The accelerating pace of technological advancement in genomic research has revolutionized the efficiency of cancer gene discovery. These advancements have enabled the use of multifaceted approaches that integrate different modalities such as array-based comparative genomic hybridization (aCGH), high-throughput sequencing and transcriptome analysis. Using these approaches, we were recently able to definitively identify the Parkinson disease (PD)-associated gene PARK2 as a targeted gene residing on chromosome 6q25.2–27, a region known to frequently harbor deletions in many human cancers and hypothesized to contain a tumor suppressor. Interestingly, PARK2 has been known for some time to be the most frequent cause of early-onset Parkinson disease, a neurodegenerative condition that results in severe movement dysfunction.
PARK2 is located within the FRA6E fragile site.1 To identify the tumor suppressor gene driving loss in this region, we compared aCGH data from several human tumor types including GBM and colon cancer and showed that there exists significant diversity of copy number loss events in this region. Interestingly, in some tumor types, a significant number of these loss events were focal, intragenic deletions in PARK2. Deletions were both homozygous and heterozygous in nature and occurred in approximately 25% of GBMs and 25% of colon cancer samples.2–4 Moreover, PARK2 protein expression was absent or significantly downregulated in 60% of GBM, lung and colon cancer cell lines we examined. Examination of expression microarray databases confirmed that PARK2 mRNA expression was significantly reduced in a wide range of cancers. Together, these data strongly suggest that PARK2 is a gene driving loss on chromosome 6q in human malignancies. To definitively determine whether PARK2 is the targeted gene, we attempted to identify somatic mutations in the gene in cancers. We sequenced PARK2 in 242 primary tumors, and identified novel PARK2 mutations in GBM, lung cancer, and colon cancer. The mutational spectrum of PARK2 was very similar to that seen in the germline of familial PD. Interestingly, two mutations, R42 and N275, involve the same amino acids as germline mutations causing early-onset PD cases.5 In order to investigate the functional consequences of PARK2 mutation, we transfected wild-type PARK2 into several cancer cell lines normally lacking PARK2 expression and observed a suppression of tumor cell growth. Consistent with this, PARK2 overexpression in a mouse xenograft model also inhibited tumor growth, an effect that was abrogated by PARK2 mutation. These observations suggested that PARK2 may function directly as a tumor suppressor.
Our findings raise the possibility that the consequences of PARK2 mutations can result in different phenotypes depending on cellular context. When PARK2 is mutated in the germline (and hence in neurons) in the absence of other mutations, neurodegeneration is the outcome. However, when PARK2 is mutated in dividing somatic cells, in the context of other oncogenic lesions, a growth advantage may result, ultimately leading to tumor formation. In fact, following the publication of our study, a survey of the landscapes of somatic copy number changes in cancer revealed that PARK2 was one of the most significant and widespread focally deleted genes in human malignancies, confirming our observations.6 Thus, PARK2 is a widely inactivated, multisite tumor suppressor that may be fundamentally important for tumorigenesis or cancer progression.
PARK2 is widely expressed in many tissues including colon, lung, brain and testis.7 It encodes a ubiquitin E3 ligase that facilitates the ubiquitination and degradation of proteins. PARK2 has been shown to serve a neuroprotective role, protecting against neuronal cell death from excitotoxicity and toxic insults. In neurons, levels of cyclin E expression are normally suppressed. Neurotoxicity can cause aberrant increases in cyclin E, which is held in check by ubiquitination and proteolysis mediated by PARK2.8,9 Inactivation of PARK2 results in an increase in cyclin E levels, and because neurons are not mitotically competent, activation of the cell cycle results in cell death. Therefore, increased cyclin E levels in neurons in the setting of PARK2 inactivation may render these cells susceptible to toxicity and manifest as neurodegeneration such as that seen in PD. Indeed, cyclin E has been shown to be elevated in the brains of PD patients.9
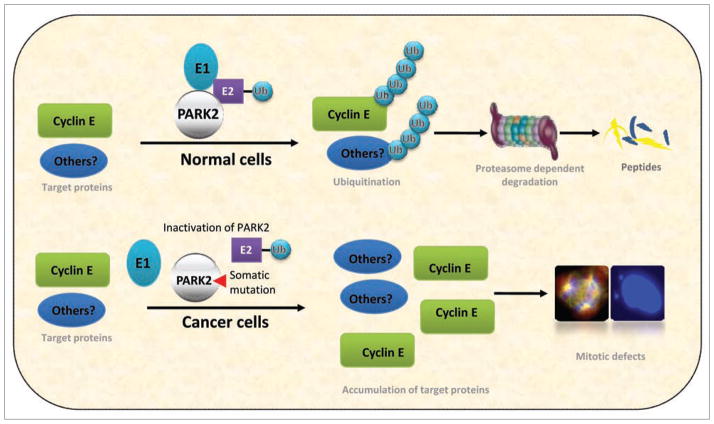
We examined the mechanisms underlying PARK2 inactivation in cancer and showed that a relevant molecular mechanism in cancer may involve the failure to ubiquitinate cyclin E in cells following PARK2 inactivation. Accumulation and dysregulation of cyclin E, a driver of cell cycle progression, is believed to be a cause of tumorigenesis but the mechanistic basis for this accumulation has been obscure.10 Increased levels of cyclin E are associated with various malignancies and constitutive expression leads to genomic instability.11 Overexpression of cyclin E has transforming activity both in vitro and in animal models. In our study, we found that wild-type PARK2 binds to and ubiquitinates cyclin E. Cancer-specific mutations in PARK2 resulted in a loss of ubiquitin ligase activity and a failure to degrade cyclin E whereas PARK2 knockdown resulted in an accumulation of cyclin E. On cell cycle analysis, PARK2 depletion increased the proportion of cells in the S and G2-M phases, and led to increased frequency of abnormal mitoses, multipolar spindles and mitotic instability (Fig. 1).
Figure 1.
Model of PARK2 function and dysfunction in human cancers. Mutation or deletion of PARK2 results in an accumulation of cyclin E and perhaps other targets of PARK2-mediated ubiquitination. This results in abnormal cell cycle progression and defects in the mitotic process.
Together, these data strongly suggest that PARK2 is a tumor suppressor gene and that somatic mutations may promote tumor growth through loss of cyclin E ubiquitination. Therefore, PARK2 inactivation appears to drive both neurodegeneration and tumorigenesis, depending on cellular context. Interestingly, epidemiologic evidence confirms a small but significant increase in the risk of cancer among PD patients. Further investigation will be needed to determine the oncogenic context in which PARK2 inactivation promotes tumorigenesis and the consequences of PARK2 inactivation-induced mitotic abnormalities on genetic stability.
Abbreviations
- PD
Parkinson disease
- CNA
copy number alteration
- GBM
glioblastoma multiforme
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/11583
Comment on: Veeriah S, et al. Nat Genet 2009; Epub ahead of print.
References
- 1.Letessier A, et al. Oncogene. 2007;26:298–307. doi: 10.1038/sj.onc.1209772. [DOI] [PubMed] [Google Scholar]
- 2.Veeriah S, et al. Nat Genet. 2010;42:77–82. doi: 10.1038/ng.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons DW, et al. Science. 2008;321:1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.TCGA CGARN. Nature. 2008;455:1061–8. [Google Scholar]
- 5.Lucking CB, et al. N Engl J Med. 2000;342:1560–7. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 6.Beroukhim R, et al. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesari R, et al. Proc Natl Acad Sci USA. 2003;100:5956–61. doi: 10.1073/pnas.0931262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Coelln R, et al. Cell Tissue Res. 2004;318:175–84. doi: 10.1007/s00441-004-0924-4. [DOI] [PubMed] [Google Scholar]
- 9.Staropoli JF, et al. Neuron. 2003;37:735–49. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 10.Shaye A, et al. Breast Cancer Res Treat. 2009;115:651–9. doi: 10.1007/s10549-008-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spruck CH, et al. Nature. 1999;401:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]