Abstract
Background: For children and adolescents with cystic fibrosis (CF) and pancreatic insufficiency, the efficacy of routine vitamin K supplementation to normalize vitamin K status remains unclear.
Objective: This study examined and determined predictors of vitamin K status in subjects aged 8–25 y with CF and pancreatic insufficiency taking various vitamin K supplements.
Design: In 97 subjects, serum 25-hydroxyvitamin D [25(OH)D], dietary intake, vitamin K supplement intake, and vitamin K statusmdashdetermined on the basis of the percentage of serum undercarboxylated osteocalcin (%ucOC; sufficient: lt 20%) and plasma proteins induced by vitamin K absence–factor II (PIVKA-II; n = 60; sufficient: le 2 mug/L)mdashwere assessed. The vitamin K supplementation groups were as follows: lt 150 mug/d (low; multivitamins or no supplement), 150–999 mug/d (middle; CF-specific vitamins), and ge 1000 mug/d (high; mephyton). %ucOC values were compared with 140 healthy subjects aged 6–21 y.
Results: In subjects with CF, the median (range) %ucOC was 35% (3%, 76%) and the median (range) for PIVKA-II was 2 (0, 42) mu g/L. Subjects with CF had a higher %ucOC with low [45% (10%, 76%)] and medium [41% (3%, 66%)] supplement intakes but not with a high supplement intake [16% (4%, 72%)] compared with healthy subjects [23% (0%, 43%); both P lt 0.05]. Supplementation group for males and females and 25(OH)D and age for males were significant predictors of vitamin K status.
Conclusions: Vitamin K status was often suboptimal despite routine supplementation. Only subjects taking high-dose vitamin K achieved a status similar to healthy subjects, and only the vitamin K supplementation dose predicted vitamin K status for males and females. These data suggest that higher doses of vitamin K are required.
See corresponding editorial on page 469.
INTRODUCTION
Vitamin K is important not only for coagulation, but also may be involved in the regulation of calcification, energy metabolism, and inflammation (1). In unsupplemented patients with cystic fibrosis (CF) and pancreatic insufficiency (PI), vitamin K deficiency is expected (2, 3), which likely reflects chronic fat malabsorption and possibly inadequate dietary intake. As late as 1992, vitamin K supplementation was only recommended for patients with CF and PI on antibiotics or with liver disease (4). Current CF care recommendations recognizing vitamin K deficiency in the absence of liver disease and the smaller or absent contribution of vitamin K by bacterial flora than previously postulated (5) support routine vitamin K supplementation, ranging from 300 to 500 μg/d, for all patients with CF and PI (6). However, the efficacy of this level of supplementation to normalize vitamin K status remains unclear. Although recent studies have found suboptimal vitamin K status with supplementation (7–12), interpretation is difficult due to small sample sizes, lack of a healthy comparison group, and inability to differentiate between a high- and low-dose vitamin K supplemental intake pattern.
The aims of this study were to examine the vitamin K status of children and young adults with CF and PI taking various vitamin K–containing supplements and to determine whether age, sex, growth status, body composition, genotype, Tanner stage, pulmonary function, serum 25-hydroxyvitamin D [25(OH)D], nutritional status, or vitamin K supplementation predict vitamin K status. It was hypothesized that 1) despite common use of CF-specific vitamin preparations, vitamin K status would be suboptimal in most subjects and 2) only those taking high doses would achieve a vitamin K status similar to healthy subjects.
SUBJECTS AND METHODS
Subjects with CF, PI, and mild-to-moderate lung disease aged 8–25 y were recruited from 3 Pennsylvania CF Centers: Children's Hospital of Philadelphia, Hospital of the University of Pennsylvania, and Milton S. Hershey Medical Center (November 2000 through March 2002). The subject's home CF Center diagnosed CF by clinical signs and duplicate quantitative pilocarpine iontophoresis sweat tests with chloride values >60 mEq/L and/or genotype analysis consistent with a diagnosis of CF. CF Centers documented PI based on a 72-h stool and diet collection with fecal fat analysis ≤90% absorption and/or a stool trypsin concentration <80 μg/g. Exclusion criteria included a forced expiratory volume in 1 s (FEV1) <40% predicted or major medical illnesses other than CF known to affect growth or nutritional status (ie, significant cardiac, renal, or gastrointestinal disease).
A contemporary comparison group of healthy subjects consisted of a subset of children aged 8–21 y from the Reference Project on Skeletal Development in Children recruited from the CHOP primary care practices and surrounding community (November 2001 through July 2007). Exclusion criteria were any disease, genetic syndrome, or use of medication known to affect growth, nutritional status, bone health, or weight or height above the 97th percentile for age and sex (13). Age, sex, race, and percentage of undercarboxylated osteocalcin (%ucOC) were obtained from healthy subjects.
This protocol was approved by the Institutional Review Boards at the Children's Hospital of Philadelphia, Hospital of the University of Pennsylvania, and Milton S. Hershey Medical Center. Written informed consent was obtained from subjects' aged 18–21 y and from parents or guardians of subjects aged <18 y. Verbal assent was obtained from subjects aged <18 y.
Anthropometric measures, body composition, maturation, pulmonary function, and genotype
Anthropometric measurements were obtained in triplicate according to standardized techniques (14), and the mean was used for analysis. Body mass index (BMI) was calculated (kg/m2) from weight by using a digital scale (Scaletronix, White Plains, NY), and standing height was measured by using a stadiometer (Holtain, Crymych, United Kingdom). Weight, height, and BMI were compared with Centers for Disease Control and Prevention 2000 reference standards to generate age- and sex-specific z scores (13). Total body fat mass, lean body mass, and percentage fat were measured by whole-body dual-energy X-ray absorptiometry (DXA; QDR4500a; Hologic Delphi, Bedford, MA) by using Discovery software version 12.4 (Hologic Delphi), which automatically adjusts the thresholds for subjects with a body weight <40 kg. Pubertal status according to the criteria of Tanner (15) was determined by using a validated self-assessment questionnaire (16). Pulmonary function was assessed by using standard methods for spirometry (17). The predicted percentage FEV1 was calculated by using the equations of Wang et al (18) and Hankinson et al (19). Genotype was obtained from medical records; if unknown, a blood sample was collected for determination (Genzyme Genetics, Pittsburgh, PA) for this study. Subjects were categorized as having DF508 homozygous genotype or other genotypes (DF508 heterozygous, combinations of other mutations).
Biochemistry
All samples were kept frozen at −70°C until assayed. Serum concentrations of total osteocalcin and ucOC were determined for both subjects with CF and PI and healthy subjects by using a hydroxyapatite-binding radioimmunoassay as described by Gundberg et al (20). Undercarboxylated osteocalcin was expressed as the percentage not bound (%ucOC) and normalized to the amount of total osteocalcin in a given sample by using equations previously described (20). The inter- and intraassay CVs were 8.7% and 5.2%, respectively. Proteins induced by vitamin K absence–factor II (PIVKA-II) were determined in citrated plasma by using an enzyme-linked immunosorbent assay (American Bioproducts, Parsippany, NJ). The inter- and intraassay CVs were <15%. Serum concentrations of 25(OH)D were measured by using a radioimmunoassay (DiaSorin, Inc, Stillwater, MN) with a radioiodinated tracer (21) (Hollis Laboratory, which participates in the DEQAS proficiency survey, Mount Pleasant, SC,) and the inter- and intraassay CVs were <10%.
Dietary intake and vitamin K supplementation
Dietary intake was assessed by using 3-d weighed food records. Families were provided with a digital food scale (Sunbeam-Oster, Schaumburg, IL), measuring cups and spoons, and detailed verbal and written instructions stressing the importance of accurate reporting and maintaining usual food patterns. A research-registered dietitian reviewed the diet records and queried families for missing or additional information when necessary. Completed records were analyzed by the Minnesota Nutrition Data System (Minneapolis, MN), and calculated nutrient intakes were compared with age- and sex-specific Dietary Reference Intakes (DRIs), expressed as a percentage of the Recommended Dietary Allowance or adequate intake, as appropriate (22). Energy intake adjusted for height, weight, age, and BMI z score was expressed as a percentage of the estimated energy requirement for active children. In preadolescent children with CF and PI, the DRI for “active” children provides the best estimate of energy requirements (23). Supplemental intake of vitamin K was assessed by a comprehensive daily vitamin and mineral supplemental intake questionnaire in which the subject or guardian reported brand, dose of vitamin K (μg), and number of tablets taken per day or per week.
Statistical analyses
For this study, subjects with CF and healthy subjects were grouped according to vitamin K status based on serum %ucOC as follows: sufficient, <20%; insufficient, 20–50%; and deficient, >50%. For adults, the literature supports <20% as sufficient status (24), and some studies suggest that >50% is indicative of deficiency (25, 26). Subjects with CF were also divided into 2 vitamin K status groups based on plasma PIVKA-II: sufficient, ≤2 ng/mL; and deficient, >2 ng/mL (see package insert) (26). Last, subjects with CF were divided into 3 groups according to reported daily vitamin K supplementation: <150 μg/d (low), 150–999 μg/d (mid), and ≥1000 μg/d (high). Groups correspond to low-dose vitamin K from multivitamin supplements or no supplement daily, CF-specific vitamin preparations daily, and high-dose vitamin K such as mephyton (commonly taken as 5000 μg twice per week, or 1429 μg/d), respectively, and were driven by clinical practice.
All variables were tested for normality, and nonparametric tests were used as appropriate. To determine associations between variables, Pearson correlation coefficients or Spearman rank correlations were performed, as appropriate. Group differences were determined by using a Student's t test or Wilcoxon's rank-sum test for continuous variables and Fisher's exact or chi-square test for categorical variables. For multiple comparisons, analysis of variance or Kruskal-Wallis tests were used. When differences were detected, Bonferroni adjustment for normally distributed variables or Wilcoxon's rank-sum test with Bonferroni correction of the alpha for non-normally distributed variables was used.
Multivariate regression models were constructed for subjects with CF by using a multistage approach to determine whether age, sex, growth status, body composition, genotype, Tanner stage, pulmonary function, 25(OH)D, nutritional status, or vitamin K supplementation predict vitamin K status (%ucOC) in this population. On the basis of the results of preliminary regression models, to account for the effect of sex, multivariate regression models were constructed separately for males and females. Selection of variables for final models was based on statistical significance, maximum R2 values, and distribution of residuals.
All statistical analyses were performed by using STATA 9.0 (Stata Corp, College Station, TX). Results were considered significant at P < 0.05 (unless otherwise indicated), and data are presented as means ± SD (normal distribution) or medians and ranges (skewed distribution).
RESULTS
For subjects with CF, 13 were classified as Tanner stage 1, 20 as stage 2, 13 as stage 3, 26 as stage 4, and 25 as stage 5. Characteristics of subjects with CF are presented per %ucOC (n = 97) group in Table 1 and PIVKA-II (n = 60) groups in Table 2. Overall, subjects with CF had mild pulmonary disease (FEV1 ≥ 70% predicted) and suboptimal growth status as indicated by negative z scores for height, weight, and BMI. Of the 45% of subjects not DF508 homozygous, 32% were DF508 heterozygous and 13% had other genotypes. Age was negatively associated with %ucOC (r = −0.30, P = 0.003) and positively associated with supplementation (r = 0.30, P < 0.01). For subjects with CF, median (range) serum osteocalcin was 22 (3, 85) ng/mL, %ucOC was 35% (3%, 76%), and PIVKA-II was 2 (0, 42) μg/L. In subjects with CF and PI, 27%, 55%, and 19% had a %ucOC value indicating sufficient, insufficient, and deficient, respectively, and 50% had PIVKA-II levels in the deficient range. Daily supplemental vitamin K intake was approximately divided into thirds: low, n = 27 (multivitamins/no supplement); middle, n = 33 (CF-specific vitamin preparations); and high, n = 37 (mephyton). Compared with recommended CF-specific intakes (4, 6), 59% of subjects did not meet ≥120% EER, 84% did not achieve ≥40% of energy from fat, and 39% were below the recommended intake for vitamin K supplementation. In addition, 59% of subjects had a dietary (not supplemented) vitamin K intake below the adequate intake for age and sex (22) of healthy children.
TABLE 1.
Characteristics of subjects with cystic fibrosis and pancreatic insufficiency by vitamin K status presented as the percentage of undercarboxylated osteocalcin (%ucOC)1
| %ucOC group |
||||
| All (n = 97) | Sufficient: <20% (n = 26) | Insufficient: 20–50% (n = 53) | Deficient: >50% (n = 18) | |
| Age (y) | 14.8 ± 4.22 | 16.4 ± 4.1 | 14.4 ± 4.4 | 13.6 ± 4.2 |
| Female sex (%) | 49 | 58 | 51 | 33 |
| DF508 homozygous (%) | 55 | 65 | 51 | 50 |
| Height z score | −0.5 ± 1.0 | −0.4 ± 1.2 | −0.6 ± 0.8 | −0.4 ± 1.0 |
| Weight z score | −0.5 ± 1.0 | −0.6 ± 1.1 | −0.5 ± 1.1 | −0.4 ± 0.6 |
| BMI z score | −0.3 ± 0.9 | −0.4 ± 0.9 | −0.3 ± 1.0 | −0.2 ± 0.6 |
| LBM (kg)3 | 37.6 ± 13.2 | 40.4 ± 12.9 | 35.9 ± 13.6 | 38.5 ± 12.3 |
| FM (kg)3 | 9.8 ± 5.4 | 10.8 ± 5.0 | 9.9 ± 6.0 | 8.2 ± 3.3 |
| Fat (%)3 | 20.4 ± 6.5 | 21.2 ± 6.8 | 20.9 ± 6.5 | 17.4 ± 5.8 |
| FEV1 (% of predicted) | 84 ± 20 | 79 ± 20 | 85 ± 21 | 90 ± 12 |
| Vitamin K status | ||||
| n | 97 | 26 | 53 | 18 |
| Osteocalcin (ng/mL) | 22 (3, 85)4 | 13 (3, 35) | 22 (5, 44)a | 31 (17, 85)c,d |
| %ucOC | 35 (3, 76) | 11 (3, 19) | 36 (21, 50) | 56 (51, 76) |
| PIVKA-II (μg/L)5 | 2 (0, 42) | 2 (0, 13) | 3 (0, 25) | 9 (2, 42)c,d |
| PIVKA-II >2 μg/L (%) | 50 | 17 | 55a | 85c |
| Supplement intake6 | ||||
| n | 97 | 26 | 53 | 18 |
| Vitamin K (μg/d) | 300 (0, 10,000) | 1578 (25, 10,000) | 300 (0, 5300)b | 21 (0, 1729)c |
| Dietary intake | ||||
| n | 69 | 17 | 38 | 14 |
| Energy (kcal) | 2673 (1531, 5293) | 2774 (1767, 5293) | 2471 (1531, 5098) | 2998 (1967, 4850) |
| EER, active (%) | 112 (65, 218) | 108 (64, 169) | 115 (65, 218) | 112 (80, 178) |
| Fat (% of energy) | 33 (23, 44) | 34 (23, 41) | 33 (23, 44) | 31 (23, 42) |
| Vitamin K (μg/d) | 61 (17, 780) | 74 (38, 273) | 60 (17, 780) | 62 (28, 131) |
| Vitamin K (% of AI) | 89 (26, 866) | 97 (37, 455) | 75 (28, 866) | 86 (26, 238) |
| Serum chemistry | ||||
| n | 97 | 26 | 53 | 18 |
| Serum 25(OH)D (ng/mL) | 20 (5, 35) | 24 (8, 35) | 19 (6, 34)a | 21 (5, 32) |
LBM, lean body mass; FM, fat mass; FEV1, forced expiratory volume in 1 s; PIVKA-II, proteins induced by vitamin K absence–factor II; EER, estimated energy requirement; AI, Adequate Intake; 25(OH)D, serum 25-hydroxyvitamin D. Significant differences were assessed by using ANOVA or Kruskal-Wallis tests. When differences were detected, Bonferroni adjustment of normally distributed variables was made, or a Wilcoxon's rank-sum test with Bonferroni correction of the α for nonnormally distributed variables was used: aP < 0.05; bP < 0.005, insufficient compared with sufficient; cP < 0.005, deficient compared with sufficient; dP < 0.005, deficient compared with insufficient.
Mean ± SD (all such values).
n = 95.
Median; range in parentheses (all such values).
n = 60 for All, n = 18 for Sufficient, n = 30 for Insufficient, and n = 12 for Deficient.
On the basis of reported vitamin K supplement intake.
TABLE 2.
Characteristics of subjects with cystic fibrosis and pancreatic insufficiency by vitamin K status presented as plasma PIVKA-II (proteins induced by vitamin K absence–factor II)1
| PIVKA-II group |
|||
| All (n = 60) | Sufficient: ≤2.0 μg/L (n = 30) | Deficient: >2.0 μg/L (n = 30) | |
| Age (y) | 15.4 ± 4.32 | 15.3 ± 4.0 | 15.5 ± 4.6 |
| Female sex (%) | 43 | 47 | 40 |
| DF508 homozygous (%) | 53 | 53 | 53 |
| Height z score | −0.3 ± 0.9 | −0.3 ± 1.0 | −0.3 ± 0.9 |
| Weight z score | −0.4 ± 0.9 | −0.7 ± 0.9 | −0.2 ± 0.8a |
| BMI z score | −0.4 ± 0.8 | −0.6 ± 0.8 | −0.1 ± 0.8a |
| LBM (kg) | 39.8 ± 13.0 | 38.0 ± 11.9 | 41.6 ± 14.1 |
| FM (kg) | 9.8 ± 5.7 | 9.1 ± 3.8 | 10.4 ± 7.1 |
| Fat (%) | 19.3 ± 6.4 | 19.4 ± 5.7 | 19.3 ± 7.1 |
| FEV1 (% of predicted) | 80 ± 19 | 75 ± 18 | 86 ± 18a |
| Vitamin K status | |||
| n | 60 | 30 | 30 |
| Osteocalcin (ng/mL) | 21 (3, 85)3 | 21 (4, 37) | 22 (3, 85) |
| ucOC (%) | 33 (4, 66) | 21 (4, 61) | 46 (12, 66) |
| <20% | 30 | 50 | 10 |
| 20–50% | 50 | 43 | 57 |
| >50% | 20 | 7 | 33a |
| PIVKA-II (μg/L) | 2 (0, 42) | 2 (0, 2) | 5 (2, 42) |
| Supplement intake4 | |||
| n | 60 | 30 | 30 |
| Vitamin K (μg/d) | 300 (0, 10,000) | 864 (0, 10,000) | 163 (0, 5300) |
| Dietary intake | |||
| n | 45 | 21 | 24 |
| Energy (kcal) | 2913 (1531, 5293) | 2965 (1531, 5098) | 2863 (1618, 5293) |
| EER, active (%) | 112 (65, 202) | 124 (65, 202) | 108 (73, 178) |
| Fat (% of energy) | 33 (23, 44) | 33 (23, 42) | 32 (23, 44) |
| Vitamin K (μg/d) | 67 (22, 780) | 74 (27, 780) | 61 (22, 410) |
| Vitamin K (% of AI) | 98 (26, 866) | 105 (37, 866) | 78 (26, 524) |
| Serum chemistry | |||
| n | 60 | 30 | 30 |
| 25(OH)D (ng/mL) | 22 (6, 35) | 23 (12, 35) | 20 (6, 34) |
LBM, lean body mass; FM, fat mass; FEV1, forced expiratory volume in 1 s; ucOC, undercarboxylated osteocalcin; EER, estimated energy requirement; AI, Adequate Intake; 25(OH)D, serum 25-hydroxyvitamin D. Significant differences were assessed by using a Student's t test or Wilcoxon's rank-sum test: aP < 0.05, deficient compared with sufficient.
Mean ± SD (all such values).
Median; range in parentheses (all such values).
On the basis of reported vitamin K supplement intake.
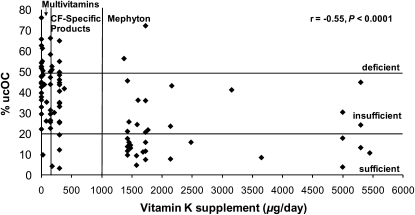
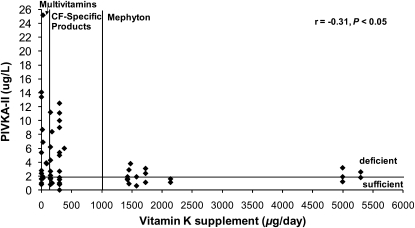
Vitamin K supplementation intake relative to vitamin K status, presented as %ucOC, is shown in Figure 1, and PIVKA-II is shown in Figure 2. Vitamin K supplementation was negatively associated with %ucOC (r = −0.55, P < 0.0001) and PIVKA-II (r = −0.30, P < 0.05). For the low, middle, and high supplemental vitamin K intake groups, supplemental vitamin K intake was 0 (0, 86), 300 (150, 380), and 1729 (1371, 10,000) μg/d, respectively (low compared with high and middle compared with high; both P < 0.001). On the basis of PIVKA-II, 67%, 58%, and 29% of subjects with CF in the low, middle, and high supplemental intake groups, respectively, were vitamin K deficient (low compared with high; P < 0.03). Correspondingly, based on %ucOC, 59%, 70%, and 38% were insufficient (middle compared with high; P < 0.01) and 37%, 18%, and 5% were deficient (low compared with high; P < 0.01). For subjects with CF in the low, middle, and high supplemental intake groups, PIVKA-II was 4 (1, 42), 3 (0, 13), and 2 (1, 4) μg/L, respectively (low compared with high; P < 0.01). There was no association between the supplemental vitamin K intake group and growth, pulmonary status, or dietary vitamin K intake.
FIGURE 1.
Vitamin K status represented as a percentage of undercarboxylated osteocalcin (%ucOC) in serum relative to vitamin K intake from supplements in 96 children and young adults with cystic fibrosis (CF) and pancreatic insufficiency. Vitamin K status is defined as sufficient (<20% ucOC), insufficient (20–50% ucOC), and deficient (>50% ucOC). Vitamin K supplementation groups are as follows: multivitamin supplements or no supplements (<150 μg/d), CF-specific supplements (150–999 μg/d), and mephyton or high-dose vitamin K supplements (>1000 μg/d). Vitamin K supplementation was negatively associated with %ucOC (r = −0.55, P < 0.0001; Spearman's rank correlation coefficient). Data for one subject with a supplemental vitamin K intake of 10,000 μg/d and a %ucOC of 13.4% are not shown.
FIGURE 2.
Vitamin K status represented as serum proteins induced by vitamin K absence–factor II (PIVKA-II) relative to vitamin K intake from supplementation in 58 children and young adults with cystic fibrosis (CF) and pancreatic insufficiency. Vitamin K status is defined as sufficient (PIVKA-II ≤ 2.0 μg/L) and deficient (PIVKA-II > 2.0 μg/L). Vitamin K supplementation groups are as follows: multivitamin supplements or no supplements (<150 μg/d), CF-specific supplements (150–999 μg/d), and mephyton or high-dose vitamin K supplements (>1000 μg/d). Vitamin K supplementation was negatively associated with PIVKA-II (r = −0.30, P < 0.05; Spearman's rank correlation coefficient). Data for 2 subjects are not shown: 1 with a supplemental vitamin K intake of 10,000 μg/d and PIVKA-II of 0.8 μg/L and 1 with an intake of 0 μg/d and a PIVKA-II of 41.6 μg/L.
For healthy subjects (48% female; age: 13.7 ± 4.2 y), vitamin K status based on %ucOC [23% (0%, 43%)] was 37% sufficient, 63% insufficient, and 0% deficient. Overall, subjects with CF had higher %ucOC [35% (3%, 76%)], which indicated poorer vitamin K status compared with healthy subjects (P < 0.0001). Specifically, subjects with CF had higher %ucOC in the low [45% (10%, 76%)] and middle [41% (3%, 66%)] but not in the high [16% (4%, 72%)] supplemental intake groups compared with healthy subjects (both P < 0.05).
Multiple regression models predicting vitamin K status represented as %ucOC in subjects with CF and PI are presented separately for males and females in Table 3. In original models with sexes combined (not shown), age, sex, vitamin K supplementation group, and 25(OH)D were significant predictors of vitamin K status (%ucOC), which together explained 43% of the variance. Girls had a 6.8% lower %ucOC than did boys (P < 0.05). As shown in Table 3, vitamin K supplementation group for both males and females in addition to 25(OH)D and age for males were significant predictors of %ucOC. Collectively, these variables explained 31% and 54% of the variance in males and females, respectively.
TABLE 3.
Multiple regression models predicting vitamin K status represented as the percentage of undercarboxylated osteocalcin in subjects with cystic fibrosis and pancreatic insufficiency1
| Coefficient | SE | t | P value | R2 | |
| % | |||||
| Males (n = 49) | 0.31 | ||||
| Age (y) | −1.0 | 0.5 | −2.1 | 0.045 | |
| Vitamin K supplementation group | −7.9 | 2.7 | −2.9 | 0.006 | |
| 25(OH)D (ng/mL) | −0.9 | 0.3 | −2.8 | 0.007 | |
| Constant | 79.8 | 11.3 | 7.1 | 0.001 | |
| Females (n = 48) | 0.54 | ||||
| Age (y) | −0.8 | 0.5 | −1.6 | 0.110 | |
| Vitamin K supplementation group | −13.5 | 2.3 | −5.8 | 0.001 | |
| 25(OH)D (ng/mL) | −0.5 | 0.3 | −1.9 | 0.066 | |
| Constant | 68.4 | 8.6 | 8.0 | 0.001 |
25(OH)D, serum 25-hydroxyvitamin D. Multivariate regression models were constructed for subjects with cystic fibrosis by using a multistage approach to determine whether age, sex, growth status, body composition, genotype, pulmonary function, 25(OH)D, nutritional status, or vitamin K supplementation predict vitamin K status (based on the percentage of undercarboxylated osteocalcin) in this population. On the basis of results of preliminary regression models, to account for the effect of sex, multivariate regression models were constructed separately for males and females. Selection of variables for final models was based on statistical significance, maximum R2 values, and distribution of residuals.
DISCUSSION
The main findings from this study were that in children and young adults with CF and PI, vitamin K status was often suboptimal despite routine supplementation with CF-specific preparations. Only subjects taking high-dose vitamin K (≥1000 μg/d) achieved a vitamin K status similar to that of healthy subjects. In addition, only vitamin K supplementation dose predicted vitamin K status for both males and females with CF and PI. Collectively, these findings suggest that routine high-dose vitamin K supplementation for this population will be required to ensure optimal response as indicated by %ucOC and PIVKA-II.
Despite advancements in CF treatment and survival over the past few decades, suboptimal growth and nutritional status remain prevalent (27, 28). In the present study, the negative height, weight, and BMI z scores of children and young adults with CF, most of whom did not meet CF-specific dietary intake recommendations, concur with previous research (29, 30). Over time, weight gain and better nutritional status in children with CF are associated with improved lung function and survival (31, 32) and remain the goal of nutritional care. Although recent behavioral intervention findings show improved caloric intake and weight gain in children with CF and document efficacious treatment options (33), future studies addressing how best to incorporate these treatments into the standard of care are needed.
Green leafy vegetables and vegetable oils are major sources of dietary vitamin K1 (phylloquinone) (1) and, surprisingly, very few studies have quantified the dietary vitamin K intake in children and young adults with CF. Using 3-d food records in 18 patients with CF aged 13–35 y, Beker et al (7), in 1997, found that the dietary contribution of vitamin K averaged 145 μg/d, ranging from 0 to 664 μg/d. However, analysis was completed with the Nutritionist III software, and the authors acknowledged that the database had incomplete vitamin K1 data for many foods. The present study showed a similarly wide range (17–780 μg/d) but a lower average (61 μg/d) in 69 subjects with CF and PI, which resulted in 59% below the vitamin K adequate intake for the age and sex (22) of healthy children. More observational studies with large sample sizes are needed to determine the dietary patterns of vitamin K intake in children and adults with CF.
Studies assessing vitamin K status using %ucOC or PIVKA-II, which are more sensitive and specific biomarkers than prothrombin time (34), have found widespread suboptimal status in unsupplemented patients with CF and PI (2, 3). Vitamin K supplementation reduced the concentration of PIVKA-II (11), %ucOC (10), and the absolute concentration of ucOC (12); however, for many studies the dose was insufficient to bring vitamin K status into the optimal range (3, 7–11). Thus, there is persistent suboptimal status despite supplementation. However, these studies were somewhat limited by small sample sizes, the lack of control subjects, and the inability to differentiate between high- and low-dose vitamin K supplemental intake patterns. Results from the present study showed that vitamin K status was often suboptimal, despite routine supplementation with CF-specific preparations. These findings agree with previous research and extend those findings to suggest that only a vitamin K supplemental dose of ≥1000 μg/d will achieve a vitamin K status similar to healthy subjects. Dose-finding studies in infants, children, and adults are required to determine optimal vitamin K supplementation.
The vitamin K supplemental dose and status presented as %ucOC and/or PIVKA-II in participants with CF and PI from different studies are presented in Table 4. Only one study (10) assessed the efficacy of a vitamin K supplemental dose ≥1000 μg/d to normalize vitamin K status. There was no difference in %ucOC between the 5000- and 1000-μg/d doses, although interpretation is difficult because only 14 subjects were evaluated for 1 mo. The 2002 CF guidelines (6), which recommend a supplemental dose of vitamin K ranging from 300 to 500 μg/d, was based on expert opinion with little evidence. As shown in Table 4, studies that reported a vitamin K supplemental dose meeting the CF recommendations still found a high percentage of subjects with suboptimal status. This, combined with results from the present study, which showed that only subjects taking high doses had a %ucOC similar to healthy subjects and only vitamin K supplementation dose predicts vitamin K status for both males and females, suggest that reformulation of CF-specific vitamin preparations may be appropriate. Prospective, randomized, dose-response studies addressing the optimal dose of supplemental vitamin K to prevent deficiencies in children and young adults with CF and PI are needed.
TABLE 4.
Summary of previous reports of supplemental vitamin K intake and vitamin K status in subjects with cystic fibrosis and pancreatic insufficiency1
| Author (reference) | Year | Country | n | Age | Vitamin K dose2 | Duration | Vitamin K status |
| y | μg/d | ||||||
| Drury et al (10) | 2008 | Canada | 14 | 8–18 | 1000 (7), 5000 (6) | 1 mo | Overall %ucOC = 29%3 (23% sufficient) |
| Gray et al (9) | 2008 | Canada | 81 | 10–164 | 111–47945 | Not reported | %ucOC = 36 ± 14%6 (total n = 78; 18% sufficient) PIVKA-II = 11 ± 24 μg/L (total n = 75; 18% sufficient) |
| Wilson et al (8) | 2001 | Canada | 72 | 0.6–46 | 100–300 | 4–17 mo | PIVKA-II = 10.2 ± 2.2 μg/L (60% sufficient) |
| Beker et al (7) | 1997 | United States | 18 | 13–35 | 7147 | 4 wk | %ucOC = 17 ± 3% (61% sufficient) PIVKA-II = 5.1 ± 3.2 μg/L (28% sufficient) |
%ucOC, percentage of undercarboxylated osteocalcin; PIVKA-II, proteins induced by vitamin K absence–factor II.
n in parentheses.
Median.
SD.
From food and supplements.
Mean ± SD (all such values).
Approximate daily dose (reported dose: 5 mg/wk).
In the present study, not all healthy subjects were classified as vitamin K sufficient based on %ucOC. In fact, most (63%) were classified as insufficient, although none were deficient. This finding supports previous research, which showed of 86 healthy children aged 6–18 y, most had high circulating concentrations of ucOC and a marked elevation in the ratio of ucOC to carboxylated osteocalcin (UCR) compared with adults, which suggests poor vitamin K status during childhood (35). In a follow-up study, modest supplementation with 45 μg vitamin K/d for 8 wk decreased ucOC and URC, which indicated an improvement in vitamin K status in healthy prepubertal children aged 6–10 y (36). The authors suggested that future dose-finding studies are needed to determine optimal osteocalcin carboxylation in healthy children.
In summary, in children and young adults with CF and PI, vitamin K status was often suboptimal or deficient despite routine supplementation with CF-specific preparations. Only subjects taking high-dose vitamin K (≥1000 μg/d) achieved a vitamin K status similar to healthy subjects. Vitamin K supplementation dose predicts vitamin K status for both males and females with CF and PI. Collectively, these findings highlight the importance of routine high-dose vitamin K supplementation for this population.
Acknowledgments
We are grateful to the subjects and their families for participating in the study and to our many colleagues who were a part of these studies. We thank Rita Herskovitz, Babette Zemel, Mary Leonard, Alisha Rovner, Dana Boctor, and Kate Temme for their technical assistance. We also thank Caren Gundberg and Sarah Booth for providing the osteocalcin and PIVKA-II analyses, respectively.
The authors' responsibilities were as follows—VAS: designed the study; VAS and JIS: collected the data; KAD, JIS, and VAS: completed the statistical analyses and interpretation; and KAD, JIS, and VAS: wrote the manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Booth SL.Roles for vitamin K beyond coagulation. Annu Rev Nutr 2009;29:89–110 [DOI] [PubMed] [Google Scholar]
- 2.Rashid M, Durie P, Andrew M, et al. Prevalence of vitamin K deficiency in cystic fibrosis. Am J Clin Nutr 1999;70:378–82 [DOI] [PubMed] [Google Scholar]
- 3.Conway SP, Wolfe SP, Brownlee KG, et al. Vitamin K status among children with cystic fibrosis and its relationship to bone mineral density and bone turnover. Pediatrics 2005;115:1325–31 [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Farrell PM, Pencharz P. Nutritional assessment and management in cystic fibrosis: a consensus report. The Consensus Committee. Am J Clin Nutr 1992;55:108–16 [DOI] [PubMed] [Google Scholar]
- 5.Shearer MJ, Vitamin K. Lancet 1995;345:229–34 [DOI] [PubMed] [Google Scholar]
- 6.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2002;35:246–59 [DOI] [PubMed] [Google Scholar]
- 7.Beker LT, Ahrens RA, Fink RJ, et al. Effect of vitamin K1 supplementation on vitamin K status in cystic fibrosis patients. J Pediatr Gastroenterol Nutr 1997;24:512–7 [DOI] [PubMed] [Google Scholar]
- 8.Wilson DC, Rashid M, Durie PR, et al. Treatment of vitamin K deficiency in cystic fibrosis: Effectiveness of a daily fat-soluble vitamin combination. J Pediatr 2001;138:851–5 [DOI] [PubMed] [Google Scholar]
- 9.Grey V, Atkinson S, Drury D, et al. Prevalence of low bone mass and deficiencies of vitamins D and K in pediatric patients with cystic fibrosis from 3 Canadian centers. Pediatrics 2008;122:1014–20 [DOI] [PubMed] [Google Scholar]
- 10.Drury D, Grey VL, Ferland G, Gundberg C, Lands LC. Efficacy of high dose phylloquinone in correcting vitamin K deficiency in cystic fibrosis. J Cyst Fibros 2008;7:457–9 [DOI] [PubMed] [Google Scholar]
- 11.van Hoorn JH, Hendriks JJ, Vermeer C, Forget PP. Vitamin K supplementation in cystic fibrosis. Arch Dis Child 2003;88:974–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaidou P, Stavrinadis I, Loukou I, et al. The effect of vitamin K supplementation on biochemical markers of bone formation in children and adolescents with cystic fibrosis. Eur J Pediatr 2006;165:540–5 [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance data from vital and health statistics; no. 314. Hyattsville, MD: National Center for Health Statistics, 2000:1–28 [PubMed] [Google Scholar]
- 14.Lohman TG, Roche AR, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics, 1988 [Google Scholar]
- 15.Tanner JM.The development of the reproductive system. 2nd ed. Growth at adolescence. Oxford, United Kingdom: Blackwell Science, 1962:28–39 [Google Scholar]
- 16.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 1980;9:271–80 [DOI] [PubMed] [Google Scholar]
- 17.Morris A, Kanner RE, Crapo R, Gardner RM. Clinical pulmonary function testing: a manual of uniform laboratory procedures. Salt Lake City, UT: Intermountain Thoracic Society, 1984 [Google Scholar]
- 18.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993;15:75–88 [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87 [DOI] [PubMed] [Google Scholar]
- 20.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 1998;83:3258–66 [DOI] [PubMed] [Google Scholar]
- 21.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 1993;39:529–33 [PubMed] [Google Scholar]
- 22.Institute of Medicine Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickle, vanadium, and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 23.Trabulsi J, Ittenbach RF, Schall JI, et al. Evaluation of formulas for calculating total energy requirements of preadolescent children with cystic fibrosis. Am J Clin Nutr 2007;85:144–51 [DOI] [PubMed] [Google Scholar]
- 24.McKeown NM, Jacques PF, Gundberg CM, et al. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr 2002;132:1329–34 [DOI] [PubMed] [Google Scholar]
- 25.Booth SL, Lichtenstein AH, O'Brien-Morse M, et al. Effects of a hydrogenated form of vitamin K on bone formation and resorption. Am J Clin Nutr 2001;74:783–90 [DOI] [PubMed] [Google Scholar]
- 26.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr 2003;133:2565–9 [DOI] [PubMed] [Google Scholar]
- 27.Cystic Fibrosis Foundation Patient Registry 2007 Annual data report. Bethesda, MD: Cystic Fibrosis Foundation, 2008 [Google Scholar]
- 28.Zemel B. The recognition and treatment of growth disorders—a 50-year retrospective. Ann Hum Biol 2009;36:496–510 [DOI] [PubMed] [Google Scholar]
- 29.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr 2000;137:374–80 [DOI] [PubMed] [Google Scholar]
- 30.Stettler N, Kawchak DA, Boyle LL, et al. Prospective evaluation of growth, nutritional status, and body composition in children with cystic fibrosis. Am J Clin Nutr 2000;72:407–13 [DOI] [PubMed] [Google Scholar]
- 31.Peterson ML, Jacobs DR, Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics 2003;112:588–92 [DOI] [PubMed] [Google Scholar]
- 32.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol 1988;41:583–91 [DOI] [PubMed] [Google Scholar]
- 33.Stark LJ, Quittner AL, Powers SW, et al. Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Arch Pediatr Adolesc Med 2009;163:915–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm 2008;78:1–22 [DOI] [PubMed] [Google Scholar]
- 35.van Summeren M, Braam L, Noirt F, Kuis W, Vermeer C. Pronounced elevation of undercarboxylated osteocalcin in healthy children. Pediatr Res 2007;61:366–70 [DOI] [PubMed] [Google Scholar]
- 36.van Summeren MJ, Braam LA, Lilien MR, Schurgers LJ, Kuis W, Vermeer C. The effect of menaquinone-7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. Br J Nutr 2009;102:1171–8 [DOI] [PubMed] [Google Scholar]