RBX1 and RBX2/SAG is that RBX1 is constitutively expressed, whereas RBX2/SAG is stress inducible [16], and that RBX1 prefers to bind with Cul2/VHL, whereas RBX2/SAG with Cul-5/SOCS [53]. In spite of these differences, we demonstrated in a biochemical ubiquitination assay that either SAG-SCFβTrCP or RBX1/ROC1-SCFβ-TrCP E3 ligase directly promotes IκBα ubiquitination to a similar extent. This result suggested that two ligases have a similar in vitro enzymatic activity toward this substrate. At the cellular levels, however, accumulation of IκBα upon Sag disruption at Rbx1 wild type background suggests that Sag deficiency was not compensated by Rbx1, and two ligases may have non-redundant functions. This is consistent to our recent observation that the in vivo functions of Sag and Rbx1 are non-redundant during mouse embryonic development [54].
In summary, our study using Sag−/− mouse ES cells supports the hypothesis that Sag appears to play a role in protection from radiation-induced cytotoxicity by modulating steady-state levels of ROS as well as by promoting IκBα degradation, leading to NFκB activation and apoptosis inhibition.
Acknowledgements
We would like to thank Dr. Thomas L. Saunders at the Transgenic Animal Model Core, Biomedical Research Core Facilities, the University of Michigan for his help in generating Sag−/− ES cells. This work is supported by the NCI grants (CA111554 and CA118762) to YS, Public Health Service grants GM61051 and CA095634 to Z-Q Pan, and NIH P30CA086862, NIH R01CA133114, and DE-SC0000830 to DRS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duan H, Wang Y, Aviram M, Swaroop M, Loo JA, Bian J, Tian Y, Mueller T, Bisgaier CL, Sun Y. SAG, a novel zinc RING finger protein that protects cells from apoptosis induced by redox agents. Mol Cell Biol. 1999;19:3145–3155. doi: 10.1128/mcb.19.4.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y, Tan M, Duan H, Swaroop M. SAG/ROC/Rbx/Hrt, a zinc RING finger gene family: molecular cloning, biochemical properties, and biological functions. Antioxid Redox Signal. 2001;3:635–650. doi: 10.1089/15230860152542989. [DOI] [PubMed] [Google Scholar]
- 3.Swaroop M, Bian J, Aviram M, Duan H, Bisgaier CL, Loo JA, Sun Y. Expression, purification, and biochemical characterization of SAG, a RING finger redox sensitive protein. Free Radicals Biol Med. 1999;27:193–202. doi: 10.1016/s0891-5849(99)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Swaroop M, Gosink M, Sun Y. SAG/ROC2/Rbx2/Hrt2, a component of SCF E3 ubiquitin ligase: genomic structure, a splicing variant, and two family pseudogenes. DNA Cell Biol. 2001;20:425–434. doi: 10.1089/104454901750361488. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Bae YS, Park JW. Thio-linked peroxidase activity of human sensitive to apoptosis gene (SAG) protein. Free Radic Res. 2002;36:73–78. doi: 10.1080/10715760210164. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Lee JH, Yang ES, Kil IS, Bae YS. Human sensitive to apoptosis gene protein inhibits peroxynitrite-induced DNA damage. Biochem Biophys Res Commun. 2003;301:671–674. doi: 10.1016/s0006-291x(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 7.He H, Tan M, Pamarthy D, Wang G, Ahmed K, Sun Y. CK2 phosphorylation of SAG at Thr10 regulates SAG stability, but not its E3 ligase activity. Mol Cell Biochem. 2007;295:179–188. doi: 10.1007/s11010-006-9287-3. [DOI] [PubMed] [Google Scholar]
- 8.Swaroop M, Wang Y, Miller P, Duan H, Jatkoe T, Madore S, Sun Y. Yeast homolog of human SAG/ROC2/Rbx2/Hrt2 is essential for cell growth, but not for germination: Chip profiling implicates its role in cell cycle regulation. Oncogene. 2000;19:2855–2866. doi: 10.1038/sj.onc.1203635. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y. Alteration of SAG mRNA in human cancer cell lines: Requirement for the RING finger domain for apoptosis protection. Carcinogenesis. 1999;20:1899–1903. doi: 10.1093/carcin/20.10.1899. [DOI] [PubMed] [Google Scholar]
- 10.Yang ES, Park JW. Regulation of nitric oxide-induced apoptosis by sensitive to apoptosis gene protein. Free Radic Res. 2006;40:279–284. doi: 10.1080/10715760500511500. [DOI] [PubMed] [Google Scholar]
- 11.Chanalaris A, Sun Y, Latchman DS, Stephanou A. SAG attenuates apoptotic cell death caused by simulated ischaemia/reoxygenation in rat cardiomyocytes. J Mol Cell Cardiol. 2003;35:257–264. doi: 10.1016/s0022-2828(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Kim MY, Mo JS, Park JW, Park HS. SAG protects human neuroblastoma SH-SY5Y cells against 1-methyl-4-phenylpyridinium ion (MPP+)-induced cytotoxicity via the downregulation of ROS generation and JNK signaling. Neurosci Lett. 2007;413:132–136. doi: 10.1016/j.neulet.2006.11.074. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Yang ES, Kim SY, Shin SW, Park JW. Regulation of heat shock-induced apoptosis by sensitive to apoptosis gene protein. Free Radic Biol Med. 2008;45:167–176. doi: 10.1016/j.freeradbiomed.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 14.He H, Gu Q, Zheng M, Normolle D, Sun Y. SAG/ROC2/RBX2 E3 ligase promotes UVB-induced skin hyperplasia, but not skin tumors, by simultaneously targeting c-Jun/AP-1 and p27. Carcinogenesis. 2008;29:858–865. doi: 10.1093/carcin/bgn021. [DOI] [PubMed] [Google Scholar]
- 15.Duan H, Tsvetkov LM, Liu Y, Song Y, Swaroop M, Wen R, Kung HF, Zhang H, Sun Y. Promotion of S-phase entry and cell growth under serum starvation by SAG/ROC2/Rbx2/Hrt2, an E3 ubiquitin ligase component: association with inhibition of p27 accumulation. Mol Carcinog. 2001;30:37–46. doi: 10.1002/1098-2744(200101)30:1<37::aid-mc1011>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Gu Q, Tan M, Sun Y. SAG/ROC2/Rbx2 is a novel activator protein-1 target that promotes c-Jun degradation and inhibits 12-O-tetradecanoylphorbol-13-acetate-induced neoplastic transformation. Cancer Res. 2007;67:3616–3625. doi: 10.1158/0008-5472.CAN-06-4020. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Duan H, Sun Y. Elevated expression of SAG/ROC2/Rbx2/Hrt2 in human colon carcinomas: SAG does not induce neoplastic transformation, but its antisense transfection inhibits tumor cell growth. Mol. Carcinog. 2001;30:62–70. [PubMed] [Google Scholar]
- 18.Tan M, Gallegos JR, Gu Q, Huang Y, Li J, Jin Y, Lu H, Sun Y. SAG/ROC-SCFbeta-TrCP E3 ubiquitin ligase promotes pro-caspase-3 degradation as a mechanism of apoptosis protection. Neoplasia. 2006;8:1042–1054. doi: 10.1593/neo.06568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia L, Yang J, Hao X, Zheng M, He H, Xiong X, Xu L, Sun Y. Validation of SAG/RBX2/ROC2 E3 Ubiquitin Ligase as an Anticancer and Radiosensitizing Target. Clin Cancer Res. 2010;16:814–824. doi: 10.1158/1078-0432.CCR-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan M, Gu Q, He H, Pamarthy D, Semenza GL, Sun Y. SAG/ROC2/RBX2 is a HIF-1 target gene that promotes HIF-1alpha ubiquitination and degradation. Oncogene. 2008;27:1404–1411. doi: 10.1038/sj.onc.1210780. [DOI] [PubMed] [Google Scholar]
- 21.Gu Q, Bowden TG, Normolle D, Sun Y. SAG/ROC2 E3 ligase regulates skin carcinogenesis by stage dependent targeting of c-Jun/AP1 and IkB/NFkB. J. Cell Biol. 2007;178:1009–1023. doi: 10.1083/jcb.200612067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang GY, Pang L, Ge HL, Tan M, Ye W, Liu XH, Huang FP, Wu DC, Che XM, Song Y, Wen R, Sun Y. Attenuation of ischemia-induced mouse brain injury by SAG, a redox- inducible antioxidant protein. J Cereb Blood Flow Metab. 2001;21:722–733. doi: 10.1097/00004647-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Kim DW, Lee SH, Jeong MS, Sohn EJ, Kim MJ, Jeong HJ, An JJ, Jang SH, Won MH, Hwang IK, Cho SW, Kang TC, Lee KS, Park J, Yoo KY, Eum WS, Choi SY. Transduced Tat-SAG fusion protein protects against oxidative stress and brain ischemic insult. Free Radic Biol Med. 2010;48:969–977. doi: 10.1016/j.freeradbiomed.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs SY, Chen A, Xiong Y, Pan ZQ, Ronai Z. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene. 1999;18:2039–2046. doi: 10.1038/sj.onc.1202760. [DOI] [PubMed] [Google Scholar]
- 25.Wu K, Fuchs SY, Chen G, Tan P, Gomez C, Ronai Z, Pan Z-Q. The SCFHOS/β-TRCP-ROC1 E3 ubiquitin ligase utilizes two distinct domains within CUL1 for substrate targeting and ubiquitin ligation. Mol. Cell. Biol. 2000;20:1382–1393. doi: 10.1128/mcb.20.4.1382-1393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beg AA, Baldwin AS., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 27.Russell JS, Tofilon PJ. Radiation-induced activation of nuclear factorkappaB involves selective degradation of plasma membrane-associated I(kappa)B(alpha) Mol Biol Cell. 2002;13:3431–3440. doi: 10.1091/mbc.E02-05-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes ED, Qu YY, Genik SJ, Lyons RH, Pacheco CD, Lieberman AP, Samuelson LC, Nasonkin IO, Camper SA, Van Keuren ML, Saunders TL. Genetic variation in C57BL/6 ES cell lines and genetic instability in the Bruce4 C57BL/6 ES cell line. Mamm Genome. 2007;18:549–558. doi: 10.1007/s00335-007-9054-0. [DOI] [PubMed] [Google Scholar]
- 30.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 31.Sun Y, Bian J, Wang Y, Jacobs C. Activation of p53 transcriptional activity by 1,10-phenanthroline, a metal chelator and redox sensitive compound. Oncogene. 1997;14:385–393. doi: 10.1038/sj.onc.1200834. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Colburn NH, Oberley LW. Decreased expression of manganese superoxide dismutase mRNA and protein after immortalization and transformation of mouse liver cells. Oncol Res. 1993;5:127–132. [PubMed] [Google Scholar]
- 33.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 34.Gazdoiu S, Yamoah K, Wu K, Pan ZQ. Human Cdc34 employs distinct sites to coordinate attachment of ubiquitin to a substrate and assembly of polyubiquitin chains. Mol Cell Biol. 2007;27:7041–7052. doi: 10.1128/MCB.00812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan WF, Sowa MB. Effects of ionizing radiation in nonirradiated cells. Proc Natl Acad Sci U S A. 2005;102:14127–14128. doi: 10.1073/pnas.0507119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 37.Du C, Gao Z, Venkatesha VA, Kalen AL, Chaudhuri L, Spitz DR, Cullen JJ, Oberley LW, Goswami PC. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol Ther. 2009;8:1962–1971. doi: 10.4161/cbt.8.20.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 40.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 41.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 44.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 45.Sato M, Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993;14:325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- 46.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 47.Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 48.Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 49.Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex [see comments] Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 50.Ohta T, Michel JJ, Schottelius AJ, Xiong Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol Cell. 1999;3:535–541. doi: 10.1016/s1097-2765(00)80482-7. [DOI] [PubMed] [Google Scholar]
- 51.Kamura T, Conrad MN, Yan Q, Conaway RC, Conaway JW. The Rbx1 subunit of SCF and VHL E3 ubiquitin ligase activates Rub1 modification of cullins Cdc53 and Cul2. Genes Dev. 1999;13:2928–2933. doi: 10.1101/gad.13.22.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan P, Fuchs SY, Chen A, Wu K, Gomez C, Ronai Z, Pan Z-Q. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IkBa. Mol Cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]
- 53.Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, Conaway JW, Nakayama KI. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan M, Davis SW, Saunders TL, Zhu Y, Sun Y. RBX1/ROC1 disruption results in early embryonic lethality due to proliferation failure, partially rescued by simultaneous loss of p27. Proc Natl Acad Sci U S A. 2009;106:6203–6208. doi: 10.1073/pnas.0812425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng M, Morgan-Lappe SE, Yang J, Bockbrader KM, Pamarthy D, Thomas D, Fesik SW, Sun Y. Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res. 2008;68:7570–7578. doi: 10.1158/0008-5472.CAN-08-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]