Abstract
Background: Vitamin D insufficiency is common in industrialized and developing nations. Recent studies have shown that vitamin D insufficiency is associated with a higher risk of active tuberculosis. Laboratory studies provided a mechanism for this link on the basis of findings that vitamin D metabolites regulate the expression of cathelicidin (LL-37), which is an endogenous antimicrobial peptide with activity against Mycobacterium tuberculosis. Little information is available on the clinical relation between vitamin D, LL-37 concentrations, and disease severity in patients with tuberculosis.
Objective: The primary objective of the study was to evaluate the relation between vitamin D nutriture, serum LL-37 concentrations, and tuberculosis by using samples stored in the Tuberculosis Trials Consortium serum repository.
Design: We measured 25-hydroxyvitamin D [25(OH)D] and LL-37 concentrations in 95 serum specimens from patients with culture-confirmed pulmonary tuberculosis and correlated these concentrations to clinical and demographic variables.
Results: The prevalence of vitamin D insufficiency [serum 25(OH)D concentration lt 30 ng/mL] in patients with active tuberculosis was 86% (n = 95) with a mean baseline serum 25(OH)D concentration of 20.4 ng/mL. Factors associated with vitamin D insufficiency were black race and indoor lifestyle. The mean ( plusmn SD) baseline LL-37 concentration was 49.5 plusmn 23.8 ng/mL. Higher LL-37 concentrations correlated with acid fast bacilli sputum smear positivity and weight gt 10% below ideal body weight. Serum vitamin D status of the study subjects did not correlate with serum LL-37 concentrations.
Conclusion: More prospectively designed studies are needed to evaluate the clinical implications of vitamin D insufficiency in patients with tuberculosis and the utility of circulating LL-37 as a potential biomarker in patients with active tuberculosis disease. The parent trial was registered at clinicaltrials.gov as NCT00023335.
INTRODUCTION
Vitamin D insufficiency is common in both industrialized and developing nations (1). Recent discoveries of novel immunoregulatory functions of vitamin D lend new complexity to this problem in public health and nutrition. Epidemiologic studies suggest that a poor host vitamin D status is associated with an increased susceptibility to tuberculosis (2–4). Laboratory studies support this link with the recent observation that vitamin D metabolites regulate the expression of cathelicidin (LL-37), which is an endogenous antimicrobial peptide with activity against Mycobacterium tuberculosis in cultured macrophages (5, 6). LL-37, an important effector molecule of innate immunity, is produced by circulating immune cells, bone marrow progenitor cells, and epithelial surfaces of immunologic barrier sites, such as the respiratory, gastrointestinal, and genitourinary epithelium, in response to infectious processes (7)
The initial preclinical investigations in vitamin D immunology and tuberculosis pathogenesis have lead to a limited number of clinical studies that attempted to evaluate the therapeutic potential of vitamin D as an adjunctive therapy in tuberculosis (8, 9). A review of vitamin D supplementation studies in active tuberculosis showed conflicting results (10). Therefore, the therapeutic implications of using vitamin D therapy to affect clinical outcomes in patients with tuberculosis remain poorly understood. A study of subjects with sepsis syndrome in the intensive care unit (ICU) suggested that vitamin D status, circulating concentrations of the antimicrobial peptide LL-37, and severity of clinical disease may be interrelated (11). However, little information is available about the clinical relation between vitamin D, antimicrobial peptide concentrations, and disease severity in patients with tuberculosis. In addition, recent data indicates that a potential clinical relation between serum concentrations of human cationic antimicrobial protein 18 [HCAP18 (a precursor of LL-37)] and vitamin D metabolites may be predictive of infectious-disease related mortality in hemodialysis patients (12). Thus, a better understanding of the clinical associations between vitamin D status and aspects of the innate immune response may prove valuable in a variety of disease states, including tuberculosis.
In the current study, we sought to delineate the relation between vitamin D nutriture, blood concentrations of LL-37, which is the antimicrobial peptide that may be regulated by a vitamin D–dependent mechanism, and active tuberculosis disease in human subjects. In our study, we used the Tuberculosis Trials Consortium (TBTC) serum repository to determine the prevalence of vitamin D insufficiency in patients who were screened for participation in a large multicenter randomized controlled trial in the treatment of tuberculosis (13) and assessed the relation between vitamin D status and serum LL-37 in this cohort. We measured serum 25-hydroxyvitamin D [25(OH)D] and circulating LL-37 concentrations in serum specimens banked in the repository and correlated these measurements to known clinical and demographic variables of the study cohort to determine whether a relation exists between 25(OH)D, circulating LL-37, and clinical measurements in patients with active tuberculosis.
SUBJECTS AND METHODS
Subjects and study design
The TBTC/US Public Health Service study 22 was a multicenter, randomized, open-label phase III clinical trial that compared the efficacy and safety of rifampin- and rifapentine-based regimens during the final 4 mo of a standard 6-mo antituberculosis therapy. Trial methods, details of informed-consent procedures and institutional review board approval have been reported elsewhere (13). Briefly, study subjects were identified at time of diagnosis (preinduction time point), but eligibility for participation in the trial depended on completion of a 2-mo induction-phase regimen. Subject enrollment continued between April 1995 and November 1998 throughout the United States and Canada. A subgroup of subjects identified at the time of diagnosis gave informed consent to enroll in a pilot serum banking project (project 157).
Project 157 enrolled a total of 139 patients whose serum was stored at project entry (ie, at the start of tuberculosis-therapy induction). A total of 101 of 139 subjects also had serum stored at 1 mo postinduction, and 37 subjects had serum stored at 2 mo postinduction. At entry into project 157, information was collected on patient demographics and other characteristics. Of 139 subjects who participated in the serum banking project 157, 38 subjects were enrolled and randomly assigned into study 22 at 2 mo postinduction of tuberculosis therapy. Reasons for nonenrollment of the remaining subjects in study 22 were noncompliance, patient preference, inconclusive microbiologic diagnosis of pulmonary tuberculosis, and failure to meet other study inclusion criteria specified elsewhere (13). Once enrolled, subjects in study 22 were followed for the duration of tuberculosis treatment (6 mo) and for 24 mo of follow-up after completion of tuberculosis therapy. The primary endpoints for study 22 were treatment failure (evidence of recurrent tuberculosis disease during treatment), death, or relapse after treatment. The primary endpoint of project 157 was to establish the feasibility of serum banking for a cohort of tuberculosis patients associated with a large clinical trial.
The current study focused on subjects who participated in project 157, whether or not they were included in study 22. This cohort of serum samples represented adult subjects who presented with signs and symptoms that were suggestive of tuberculosis, were started on a standard 4-drug therapy for tuberculosis, and whose diagnosis of tuberculosis disease was confirmed by a positive sputum culture for Mycobacterium tuberculosis. In addition, data from subjects with recorded clinical outcomes (ie, the subgroup enrolled in study 22 at 2 mo of tuberculosis therapy) were used to assess the relation between vitamin D status, LL-37 concentrations, and outcomes of tuberculosis treatment. Outcomes defined as adverse by the parent study (study 22), such as treatment failure, death, or relapse, were included in this analysis. Recent studies (13, 14) also validated the failure to convert 8-wk sputum cultures to M. tuberculosis negative as an important predictor of clinical outcomes and the propensity for relapse after tuberculosis treatment. Therefore, continued sputum-culture positivity at 8 wk of tuberculosis treatment was considered an adverse outcome for the current analysis. Study 22 was approved and overseen by institutional review boards at the Centers for Disease Control and Prevention and at every clinical site. All participants gave informed consent (13). The current retrospective research study was evaluated and deemed exempt from further review by the Emory University institutional review board (Atlanta, GA).
Study specimens
Stored serum specimens from the study 22–project 157 serum repository were obtained, and measurements of serum 25(OH)D and LL-37 concentrations were performed. Of the 139 subjects who participated in project 157, 95 subjects met the inclusion criteria for the current study. Inclusion criteria were as follows: 1) culture confirmed active pulmonary tuberculosis disease, 2) availability of ≥500 μL serum specimen stored in the serum repository, and 3) for subjects with stored serum samples at the 1 and 2 mo time points, the availability of a baseline or preinduction specimen.
Laboratory analyses
Serum samples were stored at −80°C before analysis. Serum concentrations of 25(OH)D and LL-37 were assessed with specific enzyme-linked immunosorbent assay (ELISA) kits (IDS Ltd, Fountain Hills, AZ, and Hycult Biotechnology, Uden, Netherlands, respectively). Protocols for each assay were followed per the manufacturer product manuals. LL-37 measurements were conducted in duplicate after the initial thaw of the serum samples, and the calculated mean of the measurements was used in the analyses. The intraassay CV for LL-37 measurements was ≤12%. Samples for 25(OH)D were tested in single determinations with the second thaw of the serum samples. The intra- and interassay CVs for the 25(OH)D measurements were <7% and <9%, respectively. Our laboratory is site 606 for the vitamin D external quality assessment scheme and was tested proficient for the past 3 quarters for the measurement of 25(OH)D. Laboratory personnel were blinded to all clinical information associated with the serum samples.
Statistical analyses
Data management and statistical analyses were performed with SAS software (version 9.1; SAS Institute Inc, Cary, NC). Continuous variables [eg, serum 25(OH)D and LL-37 concentrations) were summarized with standard descriptive statistics (means, SDs, and SEs). Mean baseline concentrations of serum 25(OH)D and LL-37 for concentrations of categorical prognostic factors were compared by using Wilcoxon's rank sum test or one way analysis of variance, as appropriate; the 25(OH)D and LL-37 mean concentrations were correlated to continuous prognostic factors by using Pearson's r correlation. Multiple linear regression was used to estimate the association between 25(OH)D and LL-37 mean concentrations with baseline characteristics (2 separate analyses).
We calculated that multiple regression for this study required a sample size of ≥59 subjects to detect a significant effect with an 80% power and α = 0.05. The association of each of the categorical prognostic factors with vitamin D insufficiency (<30 ng vitamin D /mL) and vitamin D deficiency (<15 ng vitamin D/mL) (1) was evaluated by using a chi-square test or Fisher's exact test (if cell counts were ≤5). Odds ratios with 95% CIs were calculated to measure the degree of association. Factors significant to P ≤ 0.20 in the univariate analyses as well as factors with a plausible biological or epidemiologic association to the outcome of interest were included in multivariate logistic regression analyses. P ≤0.05 was considered statistically significant.
RESULTS
Subject characteristics
Serum samples from 95 patients with culture-confirmed pulmonary tuberculosis were included in analyses. Seventy-two (76%) of the 95 patients from whom serum samples were obtained were men, 24 (25%) patients were black, and 23 (24%) patients were Hispanic. The mean age of the study population was 42.7 ± 15.5 y (range: 18–77 y). Baseline demographic, social, and clinical characteristics of patients are presented in Table 1. Alcohol consumption and unemployment were the most frequently cited socioeconomic or epidemiologic risk factors for active tuberculosis disease (41.1% and 35.1% of subjects, respectively). The highest proportion of subjects were enrolled during the spring (34.7% of subjects), and the lowest proportion of subjects were enrolled during the winter (15.8% of subjects).
TABLE 1.
Baseline demographic characteristics of 95 subjects with active pulmonary tuberculosis1
| Demographic characteristic | Values |
| Age (y) | 42.7 ± 15.52 |
| Sex [n (%)] | |
| M | 72 (75.8) |
| F | 23 (24.2) |
| Race [n (%)] | |
| White | 22 (23.2) |
| Black | 24 (25.3) |
| Hispanic | 23 (24.2) |
| Asian or Pacific Islander | 20 (21.1) |
| American Indian or Alaskan Native | 6 (6.3) |
| Season enrolled [n (%)] | |
| Winter | 15 (15.8) |
| Spring | 33 (34.7) |
| Summer | 25 (26.3) |
| Fall | 22 (23.2) |
| Social characteristics and risk factors for tuberculosis [n (%)] | |
| Homeless or living in a shelter | 17 (18.1) |
| Unemployed >1 y | 33 (35.1) |
| Illicit drug use | 21 (22.3) |
| Use of alcohol >1 drink/d | 39 (41.1) |
| Incarcerated >1 mo | 7 (7.5) |
| Close contact with active tuberculosis case | 26 (27.7) |
| Comorbidies at tuberculosis diagnosis [n (%)] | |
| Diabetes mellitus (type 1 or 2) | 14 (14.7) |
| Malignancy | 1 (1.1) |
| Prior treatment of active tuberculosis | 6 (6.3) |
| Clinical characteristics [n (%)] | |
| ≥10% below ideal body weight at tuberculosis diagnosis | 13 (13.7) |
| Abnormal chest X-ray | 94 (98.9) |
| Cavitary disease on chest X-ray | 37 (39) |
| Bilateral disease on chest X-ray | 45 (47.4) |
| Positive AFB smear | 53 (56.4) |
| Isoniazid resistance (tested n = 94) | 12 (12.8) |
| Rifampin resistance | 1 (1.0) |
| Pyrazinamide resistance (tested n = 60) | 1 (1.6) |
| Ethambutol resistance | 0 (0) |
| Streptomycin resistance (tested n = 75) | 5 (6.7) |
AFB, acid fast bacilli.
Mean ± SD.
Of 95 study subjects, 6 (6.3%) subjects reported a prior episode of treatment of active tuberculosis. Susceptibility testing to first-line antituberculosis drugs showed that 12 (12.8%) of 94 isolates were resistant to isoniazid, 1 (1.0%) of 95 isolates had a resistance to rifampin, 1 (1.6%) of 60 isolates had resistance to pyrazinamide, 5 (6.7%) of 75 isolates were resistant to streptomycin, and all 95 isolates were susceptible to ethambutol. Data on current tuberculosis episode treatment outcomes and follow-up were available for 35 (36.8%) of 95 subjects. Thirty-three (94.3%) of 35 subjects had a sputum smear conversion at 8 wk of treatment, and 30 (85.7%) of 35 subjects had a sputum-culture conversion at 8 wk. In 35 subjects with known outcome and treatment data, 34 (97.1%) subjects completed a full course of tuberculosis treatment, and 29 subjects (85.3%) achieved a clinical cure. The recorded adverse outcomes were as follows: 1) death (n = 1), 2) persistent sputum-culture positivity at 8 wk and relapse (n = 2), 3) persistent sputum-culture positivity at 8 wk with a clinical cure (n = 2), and 4) persistent sputum-culture positivity at 8 wk and subsequent loss to follow-up (n = 1).
Serum 25(OH)D concentrations in study subjects with tuberculosis
The mean baseline serum 25(OH)D concentration for 95 subjects with culture-confirmed pulmonary tuberculosis was 20.4 ng/mL (SD: 9.7 ng/mL; range: 6.9–53.1 ng/mL; n = 95). Mean serum 25(OH)D concentrations at 1 and 2 mo after initiation of tuberculosis therapy were 19.3 ng/mL (SD: 7.2 ng/mL; range: 7.1–39.6 ng/mL; n = 79) and 20.8 ng/mL (SD: 9.2 ng/mL; range: 7.6–49.8 ng/mL; n = 35), respectively. At the initiation of tuberculosis treatment, 82 of 95 patients (86.3%; 95% CI: 77.7%, 92.5%) were vitamin D insufficient [serum 25(OH)D concentration <30 ng/mL) (1) and 33 of 95 patients (34.7%; 95% CI: 25.3%, 45.2%) were vitamin D deficient [serum 25(OH)D concentration <15 ng/mL]. Prospective tuberculosis treatment outcomes were available in a subset of subjects (ie, those subjects randomly assigned in study 22). These subjects had a mean baseline serum 25(OH)D concentration of 20.2 ng/mL (SD: 9.8 ng/mL; range: 6.9–53.1 ng/mL; n = 29). There was no significant change in 25(OH)D concentrations after 1 and 2 mo of antituberculosis therapy [mean 25(OH)D concentrations were 19.2 ng/mL (SD: 6.8 ng/mL; range: 8.5–39.6 ng/mL; n = 27) and 20.8 ng/mL (SD: 9.7 ng/mL; range: 7.6–49.8 ng/mL; n = 26), respectively].
In univariate analyses, baseline mean serum 25(OH)D concentrations were not significantly associated with any variables related to the clinical and socioeconomic demographics or clinical laboratory values of subjects (Tables 2 and 3). We selected associations of 25(OH)D with LL-37, black race, illicit drug use, history of imprisonment, platelet count (103/mm3), and total white blood cell count (103/mm3) as factors for multivariate linear regression. In linear-regression modeling, black race (β = −5.61, P = 0.01) and illicit drug use (β = 6.62, P = 0.004) were significantly associated with 25(OH)D when LL-37 and platelet count were controlled for (Table 4).
TABLE 2.
Univariate analysis of categorical variables associated with mean baseline concentrations of serum 25-hydroxyvitamin D [25(OH)D] and cathelicidin (LL-37) in 95 patients with active pulmonary tuberculosis1
| Categorical variablec | |||||
| 25(OH)D |
LL-37 |
||||
| Demographic characteristic | n (%) | Mean ± SE | P | Mean ± SE | P |
| ng/mL | ng/mL | ||||
| Sex | |||||
| M | 72 (75.8) | 20.9 ± 1.2 | 0.602 | 50.2 ± 2.7 | 0.422 |
| F | 23 (24.2) | 18.9 ± 1.6 | 47.5 ± 5.7 | ||
| Race | |||||
| White | 22 (23.2) | 22.8 ± 2.7 | 0.453 | 48.6 ± 6.6 | 0.523 |
| Black | 24 (25.3) | 17.9 ± 4.4 | 46.6 ± 10.9 | ||
| Hispanic | 23 (24.2) | 20.6 ± 4.4 | 45.9 ± 11.0 | ||
| Asian or Pacific Islander | 20 (21.1) | 21.5 ± 4.5 | 57.7 ± 11.1 | ||
| American Indian or Alaskan Native | 6 (6.3) | 17.6 ± 5.3 | 51.4 ± 13.2 | ||
| Black | 24 (25.3) | 17.9 ± 1.8 | 0.132 | 46.6 ± 4.7 | 0.622 |
| Other | 71 (74.7) | 21.6 ± 1.2 | 50.5 ± 2.9 | ||
| Place of birth | |||||
| Foreign | 35 (36.8) | 20.5 ± 1.4 | 0.522 | 54.5 ± 4.5 | 0.242 |
| United States or Canada | 60 (63.2) | 20.3 ± 1.4 | 46.7 ± 02.8 | ||
| Education | |||||
| Grade ≤12 | 48 (53.9) | 19.2 ± 1.4 | 0.232 | 47.1 ± 3.2 | 0.252 |
| Completed high school or higher | 41 (46.1) | 21.0 ± 1.4 | 52.6 ± 4.2 | ||
| Season | |||||
| Winter | 15 (15.8) | 17.4 ± 3.5 | 0.283 | 52.6 ± 8.7 | 0.833 |
| Spring | 33 (34.7) | 20.1 ± 3.1 | 46.6 ± 7.7 | ||
| Summer | 25 (26.3) | 19.9 ± 3.1 | 51.6 ± 8.0 | ||
| Fall | 22 (23.2) | 23.5 ± 1.6 | 49.5 ± 3.2 | ||
| Winter and spring | 48 (50.5) | 19.3 ± 1.3 | 0.252 | 48.5 ± 3.1 | 0.952 |
| Summer and fall | 47 (49.5) | 21.6 ± 1.5 | 50.6 ± 3.8 | ||
| Social characteristics and risk factors for tuberculosis (≤5 y before current episode of tuberculosis) | |||||
| Homeless or living in a shelter or SRO hotel >6 mo | 17 (18.1) | 22.3 ± 2.6 | 0.292 | 49.9 ± 5.6 | 0.832 |
| Unemployed >1 y | 33 (35.1) | 20.4 ± 1.6 | 0.912 | 48.1 ± 4.4 | 0.712 |
| Illicit drug use | 21 (22.3) | 23.8 ± 2.7 | 0.122 | 42.5 ± 5.7 | 0.092 |
| Use of alcohol >1 drink/d | 39 (41.1) | 21.0 ± 1.7 | 0.722 | 45.9 ± 3.5 | 0.292 |
| Incarcerated >1 mo | 7 (7.5) | 25.7 ± 4.5 | 0.172 | 36.6 ± 7.9 | 0.152 |
| Close contact with active tuberculosis case | 26 (27.7) | 20.5 ± 1.2 | 0.282 | 43.8 ± 4.1 | 0.112 |
| Previous tuberculosis diagnosis and treatment | |||||
| Prior course of INH preventive therapy for tuberculosis | 7 (7.4) | 22.8 ± 3.2 | 0.402 | 43.6 ± 10.4 | 0.372 |
| Prior treatment of active tuberculosis | 6 (6.3) | 21.5 ± 4.8 | 0.982 | 46.3 ± 4.6 | 0.962 |
| Clinical information | |||||
| Diabetes mellitus (type 1 or 2) | 14 (14.7) | 17.4 ± 1.8 | 0.282 | 48.6 ± 7.2 | 0.832 |
| Malignancy | 1 (1.1) | 20.0 | 0.812 | 42.8 | 0.872 |
| Abnormal chest X-ray | 94 (98.9) | 20.4 ± 0.9 | 0.952 | 49.6 ± 2.5 | 0.902 |
| Cavities on chest X-ray | 37 (39.0) | 19.7 ± 1.4 | 0.942 | 53.5 ± 4.1 | 0.172 |
| Bilateral disease chest X-ray | 45 (47.4) | 19.3 ± 1.4 | 0.312 | 51.8 ± 3.8 | 0.472 |
| Positive AFB smear | 53 (56.4) | 20.2 ± 1.4 | 0.482 | 54.1 ± 3.4 | 0.022,4 |
SRO, single room occupancy; INH, isoniazid; AFB, acid fast bacilli.
Determined by using Wilcoxon's rank sum test.
Determined by using ANOVA.
P ≤ 0.05.
TABLE 3.
Univariate analysis of continuous variables associated with mean baseline concentrations of serum 25-hydroxyvitamin D [25(OH)D] (ng/mL) and cathelicidin (LL-37) (ng/mL) in 95 patients with active pulmonary tuberculosis by using Pearson's r correlation1
| 25(OH)D |
LL-37 |
||||
| Continuous variables | Mean factor ± SD (range) | Pearson's r | P | Pearson's r | P |
| Age (y)2 | 42.7 ± 15.5 (18–77) | −0.10 | 0.33 | −0.11 | 0.29 |
| Serum LL-37 (ng/mL)2 | 49.5 ± 23.8 (8.2–111.2) | −0.14 | 0.17 | 1.00 | — |
| Creatinine (mg/dL)3 | 0.9 ± 0.7 (0.30–5.0) | −0.08 | 0.42 | 0.07 | 0.52 |
| AST/SGOT (U/L)3 | 32.8 ± 26.8 (7.0–199.0) | −0.01 | 0.92 | 0.07 | 0.53 |
| ALT/SGPT (U/L)4 | 29.5 ± 22.3 (6.0–121.0) | 0.02 | 0.86 | 0.10 | 0.36 |
| Total bilirubin (mg/dL)5 | 2.3 ± 4.6 (0.1–35.0) | −0.10 | 0.35 | −0.09 | 0.42 |
| Alkaline phosphatase (U/L)6 | 109.9 ± 70.0 (49.0–481.0) | −0.04 | 0.74 | 0.237 | 0.037 |
| Hemoglobin (g/dL)3 | 13.3 ± 1.9 (7.9–17.5) | 0.06 | 0.58 | −0.20 | 0.06 |
| Hematocrit (%)3 | 39.0 ± 5.3 (23.0–54.0) | 0.06 | 0.54 | −0.18 | 0.09 |
| Platelets (103/mm3)8 | 323.5 ± 129.4 (65.0–704.0) | −0.20 | 0.06 | 0.327 | 0.0027 |
| WBCs (103/mm3)3 | 8.3 ± 2.6 (2.8–17.2) | −0.14 | 0.18 | 0.237 | 0.037 |
| Lymphocytes (103/mm3)9 | 2.2 ± 4.4 (0.1–38.0) | 0.01 | 0.90 | 0.002 | 0.99 |
AST/SGOT, aspartate transaminase/serum glutamic oxaloacetic transaminase; ALT/SGPT, alanine transaminase/serum glutamic-pyruvic transaminase; WBCs, white blood cells.
Numbers of patients: 295, 393, 483, 592, 687, 890, 991.
P ≤ 0.05.
TABLE 4.
Risk factors associated with mean baseline concentrations of serum 25-hydroxyvitamin D in 95 patients with active pulmonary tuberculosis by using multivariate linear regression1
| Factor | Regression coefficient | SE | P |
| Black race | −5.61 | 2.20 | 0.012 |
| Illicit drug use | 6.62 | 2.26 | 0.0042 |
| Platelets (103/mm3) | −0.01 | 0.01 | 0.07 |
| Serum cathelicidin, LL-37 (ng/mL) | −0.02 | 0.01 | 0.58 |
For the final model, R2 = 0.18, F = 4.49, and P = 0.002.
Statistically significant.
In univariate analysis, risk factors for having vitamin D insufficiency [defined as a serum 25(OH)D concentration <30 ng/mL] included a lower mean serum creatinine concentration (the mean creatinine in subjects with vitamin D insufficiency was 0.87 mg/dL compared with 0.97 mg/dL in subjects without insufficiency; P = 0.02) (Tables 5 and 6). In multivariate analyses, no risk factors were significantly associated with the presence or absence of vitamin D insufficiency (results not shown). In univariate and multivariate analyses, there were no risk factors associated with the presence or absence of vitamin D deficiency [serum 25(OH)D concentration <15 ng/mL] (results not shown). Further evaluation of the relation between serum 25(OH)D concentrations and clinical outcomes was limited because of the low overall observed event rate of adverse outcomes (n = 6) in study subjects who participated in project 157.
TABLE 5.
Univariate analysis of categorical variables associated with baseline vitamin D insufficiency (serum 25-hydroxyvitamin D concentration <30 ng/mL) in 95 patients with active pulmonary tuberculosis1
| Categorical variable |
||||
| Demographic characteristic | Subjects with vitamin D insufficiency (n = 82) | Subjects without vitamin D insufficiency (n = 13) | OR (95% CI) | 2 |
| n (%) | n (%) | |||
| Sex | ||||
| M | 60 (73.2) | 12 (92.3) | 0.23 (0.03, 1.85) | 0.18 |
| F | 22 (26.8) | 1 (7.7) | 1.00 | — |
| Race | ||||
| White | 17 (20.7) | 5 (38.5) | 1.00 | — |
| Black | 22 (26.8) | 2 (15.4) | 3.24 (0.56, 18.76) | 0.23 |
| Hispanic | 22 (26.8) | 1 (7.7) | 6.47 (0.69, 60.68) | 0.10 |
| Asian or Pacific Islander | 15 (18.3) | 5 (38.5) | 0.88 (0.21, 3.65) | 1.00 |
| American Indian or Alaskan Native | 6 (7.3) | 0 (0) | Undefined | 0.55 |
| Black | 22 (26.8) | 2 (15.4) | 2.02 (0.41, 9.83) | 0.51 |
| Other | 60 (73.2) | 11 (84.6) | 1.00 | — |
| Place of birth | ||||
| Foreign | 30 (36.6) | 5 (38.5) | 0.92 (0.28, 3.08) | 1.00 |
| United States or Canada | 52 (63.4) | 8 (61.5) | 1.00 | — |
| Education | ||||
| Grade ≤12 | 44 (55.7) | 4 (40.0) | 1.89 (0.49, 7.21) | 0.50 |
| Completed high school or higher | 35 (44.3) | 6 (60.0) | 1.00 | — |
| Season | ||||
| Winter | 13 (15.9) | 2 (15.4) | 1.03 (0.15, 7.02) | 1.00 |
| Spring | 29 (35.4) | 4 (30.8) | 1.14 (0.23, 5.70) | 1.00 |
| Summer | 21 (25.6) | 4 (30.7) | 0.83 (0.16, 4.19) | 1.00 |
| Fall | 19 (23.1) | 3 (23.1) | 1.00 | — |
| Winter and spring | 42 (51.3) | 6 (46.2) | 1.23 (0.38, 3.96) | 0.74 |
| Summer and fall | 40 (48.7) | 7 (53.8) | 1.00 | — |
| Social characteristics and risk factors for tuberculosis (≤5 y before current episode of tuberculosis) | ||||
| Homeless or living in a shelter or SRO hotel >6 mo | 14 (17.1) | 3/12 (25.0) | 0.62 (0.15, 2.57) | 0.45 |
| Unemployed >1 y | 29/81 (35.8) | 4 (30.8) | 1.25 (0.36, 4.43) | 1.00 |
| Illicit drug use | 17 (20.7) | 4/12 (33.3) | 0.52 (0.14, 1.95) | 0.46 |
| Use of alcohol >1 drink/d | 34 (41.5) | 5 (38.5) | 1.13 (0.34, 3.77) | 1.00 |
| Incarcerated >1 mo | 5 (6.1) | 2/11 (18.2) | 0.29 (0.05, 1.73) | 0.19 |
| Close contact with active tuberculosis case | 24 (29.3) | 2/12 (16.7) | 2.07 (0.42, 10.16) | 0.50 |
| Previous tuberculosis diagnosis and treatment | ||||
| Prior course of INH preventive therapy | 5 (6.1) | 2 (15.4) | 0.36 (0.06, 2.07) | 0.24 |
| Prior treatment of active tuberculosis | 4 (4.9) | 2 (15.4) | 0.28 (0.05, 1.73) | 0.19 |
| Clinical information | ||||
| Diabetes mellitus (type 1 or 2) | 13 (15.9) | 1 (7.7) | 2.26 (0.27, 18.92) | 0.68 |
| Malignancy | 1 (1.2) | 0 (0) | Undefined | 1.00 |
| ≥10% below ideal body weight | 11 (13.4) | 2 (15.4) | 0.85 (0.17, 4.37) | 1.00 |
| Abnormal chest X-ray | 81 (98.8) | 13 (100.0) | Undefined | 1.00 |
| Cavities on chest X-ray | 34 (41.5) | 3 (23.1) | 2.36 (0.60, 9.23) | 0.24 |
| Bilateral disease chest X-ray | 40 (48.8) | 5 (38.5) | 1.52 (0. 46, 5.05) | 0.56 |
| Positive AFB smear | 46/81 (56.8) | 7 (53.9) | 1.13 (0.35, 3.65) | 0.84 |
OR, odds ratio; SRO, single room occupancy; INH, isoniazid; AFB, acid fast bacilli.
Determined by using Fisher's exact test.
TABLE 6.
Univariate analysis of continuous variables associated with baseline vitamin D insufficiency (serum 25-hydroxyvitamin D concentration <30 ng/mL) in 95 patients with active pulmonary tuberculosis1
| Subjects with vitamin D insufficiency (n = 82) |
Subjects without vitamin D insufficiency (n = 13) |
||||
| Continuous variables | n | Mean factor ± SE | n | Mean factor ± SE | p2 |
| Age (y) | 82 | 43.1 ± 1.8 | 13 | 40.2 ± 3.0 | 0.65 |
| Serum LL-37 (ng/mL) | 82 | 50.1 ± 5.6 | 13 | 45.9 ± 6.5 | 0.52 |
| Creatinine (mg/dL) | 81 | 0.87 ± 0.1 | 12 | 0.97 ± 0.1 | 0.023 |
| AST/SGOT (U/L) | 81 | 33.0 ± 3.1 | 12 | 31.4 ± 4.6 | 0.66 |
| ALT/SGPT (U/L) | 72 | 28.5 ± 2.6 | 11 | 36.5 ± 6.7 | 0.19 |
| Total bilirubin (mg/dL) | 80 | 2.4 ± 0.5 | 12 | 1.5 ± 1.0 | 0.51 |
| Alkaline phosphatase (U/L) | 76 | 111.9 ± 7.9 | 11 | 95.9 ± 15.6 | 0.34 |
| Hemoglobin (g/dL) | 82 | 13.2 ± 0.2 | 11 | 13.5 ± 0.6 | 0.51 |
| Hematocrit (%) | 82 | 38.8 ± 0.6 | 11 | 40.2 ± 1.8 | 0.26 |
| Platelets (103/mm3) | 79 | 330.1 ± 14.6 | 11 | 276.4 ± 35.6 | 0.18 |
| WBCs (103/mm3) | 82 | 8.3 ± 0.3 | 11 | 8.2 ± 0.7 | 0.87 |
| Lymphocytes (103/mm3) | 80 | 2.2 ± 0.5 | 11 | 1.9 ± 0.2 | 0.11 |
AST/SGOT, aspartate transaminase/serum glutamic oxaloacetic transaminase; ALT/SGPT, alanine transaminase/serum glutamic-pyruvic transaminase; WBCs, white blood cells.
Determined by using Wilcoxon's rank sum test.
P ≤ 0.05.
Serum LL-37 concentrations in eligible subjects with tuberculosis
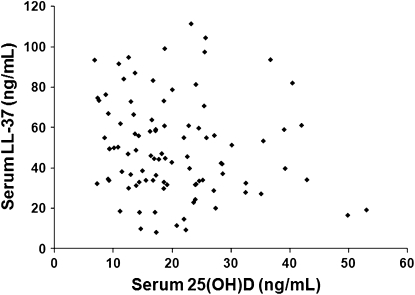
The mean baseline serum LL-37 concentration for patients with culture-confirmed tuberculosis in the project 157 cohort was 49.5 ng/mL (SD: 23.8 ng/mL; range: 8.2–111.2 ng/mL; n = 95). Follow-up serum LL-37 concentrations subjects with stored samples at 1 and 2 mo of tuberculosis treatment were decreased compared with baseline, with means of 46.0 ng/mL (SD: 27.5 ng/mL; range: 5.1–116.4 ng/mL; n = 79), and 42.1 ng/mL (SD: 23.4 ng/mL; range: 12.6–95.2 ng/mL; n = 35). We did not observe any significant relation between baseline serum 25(OH)D and LL-37 concentrations (Pearson's r = −0.14, P = 0.17) (Figure 1). No further correlation between vitamin D status and LL-37 concentrations was identified after adjustment for covariates identified during statistical analyses.
FIGURE 1.
Serum 25-hydroxyvitamin D [25(OH)D] and cathelicidin (LL-37) concentrations in 95 subjects with active pulmonary tuberculosis. No significant correlation between serum 25(OH)D and systemic LL-37 concentrations was observed as measured in stored serum samples from patients with active pulmonary tuberculosis. The analysis was not changed after adjustment for differences in race and other baseline and clinical characteristics (R2 = −0.14, P = 0.17).
Subjects whose follow-up outcome was completed, (ie, the subgroup enrolled in study 22 including 29 study subjects) had a mean (±SD) baseline LL-37 concentration of 50.8 ± 25.3 ng/mL. Follow-up mean LL-37 concentrations after 1 and 2 mo of treatment were not significantly different from baseline [mean ± SD: 41.3 ± 25.4 ng/mL (range: 12.0–101.0 ng/mL; n = 27) and 40.9 ± 23.6 ng/mL (range: 12.6–95.2 ng/mL; n = 26), respectively].
In univariate analyses in all study subjects with baseline samples (n = 95), significantly higher baseline serum concentrations of LL-37 were detected in study subjects with a baseline positive acid fast bacilli (AFB) smear of sputum (mean ± SE: 54.1 ± 3.4 ng/mL; n = 53) than in AFB smear-negative subjects (mean ± SE: 43.5 ± 3.4 ng/mL; n = 41) (P = 0.02; Table 2). Higher LL-37 concentrations were also significantly correlated with higher serum alkaline phosphatase concentrations (r = 0.23, P = 0.03), a higher total platelet count (r = 0.32, P = 0.002), and a higher total white blood cell count (r = 0.23, P = 0.03) (Table 3). In multivariate linear regression, the mean baseline serum LL-37 concentration was significantly associated with illicit drug use (β = −15.69, P = 0.01), body weight <10% below ideal (β = 16.71, P = 0.02), a positive baseline AFB smear (β = 12.52, P = 0.02), and alkaline phosphatase concentrations (β = 0.09, P = 0.02) when 25(OH)D concentrations and total platelet count were controlled for (Table 7). An analysis of LL-37 as a categorical variable was not performed, as no cutoffs for a normal serum LL-37 concentration have been established in the literature to our knowledge. The relation between LL-37 concentrations and the eventual clinical outcome of tuberculosis treatment was not evaluated because of limited numbers of subjects in the subgroup who experienced adverse outcomes (n = 6).
TABLE 7.
Multivariate analysis of risk factors associated with baseline concentrations of cathelicidin (LL-37) in patients with active pulmonary tuberculosis by using multivariate linear regression1
| Factor | Regression coefficient ± SE | P |
| Illicit drug use | −15.69 ± 6.14 | 0.01 |
| ≥10% below ideal body weight | 16.71 ± 7.25 | 0.02 |
| Positive baseline AFB smear | 12.52 ± 5.25 | 0.02 |
| Alkaline phosphatase (U/L) | 0.09 ± 0.04 | 0.02 |
| Platelets (103/mm3) | 0.03 ± 0.02 | 0.14 |
| Serum 25(OH)D (ng/mL) | 0.10 ± 0.29 | 0.74 |
AFB, acid fast bacilli; 25(OH)D, 25-hydroxyvitamin D. For the final model, R2 = 0.29, F = 4.97, and P < 0.001.
DISCUSSION
We observed a markedly high prevalence of vitamin D insufficiency in our cohort of patients with active pulmonary tuberculosis, with 86% of our study subjects with measured concentrations of serum 25(OH)D <30 ng/mL. Other important findings in this analysis included a limited association between vitamin D concentrations and other extrinsic factors, such as skin pigmentation and season of enrollment, which have been shown to significantly affect vitamin D synthesis and metabolism in other patient groups (1, 15–17). These findings echo the observations of Sita-Lumsden et al (18) who showed that tuberculosis patients have a higher prevalence of vitamin D insufficiency than do healthy household contacts and also experience diminished seasonal variation in vitamin D status and a lack of correlation between vitamin D concentrations and skin pigmentation, which suggests that additional confounding factors may affect the handling of vitamin D in active tuberculosis.
Another important finding of this study was the lack of clear correlation between systemic concentrations of 25(OH)D and LL-37 (ie, the antimicrobial peptide regulated by vitamin D metabolites) (19). These findings are in contrast to our previous observations in septic and nonseptic ICU patients, in which we were able to demonstrate a correlation between vitamin D status and systemic concentrations of antimicrobial peptides (11). These conflicting results suggest that differences in underlying disease states may alter the potential relation between blood vitamin D concentrations and concentrations of LL-37. The regulatory functions of vitamin D within the innate immune system appear to involve localized up-regulation of antimicrobial peptide expression through the direct stimulation of immune cells by circulating vitamin D metabolites and co-stimulation of Toll-like receptor by the pathogen-derived ligand (19). A recent study of healthy volunteers who received vitamin D supplementation showed that HCAP18 (the precursor to LL-37) expression in primary ex vivo cultures of human monocytes correlated to serum 25(OH)D concentrations, whereas systemic concentrations of HCAP18 did not correlate with serum vitamin D concentrations (20). Therefore, immunoregulatory consequences of vitamin D deficiency may not be readily apparent at the systemic concentration. More studies are needed to look at the interaction of vitamin D status and localized LL-37 responses within immune cells and at localized immunologic barrier sites such as the alveolar epithelium, gut mucosa, and intact skin.
Early epidemiologic studies showed that the presence of vitamin D deficiency may correlate with increased disease severity in tuberculosis patients (21); however, little contemporary information is available regarding the relation between vitamin D status, clinical indicators of advanced disease, and outcomes of tuberculosis treatment. Although vitamin D concentrations were low in the majority of tuberculosis patients in the current study, we observed no association between the degree of vitamin D insufficiency and disease severity by clinical, radiographic, or microbiological assessments. In addition, the presence of vitamin D deficiency or insufficiency was not predictive of poor tuberculosis treatment outcomes in our cohort of tuberculosis patients.
However, the association between systemic LL-37 concentrations and clinically significant variables of disease severity and immune activation in tuberculosis patients (ie, AFB smear positivity, presence of malnutrition, and total cell counts) suggest that serum LL-37 may serve as a marker of systemic immune activation and the inflammatory milieu of the host. Cells of the immune system, such as neutrophils and monocytes, are known sources of LL-37 (7); thus, elevations in total cell counts in the setting of infection may lead to higher circulating concentrations of LL-37, as suggested by our study. The positive correlation between serum alkaline phosphatase, platelet counts, and circulating LL-37 is less clear outside of the supposition that all 3 indicators may be markers of inflammatory activation (22). An important aspect of this study is its further characterization of the range of serum LL-37 concentrations in various disease states. There is a paucity of information on this topic in the literature, and our study is unique in terms of its use of a commercial assay for LL-37, which is the predominant extracellular form of the antimicrobial peptide. Previous determinations of HCAP18 (the LL-37 precursor molecule) concentrations in healthy patients by ELISA reported an average circulating concentration of 1180 mg/mL (n = 58) (23), which is a significantly higher range of values than the LL-37 concentrations (mean ± SD: 49.5 ± 23.8 ng/mL; range 8.2–111.2 ng/mL; n = 95) observed in the patients with tuberculosis in the current study. Our previous study of LL-37 concentrations in septic and nonseptic patients, which used the same ELISA methodology presented in the current study, may offer a better comparison because of the limited available information that correlates HCAP18 and LL-37 concentrations in a human population. We previously reported mean LL-37 concentrations of 13.7 ng/mL (SD: 2.12 ng/mL; n = 24) and 10.6 ng/mL (SD: 1.38 ng/mL; n = 25) for 2 groups of critically ill patients and a mean LL-37 concentration of 27.21 ng/mL (SD: 4.87 ng/mL; n = 21; ) in healthy outpatient control subjects (11). Although the range of values generated in the current study appears consistent with the results of our prior studies, it is remarkable that our population of tuberculosis patients was shown to have higher LL-37 concentrations (49.5 ± 23.8 ng/mL) than either the ICU patients or the normal control subjects reported in our prior studies. These findings appear consistent with our conclusions that LL-37 concentrations may serve as a marker of heightened immune activation in a chronic infectious process such as tuberculosis and, in the context of our previous studies, may also be reflective of the recently described phenomenon of autoimmunosuppression that can accompany acute sepsis syndrome (24, 25).
A potential limitation of the current study is its retrospective design, which left us unable to collect and examine additional samples from the study subjects. Further evaluation of LL-37 in sputum or bronchoscopy specimens would complement this analysis by allowing for the study of localized immune responses and their relation to circulating concentrations of vitamin D metabolites. Another limitation stemming from the retrospective design of the study is the inability to solicit additional information regarding sunlight exposure and dietary intakes of vitamin D–containing foods for subjects who participated in project 157 to further characterize the nutritional and socioeconomic variables that may affect vitamin D status in tuberculosis patients. The small sample size of the current study limited our analysis of vitamin D status and LL-37 as potential biomarkers of treatment response and their relation to adverse outcomes after tuberculosis therapy. More research, perhaps conducted in the context of ongoing prospective studies of vitamin D as an adjunctive therapy for tuberculosis, is needed to illustrate the clinical relation between vitamin D repletion and the innate immune response in the setting of active tuberculosis and other infectious diseases.
In conclusion, we observed a high prevalence of vitamin D insufficiency (86%) in patients with active tuberculosis who were undergoing screening for a large TBTC tuberculosis treatment trial. No relation between vitamin D status and systemic serum concentrations of LL-37, which is an antimicrobial peptide synthesized by immune cells in a vitamin D–dependent manner, was observed, although localized or intracellular LL-37 concentrations were not analyzed. Higher LL-37 concentrations did correspond to indicators of an exaggerated immune activation and increased disease burden in subjects with active tuberculosis. The results of this study provide an important background to inform additional larger-scale, prospectively designed studies that evaluate the relation between vitamin D status, localized and systemic innate immune responses, and the clinical management of tuberculosis and other infectious diseases.
Acknowledgments
We acknowledge and thank the Centers for Disease Control TBTC for use of the TBTC project 157 serum repository and database for these analyses.
The authors’ responsibilities were as follows—AVY, VT, and SMR: design of the experiments; AVY and MK: collection of experimental data; EVK and AVY: statistical analyses; AVY and VT: drafting of the manuscript; and EVK, VT, SMR, HMB, and TRZ: review and revision of the manuscript. None of the authors reported a conflict of interest.
REFERENCES
- 1.Holick MF.Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 2.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 2008;37:113–9 [DOI] [PubMed] [Google Scholar]
- 3.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 2007;176:208–13 [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. [see comment]Lancet 2000;355:618–21 [DOI] [PubMed] [Google Scholar]
- 5.Wang T-T, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004;173:2909–12 (Published erratum appears in J Immunol 2004;173:following 6489.). [DOI] [PubMed] [Google Scholar]
- 6.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005;19:1067–77 [DOI] [PubMed] [Google Scholar]
- 7.Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect 2002;4:361–72 [DOI] [PubMed] [Google Scholar]
- 8.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5 [PubMed] [Google Scholar]
- 9.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial Am J Respir Crit Care Med (Epub ahead of print 29 January 2009. [DOI] [PubMed] [Google Scholar]
- 10.Vitamin D.for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocrine Pract (Epub ahead of print 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 2009;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gombart AF, Bhan I, Borregaard N, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis 2009;48:418–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002;360:528–34 [DOI] [PubMed] [Google Scholar]
- 14.Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006;174:331–8 [DOI] [PubMed] [Google Scholar]
- 15.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982;1:74–6 [DOI] [PubMed] [Google Scholar]
- 16.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–9 [DOI] [PubMed] [Google Scholar]
- 17.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69:374–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sita-Lumsden A, Lapthorn G, Swaminathan R, Milburn HJ. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax 2007;62:1003–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response [see comment] Science 2006;311:1770–3 [DOI] [PubMed] [Google Scholar]
- 20.Adams JS, Ren S, Liu PT, et al. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 2009;182:4289–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange JM, Davies PD, Brown RC, Woodhead JS, Kardjito T. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle 1985;66:187–91 [DOI] [PubMed] [Google Scholar]
- 22.Germolec DR, Frawley RP, Evans E. Markers of inflammation. Methods Mol Biol 2010;598:53–73 [DOI] [PubMed] [Google Scholar]
- 23.Sorensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for HCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods 1997;206:53–9 [DOI] [PubMed] [Google Scholar]
- 24.Bone RC.Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 1996;24:1125–8 [DOI] [PubMed] [Google Scholar]
- 25.Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med 2008;29:617–25, viii [DOI] [PMC free article] [PubMed] [Google Scholar]