Abstract
Purpose
Juvenile polyposis (JPS) can be caused by a germline defect of the SMAD4 gene. Somatic inactivation of SMAD4 occurs in pancreatic and colorectal cancer and is reflected by loss of SMAD4 immunohistochemistry. Here, SMAD4 immunohistochemistry as a marker of SMAD4 gene status and the role of SMAD4 in the adenoma-carcinoma sequence in neoplastic progression in JPS are studied.
Experimental Design
20 polyps with a SMAD4 germline defect and 38 control polyps were studied by SMAD4 immunohistochemistry. Inactivation of the SMAD4 wild-type allele was studied in dysplastic epithelium and in areas with aberrant SMAD4 expression. APC, ß-catenin, p53 and K-ras were studied to evaluate the adenoma-carcinoma sequence.
Results
9/20 polyps with a SMAD4 germline defect showed loss of epithelial SMAD4 expression. LOH of SMAD4 was found in 5 polyps and a somatic stop codon mutation was found in 2 polyps without LOH. Remarkably, somatic inactivation of epithelial SMAD4 did not always coincide with dysplasia and aberrant p53 staining was found in 4 of 6 dysplastic polyps with normal SMAD4 staining. One K-ras mutation was found in 9 juvenile polyps with dysplasia. No evidence for Wnt activation was found.
Conclusions
SMAD4 immunohistochemistry mirrors genetic status and provides a specific adjunct in the molecular diagnosis of JPS. However, epithelial SMAD4 inactivation is not required for polyp formation and not obligatory for neoplastic progression in JPS. Instead, different routes to neoplasia in JPS caused by germline SMAD4 mutation appear operative, including somatic loss of SMAD4 and p53 inactivation without somatic loss of SMAD4.
Keywords: Juvenile Polyposis, colorectal cancer, SMAD4, BMPR1A, LOH
Introduction
Juvenile polyposis syndrome (JPS) is an autosomal dominant disorder characterized by the presence of distinct juvenile polyps in the gastrointestinal tract and an increased colorectal cancer risk.(1-3) On histology, juvenile polyps have a prominent stromal compartment containing distorted and cystically dilated crypts often lined by reactive epithelium.(4) A germline mutation in the SMAD4 or BMPR1A gene is found in 50% of patients.(5, 6) Both genes are involved in the Transforming Growth Factor–Beta/Bone Morphogenic Protein (TGF-ß/BMP) signaling pathway, regulating cell proliferation and differentiation. SMAD4 is a cytoplasmic co-mediator which forms heteromeric complexes with various receptor dependant SMADs. These complexes are translocated to the nucleus and regulate DNA transcription.(7, 8) Somatic inactivation of the SMAD4 tumor suppressor gene occurs in up to 55% of pancreatic cancers, and in other malignancies including colorectal cancer. This occurs either through somatic intragenic mutation with loss of the second allele (loss of heterozygosity, LOH) or deletion of both alleles (homozygous deletion).(9-11)
In JPS the mechanism leading to polyp formation and the role of SMAD4 and BMPR1A is poorly understood. One hypothesis is that juvenile polyps develop through a ‘landscaper defect’ in which the defective cell population lies in the stromal compartment. Neoplasia of the epithelial cells may take place as a result of an abnormal microenvironment.(12, 13) Others suggest that inactivation of the second allele in the epithelial cell compartment is likely to initiate polyp formation.(14-16) Different mechanisms of polyp formation may exist for individuals with either a SMAD4 or BMPR1A germline mutation.(12)
In pancreatic cancer, somatic inactivation of SMAD4 is accurately mirrored by loss of immunohistochemical staining.(17) Similarly, SMAD4 immunohistochemistry may prove a valuable tool in the molecular diagnosis of JPS. Also, this analysis could clarify the role of this gene in juvenile polyp development and disease progression. This understanding has been hampered by lack of studies systematically demonstrating a correlation between SMAD4 immunohistochemistry and SMAD4 gene status in JPS. Therefore, we investigated SMAD4 protein expression by immunohistochemistry and correlated this result with SMAD4 gene status in juvenile polyps carrying a SMAD4 germline defect. In addition, we addressed the role and timing of somatic loss of the wild type SMAD4 allele and the conventional adenoma-carcinoma sequence in neoplastic progression in JPS.
Material and Methods
Patients and tissue
Archival material from patients with one or more juvenile polyps was collected from The Johns Hopkins Polyposis Registry and clinic (Baltimore, MD, USA) and two academic hospitals in the Netherlands (Academic Medical Center, Amsterdam, and University Medical Center, Utrecht). The study was carried out according to the guidelines of the ethical committee of these institutions and with their approval. Clinical and family history data were examined and polyps were carefully reviewed by an experienced GI pathologist (GJAO) to confirm the diagnosis of JPS or sporadic juvenile polyps. All JPS patients previously underwent genetic analysis through direct sequencing and MLPA analysis.(5) Forty-one patients were included in this study, including 8 patients with a SMAD4 germline defect, 6 with a BMPR1A germline defect and 27 with sporadic juvenile polyps. Polyp tissue was formalin-fixed and paraffinized according to standard procedures.
Immunohistochemistry
Immunohistochemistry was performed using a monoclonal antibody against SMAD4 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA, Cat.no. sc-7966, 1:400), p53 (Neomarkers DO7+BP53-12, Cat.no. MS-738-P, 1:2000) and ß-catenin (BD Transduction Laboratories clone 14, Cat.no. 610154, 1:5000). Briefly, 4 μm sections were deparaffinized, blocked for endogenous peroxidase activity by immersion in 0.3% H2O2 in methanol for 20 min. Antigen retrieval was performed in Tris/EDTA buffer (10 mM/1 mM; pH 9.0) for 10 min at 120°C. Nonspecific binding sites were blocked in PBS with 10% normal goat serum for SMAD4, and in 5% normal goat serum for p53 and ß-catenin, for 10 minutes. This was followed by antibody incubation of 1hour for SMAD4 and p53 at room temperature, and an overnight incubation for ß-catenin at 4°C. Antibody binding was visualized using the Powervision+poly-HRP detection system (ImmunoVision Technologies, Co, Daly City, CA, USA) and PowerDAB (Immunologic, Duiven, The Netherlands, Cat. no. BS03-25) for SMAD4, Powervision+poly-HRP detection system and 3,3-diamino-benzidine (DAB, Sigma D5637) were used for p53 and ß-catenin. Sections were counterstained with haematoxylin.
Scoring of immunohistochemistry
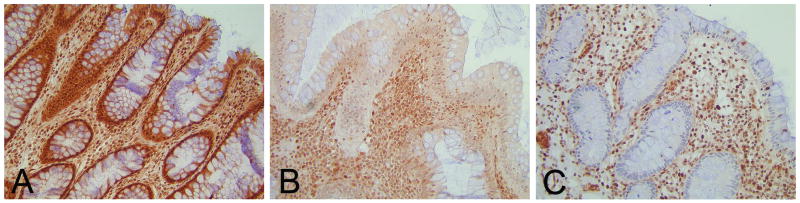
On examination, slides were scored as having either normal, reduced or loss of expression of SMAD4. Normal nuclear staining in the epithelial cells lining normal crypts, or inflammatory cells in the mesenchymal stroma on the same section served as an internal control, i.e. normal expression refers to the same expression as seen in these control cells. Loss of expression was defined as absence of nuclear staining. Reduced expression was graded when a weaker expression, but not a complete absence of nuclear staining, was noted compared to the control cells (Figure 1). p53 immunohistochemistry was scored as either normal constitutive immunoreactivity or as a staining pattern suggesting mutation of the p53 gene, which can be reflected by either very intense immunostaining suggesting a stabilizing p53 mutation or total absence of p53 immunoreactivity consistent with a stopcodon mutation in the p53 gene.(18) ß-catenin immunohistochemistry was scored as either normal membranous or nuclear staining, indicating activation of Wnt signaling.(19) Also, all sections were reviewed for dysplasia (GJAO and FJWK) using standard H&E stained reference slides: Dysplasia was graded according to the standard criteria.(20)
Figure 1.
SMAD4 Immunohistochemical scoring. SMAD4 IHC was scored as normal (A), reduced (B) or loss of SMAD4 expression (C). Nuclear staining in the epithelial cells lining normal crypts or inflammatory cells in the mesenchymal stroma on the same section served as internal control. Note loss of SMAD4 expression in non-neoplastic epithelium in C. Magnification 20X.
Laser microdissection and DNA isolation
Epithelium of interest was isolated by laser capture microdissection (LCM) using the PALM® Laser Microbeam Microdissection System (Microlaser Technologies, Bernried, Germany) on 8 μm sections counterstained with haematoxylin. DNA was obtained using TK buffer (400 μg/ml of proteinase K and 0.5% Tween 20, 50 mmol/l Tris (pH 9), 1 mmol/l NaCl, 2 mmol/L EDTA). After overnight incubation in 50 μl TK buffer at 56°C, tubes were incubated at 95°C for 10 minutes to inactivate the proteinase K.(21)
LOH analysis
Loss of heterozygosity was assessed using fluorescently labeled primers for the following microsatellites: D18S46, D18S474, D18S858 and D18S64.(16, 22, 23) Epithelium with aberrant SMAD4 expression was separated from normal SMAD4 stained epithelium using LCM. After PCR amplification the products were separated using the ABI Prism® 310 genetic analyzer (Applied Biosystems, Foster City, CA, USA). One μl of the PCR product was mixed with 23 μl formamide and 0.5 μl GeneScanTM ROX-500 (Invitrogen, Carlsbad, CA, USA) as a size marker.
Samples with two distinctly sized alleles of a particular marker were termed informative. For all informative markers, the allelic imbalance factor was calculated as described by Cawkwell et al.(24) LOH was assumed if the allelic imbalance factor was greater than 1.6 or less than 0.6. Observed losses were confirmed to exclude induced LOH. If retention of heterozygosity was found, microdissected material was sequenced to establish whether a somatic point mutation of the SMAD4 gene had occurred.
Mutation analysis
Sequencing of SMAD4 was performed as described previously.(5) For APC and K-ras mutation analysis, DNA was isolated from polyps with dysplasia and PCR amplified using Platinum®Taq DNA Polymerase (Invitrogen Corporation, Carsbad, California, USA). Four primers sets covering the mutation cluster region (MCR) in exon 15 of the APC gene (25) were used (1For-GAAATAGGATGTAATCAGACG, 1Rev-CGCTCCTGAAGAAAATTCAAC, 2For-ACTGCAGGGTTCTAGTTTATC, 2Rev-GAGCTGGCAATCGAACGACT, 3For-TACTTCTGTCAGTTCACTTGATA, 3Rev-ATTTTTAGGTACTTCTCGCTTG, 4For-AAACACCTCCACCACCTCC, 4Rev-GCATTATTCTTAATTCCACATC). Two primer sets were used for K-ras mutation analysis for exon 1 and 2 containing mutational hotspot codons 12, 13 and 61 (Exon1For-CTGGTGGAGTAT TTGATAGT, Exon1Rev-ATG GTCCTGCACCAGTAATA, Exon2For-GTGCACTGTAATAATCCAGAC, Exon2Rev-CCACCTATAATGGTGAATATCT). PCR products were subsequently sequenced using the ABI Prism® 3130 genetic analyzer.
Results
SMAD4 Immunohistochemistry
A total of 58 polyps, including 20 polyps from 8 patients with a SMAD4 germline defect, 11 polyps from 6 patients with a BMPR1A germline defect and 27 sporadic juvenile polyps from 27 patients, were assessed for SMAD4 protein expression using immunohistochemistry (Figure 1). Of 20 polyps with a SMAD4 germline defect, 9 showed focal reduction or loss of nuclear SMAD4 protein expression in the epithelium (Table 1). In contrast, none of the 11 polyps carrying a BMPR1A germline mutation or any of the 27 sporadic juvenile polyps had aberrant SMAD4 expression (data not shown).
Table 1.
SMAD4 immunohistochemistry in juvenile polyps from patients with a SMAD4 germline mutation.
| Patient | polyp | Exon | Mutation | Effect | SMAD4 IHC | ||
|---|---|---|---|---|---|---|---|
| normal | reduced | loss | |||||
| 1 | 1.1 | 10 | c.1411-1435del25 | p.G471FfsX25 | |||
| 1.2 | 10 | c.1411-1435del25 | p.G471FfsX25 | ||||
| 2* | 2.1 | 8 | c.970 T>C | p.C324R | |||
| 2.2 | 8 | c.970 T>C | p.C324R | ||||
| 2.3 | 8 | c.970 T>C | p.C324R | ||||
| 3* | 3.1 | 8 | c.970 T>C | p.C324R | |||
| 3.2 | 8 | c.970 T>C | p.C324R | ||||
| 4† | 4.1 | 1-11 | hemizygous deletion | ||||
| 5† | 5.1 | 1-11 | hemizygous deletion | ||||
| 5.2 | 1-11 | hemizygous deletion | |||||
| 6 | 6.1 | 8 | c.989 A>G | p.E330G | |||
| 6.2 | 8 | c.989 A>G | p.E330G | ||||
| 6.3 | 8 | c.989 A>G | p.E330G | ||||
| 7 | 7.1 | 9 | c.1193 G>A | p.W398X | |||
| 7.2 | 9 | c.1193 G>A | p.W398X | ||||
| 7.3 | 9 | c.1193 G>A | p.W398X | ||||
| 8 | 8.1 | 8 | 971delG | p.C324FfsX12 | |||
| 8.2 | 8 | 971delG | p.C324FfsX12 | ||||
| 8.3 | 8 | 971delG | p.C324FfsX12 | ||||
| 8.4 | 8 | 971delG | p.C324FfsX12 | ||||
Patient 2 and 3 and
patient 4 and 5 were from the same family
LOH and mutation analysis
To assess the implication of aberrant epithelial SMAD4 protein expression, we investigated whether reduction or loss of SMAD4 expression correlated with occurrence of a somatic event in SMAD4, i.e. LOH or a somatic point mutation in polyps with a SMAD4 germline mutation. LOH analysis of the SMAD4 locus was performed using 4 microsatellite markers. Nine polyps were assessed, all carrying a germline mutation in SMAD4, and all had aberrant SMAD4 expression. Results are summarized in Table 2.
Table 2.
SMAD4 LOH and mutation analysis in juvenile polyps from patients with a SMAD4 germline mutation.
| Patient | Polyp | SMAD4 IHC | LOH analysis | Somatic mutation | ||||
|---|---|---|---|---|---|---|---|---|
| D18s46 | D18s474 | D18s858 | D18s64 | |||||
| 1 | 1.1 | reduced | S M A D 4 | c.170 T>A p.L57X | ||||
| 2* | 2.3 | reduced | ||||||
| 3* | 3.1 | reduced | ||||||
| 4† | 4.1 | loss | ||||||
| 5† | 5.1 | loss | ||||||
| 6 | 6.2 | normal | ||||||
| 7 | 7.1 | normal | ||||||
| 7.3 | loss | c.403 C>T p.R135X | ||||||
| 8 | 8.1 | loss | ||||||
| 8.2a | loss | |||||||
| 8.2b | normal | |||||||
| 8.4a | loss | |||||||
| 8.4b | normal | |||||||
Patient 2 and 3 and
patient 4 and 5 are from the same family
 LOH
LOH
 Non-informative
Non-informative
 Retention
Retention
Polyp 2.3, 3.1, 8.1, 8.2a and 8.4a with reduction or loss of nuclear SMAD4 expression showed LOH in two or more markers surrounding SMAD4, including at least one of two markers closest to the SMAD4 locus. Retention of heterozygosity was found in polyp 1.1 and 7.3 even though SMAD4 expression was reduced or lost. Subsequent sequence analysis revealed a somatic stop codon mutation in exon 1 (1.1) and exon 2 (7.3) of SMAD4, likely resulting in truncation of the protein. In polyp 4.1 and 5.1 with a hemizygous germline deletion of SMAD4 and immunohistochemical loss of the SMAD4 protein, LOH markers closest to SMAD4 were non-informative, although more distant markers did show LOH.
Dysplasia and genetic status of SMAD4
With aberrant epithelial SMAD4 protein expression reflecting the occurrence of a somatic event in the SMAD4 tumor suppressor gene, we investigated the association of these phenomena to neoplastic change in juvenile polyps by reviewing all corresponding H&E slides for dysplasia. In 9 of 20 polyps with a SMAD4 germline defect foci of low-grade dysplasia were found, two of which contained focal high-grade dysplasia. Four polyps were graded indefinite for dysplasia and 7 negative for dysplasia. (Table 3)
Table 3.
Dysplasia in juvenile polyps and SMAD4, APC, ß-catenin, K-ras and p53 status.
| Patient | polyp | Dysplasia | SMAD4 IHC | APC mutation | K-ras mutation | ß-catenin IHC | p53 IHC |
|---|---|---|---|---|---|---|---|
| 1 | 1.1 | LGD | reduced | NM | failed | M | N |
| 1.2 | Neg. | normal | - | - | M | N | |
| 2* | 2.1 | Indef. | normal | - | - | M | N |
| 2.2 | Neg. | normal | - | - | M | N | |
| 2.3 | Indef. | reduced | - | - | M | N | |
| 3* | 3.1 | LGD | reduced | NM | NM | M | N |
| 3.2 | Indef. | normal | - | - | M | N | |
| 4† | 4.1 | Neg. | loss | - | - | M | N |
| 5† | 5.1 | Neg. | loss | - | - | M | N |
| 5.2 | Neg. | normal | - | - | M | N | |
| 6 | 6.1 | Neg. | normal | - | - | M | N |
| 6.2 | HGD | normal | NM | NM | M | A | |
| 6.3 | HGD | normal | NM | NM | M | A | |
| 7 | 7.1 | LGD | normal | NM | NM | M | A |
| 7.2 | LGD | normal | NM | NM | M | N | |
| 7.3 | LGD | loss | NM | Codon 12 | M | A | |
| 8 | 8.1 | Indef. | loss | - | - | M | A |
| 8.2a | Neg. | loss | - | - | M | N | |
| 8.2b | LGD | normal | NM | NM | M | A | |
| 8.3 | Neg. | normal | - | - | M | N | |
| 8.4a | Neg. | loss | - | - | M | N | |
| 8.4b | LGD | normal | NM | NM | M | N |
Patient 2 and 3 and
patient 4 and 5 were from the same family
LGD: low-grade dysplasia. HGD: high-grade dysplasia. Indef.: indefinite for dysplasia. Neg.: negative for dysplasia. NM: no mutation found. M: membranous ß-catenin staining pattern. N: normal p53 staining pattern. A: aberrant p53 staining pattern (i.e. overexpression of absent staining).
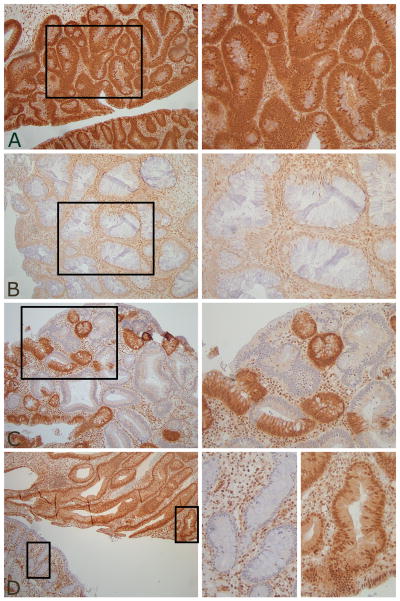
Intriguingly, the presence of dysplasia did not consistently correlate with reduction or loss of nuclear SMAD4 protein expression in juvenile polyps (Table 3 and 4). Polyp 6.2, 6.3 and 7.1 showed dysplasia even though nuclear SMAD4 expression of the epithelium was normal (Figure 2a), whereas, polyp 4.1 and 5.1 showed loss of epithelial SMAD4 expression but had no dysplasia (Figure 1c). Polyp 1.1 and 3.1 had foci of low-grade dysplasia within a larger area of reduced epithelial nuclear SMAD4 expression (Figure 2b) but in polyp 7.3 areas of low-grade dysplasia extended beyond the area showing loss of expression of SMAD4 (Figure 2c).
Table 4.
Correlation between dysplasia and SMAD4 immunostaining in juvenile polyps.
| Normal SMAD4 IHC | Reduced/lost SMAD4 IHC | Total | |
|---|---|---|---|
| No dysplasia | 5 (56%) | 4 (44%) | 9 |
| Indefinite for dysplasia | 2 (50%) | 2 (50%) | 4 |
| Dysplasia (LGD and HGD) | 6 (66%) | 3 (33%) | 9 |
Figure 2.
SMAD4 IHC and dysplasia. A: Dysplasia with normal epithelial SMAD4 expression. B: Dysplasia within area of reduced SMAD4 expression. C: Dysplasia extending beyond area of SMAD4 loss. E: Dysplasia with normal SMAD4 expression and non-neoplastic epithelium with loss of SMAD4 expression adjacent on one section. Magnification left panel 10X (figure 2E 5X) with 20X zoom (right panel).
Remarkably, polyp 8.2 and 8.4 both showed loss of SMAD4 expression in non-neoplastic epithelium (8.2a and 8.4a) but the same sections also contained low-grade dysplasia with normal SMAD4 expression (8.2b and 8.4b) (Figure 2d).
To confirm that SMAD4 immunohistochemistry accurately mirrors the molecular status of SMAD4, we aimed to exclude somatic inactivation of SMAD4 in juvenile polyp tissue with dysplasia and a normal SMAD4 staining pattern. Therefore, dysplastic epithelium with normal nuclear SMAD4 expression was microdissected and analyzed for LOH and somatic mutation using non-dysplastic epithelium with normal nuclear SMAD4 expression as a reference. As shown in Table 2, polyp 6.2, 7.1, 8.2b and 8.4b all had retention of heterozygosity of the SMAD4 locus and no somatic mutations were found.
Role of APC, ß-catenin, K-ras, and p53 in neoplastic progression in JPS
To investigate whether mutations in the conventional adenoma-carcinoma sequence underlie neoplastic change in juvenile polyps without loss of the wild-type SMAD4 allele (i.e. with normal SMAD4 protein expression), APC (MCR) and K-ras mutation analysis, as well as p53 and ß-catenin immunohistochemistry were performed. Results are summarized in Table 3. One somatic K-ras mutation was found in codon 12 (GGT → GAT) in polyp 7.3 with low-grade dysplasia and loss of SMAD4 immunostaining. Non-neoplastic areas from the same polyp did not show this K-ras mutation. Aberrant p53 staining, suggesting a p53 mutation, was found in 6 polyps. Five of these polyps contained dysplasia and 1 was graded indefinite for dysplasia. Interestingly four of these polyps (66%) showed normal SMAD4 expression. No mutations were found in the MCR of the APC gene and no aberrant ß-catenin expression was found.
Discussion
SMAD4 is one of two known genes responsible for juvenile polyposis syndrome when mutated in the germline. SMAD4 is a tumor suppressor gene and is frequently inactivated in advanced stages of pancreatic and colorectal cancer. In pancreatic cancer, loss of immunohistochemical labeling in tumor cells reflects genetic status of SMAD4 with high accuracy.(17)
The role of SMAD4 in JPS polyp formation is poorly understood. Investigators supporting the landscaper theory postulate that juvenile polyps arise primarily due to a stromal defect. The abnormal stroma causes disruption of normal development and regeneration of the overlying epithelium.(12, 13) In contrast, other studies provide evidence that LOH of SMAD4 in the epithelium initiates polyp growth suggesting that SMAD4 acts as a classic tumor suppressor protein in JPS polyps.(14-16) In fact, it is deemed likely that a second hit of the wild type allele initiates growth and neoplastic progression of JPS polyps, which fits the classic tumor suppressor model.(16)
In this study we illuminate the role of SMAD4 in juvenile polyp formation by investigating SMAD4 protein expression and SMAD4 gene status in juvenile polyps from 8 patients with a germline SMAD4 mutation. In almost half of all polyps from patients with a SMAD4 germline defect, focal reduction or loss of nuclear SMAD4 expression in the epithelium was seen. In contrast, no aberrant SMAD4 expression was noted in polyps from patients with a BMPR1A mutation, or in any of the sporadic juvenile polyps.
Aberrant SMAD4 immunostaining in JPS showed clear correlation with somatic inactivation of the SMAD4 gene. A second hit of the wild-type SMAD4 allele was found in 7 of 9 polyps with aberrant SMAD4 expression. This included LOH in five polyps and a somatic stop codon mutation resulting in truncation of the SMAD4 protein in two others. It proved difficult to assess LOH status using the microsatellite technique in two polyps (4.1 and 5.1) from two patients with a hemizygous germline deletion of all 11 exons of SMAD4. LOH analysis gave unreliable or non-informative results because the full extent of the germline deletion was not known. However, markers located further away from the SMAD4 gene locus did show LOH in these polyps.
These results clearly demonstrate that aberrant nuclear SMAD4 protein expression in JPS patients is indicative of somatic inactivation through LOH or somatic mutation, as has previously been shown in pancreatic cancer.(17) Furthermore, reduction or loss of epithelial SMAD4 expression in the polyps of individuals with JPS is specific for the presence of a SMAD4 germline defect, ranging from missense mutations to hemizygous deletions. Therefore, SMAD4 immuunohistochermistry can be used as a first screening method in the molecular diagnosis of JPS. An underlying germline SMAD4 mutation is likely if reduced (compared to surrounding stroma) or absent SMAD4 expression is found in the epithelial component of a juvenile polyp. However, normal SMAD4 expression is less predictive of germline status.
Moreover, since focal loss of epithelial SMAD4 expression was found only in a subset of juvenile polyps with a SMAD4 germline mutation, inactivation of the wild type SMAD4 allele in the epithelium is not required for polyp initiation and formation, but rather occurs as a late event during polyp growth and neoplastic progression. This concurs with previous observations in mouse models of juvenile polyposis reporting that haploinsufficiency is sufficient for polyp initiation.(26, 27)
One study by Kim et al. reported that targeted inactivation of Smad4 in stromal T-cells leads to a JPS-like phenotype and epithelial cancers of the gastrointestinal tract in mice, whereas inactivation in the epithelium does not.(28) Although our results argue that inactivation of SMAD4 occurs in the epithelium and not in the stroma of juvenile polyps, we cannot eliminate the concept that haploinsufficiency of SMAD4 in cells of the stromal compartment contributes to juvenile polyp initiation as per the landscaper theory. In fact, our finding that epithelial inactivation of SMAD4 is not required for polyp initiation suggests that this may indeed be the case.
Surprisingly, we found that the majority of polyps with dysplasia showed normal SMAD4 protein expression (66%), whereas loss of SMAD4 expression was slightly more common in non-neoplastic polyps than in juvenile polyps with dysplasia (44% vs. 33%) (Table 4). With regard to SMAD4 in neoplastic progression, this finding suggests that neoplastic change of the epithelium in juvenile polyps with a SMAD4 germline defect is not necessarily initiated by inactivation of the wild-type SMAD4 allele (Figure 2a), conflicting with the proposed gatekeeper function of SMAD4 in JPS.(16) Rather, these results suggest an alternative pathway leading to neoplasia in JPS with somatic inactivation of SMAD4 as a late event during neoplastic progression, in accordance with its role in the conventional adenoma-carcinoma sequence in colorectal cancer.(29) Although in our study evidence for (early) Wnt-pathway activation was not found p53 accumulation occurred in 4 of 6 polyps with dysplasia and normal SMAD4 immunostaining. Others did report somatic APC mutations in dysplastic juvenile polyps (30), and our study used only APC MCR mutation analysis and ß-catenin immunohistochemistry; however also others did not find support for a major role for Wnt pathway activation in early neoplastic development in JPS.(14, 27, 31)
On the other hand, somatic inactivation of SMAD4 also occurred in epithelium without morphological features of dysplasia in 44% of juvenile polyps (Table 4, Figure 1c). In some cases this was observed on the very same section containing areas of low-grade dysplasia with normal SMAD4 expression (Figure 2d).
Consequently, the role of SMAD4 in neoplastic progression of juvenile polyps remains unclear. Although SMAD4 inactivation is seen in a clonal pattern it occurs seemingly independent of microscopically evident neoplastic change. Perhaps the most likely scenario is that two pathways causing neoplasia occur. In juvenile polyps carrying a SMAD4 germline defect, an increased selective pressure leading to early stage inactivation of this gene may exist. This molecular marker of neoplasia can be visualized by loss of SMAD4 immunohistochemical staining but may on microscopy of the H&E section not yet be recognizable as dysplasia. Alternatively, selective pressure may also be increased on other genes capable of initiating neoplastic change, such as p53. This could be a direct result of the SMAD4 germline defect or from the abnormal microenvironment present in juvenile polyps. In addition, epigenetic silencing of genes may be important in this model. Somatic inactivation of SMAD4 may then occur at a later stage, possibly leading to acceleration of the neoplastic progression. Alternatively, retention of a wild type SMAD4 allele may also enable polyps to benefit from the tumor promoting actions by the TGF-ß signalling pathway.(8) Although this study was performed with a limited number of patients, several important conclusions can be drawn. First, we found that SMAD4 immunohistochemistry accurately reflects SMAD4 status in polyps of the juvenile polyposis syndrome and can be a useful and specific adjunct to the molecular diagnosis of JPS. Second, somatic inactivation of SMAD4 occurs in the epithelium but is presumably not a prerequisite for neoplastic change. Our results suggest that various pathways can lead to neoplasia in juvenile polyposis caused by germline mutation of SMAD4. One pathway initiated by somatic loss of SMAD4 and another characterized by p53 inactivation with retention of the wild-type SMAD4 allele.
Statement of Translational Relevance.
The current study shows that SMAD4 immunohostochemistry mirrors genetic status and can be used as a first screening method in the molecular diagnosis of JPS. A germline SMAD4 mutation is likely if absent or reduced SMAD4 expression is found in a juvenile polyp. In addition, this study increases our understanding of juvenile polyposis pathogenesis. It is shown that biallelic SMAD4 inactivation is not required for polyp formation and not obligatory for neoplastic progression in juvenile polyps. Moreover, different routes to neoplasia in juvenile polyposis caused by germline SMAD4 mutation appear operative, including somatic loss of SMAD4 and p53 inactivation without somatic loss of SMAD4.
Acknowledgments
Funding: Supported by The Netherlands Digestive Disease Foundation (MLDS WS 04-06), The John G. Rangos, Sr. Charitable Foundation, The Clayton Fund, and NIH grants CA 53801, 63721, 51085, and P50 CA 93-16. The study sponsors were not involved in study design, collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Abbreviations
- JPS
juvenile polyposis syndrome
- LOH
loss of heterozygosity
- MCR
mutation cluster region
Footnotes
Competing interests: No competing interests to declare.
References
- 1.Giardiello FM, Offerhaus GJ, Krush AJ, et al. Risk of hepatoblastoma in familial adenomatous polyposis. J Pediatr. 1991;119:766–8. doi: 10.1016/s0022-3476(05)80297-5. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR, Williams CB, Bussey HJ, Morson BC. Juvenile polyposis--a precancerous condition. Histopathology. 1988;13:619–30. doi: 10.1111/j.1365-2559.1988.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 3.Brosens LA, van Hattem A, Hylind LM, et al. Risk of colorectal cancer in juvenile polyposis. Gut. 2007;56:965–7. doi: 10.1136/gut.2006.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaltonen LA, Jass JR, Howe JR. Juvenile Polyposis. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 130–2. [Google Scholar]
- 5.van Hattem WA, Brosens LA, de Leng WW, et al. Large genomic deletions of SMAD4, BMPR1A and PTEN in juvenile polyposis. Gut. 2008;57:623–7. doi: 10.1136/gut.2007.142927. [DOI] [PubMed] [Google Scholar]
- 6.Aretz S, Stienen D, Uhlhaas S, et al. High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome. J Med Genet. 2007;44:702–9. doi: 10.1136/jmg.2007.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 8.Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 9.Schutte M, Hruban RH, Hedrick L, et al. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527–30. [PubMed] [Google Scholar]
- 10.Thiagalingam S, Lengauer C, Leach FS, et al. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996;13:343–6. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 11.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 12.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–6. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 13.Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–7. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- 14.Takaku K, Miyoshi H, Matsunaga A, et al. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 1999;59:6113–7. [PubMed] [Google Scholar]
- 15.Woodford-Richens K, Bevan S, Churchman M, et al. Analysis of genetic and phenotypic heterogeneity in juvenile polyposis. Gut. 2000;46:656–60. doi: 10.1136/gut.46.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodford-Richens K, Williamson J, Bevan S, et al. Allelic loss at SMAD4 in polyps from juvenile polyposis patients and use of fluorescence in situ hybridization to demonstrate clonal origin of the epithelium. Cancer Res. 2000;60:2477–82. [PubMed] [Google Scholar]
- 17.Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J Pathol. 2000;156:37–43. doi: 10.1016/S0002-9440(10)64703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baas IO, Mulder JW, Offerhaus GJ, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto M, Ahnen DJ, Franklin WA, Maltzman TH. Expression of beta-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis. 2000;21:1935–40. doi: 10.1093/carcin/21.11.1935. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SR, Vogelstein B, Kudo S, et al. Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen LA, editors. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. pp. 101–19. [Google Scholar]
- 21.Brosens LA, Iacobuzio-Donahue CA, Keller JJ, et al. Increased cyclooxygenase-2 expression in duodenal compared with colonic tissues in familial adenomatous polyposis and relationship to the -765G -> C COX-2 polymorphism. Clin Cancer Res. 2005;11:4090–6. doi: 10.1158/1078-0432.CCR-04-2379. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho R, Milne AN, van Rees BP, et al. Early-onset gastric carcinomas display molecular characteristics distinct from gastric carcinomas occurring at a later age. J Pathol. 2004;204:75–83. doi: 10.1002/path.1602. [DOI] [PubMed] [Google Scholar]
- 23.Hirata H, Matsuyama H, Matsumoto H, et al. Deletion mapping of 18q in conventional renal cell carcinoma. Cancer Genet Cytogenet. 2005;163:101–5. doi: 10.1016/j.cancergencyto.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Cawkwell L, Bell SM, Lewis FA, et al. Rapid detection of allele loss in colorectal tumours using microsatellites and fluorescent DNA technology. Br J Cancer. 1993;67:1262–7. doi: 10.1038/bjc.1993.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–33. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 26.Alberici P, Jagmohan-Changur S, De Pater E, et al. Smad4 haploinsufficiency in mouse models for intestinal cancer. Oncogene. 2006;25:1841–51. doi: 10.1038/sj.onc.1209226. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Brodie SG, Yang X, et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene. 2000;19:1868–74. doi: 10.1038/sj.onc.1203504. [DOI] [PubMed] [Google Scholar]
- 28.Kim BG, Li C, Qiao W, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–9. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 29.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 30.Wu TT, Rezai B, Rashid A, et al. Genetic alterations and epithelial dysplasia in juvenile polyposis syndrome and sporadic juvenile polyps. Am J Pathol. 1997;150:939–47. [PMC free article] [PubMed] [Google Scholar]
- 31.Fogt F, Brown CA, Badizadegan K, Zimmerman RL, Odze R. Low prevalence of loss of heterozygosity and SMAD4 mutations in sporadic and familial juvenile polyposis syndrome-associated juvenile polyps. Am J Gastroenterol. 2004;99:2025–31. doi: 10.1111/j.1572-0241.2004.30502.x. [DOI] [PubMed] [Google Scholar]