Abstract
Using the isolated, perfused canine pancreas preparation, previously described, the interrelationship of the secretion of pancreatic glucagon and insulin was studied after stimulation with glucose, gastrointestinal hormones, and the amino acid arginine.
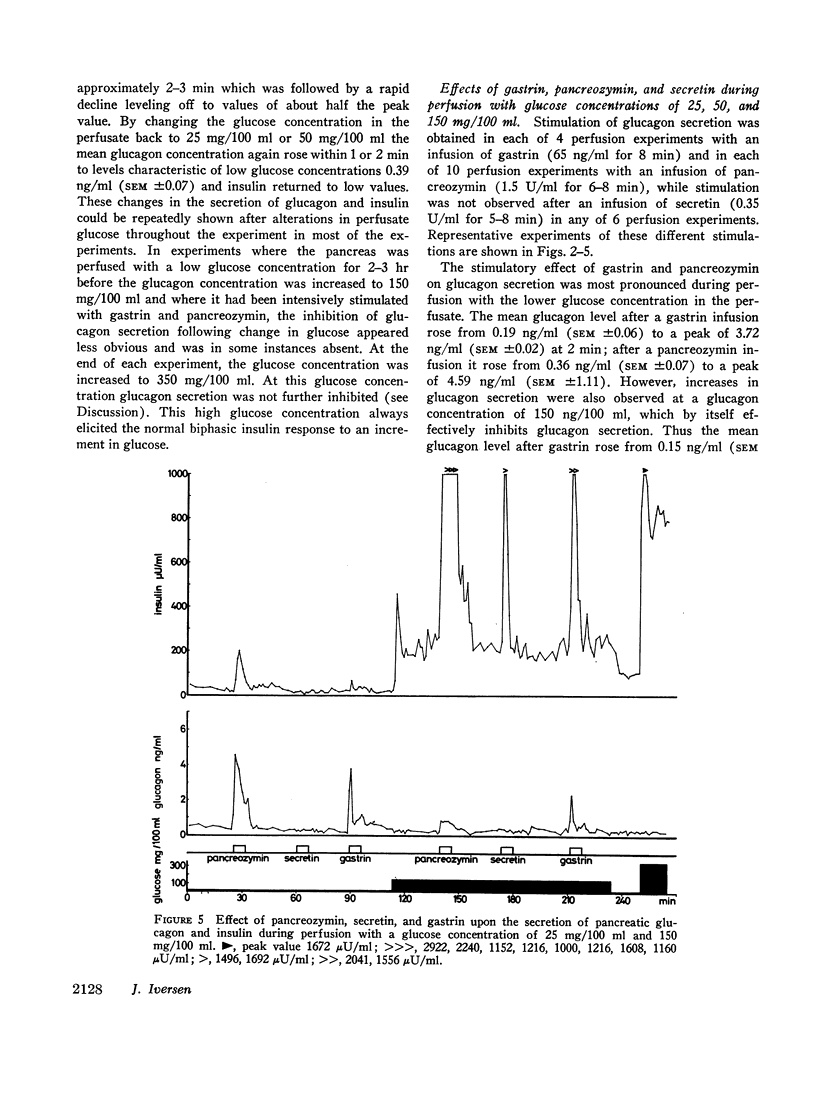
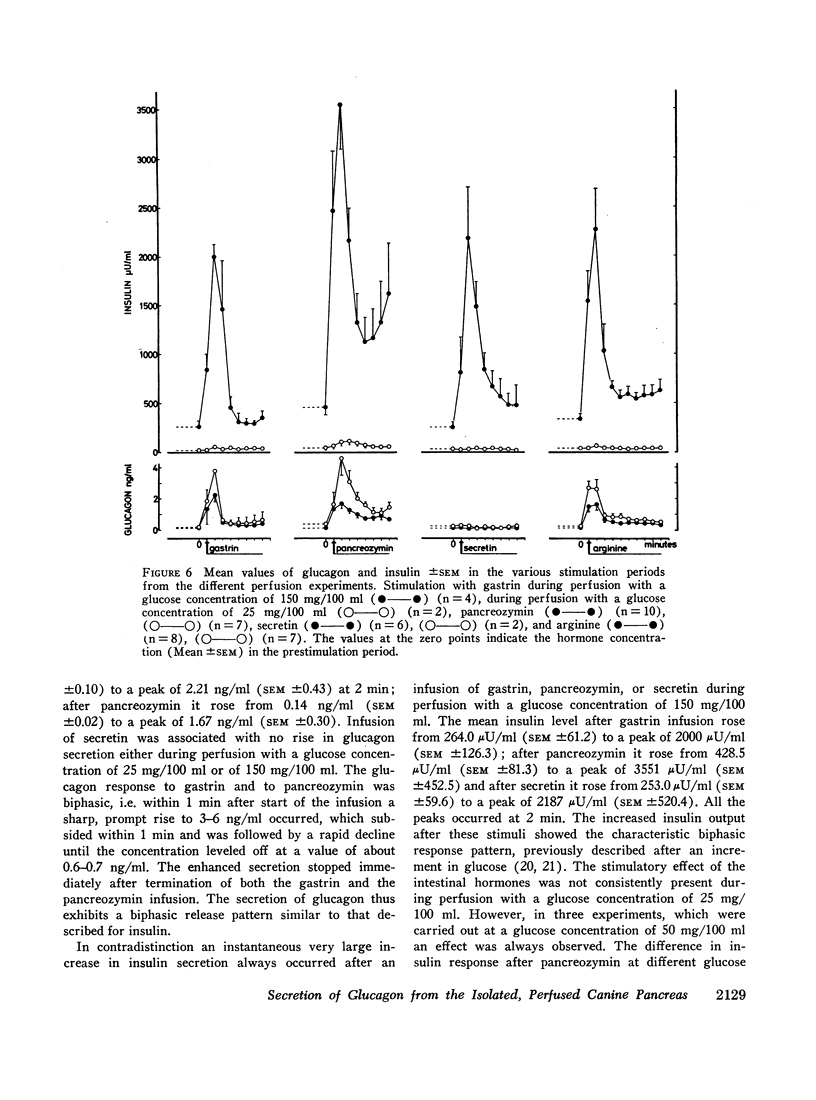
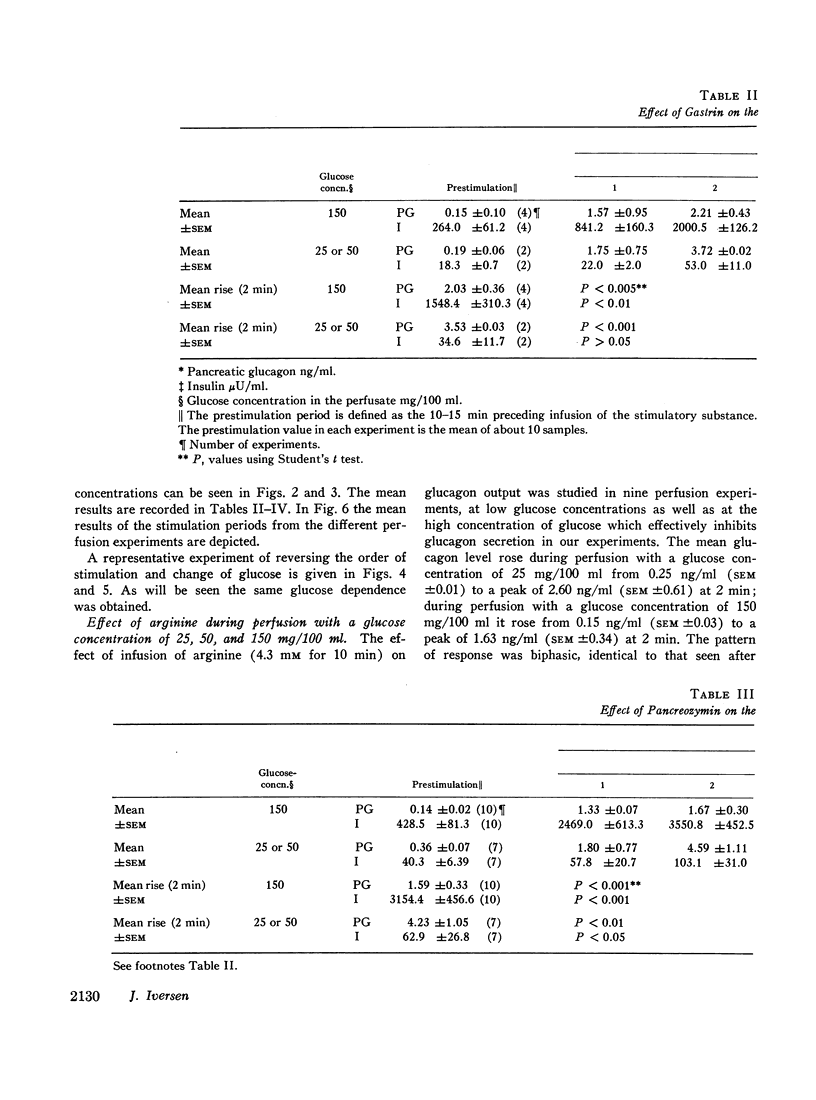
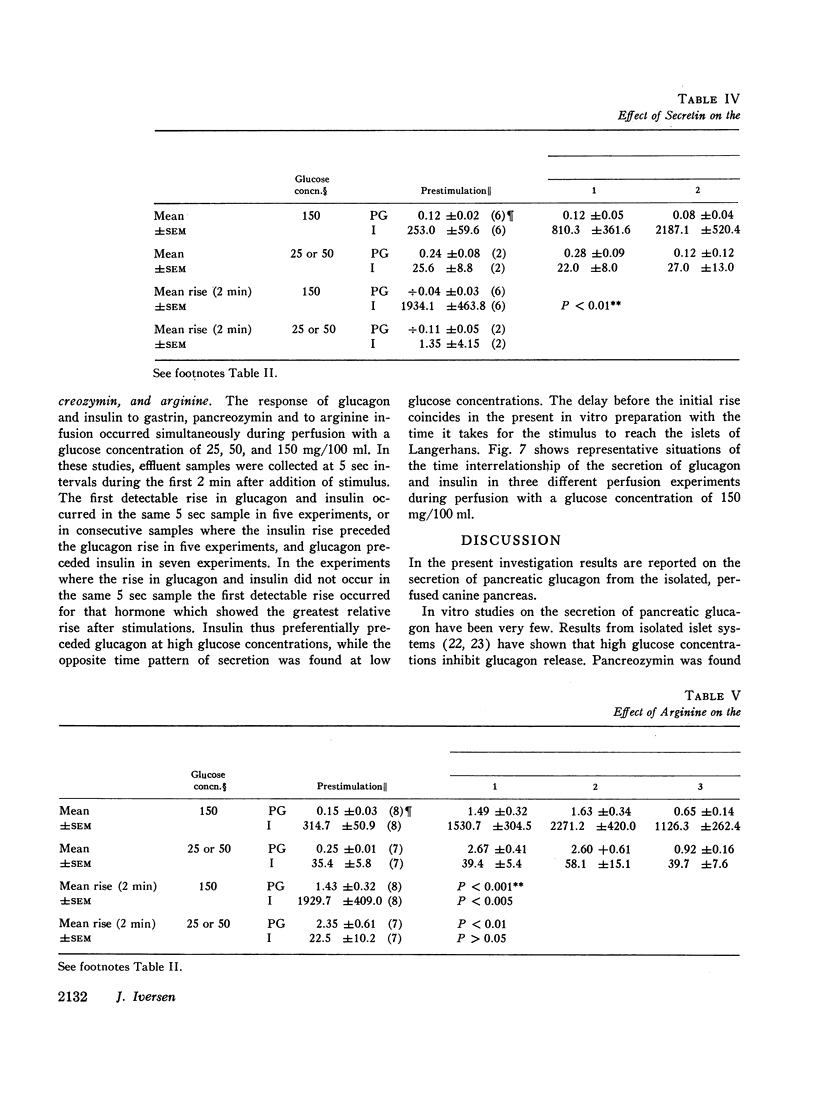
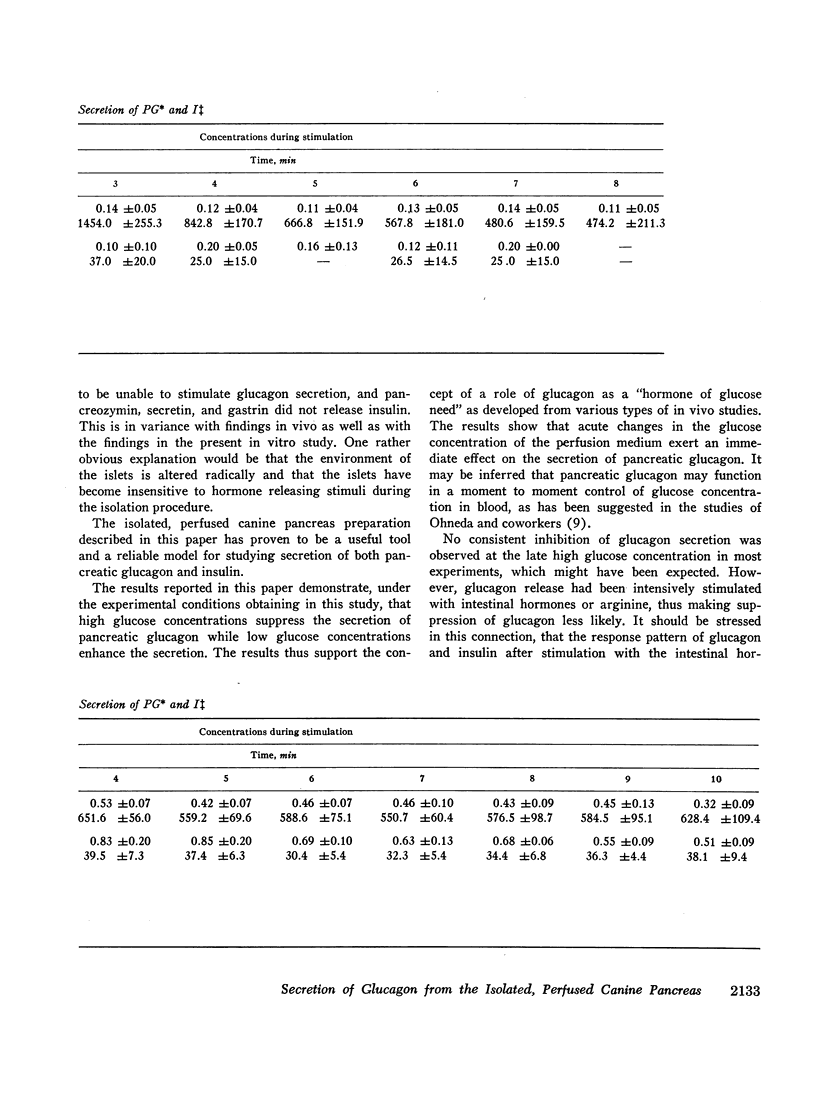
The results confirm the concept that pancreatic glucagon is a hormone of “glucose need” and suggest that it may be important in a moment to moment control of glucoregulation. The secretion of pancreatic glucagon was stimulated after infusion of gastrin, pancreozymin, and arginine, while no increase was associated with secretin infusion. The magnitude of the increase was closely related to the glucose concentration present in the perfusion medium, being higher and more pronounced during perfusion with low concentrations of glucose (25 mg/100 ml or 50 mg/100 ml).
Stimulation of insulin secretion was seen after glucose, gastrin, pancreozymin, secretin, and arginine. The magnitude of the increase was again closely related to the glucose concentration present, this time being higher and more pronounced during perfusion with high glucose concentrations (150 mg/100 ml).
Secretion of both pancreatic hormones always followed a biphasic response pattern after the stimuli mentioned, similar to the characteristic release pattern previously described for insulin after an increment in glucose concentration.
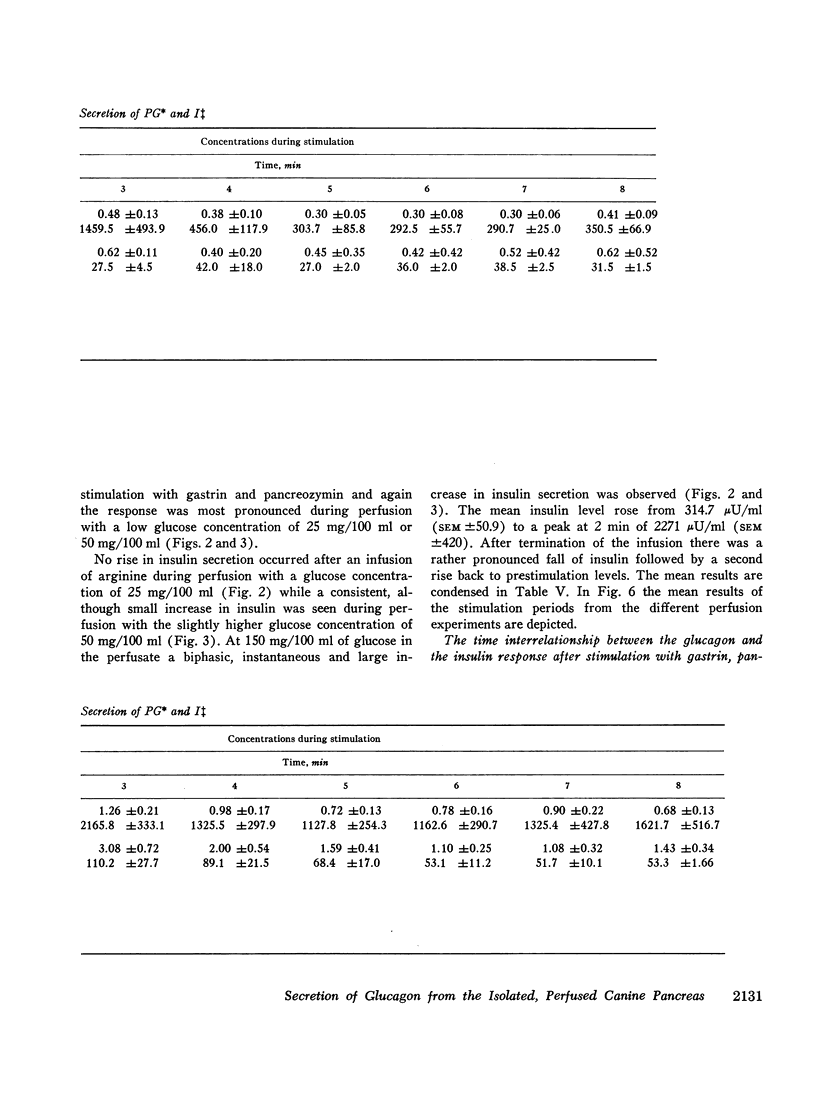
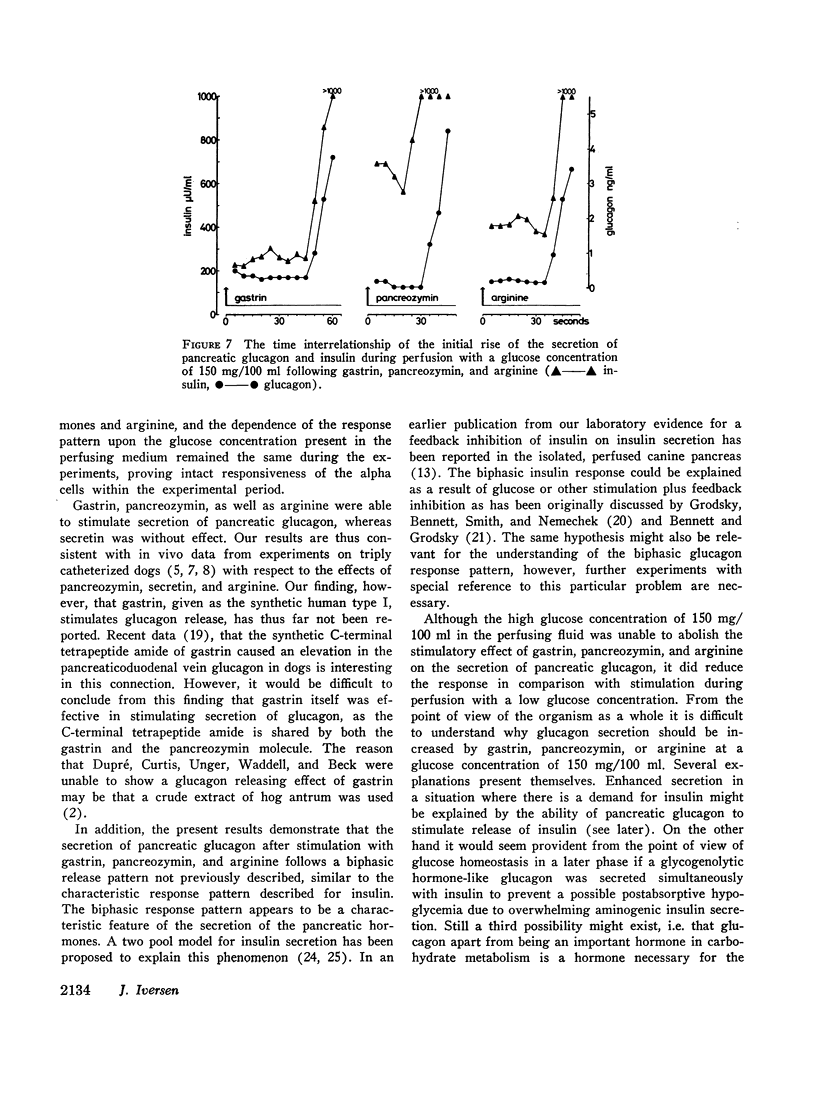
In order to elucidate whether endogenous pancreatic glucagon possesses an insulinogenic action, as it has been shown to be the case with the administration of exogenous pancreatic glucagon, the time interrelationship of the secretion of pancreatic glucagon and insulin was investigated by determining the initial rise of the hormones after stimulation with gastrin, pancreozymin, and arginine. The rise of glucagon and insulin occurred simultaneously, i.e. inside a 10 sec period. This does not, however, exclude with certainty an insulinogenic role of pancreatic glucagon.
Full text
PDF
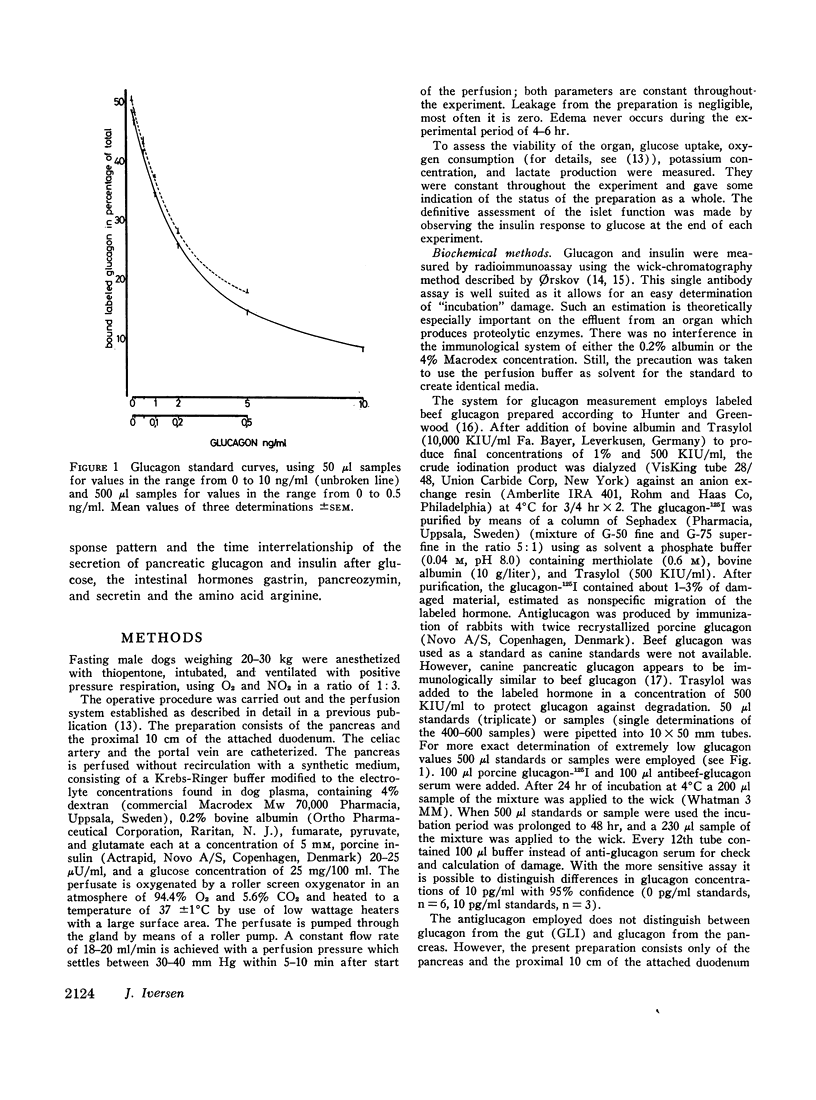
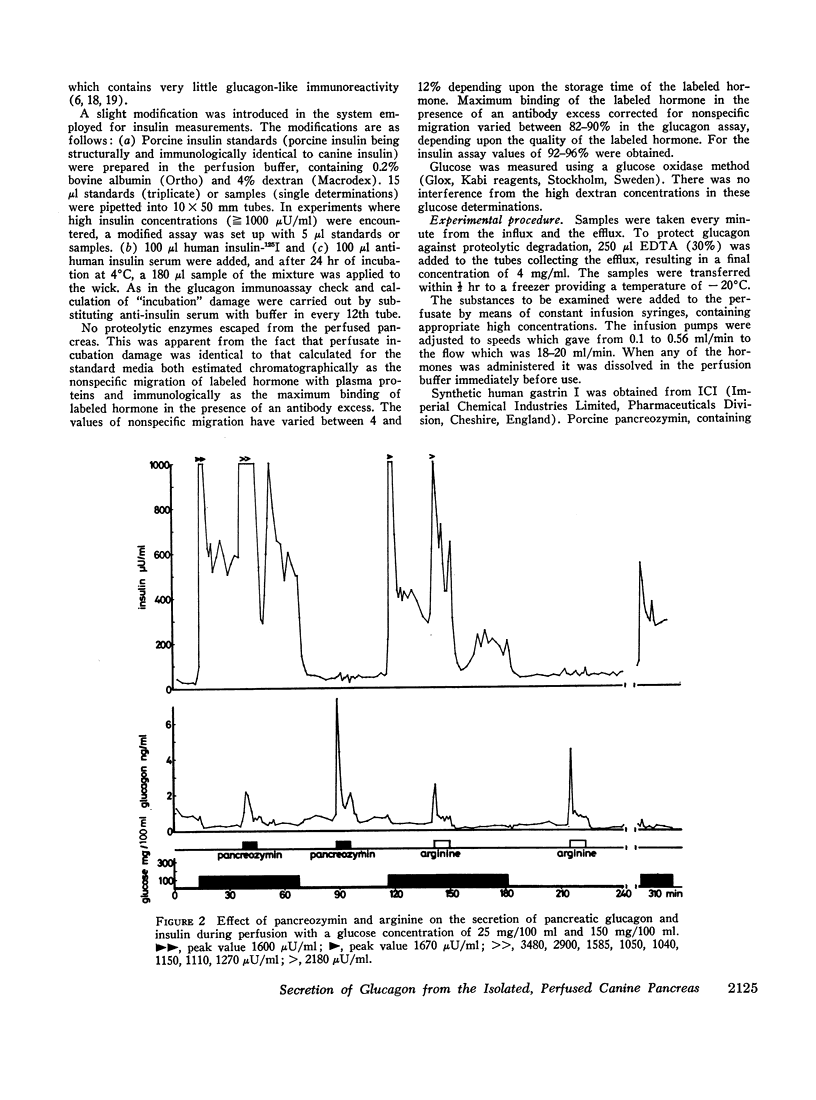
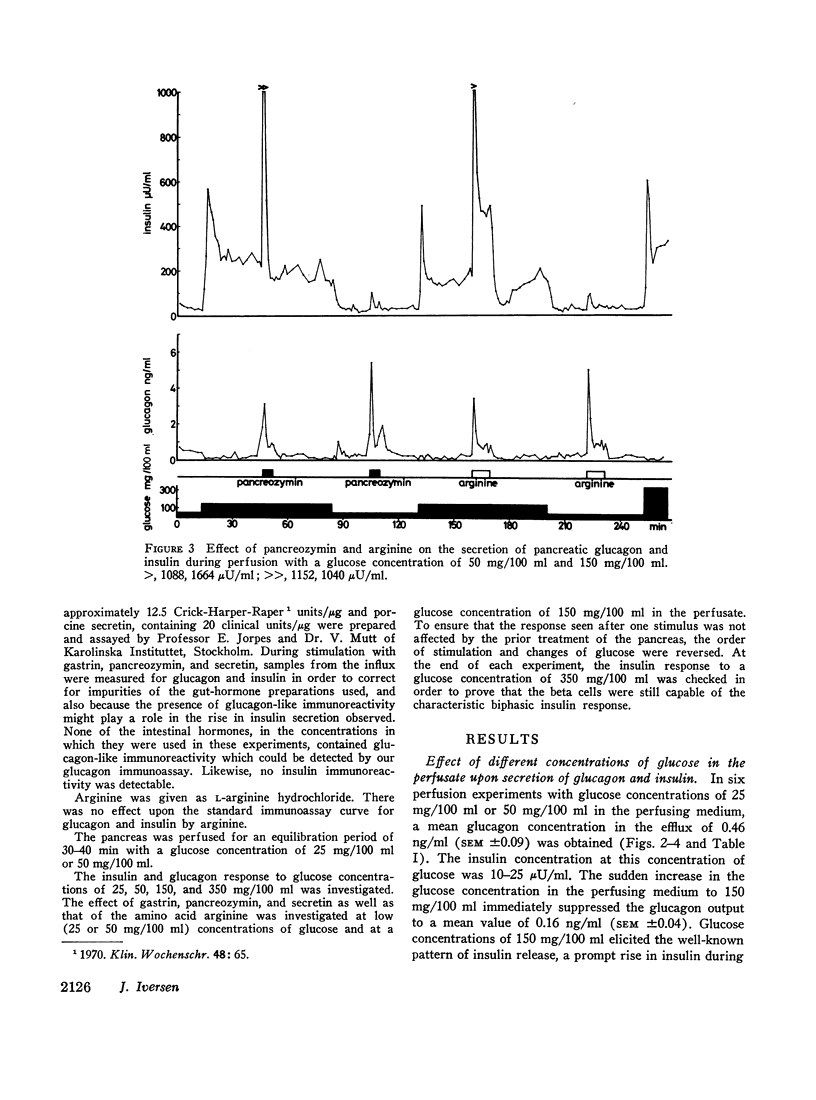
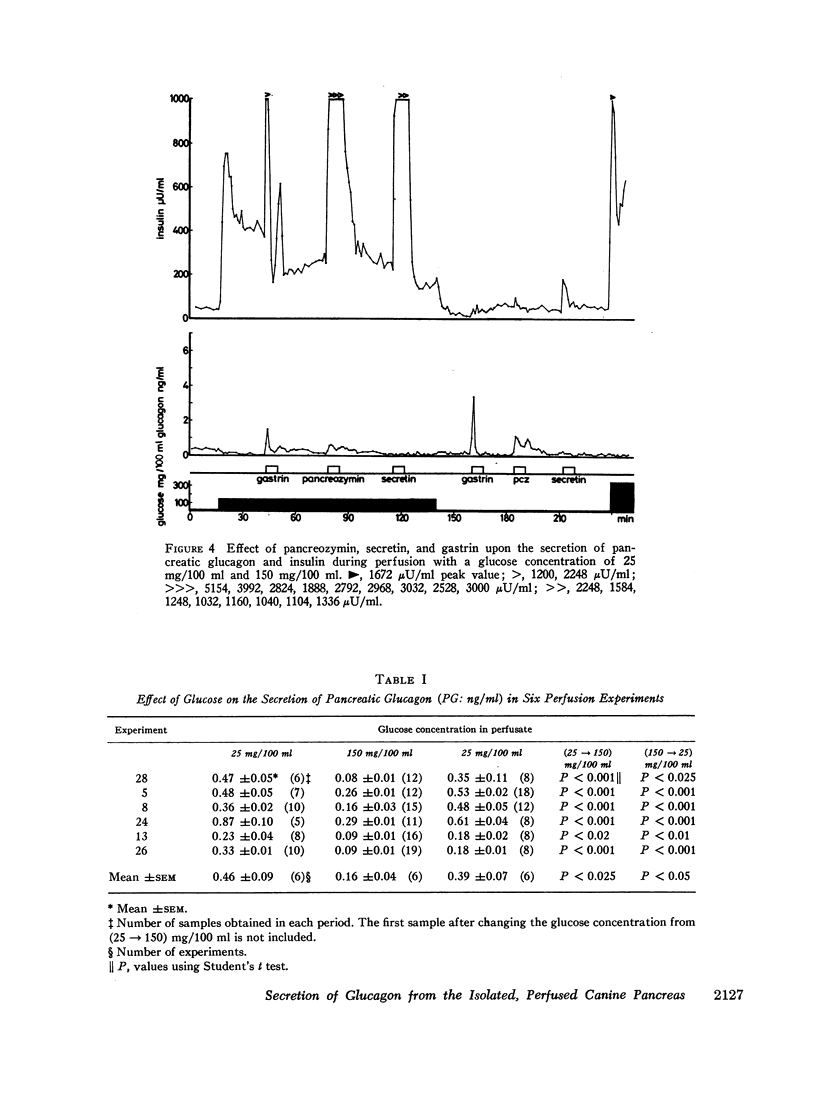


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan K. D., Vance J. E., Dinstl K., Williams R. H. Effect of blood glucose on glucagon secretion in anesthetized dogs. Diabetes. 1969 Jan;18(1):11–18. doi: 10.2337/diab.18.1.11. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Vance J. E., Williams R. H. Insulin and glucagon release from isolated islets of Langerhans. Effect of enteric factors. Diabetes. 1969 Jun;18(6):381–386. doi: 10.2337/diab.18.6.381. [DOI] [PubMed] [Google Scholar]
- Chambers J. W., Georg R. H., Bass A. D. Effect of glucagon, cyclic 3',5'-adenosine monophosphate and its dibutyryl derivative on amino acid uptake by the isolated perfused rat liver. Endocrinology. 1970 Aug;87(2):366–370. doi: 10.1210/endo-87-2-366. [DOI] [PubMed] [Google Scholar]
- Chesney T. M., Schofield J. G. Studies on the secretion of pancreatic glucagon. Diabetes. 1969 Sep;18(9):627–632. doi: 10.2337/diab.18.9.627. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Curry D. L. Glucagon potentiation of insulin secretion by the perfused rat pancreas. Diabetes. 1970 Jun;19(6):420–428. doi: 10.2337/diab.19.6.420. [DOI] [PubMed] [Google Scholar]
- Dupre J., Curtis J. D., Unger R. H., Waddell R. W., Beck J. C. Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J Clin Invest. 1969 Apr;48(4):745–757. doi: 10.1172/JCI106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussgänger R. D., Straub K., Goberna R., Jaros P., Schröder K. E., Raptis S., Pfeiffer E. F. Primary secretion of insulin and secondary release of glucagon from the isolated perfused rat pancreas following stimulation with pancreozymin. Horm Metab Res. 1969 Jul;1(4):224–227. doi: 10.1055/s-0028-1095139. [DOI] [PubMed] [Google Scholar]
- Grossman M. I. Gastrin and its activities. Nature. 1970 Dec 19;228(5277):1147–1150. doi: 10.1038/2281147a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Iversen J., Miles D. W. Evidence for a feedback inhibition of insulin on insulin secretion in the isolated, perfused canine pancreas. Diabetes. 1971 Jan;20(1):1–9. doi: 10.2337/diab.20.1.1. [DOI] [PubMed] [Google Scholar]
- Kaneto A., Mizuno Y., Tasaka Y., Kosaka K. Stimulation of glucagon secretion by tetragastrin. Endocrinology. 1970 May;86(5):1175–1180. doi: 10.1210/endo-86-5-1175. [DOI] [PubMed] [Google Scholar]
- Ketterer H., Eisentraut A. M., Unger R. H. Effect upon insulin secretion of physiologic doses of glucagon administered via the portal vein. Diabetes. 1967 May;16(5):283–288. doi: 10.2337/diab.16.5.283. [DOI] [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov H., Thomsen H. G., Yde H. Wick chromatography for rapid and reliable immunoassay of insulin, glucagon and growth hormone. Nature. 1968 Jul 13;219(5150):193–195. doi: 10.1038/219193b0. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Pupo A. A. Insulin responses to glucose: evidence for a two pool system in man. J Clin Invest. 1969 Dec;48(12):2309–2319. doi: 10.1172/JCI106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMOLS E., MARRI G., MARKS V. PROMOTION OF INSULIN SECRETION BY GLUCAGON. Lancet. 1965 Aug 28;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966 Dec;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- Samols E., Tyler J., Megyesi C., Marks V. Immunochemical glucagon in human pancreas, gut, and plasma. Lancet. 1966 Oct 1;2(7466):727–729. doi: 10.1016/s0140-6736(66)92982-5. [DOI] [PubMed] [Google Scholar]
- Sokal J. E., Ezdinli E. Z. Basal plasma glucagon levels of man. J Clin Invest. 1967 May;46(5):778–785. doi: 10.1172/JCI105578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. S., McIntyre N. Stimulation by glucagon of insulin release from rabbit pancreas in vitro. Lancet. 1966 Feb 12;1(7433):351–352. doi: 10.1016/s0140-6736(66)91327-4. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Aguilar-Parada E., Eisentraut A. M. The role of aminogenic glucagon secretion in blood glucose homeostasis. J Clin Invest. 1969 May;48(5):810–822. doi: 10.1172/JCI106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Valverde I., Eisentraut A. M., Exton J. Characterization of the responses of circulating glucagon-like immunoreactivity to intraduodenal and intravenous administration of glucose. J Clin Invest. 1968 Jan;47(1):48–65. doi: 10.1172/JCI105714. [DOI] [PMC free article] [PubMed] [Google Scholar]


