Abstract
Background
Prolonged disruption of sleep in animal studies is associated with decreased neurogenesis in the dentate gyrus. Our objective was to determine if insomnia severity in a sample of PTSD and controls was associated with decreased volume in the CA3/dentate hippocampal subfield.
Methods
Volumes of hippocampal subfields in seventeen veteran males positive for PTSD (41 ±12 years) and nineteen age-matched male veterans negative for PTSD were measured using 4 Tesla MRI. Subjective sleep quality was measured by the Insomnia Severity Index (ISI) and the Pittsburgh Sleep Quality Index (PSQI).
Results
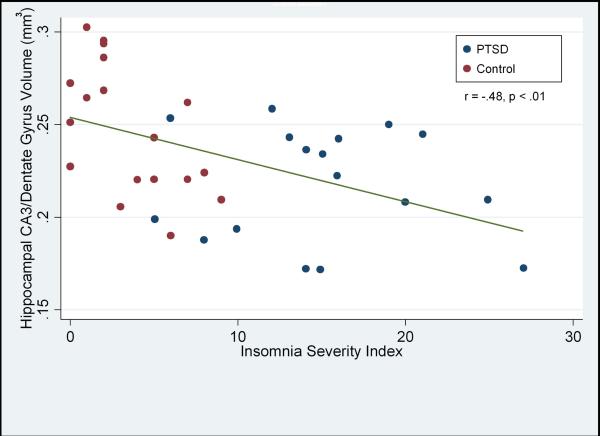
Higher scores on the ISI, indicating worse insomnia, were associated with smaller volumes of the CA3/dentate subfields (r= −.48, p < 0.01) in the combined sample. Adding the ISI score as a predictor for CA3/dentate volume to a hierarchical linear regression model after first controlling for age and PTSD symptoms accounted for a 13 % increase in incremental variance (t= −2.47, p= 0.02).
Conclusions
The findings indicate for the first time in humans that insomnia severity is associated with volume loss of the CA3/dentate subfields. This is consistent with animal studies showing that chronic sleep disruption is associated with decreased neurogenesis and dendritic branching in these structures.
Keywords: sleep, hippocampus, magnetic resonance imaging, neurogenesis, dentate gyrus, posttraumatic stress disorder
Introduction
One of the most exciting discoveries in neurosciences is that the adult mammalian brain has the capacity for neurogenesis, or growth of new neurons (1). Multiple studies have suggested that neurogenesis is restricted to a few areas in the brain including the hippocampal dentate gyrus (DG) and the subventricular zone of the lateral ventricles (For review see (2)). The hippocampus has been implicated also in PTSD, showing smaller volumes on MRI. (For review see (3)). Recently, our group has found with 4 Tesla MRI that hippocampal volume loss in PTSD is specific to CA3/dentate subfields using high field strength MRI (4 Tesla) (4).
A growing number of studies have shown that prolonged disruption of sleep in rats results in a decrease in neurogenesis and hippocampal cell survival (5–8) (For review see (9)). Although the pathophysiologic link between chronic sleep disturbance and diminished neurogenesis is not known, it does appear to be related to sleep per se and not to other variables such as elevated glucocorticoids (8). This linkage fits with the theory that sleep has trophic effects on neurons and is vitally important for healthy brain function. Interestingly, a recent study showed that subjects with primary insomnia have reduced hippocampal volume (10), similar in magnitude to findings published in PTSD patients. This suggests that sleep is critical for the hippocampus and perhaps more specifically, to hippocampal subfields associated with neurogenesis. We developed a reliable measure of hippocampal subfields in humans using high field strength MRI (11, 12). Our primary objective in this study was to examine the relationship of insomnia severity to the CA3/dentate subfield.
Methods and Materials
The details of the recruitment and characteristics of the PTSD and control sample is described in detail in (4). To summarize, the sample included healthy veteran subjects with PTSD (N=17, mean age 41± 12 SD) and veteran controls (N= 19, mean age 38± 15) with no history of traumatic brain injury, history of psychotic or bipolar disorders, alcohol abuse or dependence within the previous 12 months, and drug abuse or dependence within the previous six months. The PTSD group had a mean score of 61 (SD= 14) on the Clinician Administered PTSD Scale (CAPS) (13) compared to a mean of 8 (SD= 7) in the controls. Six subjects in the PTSD group and 3 subjects in the control group had a past history of alcohol abuse or dependence. Two subjects in the PTSD group and 3 subjects in the control group had a past history of drug abuse or dependence. Insomnia severity was measured with the Insomnia Severity Index (ISI) (14) and global subjective sleep quality was measured by the Pittsburgh Sleep Quality Index (PSQI) (15). In addition, we examined the 3 factor structure of the PSQI (16) which correspond to measures of sleep efficiency, perceived sleep quality, and daily disturbances.
The MRI acquisition and data processing are described in detail in (4). To summarize, the hippocampal subfields, entorhinal cortex (ERC), subiculum, CA1, CA1&CA2 transition zone (CA1&CA2), CA3 & dentate gyrus (CA3&DG), were marked on 5 consecutive slices using a standardized marking scheme based on a hypointense line representing myelinated fibers in the stratum moleculare/lacunosum (17) which can be reliably visualized on these high resolution images. The volumes of the subfields were determined by multiplying the number of pixels within the markings by pixel size and slice thickness and then by adding the volumes on all marked slices. The intracranial volume was determined from the 3D T2 weighted image using the BET program (FMRIB Image Analysis) for skull stripping.
Statistical Analysis
Pearson correlation coefficients were calculated between ISI and PSQI scores with ICV-corrected total hippocampal volume and the following hippocampal subfields: CA3/dentate. CA1, CA2, subiculum, and entorhinal cortex (ERC). A hierarchical linear regression was conducted to predict CA3/dentate volume, with Age entered in the first step, PTSD symptoms (CAPS score) entered in the second step, and insomnia severity (ISI score) entered in the third step.
Results
There was considerable overlap in sleep quality in the two groups: 5 PTSD subjects had sleep quality scores within the range of the comparison group. There was a strong inverse correlation between the mean volumes of the CA3/dentate subfields and the ISI score (r= −.48, p < 0.01) in the combined sample. See Figure. There was a smaller, but significant correlation of the ISI score with total hippocampal volume (r= −.36, p= .02) and a trend for the CA1 subfield (r= −.29, p= .09). There were no significant correlations between subjective sleep quality and CA2, ERC, or subiculum. A hierarchical linear regression model for the CA3/dentate subfield showed that after controlling for age and PTSD symptoms the ISI score accounted for a 13 % increase in incremental variance (t= −2.47, p= 0.02). See Table. Despite the high correlation between PTSD symptoms and the ISI score (r= .81, p< .001), insomnia severity was the best predictor of the CA3/dentate volume and accounted for unique variance beyond PTSD. Past history of drug or alcohol use was not significantly related to the hippocampal subfields and had no effect on the relationship between the ISI score and the CA3/dentate volume.
Figure.
Scatterplot of CA3/Dentate Gyrus volume (mm3) and subjective sleep quality. Higher values on the ISI correspond to worse sleep. PTSD cases are depicted with red dots. Control subjects are depicted with blue dots.
Table.
A hierarchical linear regression model for the CA3/dentate subfield accounting for the effects of age, PTSD symptoms (CAPS score), and severity of insomnia (ISI score).
| Step & Variable | R2 | ΔR2 | β Step 1 | β Step 2 | β Step 3 |
|---|---|---|---|---|---|
| Step 1- Age | .08 | - | β = −.29 t = −1.8 p = .08 |
β = −.26 t = −1.6 p = .11 |
β = −.29 t = −1.9 p = .06 |
| Step 2-CAPS | .18 | .10 | β = −.32 t = −2.0 p = .05 |
β = .16 t = 0.7 p = .51 |
|
| Step 3- ISI | .31 | .13 | β = −.60 t = −2.5 p = .02 |
The PSQI produced nearly identical results. The global PSQI score was significantly correlated with the CA3/dentate volume (r= −.45, p < .01). Further, we examined the 3 factor structure of the PSQI (16) and found that the CA3/dentate gyrus volume was correlated with sleep efficiency (r= −.40, p= .01), perceived sleep quality r= −.47, p < .01), and daily disturbances (r= −.27, p= .10).
Discussion
The findings indicate for the first time in humans that subjective insomnia severity is associated with volume loss of the CA3/dentate subfields. The strong association of insomnia to the CA3/dentate subfield volume is consistent with animal models which have shown that chronic sleep disruption is associated with decreased neurogenesis and dendritic branching in these structures. New neurons in the dentate send axonal projections along the mossy fiber track to the CA3 region (Reviewed in (2)) and then onward through the Schaffer collaterals to CA1 and subiculum, and then to the ERC (18). Biological factors which have relatively large effects on neurogenesis in the dentate could produce both local volumetric changes in the CA3/dentate region as well as downstream effects to other subfields which could potentially influence total hippocampal volume. However, it is still not known in humans if the growth of new granule cells in the dentate gyrus account for the increase in hippocampal volume that have been described in some treatment studies (19, 20).
Overall our results show that insomnia has a stronger relationship to the hippocampal subfields than PTSD symptom severity. These findings will need to be replicated in larger samples. Longitudinal studies with nonpharmacologic treatment of insomnia would help to build a stronger causal connection between disrupted sleep and reduced hippocampal volume. For example, if effective and specific insomnia treatment produces a measurable increase in the volume of the CA3/dentate hippocampal subfield volume, than this would strengthen the inference that sleep has role in neurogenesis and that insomnia may account for volume differences across groups. Cognitive behavior treatment for insomnia would be a particularly attractive choice because if would avoid the potential confound that medication may have on the hippocampus.
Acknowledgments
Funding/Support: This research was supported in part by grants from the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration, Office of Research and Development, the Department of Defense, the National Center for Research Resource of NIH (RR23953), and the National Institute for Mental Health (TCN: MH057157). This material is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center, San Francisco California.
Financial disclosures: None of the authors have financial interests over the past two years that are relevant to the subject matter of this manuscript. Dr. Neylan has served on an advisory board for Pfizer, and has received research support from Actelion and Glaxo Smith Kline. Dr. Marmar has served on an advisory board for Pfizer. Dr. Weiner has received research support from Merck and Avid, has stock options in Synarc and Elan, and has been on scientific advisory boards for Bayer Schering Pharma, Lilly, CoMentis, Neurochem, Eisai, Avid, Aegis, Genentech, Allergan, Lippincott, Bristol Myers, Forest, Pfizer, McKinsey, Mitsubishi, and Novartis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 3.Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, et al. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman-Marin R, Bashir T, Suntsova N, Szymusiak R, McGinty D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neuroscience. 2007;148:325–333. doi: 10.1016/j.neuroscience.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman-Marin R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol. 2003;549:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tung A, Takase L, Fornal C, Jacobs B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience. 2005;134:721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Mueller AD, Pollock MS, Lieblich SE, Epp JR, Galea LA, Mistlberger RE. Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1693–1703. doi: 10.1152/ajpregu.00858.2007. [DOI] [PubMed] [Google Scholar]
- 9.Meerlo P, Mistlberger RE, Jacobs BL, Craig Heller H, McGinty D. New neurons in the adult brain: The role of sleep and consequences of sleep loss. Sleep Med Rev. 2008 doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–958. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, et al. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28:719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4 T. Neuroimage. 2008;42:42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 14.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29:112–116. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson SH, Thom M, Bartlett PA, Symms MR, McEvoy AW, Sisodiya SM, et al. PROPELLER MRI visualizes detailed pathology of hippocampal sclerosis. Epilepsia. 2008;49:33–39. doi: 10.1111/j.1528-1167.2007.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 19.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]