Abstract
Polo-like kinase 1 (Plk1) and Aurora A play key roles in centrosome maturation, spindle assembly, and chromosome segregation during cell division. Here we show that the functions of these kinases during early mitosis are coordinated through Bora, a partner of Aurora A first identified in Drosophila. Depletion of human Bora (hBora) results in spindle defects, accompanied by increased spindle recruitment of Aurora A and its partner TPX2. Conversely, hBora overexpression induces mislocalization of Aurora A and monopolar spindle formation, reminiscent of the phenotype seen in Plk1-depleted cells. Indeed, Plk1 regulates hBora. Following Cdk1-dependent recruitment, Plk1 triggers hBora destruction by phosphorylating a recognition site for 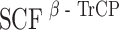 . Plk1 depletion or inhibition results in a massive accumulation of hBora, concomitant with displacement of Aurora A from spindle poles and impaired centrosome maturation, but remarkably, co-depletion of hBora partially restores Aurora A localization and bipolar spindle formation. This suggests that Plk1 controls Aurora A localization and function by regulating cellular levels of hBora.
. Plk1 depletion or inhibition results in a massive accumulation of hBora, concomitant with displacement of Aurora A from spindle poles and impaired centrosome maturation, but remarkably, co-depletion of hBora partially restores Aurora A localization and bipolar spindle formation. This suggests that Plk1 controls Aurora A localization and function by regulating cellular levels of hBora.
Electronic supplementary material
The online version of this article (doi:10.1007/s00412-008-0165-5) contains supplementary material, which is available to authorized users.
Introduction
The precise regulation of the spindle apparatus, a bipolar array of highly dynamic microtubules (MTs), is indispensable for accurate sister chromatid segregation and genome stability. In somatic animal cells, centrosomes control spindle assembly in time and space. At the G2 to M transition, centrosomes undergo a maturation process that is reflected in the enhanced recruitment of γ-tubulin ring complexes (γ-TuRCs) and other MT regulatory factors. Concomitantly, the two centrosomes separate from each other to form the spindle poles. These crucial events are controlled by three highly conserved serine/threonine kinases, cyclin-dependent kinase 1 (Cdk1), polo-like kinase 1 (Plk1), and Aurora A (Barr et al. 2004; Blagden and Glover 2003; Nigg 2001; Vagnarelli and Earnshaw 2004). Cdk1 is initially activated at the centrosome (Jackman et al. 2003) and required for centrosome separation (Blangy et al. 1995; Sawin and Mitchison 1995). Likewise, Plk1 and Aurora A localize to centrosomes and are activated at the G2/M transition, but the coordination of their activities is not presently understood. When Plk1 activity is impaired by antibody injection (Lane and Nigg 1996), RNAi-mediated Plk1 depletion (Hanisch et al. 2006; Sumara et al. 2004; van Vugt et al. 2004), or small molecule inhibitors (Lenart et al. 2007; McInnes et al. 2006; Peters et al. 2006; Santamaria et al. 2007), cells fail to recruit γ-TuRCs and often form monoastral spindles. Similarly, RNAi-mediated depletion of Aurora A results in the formation of monopolar spindles, decreased accumulation of γ-tubulin, and reduced density of centrosomal MTs (Ducat and Zheng 2004). Thus, both Plk1 and Aurora A clearly play important roles in centrosome maturation and bipolar spindle formation, and so one would expect that the functions of these kinases should be coordinated. However, although Plk1 is required for centrosomal localization of Aurora A (De Luca et al. 2006; Hanisch et al. 2006), we presently lack a mechanistic understanding of the links between these kinases.
Plk1 and Aurora A are activated through phosphorylation within their respective activation loops (T210 in human Plk1, T288 in human Aurora A; Jang et al. 2002; Littlepage et al. 2002), and both kinases are also controlled by additional mechanisms. In the case of Plk1, the C-terminal end domain (the so-called polo-box domain; PBD) functions as a phospho-peptide-binding module that mediates the binding of Plk1 to proteins pre-phosphorylated by ‘priming’ kinases (Cheng et al. 2003; Elia et al. 2003a, b). In the case of Aurora A, regulation appears to be conferred primarily through interactions with binding partners. Of several Aurora A interactors (Chen et al. 2002; Farruggio et al. 1999; Hirota et al. 2003; Mori et al. 2007; Ouchi et al. 2004), the role of the MT-binding protein TPX2 (targeting protein for XKlp2) is understood best (Eyers et al. 2003; Kufer et al. 2003; Tsai et al. 2003). Binding of the N terminus of TPX2 triggers kinase activation through a conformational change that protects the activation loop of Aurora A from dephosphorylation by protein phosphatase 1 (Bayliss et al. 2003; Eyers et al. 2003). Moreover, TPX2 targets Aurora A to the mitotic spindle (Kufer et al. 2002) and confers regulation by the Ran GTPase spindle assembly pathway (Tsai et al. 2003).
Bora was first identified as a binding partner of Aurora A in Drosophila (Hutterer et al. 2006). Its overexpression suppressed the centrosome maturation and asymmetric division defects seen in Aurora A mutants. When examined in vitro, Bora was able to activate Aurora A, albeit to a modest extent. Activation of Cdk1 at the onset of mitosis triggered the translocation of Bora from the nucleus to the cytoplasm, providing an attractive mechanism for the activation of cytoplasmic Aurora A in Drosophila. Here, we have explored the regulation and function of hBora in human cells. We show that both depletion and overexpression of hBora interfere with spindle formation. Furthermore, we find that hBora binds not only to Aurora A but also to Plk1. This latter interaction requires phosphorylation of hBora on a Cdk1 site and results in a  -dependent degradation of the protein, in agreement with a recent independent study (Seki et al. 2008). Most interestingly, the simultaneous depletion of hBora and Plk1 partially restores Aurora A localization to the centrosome and bipolar spindle formation, suggesting that the monoastral spindles typically seen upon interference with Plk1 activity could be due, at least in part, to the upregulation of hBora. Taken together, our data indicate that Plk1 regulates Aurora A localization and function through its ability to adjust cellular levels of hBora. This implies that hBora contributes to integrate the functions of three major mitotic kinases, Cdk1, Plk1, and Aurora A.
-dependent degradation of the protein, in agreement with a recent independent study (Seki et al. 2008). Most interestingly, the simultaneous depletion of hBora and Plk1 partially restores Aurora A localization to the centrosome and bipolar spindle formation, suggesting that the monoastral spindles typically seen upon interference with Plk1 activity could be due, at least in part, to the upregulation of hBora. Taken together, our data indicate that Plk1 regulates Aurora A localization and function through its ability to adjust cellular levels of hBora. This implies that hBora contributes to integrate the functions of three major mitotic kinases, Cdk1, Plk1, and Aurora A.
Results
Cell-cycle expression of hBora
To study the cell-cycle regulation of hBora expression, lysates from synchronized HeLa S3 cells were analyzed by Western blotting using an antibody raised against full-length hBora. For comparison, levels of Wee1, Plk1, Cyclin B1, Aurora A, and α-tubulin were determined in parallel. After release from a thymidine block, hBora levels increased gradually as cells approached mitosis but began to decrease before the onset of cyclin B degradation (Fig. 1). Moreover, several hBora forms with retarded electrophoretic mobility could be seen (see also ESM Fig. S1A left panel). These largely collapsed upon treatment of samples with alkaline phosphatase, indicating that they partly reflect phosphorylation (Fig. 1). Indeed, when tested as a substrate for mitotic kinases in vitro, hBora could be phosphorylated by Cdk1/cyclin B, Plk1, and Aurora A but not Aurora B (see also Hutterer et al. 2006; ESM Fig. S1B). Both Cdk1/cyclin B and Plk1 retarded the electrophoretic mobility of hBora, albeit to different extents (ESM Fig. S1C).
Fig. 1.
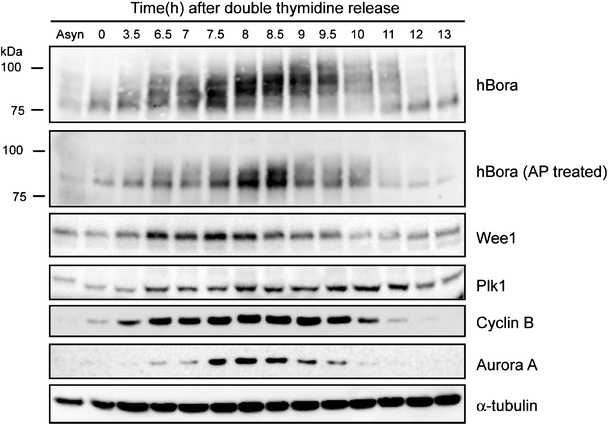
hBora is cell-cycle regulated and phosphorylated during mitosis. HeLa S3 cells were synchronized by double thymidine block (G1/S phase, indicated as time 0) and released. Samples were collected at the indicated times and probed by Western blotting, using the indicated antibodies. The first lane shows a lysate from asynchronously growing cells (Asyn). Part of the lysates was treated for 30 min with alkaline phosphatase (AP) to confirm that the retarded electrophoretic mobility of hBora was phosphorylation dependent
Depletion of hBora causes aberrant spindle formation
Two siRNA duplexes targeting hBora were used to explore the consequences of hBora depletion. Western blots revealed extensive depletion of hBora after 72 h of siRNA treatment, whereas levels of Aurora A and Plk1 remained unchanged (Fig. 2a). Compared to a GL2 control duplex (Elbashir et al. 2001), the two hBora siRNA duplexes produced similar increases in mitotic indices (ESM Fig. S2A) and a range of spindle abnormalities (Fig. 2b and ESM Fig. S2B). Specifically, many cells displayed bipolar spindles that were larger and denser than normal spindles (Fig. 2c). In addition, we observed cells with long, wavy spindles and lagging chromosomes and, as reported previously (Hutterer et al. 2006), occasional multipolar spindles (Fig. 2b and ESM Fig. S2B). Long, wavy spindles occasionally displayed fragmented poles (not shown), suggesting that they evolved to form multipolar spindles, a conclusion supported by live cell microscopy (data not shown).
Fig. 2.
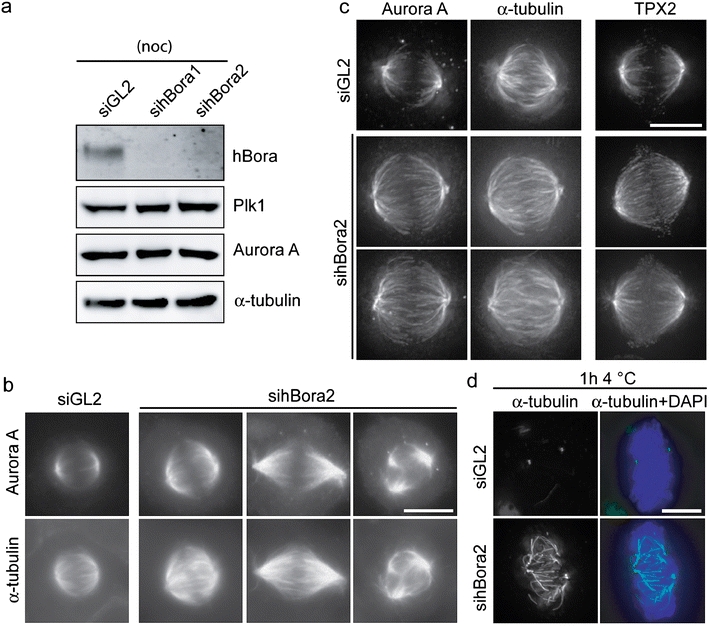
Aberrant spindle formation in hBora-depleted cells. a Western blotting of mitotic HeLa S3 cells treated for 72 h with GL2 (control) siRNA oligonucleotide or two independent oligonucleotides targeting hBora (sihBora1 and 2, respectively). Membranes were probed for hBora, Plk1, Aurora A, and for α-tubulin as loading control. b HeLa S3 cells treated with GL2 (control) or hBora2 siRNA for 72 h were fixed and permeabilized with methanol. Cells were stained to detect Aurora A and α-tubulin by immunofluorescence. Bar 10 μm. c Cells (treated as in b) were processed for immunofluorescence staining with antibodies against Aurora A, α-tubulin, and TPX2. Delta Vision pictures are shown. Note increased microtubule density and increased Aurora A and TPX2 (shown in separate cells) staining on the spindles of hBora-depleted cells. Bar 10 μm. d Cells (treated as in b) were placed on ice for 1 h and then fixed and permeabilized. Cells were stained with anti-α-tubulin (green), and DNA was visualized using DAPI (blue). Bar 10 μm
In line with the original identification of Bora as a partner of Aurora A (Hutterer et al. 2006), co-immunoprecipitation between the N terminus of hBora and endogenous Aurora A could readily be confirmed (ESM Fig. S3A). Additionally, we found that phosphorylation of hBora was required for binding to Aurora A (ESM Fig. S3A) and that alkaline phosphatase treatment of lysates abolished the interaction (ESM Fig. S3B). Having confirmed the Aurora A–hBora interaction, we next asked whether hBora depletion would affect Aurora A localization. To facilitate comparisons with controls, we focused on those hBora-depleted cells that showed relatively normal bipolar spindles. Even though such cells may not reflect the most severe phenotype of hBora depletion, immunofluorescence microscopy readily revealed an enrichment of both Aurora A and TPX2 on the spindles (Fig. 2c). In addition, the density of spindle MT was increased in hBora-depleted cells (Fig. 2c), whereas other proteins, notably pericentrin or Plk1, were not detectably affected (data not shown).
In view of evidence implicating the Aurora A–TPX2 complex in the stabilization of kinetochore MTs (K-fibers) in Caenorhabditis elegans (Ozlu et al. 2005), we asked, using cold treatment (Rieder and Borisy 1981), whether the increase in MT density in the spindles of hBora-depleted cells was accompanied by an increased stability of K-fibers. After 1 h of cold treatment, only centrosomal tubulin remained visible in control cells, as expected. In stark contrast, K-fibers remained largely intact in hBora-depleted cells (Fig. 2d and ESM Fig. S2C). Taken together, the above results indicate that hBora depletion results in an increased association of Aurora A with the spindle apparatus, which probably contributes to explain the observed spindle aberrations. Independently, a role for hBora in the stabilization of spindle microtubules was also observed by Seki et al. (2008).
Excess hBora causes Aurora A mislocalization and monoastral spindle formation
Staining of mitotic HeLa S3 cells with anti-hBora antibodies failed to highlight any specific structures (not shown), and immunostaining of ectopically expressed Myc-tagged hBora confirmed a diffuse localization of hBora (Fig. 3a). Remarkably, nearly all mitotic cells overexpressing hBora showed monoastral spindles (Fig. 3a), indicating that excess hBora interferes with bipolar spindle formation. The same phenotype was also observed upon overexpression of the N terminus of hBora (1–379; hBoraN), whereas the C terminus (377–599; hBoraC) produced no effect (Fig. 3a). Since it is the N terminus of hBora that binds Aurora A, this result suggested that the induction of monoastral spindles by excess hBora could reflect interference with Aurora A function. Indeed, overexpression of either hBora or hBoraN resulted in displacement of Aurora A from the spindle, whereas the kinase localized normally to the bipolar spindles of untransfected cells or cells expressing hBoraC (Fig. 3b). The most straightforward interpretation of these results is that excess cytoplasmic hBora interferes with bipolar spindle formation through the sequestration of Aurora A away from the spindle.
Fig. 3.
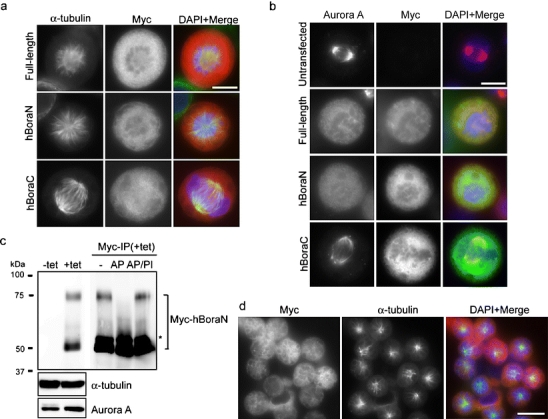
Overexpression of hBora leads to monoastral spindle formation and Aurora A mislocalization. a, b HeLa S3 cells were transfected with the indicated Myc-tagged hBora constructs, fixed and stained with 9E10 anti-Myc (red) and α-tubulin (green; a) or with 9E10 anti-Myc (green) and Aurora A (red) antibodies (b). DNA was visualized using DAPI (blue). Bars 10 μm. c Lysates prepared from HEK293T cells expressing Myc-hBoraN upon induction with 1 μg/ml tetracyclin (+tet) for 48 h were subjected to immunoprecipitation with 9E10 anti-Myc antibody and treated with alkaline phosphatase (AP) in the presence or absence of phosphatase inhibitors (PI). Lysates and immunoprecipitated proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and probed by Western blotting with 9E10 anti-Myc, anti-Aurora A, and α-tubulin antibodies. Note the absence of the upper band in the sample treated with AP. The asterisk denotes the IgG heavy chain. d Cells (induced as in c) were fixed and stained with 9E10 anti-Myc (red) and α-tubulin (green) antibodies. DNA was visualized using DAPI (blue). Bar 10 μm
To corroborate the above conclusion, we generated a tetracycline (tet)-inducible HEK293T cell line that allows the controlled expression of Myc-tagged hBoraN. As shown by Western blotting, tet induction of hBoraN for 48 h resulted in two Myc-immunoreactive bands (Fig. 3c). The upper band was sensitive to alkaline phosphatase treatment, confirming that it represents a phosphorylated form of Myc-hBoraN (Fig. 3c). Strikingly, more than 80% of the cells expressing hBoraN were arrested in mitosis and mostly displayed monoastral spindles (Fig. 3d), and as seen before (Fig. 3a,b), Aurora A was displaced from these spindles (data not shown). As expected, in view of the role of Aurora A in centrosome maturation, the recruitment of γ-tubulin was also drastically impaired, whereas TPX2 and Aurora B analyzed for control were not displaced (data not shown).
hBora interacts with Plk1 during mitosis
Intrigued by the remarkable similarity between the phenotype induced by excess hBora and that displayed by cells that lack Plk1 protein or activity (De Luca et al. 2006; Hanisch et al. 2006), we explored a potential link between hBora and Plk1. First, we asked whether hBora overexpression would perturb Plk1 localization. We found that induction of Myc-hBoraN led to Plk1 displacement from both the spindle poles and the kinetochores (Fig. 4a), and as described above for Aurora A, this effect was induced by hBora and hBoraN but not hBoraC (Fig. 4b). Next, we asked whether Plk1 and hBora might interact with each other in vivo. When Myc-hBoraN was immunoprecipitated from the tet-inducible cell line, both Plk1 and Aurora A could readily be detected in the immunoprecipitates, whereas TPX2 was absent (Fig. 4c). Plk1 was also precipitated when isolating the Aurora A/hBoraN complex with anti-Aurora A antibodies (data not shown). Thus, hBoraN and TPX2 do not bind to the same pool of Aurora A, but Plk1 and Aurora A are able to form a ternary complex with hBoraN. Interestingly, when these samples were probed with an antibody recognizing phosphorylated Threonine 288 (pT288) within the activation loop of Aurora A, the corresponding signal was clearly reduced in the lysate harboring excess hBoraN. Furthermore, whereas the Aurora A co-precipitating with TPX2 was readily recognized by the anti-pT288 antibody, the Aurora A co-precipitating with hBoraN did not react. Taken at face value, these results argue that the Aurora A in the TPX2 complex is more active than the kinase associated with hBoraN (Fig. 4c).
Fig. 4.
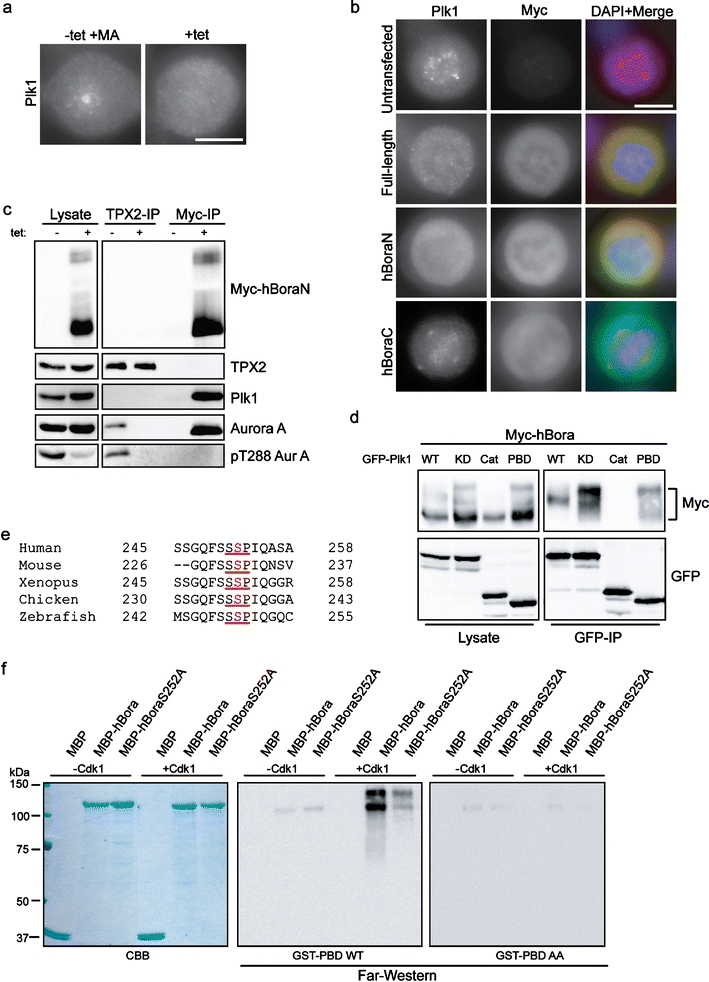
hBora interacts with Plk1 during mitosis. a Cells (induced as in Fig. 3c) were fixed and stained with an antibody against Plk1. DNA was visualized using DAPI (blue). Bar 10 μm. b HeLa S3 cells transfected with the indicated Myc-tagged hBora constructs were fixed and stained with 9E10 anti-Myc (green) and anti-Plk1 (red) antibodies. DNA was visualized using DAPI (blue). Bar 10 μm. c Lysates from HEK293T (prepared as in Fig. 3c) were subjected to immunoprecipitation with TPX2 or 9E10 anti-Myc antibodies. Lysates and immunoprecipitated proteins were separated by SDS-PAGE and probed by Western blotting with 9E10 anti-Myc, anti-TPX2 anti-Aurora A, anti-pT288 Aurora A, and α-tubulin antibodies. d HEK293T cells were co-transfected with Myc-hBora and GFP-tagged-Plk1 constructs [wild-type (WT); kinase dead (KD); catalytic domain (Cat), polo-box domain (PBD)] for 48 h, and cells were arrested with nocodazole for the last 16 h. The presence of Myc-hBora in GFP immunoprecipitates was assessed by Western blotting. e Evolutionary conservation of the putative PBD binding site in hBora (underlined). The phosphorylated serine is marked in red. Numbers refer to their position. f In vitro kinase assay was performed with Cdk1-cyclin B (or buffer for control) and the indicated proteins as substrates. The samples were subjected to SDS-PAGE, and subsequently, a far-Western ligand blotting assay using GST-PBD and GST-PBD-AA was performed. Coomassie blue staining (CBB) showed protein loading
To determine which domain of Plk1 interacts with hBora, co-immunoprecipitation experiments were performed on HEK293T cells after co-transfection with Myc-hBora and various green fluorescent protein (GFP)-Plk1 constructs. As shown in Fig. 4d, phosphorylated forms of hBora were co-precipitated with both wild-type (WT) and catalytically inactive (KD) versions of Plk1, indicating that Plk1 activity is not required for this interaction. However, the hBora proteins co-precipitated by active and inactive versions of Plk1 displayed different electrophoretic mobilities, suggesting that they differed in phosphorylation state. Most importantly, the GFP-tagged C-terminal end domain of Plk1 (GFP-Plk1 PBD) also bound to hBora, whereas the catalytic domain (GFP-Plk1Cat) did not (Fig. 4d). Taken together, the above results suggested that Plk1 interacts via its PBD with a phosphorylated form of hBora and that this binding prompts hBora phosphorylation by Plk1. Given that both Drosophila and human Bora can be phosphorylated by Cdk1 (Hutterer et al. 2006; ESM Fig. S1B and S1C) and a potential Cdk1-induced PBD-docking site in hBora (S252) has been conserved in evolution (Fig. 4e), we tested the functionality of this candidate PBD docking site in a far-Western ligand-binding assay (Neef et al. 2003). Without pre-phosphorylation by Cdk1, wild-type hBora showed very little binding to the glutathione-S-transferase (GST)-PBD, but strong binding was seen after phosphorylation (Fig. 4f, central panel). PBD binding to a S252A mutant of hBora was markedly reduced, albeit not completely abolished, even after pre-phosphorylation by Cdk1 (Fig. 4f, central panel). Virtually no binding to any hBora protein was seen with a PBD mutant (GST-PBD AA) that is unable to bind to phosphopeptides (Elia et al. 2003a, b; Fig. 4f, right panel). Coomassie Blue staining confirmed the presence of equal amounts of hBora (Fig. 4, left panel). These results identify the motif centered on S252 as a major Cdk1-dependent Plk1-PBD binding site in hBora.
Plk1 triggers the 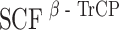 -mediated degradation of hBora
-mediated degradation of hBora
Having shown that hBora is a binding partner (Fig. 4) and potential substrate of Plk1 (ESM Fig. S1B and S1C), we next explored the physiological significance of this interaction. We found that siRNA-mediated depletion of Plk1 led to a striking accumulation of hBora (Fig. 5a), and a similar increase in hBora levels was seen upon inhibition of Plk1 by the small molecule inhibitor ZK–thiazolidinone (TAL) (Santamaria et al. 2007; data not shown). Plk1 is well known to trigger the degradation of several cell-cycle regulators through phosphorylation of a phosphodegron (DpSGxxpT), which then leads to the recruitment of the β-transducin repeat-containing protein (β-TrCP) component of a Skp1-Cul1-F-box-protein ubiquitin ligase, followed by polyubiquitination and proteasomal degradation (Liu and Maller 2005; Mamely et al. 2006; Moshe et al. 2004; Rauh et al. 2005; Tung et al. 2005; Watanabe et al. 2004). Since examination of the hBora sequence revealed an evolutionarily conserved potential β-TrCP-binding motif within the C terminus (ESM Fig. S4A), we asked whether Plk1 might act through this putative phosphodegron to control cellular levels of hBora. First, we established that Plk1 phosphorylated full-length hBora, as well as N- and C-terminal fragments, but that phosphorylation was reduced upon mutation of the putative phospho-degron (S497A/T501A; data not shown). Next, we tested the ability of Plk1 KD to interfere with hBora degradation by acting as a dominant negative mutant. In response to co-expression of full-length Myc-hBora with Plk1 KD, a significant increase in hBora (phospho-) protein levels could be seen, whereas the co-expression of WT Plk1 had no obvious effect (ESM Fig. S4B). Mutation of the phosphodegron (S497/T501) or the PBD-docking site (S252A) both abolished responsiveness of the mutant hBora proteins to alterations in Plk1 activity (ESM Fig. S4B). Finally, the phosphorylated form of wild-type hBora could readily be co-immunoprecipitated with β-TrCP, whereas both the phosphodegron and the PBD-docking site mutants failed to interact (ESM Fig. S4C). Moreover, siRNA-mediated depletion of β-TrCP-1/2 resulted in the concomitant upregulation of both hBora and Wee1, a known β-TrCP target (ESM Fig. S1A). Taken together, these results demonstrate that the phosphodegron identified in hBora is functional and that Plk1 binding is essential for triggering the β-TrCP-mediated degradation of hBora. As a consequence, interference with Plk1 activity results in the accumulation of hBora (phospho-) protein. These conclusions are in excellent agreement with a recent independent study (Seki et al. 2008), in which human Bora was also identified as a Plk1-dependent substrate for β-TrCP-mediated proteolysis.
Fig. 5.
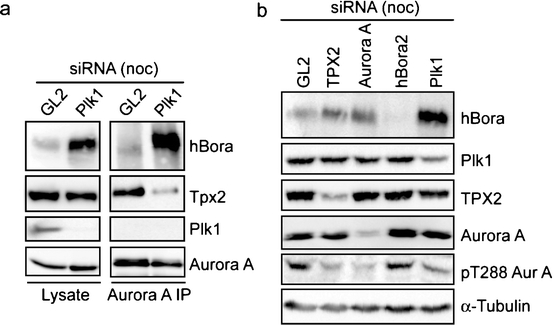
Plk1 controls hBora levels. a Western blotting of mitotic HeLa S3 cells treated with Plk1 or GL2 (control) siRNA for 36 h. Lysates were subjected to immunoprecipitation with Aurora A antibody. Lysates and immunoprecipitated proteins were separated by SDS-PAGE and probed by Western blotting with anti-hBora, anti-TPX2 anti-Plk1, and anti-Aurora A antibodies. b Western blotting of mitotic HeLa S3 cells treated for 72 h with GL2 (control), TPX2, Aurora A, hBora2 siRNA oligonucleotides, or for 36 h with Plk1 siRNA oligonucleotide. Membranes were probed for hBora, Plk1, TPX2, Aurora A, pT288 Aurora A, and α-tubulin for loading control
Could Plk1 regulate Aurora A by controlling hBora levels?
Considering that hBora interacts with both Aurora A and Plk1 but is itself controlled by Plk1, it follows that hBora could contribute to coordinate the functions of these two key regulators of mitotic progression. One possibility is that Plk1 may determine Aurora A localization and/or activity through its ability to regulate the intracellular levels of hBora. Several experiments were carried out to test this possibility. First, when Aurora A was immunoprecipitated from Plk1-depleted cells, most Aurora A was complexed to hBora, reflecting the increased levels of hBora in such cells (Fig. 5a). In contrast, the amounts of TPX2 co-precipitating with Aurora A were substantially reduced, although total levels of TPX2 were not changed (Fig. 5a). Second, HeLa S3 cells were depleted of TPX2, Aurora A, hBora, or Plk1, and lysates were then probed with the activation-state specific anti-pT288 antibody (Fig. 5b). Depletion of either TPX2 or Aurora A caused a clear reduction in the level of active Aurora A, as reported by this antibody. A similar reduction was also seen when hBora levels were increased through depletion of Plk1, whereas depletion of hBora did not significantly affect Aurora A activity, when compared to a GL2-control (Fig. 5b). Together, these results indicate that hBora competes with TPX2 for Aurora A binding. As a consequence, levels of hBora are expected to determine how much Aurora A is available for binding to TPX2 and perhaps other activating partners.
Our findings suggest a plausible explanation for the observation that Plk1 is required for Aurora A localization to spindle poles (De Luca et al. 2006; Hanisch et al. 2006). Specifically, the requirement for Plk1 during centrosome maturation and spindle formation could in principle reflect its role in lowering hBora levels below a threshold, such as to allow Aurora A to exert its functions on the centrosome and spindle. This model predicts that a reduction of hBora levels might alleviate at least some of the early mitotic defects that are typically seen upon depletion of Plk1 or inhibition of Plk1 activity. To explore this possibility, we treated cells with siRNA duplexes targeting Plk1 and hBora, either in combination or singly (paired with GL2 for control), and asked whether co-depletion of hBora and Plk1 would restore Aurora A localization to the centrosome and spindle bipolarity. Co-depletion of hBora with Plk1 suppressed the accumulation of hBora that normally results from Plk1 depletion, as expected (Fig. 6a). Yet, Plk1 depletion and Plk1/hBora co-depletion both led to marked increases in mitotic indices (Fig. 6b), indicating that not all early mitotic functions of Plk1 can be attributed to its interaction with hBora. Interestingly, the two cell populations displayed remarkably different phenotypes. Whereas the Plk1-depleted cells displayed mostly monopolar spindles, as expected, the Plk1/hBora co-depleted cells showed mostly bipolar spindles, albeit with uncongressed chromosomes (Fig. 6c,d). Moreover, Aurora A was displaced from spindle poles in Plk1-depleted cells, consistent with previous data (De Luca et al. 2006; Hanisch et al. 2006), but largely restored to these structures in Plk1/hBora co-depleted cells (Fig. 7a lower panel). To facilitate the comparison with Plk1-depleted cells, Plk1/hBora co-depleted cells were also treated with monastrol to induce monopolar spindles (Fig. 7a middle panel), and furthermore, both cell populations were exposed to 4°C in order to depolymerize spindle MTs and better visualize centrosome-associated Aurora A (Fig. 7b). A similar, albeit partial, rescue of spindle bipolarity could also be observed upon hBora depletion from cells treated with the Plk1 inhibitor TAL (data not shown). Taken together, these results demonstrate that co-depletion of hBora partially rescued the defects in bipolar spindle formation and Aurora A localization that normally result from Plk1 depletion.
Fig. 6.
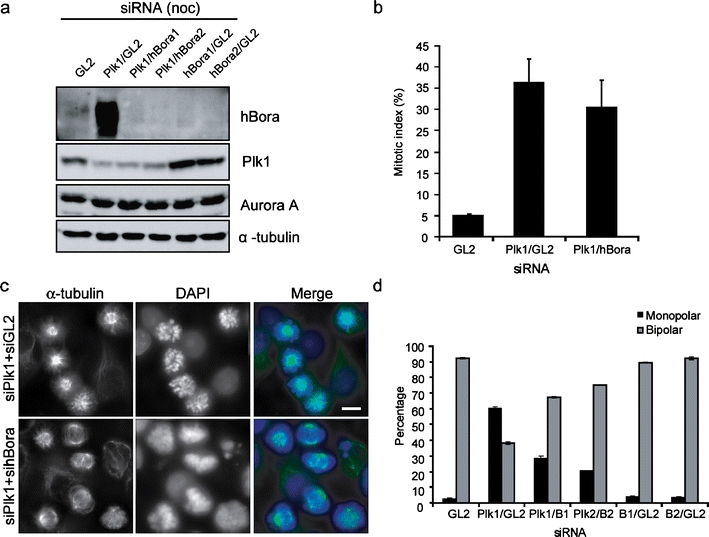
hBora levels are critical for proper spindle assembly. a Western blotting of mitotic HeLa S3 cells treated for 72 h with different combinations of siRNA. Plk1 siRNA was added after 36 h of GL2 (control) or hBora depletion, and nocodazole was added for the last 12 h. Total amount of siRNA was held constant at 100 nM. Membranes were probed for hBora, Plk1, Aurora A, and for α-tubulin as loading control. b Histogram showing the mitotic indices of HeLa S3 cells (treated as in a). Results are from three individual experiments (300–350 per experiment), and bars indicate SD. c Hela S3 cells were treated with different combinations of siRNA, fixed and permeabilized, and stained with anti-α-tubulin antibody. DNA was visualized using DAPI. Bar 10 μm. Total amount of siRNA was held constant at 100 nM. d Histogram showing the percentage of mitotic cells with monopolar/ bipolar spindle in experiments performed as in c. (N = 3,150 cells per experiment), bars indicate SD
Fig. 7.
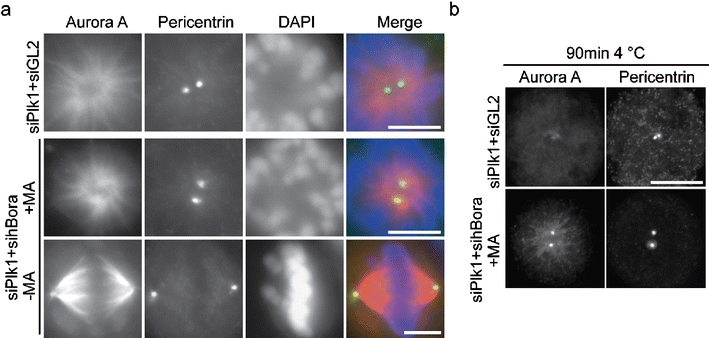
Plk1 regulates Aurora A localization by modulating hBora. a HeLa S3 cells, treated as in Fig. 6c and treated with or without 150 μM monastrol (MA) were fixed and stained for Aurora A (red) and pericentrin (green). DNA was visualized using DAPI. Bar 10 μm. b Same as a but cells were subjected to cold treatment for 90 min before fixation. Cells were stained with anti-Aurora A and anti-pericentrin antibodies. Note that the frequency of the observed rescue (restoration of Aurora A to spindle poles) was comparable to the frequency of restoration of bipolar spindle formation (Fig. 6d). Bar 10 μm
Discussion
The results reported here indicate that precise levels of hBora are critical for correct Aurora A localization, spindle assembly, and accurate chromosome segregation. In particular, we demonstrate that hBora binds not only to Aurora A but also to Plk1 and that Plk1 regulates hBora levels through 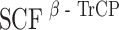 -mediated degradation. While these results are in excellent agreement with findings recently reported by Seki et al. (2008), our data additionally identify the Cdk1 site S252 as critical for the recruitment of Plk1 to hBora. It is most interesting to note that the interference with Plk1 activity resulted not only in a drastic upregulation of hBora but in a concomitant sequestration of Aurora A within the cytosol. So, by virtue of its ability to regulate the abundance of a cytoplasmic hBora–Aurora A complex, Plk1 controls the availability of Aurora A for interactions with spindle-associated partners such as TPX2. Collectively, our findings lead us to propose that hBora contributes to integrate the functions of three major mitotic kinases, Cdk1, Plk1, and Aurora A.
-mediated degradation. While these results are in excellent agreement with findings recently reported by Seki et al. (2008), our data additionally identify the Cdk1 site S252 as critical for the recruitment of Plk1 to hBora. It is most interesting to note that the interference with Plk1 activity resulted not only in a drastic upregulation of hBora but in a concomitant sequestration of Aurora A within the cytosol. So, by virtue of its ability to regulate the abundance of a cytoplasmic hBora–Aurora A complex, Plk1 controls the availability of Aurora A for interactions with spindle-associated partners such as TPX2. Collectively, our findings lead us to propose that hBora contributes to integrate the functions of three major mitotic kinases, Cdk1, Plk1, and Aurora A.
hBora levels are critical for proper spindle assembly
In response to siRNA-mediated depletion of hBora, we observed the formation of long and wavy spindles, which eventually progressed to form multipolar spindles. In addition, we observed unusually ‘fat’ spindles that were characterized by increased MT density, increased amounts of spindle-associated Aurora A, and increased cold stability of K-fibers. The exact mechanisms underlying these spindle defects remain to be unraveled, but since hBora and TPX2 do not bind simultaneously to Aurora A, any reduction in hBora levels is expected to favor complex formation between Aurora A and spindle-associated activators such as TPX2 (Eyers et al. 2003; Kufer et al. 2002; Tsai et al. 2003). In turn, the increased abundance of Aurora A on the spindle is likely to cause enhanced activity of downstream effectors, notably chTOG, the human homolog of Xenopus XMAP215, and Drosophila minispindles (Barros et al. 2005; Giet et al. 2002; Kinoshita et al. 2005; Peset et al. 2005). Consistent with the above interpretation, the phenotypes seen in hBora-depleted cells resemble those of cells overexpressing Aurora A (Anand et al. 2003; Meraldi et al. 2002). Although modest overexpression of hBora did not produce a significant phenotype (data not shown; Seki et al. 2008), striking spindle defects were observed upon expression of hBora to high levels. In particular, excess hBora severely impaired the recruitment of both Aurora A and γ-tubulin to centrosomes, so that centrosome maturation and separation failed, resulting in the formation of monopolar spindles. Importantly, this phenotype was dependent on the ability of hBora to bind Aurora A. While excess hBora caused the sequestration of Aurora A into a diffusely localized pool, TPX2 remained on the spindle apparatus, suggesting that cytoplasmic hBora determines the size of the Aurora A pool that is available for interactions with spindle-associated binding partners. In support of this conclusion, a rapid exchange of Aurora A between the spindle and the cytosol has previously been observed in photobleaching experiments (Stenoien et al. 2003). Considering that Aurora A displays activity when bound to hBora (Hutterer et al. 2006; this study), it is possible that this diffusely localized complex carries out important functions by acting on cytosolic substrates, perhaps regulating cell-cycle progression. However, when hBora is deregulated, it perturbs Aurora A functions that are important for spindle assembly. Thus, the phenotypic consequences of hBora depletion and overexpression can be explained, at least in part, by deregulation of Aurora A complexes on mitotic structures. In future, it will be interesting to investigate whether hBora also regulates the function of other interaction partners, notably the activity of Plk1.
Plk1 regulates hBora stability
As shown here and elsewhere (Seki et al. 2008), hBora interacts not only with Aurora A but also with Plk1, in both cases through its N-terminal domain. The interaction between hBora and Plk1 requires the Plk1 PBD and prior phosphorylation of hBora on a Cdk1 site, S252, in line with a well-established docking model (Elia et al. 2003a, b). After its recruitment to hBora, Plk1 phosphorylates a conserved phosphodegron, which then serves as a recognition motif for the ubiquitin ligase 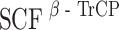 , leading to the proteasomal degradation of hBora. We consistently observed significant amounts of hBora in nocodazole-arrested cells. This indicates that not all hBora gets degraded when Cdk1 and Plk1 are activated at the G2/M transition, suggesting that a population of hBora is protected against β-TrCP-mediated degradation during early mitosis, perhaps through phosphorylation at particular sites and/or the binding of interaction partners (perhaps Aurora A itself). Furthermore, phosphatases counteracting Cdk1 and/or Plk1 are likely to contribute to the establishment of a steady-state level of hBora. Complex regulation of protein stability is not without precedent (Mailand et al. 2002), and so we anticipate the existence of multiple mechanisms to ensure physiological levels of hBora and appropriate timing of hBora degradation. As an extension of this view, we also note that hBora carries within its N terminus potential destruction motifs for yet another ubiquitin ligase, the anaphase-promoting complex/cyclosome. So, although our data point to
, leading to the proteasomal degradation of hBora. We consistently observed significant amounts of hBora in nocodazole-arrested cells. This indicates that not all hBora gets degraded when Cdk1 and Plk1 are activated at the G2/M transition, suggesting that a population of hBora is protected against β-TrCP-mediated degradation during early mitosis, perhaps through phosphorylation at particular sites and/or the binding of interaction partners (perhaps Aurora A itself). Furthermore, phosphatases counteracting Cdk1 and/or Plk1 are likely to contribute to the establishment of a steady-state level of hBora. Complex regulation of protein stability is not without precedent (Mailand et al. 2002), and so we anticipate the existence of multiple mechanisms to ensure physiological levels of hBora and appropriate timing of hBora degradation. As an extension of this view, we also note that hBora carries within its N terminus potential destruction motifs for yet another ubiquitin ligase, the anaphase-promoting complex/cyclosome. So, although our data point to 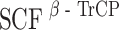 as the ubiquitin ligase responsible for Plk1-induced hBora degradation, other mechanisms are likely to contribute to the regulation of hBora levels, particularly during later stages of mitosis and the subsequent G1 phase.
as the ubiquitin ligase responsible for Plk1-induced hBora degradation, other mechanisms are likely to contribute to the regulation of hBora levels, particularly during later stages of mitosis and the subsequent G1 phase.
Does hBora confer Aurora A regulation through Plk1?
Both Plk1 and Aurora A regulate key events during late G2 and early mitosis, including centrosome maturation and spindle assembly (see “Introduction”). Although no direct interactions between these kinases have so far been established, Plk1 was found to be required for Aurora A localization to centrosomes (De Luca et al. 2006; Hanisch et al. 2006). Our present data, centered on hBora, suggest an attractive explanation for this observation. Specifically, Plk1 activity might be required to keep hBora levels below a threshold, such as to limit the extent of sequestration of Aurora A into cytoplasmic complexes with hBora. In support of this view, several of the early mitotic defects typically seen in cells deprived of Plk1 protein and/or activity could be rescued by hBora co-depletion. It is possible, therefore, that the centrosome maturation and separation defects seen in Plk1-depleted cells may stem, at least in part, from the hBora-mediated impairment of Aurora A function. Alternatively (or in addition), hBora might negatively regulate Plk1. We emphasize that not all early mitotic functions of Plk1 can be explained through hBora-mediated regulation of Aurora A. In particular, co-depletion of hBora did not rescue the chromosome congression defect or the mitotic arrest typically seen in Plk1-depleted cells. On the other hand, we note that high levels of hBora produced a more severe effect on Aurora A localization (displacement from the entire spindle) than Plk1 inhibition or depletion (displacement primarily from the spindle poles). This notwithstanding, our studies on hBora have uncovered an important mechanistic relationship between Plk1 and Aurora A. Thus, it is interesting to consider the possible implications of this relationship for the proposed roles of mitotic kinases in tumorigenesis. Both Plk1 and Aurora A are often overexpressed in tumors (Knecht et al. 1999; Sen et al. 1997), and Aurora A is considered a cancer susceptibility gene (Ewart-Toland et al. 2003; Meraldi et al. 2004). It is intriguing, therefore, that hBora also maps to a chromosomal region (13q21) that is often altered in tumors (Rozenblum et al. 2002). In consideration of the data reported here, one would predict that deregulation of hBora should lead to similar cellular phenotypes as the aberrant expression of either Plk1 or Aurora A.
Experimental procedures
Plasmids, cells, and immunofluorescence microscopy
Plasmid constructions, site-directed mutagenesis, transfections, cell-cycle synchronization, and production of full-length hBora are described in Electronic Supplementary Material. HEK293T Tet-on inducible cell lines for expression of full-length Myc-hBora and Myc-hBoraN were generated as described (Chalamalasetty et al. 2006), and transgene expression was induced by addition of 1 μg/ml tetracycline for 48 h. Immunofluorescence microscopy was carried out as described in Electronic Supplementary Material.
Transient transfections and siRNA
Plasmid transfections were performed using Fugene 6 reagent (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. siRNA duplexes were transfected using Oligofectamine (Invitrogen, Carlsbad, CA, USA), using the GL2 duplex for control (Elbashir et al. 2001). hBora siRNA duplexes (sihBora1: 5′-CCGGTTGATAATGGCAGTTTA-3′ and sihBora2 5′-TAACTAGTCCTTCGCCTATTT-3′) and β-TrCP1/2 siRNA duplex (5′-AAGTGGAATTTGTGGAACATC-3′) were purchased from Qiagen (Hilden, Germany). Plk1 and TPX2 were depleted using previously published siRNA duplexes (Hanisch et al. 2006; Kufer et al. 2002). For co-depletion experiment, HeLa S3 cells were treated with GL2 or hBora siRNA for 36 h. Subsequently, fresh media were replaced containing combinations of Plk1/GL2 or Plk1/hBora siRNA duplexes for another 36 h.
Biochemical assays
Immunoprecipitations, Western blots, and kinase assays are described in Electronic Supplementary Material. Far-Western ligand binding assays were carried out as described (Neef et al. 2003), using GST-tagged PBD (1 μg/ml) for 2 h at 4°C, followed by detection of bound protein with monoclonal anti-Plk1 antibody.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Anja Wehner for excellent technical assistance and Xiuling Li and Stefan Hümmer for help with mass spectrometry and live-cell imaging. We are also grateful to Andrea Hutterer and Juergen A. Knoblich (IMBA, Vienna) for communicating results prior to publication and to Xiumin Yan, Francis Barr, Rüdiger Neef, Tobias Oelschlägel, and Thomas Mayer for helpful discussion. This work was supported by the Max Planck Society, the ‘Deutsche Forschungsgemeinschaft (SFB 646), and the ‘Fonds der Chemischen Industrie’. E.H.Y. Chan was supported by the Hong Kong Croucher Foundation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/S1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Barros TP, Kinoshita K, Hyman AA, Raff JW. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J Cell Biol. 2005;170:1039–1046. doi: 10.1083/jcb.200504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell. 2003;12:851–862. doi: 10.1016/S1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- Blagden SP, Glover DM. Polar expeditions—provisioning the centrosome for mitosis. Nat Cell Biol. 2003;5:505–511. doi: 10.1038/ncb0603-505. [DOI] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Chalamalasetty RB, Hummer S, Nigg EA, Sillje HH. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci. 2006;119:3008–3019. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- Chen SS, Chang PC, Cheng YW, Tang FM, Lin YS. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J. 2002;21:4491–4499. doi: 10.1093/emboj/cdf409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KY, Lowe ED, Sinclair J, Nigg EA, Johnson LN. The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J. 2003;22:5757–5768. doi: 10.1093/emboj/cdg558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Lavia P, Guarguaglini G. A functional interplay between Aurora-A, Plk1 and TPX2 at spindle poles: Plk1 controls centrosomal localization of Aurora-A and TPX2 spindle association. Cell Cycle. 2006;5:296–303. doi: 10.4161/cc.5.3.2392. [DOI] [PubMed] [Google Scholar]
- Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301:60–67. doi: 10.1016/j.yexcr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/S0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, MacCarthy-Morrogh L, Ponder BA, Nagase H, Burn J, Ball S, Almeida M, Linardopoulos S, Balmain A. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34:403–412. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- Eyers PA, Erikson E, Chen LG, Maller JL. A novel mechanism for activation of the protein kinase Aurora A. Curr Biol. 2003;13:691–697. doi: 10.1016/S0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- Farruggio DC, Townsley FM, Ruderman JV. Cdc20 associates with the kinase aurora2/Aik. Proc Natl Acad Sci U S A. 1999;96:7306–7311. doi: 10.1073/pnas.96.13.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R, McLean D, Descamps S, Lee MJ, Raff JW, Prigent C, Glover DM. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J Cell Biol. 2002;156:437–451. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch A, Wehner A, Nigg EA, Sillje HH. Different Plk1 functions show distinct dependencies on polo-box domain-mediated targeting. Mol Biol Cell. 2006;17:448–459. doi: 10.1091/mbc.E05-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–598. doi: 10.1016/S0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell. 2006;11:147–157. doi: 10.1016/j.devcel.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Ma S, Terada Y, Erikson RL. Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase. J Biol Chem. 2002;277:44115–44120. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Noetzel TL, Pelletier L, Mechtler K, Drechsel DN, Schwager A, Lee M, Raff JW, Hyman AA. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J Cell Biol. 2005;170:1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–2797. [PubMed] [Google Scholar]
- Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufer TA, Nigg EA, Sillje HH. Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma. 2003;112:159–163. doi: 10.1007/s00412-003-0265-1. [DOI] [PubMed] [Google Scholar]
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Littlepage LE, Wu H, Andresson T, Deanehan JK, Amundadottir LT, Ruderman JV. Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc Natl Acad Sci U S A. 2002;99:15440–15445. doi: 10.1073/pnas.202606599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Mailand N, Podtelejnikov AV, Groth A, Mann M, Bartek J, Lukas J. Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002;21:5911–5920. doi: 10.1093/emboj/cdf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, Perrakis A, Medema RH, Freire R. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol. 2006;16:1950–1955. doi: 10.1016/j.cub.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McInnes C, Mazumdar A, Mezna M, Meades C, Midgley C, Scaerou F, Carpenter L, Mackenzie M, Taylor P, Walkinshaw M, Fischer PM, Glover D. Inhibitors of Polo-like kinase reveal roles in spindle-pole maintenance. Nat Chem Biol. 2006;2:608–617. doi: 10.1038/nchembio825. [DOI] [PubMed] [Google Scholar]
- Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Honda R, Nigg EA. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004;14:29–36. doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Mori D, Yano Y, Toyo-oka K, Yoshida N, Yamada M, Muramatsu M, Zhang D, Saya H, Toyoshima YY, Kinoshita K, Wynshaw-Boris A, Hirotsune S. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol Cell Biol. 2007;27:352–367. doi: 10.1128/MCB.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshe Y, Boulaire J, Pagano M, Hershko A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci U S A. 2004;101:7937–7942. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, Barr FA. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863–875. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Ouchi M, Fujiuchi N, Sasai K, Katayama H, Minamishima YA, Ongusaha PP, Deng C, Sen S, Lee SW, Ouchi T. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J Biol Chem. 2004;279:19643–19648. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- Ozlu N, Srayko M, Kinoshita K, Habermann B, O’Toole ET, Muller-Reichert T, Schmalz N, Desai A, Hyman AA. An essential function of the C. elegans ortholog of TPX2 is to localize activated aurora A kinase to mitotic spindles. Develop Cell. 2005;9:237–248. doi: 10.1016/j.devcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Peset I, Seiler J, Sardon T, Bejarano LA, Rybina S, Vernos I. Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J Cell Biol. 2005;170:1057–1066. doi: 10.1083/jcb.200504037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters U, Cherian J, Kim JH, Kwok BH, Kapoor TM. Probing cell-division phenotype space and polo-like kinase function using small molecules. Nat Chem Biol. 2006;2:618–626. doi: 10.1038/nchembio826. [DOI] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Borisy GG. The attachment of kinetochores to the pro-metaphase spindle in PtK1 cells. Recovery from low temperature treatment. Chromosoma. 1981;82:693–716. doi: 10.1007/BF00285776. [DOI] [PubMed] [Google Scholar]
- Rozenblum E, Vahteristo P, Sandberg T, Bergthorsson JT, Syrjakoski K, Weaver D, Haraldsson K, Johannsdottir HK, Vehmanen P, Nigam S, Golberger N, Robbins C, Pak E, Dutra A, Gillander E, Stephan DA, Bailey-Wilson J, Juo SH, Kainu T, Arason A, Barkardottir RB, Nevanlinna H, Borg A, Kallioniemi OP. A genomic map of a 6-Mb region at 13q21-q22 implicated in cancer development: identification and characterization of candidate genes. Hum Genet. 2002;110:111–121. doi: 10.1007/s00439-001-0646-6. [DOI] [PubMed] [Google Scholar]
- Santamaria A, Neef R, Eberspacher U, Eis K, Husemann M, Mumberg D, Prechtl S, Schulze V, Siemeister G, Wortmann L, Barr FA, Nigg EA. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell. 2007;18:4024–4036. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci U S A. 1995;92:4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki A, Coppinger JA, Du H, Jang CY, Yates JR, 3rd, Fang G. Plk1- and beta-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol. 2008;181:65–78. doi: 10.1083/jcb.200712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Zhou H, White RA. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14:2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- Stenoien DL, Sen S, Mancini MA, Brinkley BR. Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle. Cell Motil Cytoskelet. 2003;55:134–146. doi: 10.1002/cm.10120. [DOI] [PubMed] [Google Scholar]
- Sumara I, Gimenez-Abian JF, Gerlich D, Hirota T, Kraft C, de la Torre C, Ellenberg J, Peters JM. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol. 2004;14:1712–1722. doi: 10.1016/j.cub.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR, 3rd, Jackson PK. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci U S A. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–222. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- van Vugt MA, van de Weerdt BC, Vader G, Janssen H, Calafat J, Klompmaker R, Wolthuis RM, Medema RH. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J Biol Chem. 2004;279:36841–36854. doi: 10.1074/jbc.M313681200. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci U S A. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


