Abstract
Context
Multiple complex symptoms from cancer treatment can interfere with functioning.
Objectives
To evaluate the efficacy of an “energy and sleep enhancement” (EASE) intervention to relieve fatigue and sleep disturbance and improve health-related functional status.
Methods
Individuals receiving chemotherapy were randomized to the EASE (n = 153) or a control intervention (n = 139). The EASE intervention included information and behavioral skills taught by an oncology nurse in three telephone sessions. The primary outcomes of fatigue, sleep disturbance and functional status were measured before chemotherapy, Day 4 after first treatment (baseline) and 43-46 or 57-60 days later (follow-up) depending on chemotherapy cycle length.
Results
The sample was primarily female (82%) and non-Hispanic white (89%) with mean age of 53.9 years. Fatigue and patient-reported sleep disturbance were elevated in both groups at baseline and follow-up. Actigraphy revealed total sleep time was almost eight hours and sleep percent was over 85% for both groups at both time points (normal range). Physical functioning was diminished and at the same level as a sample with serious illness. Mental functioning was in normal range. A repeated measures analysis of variance revealed no statistically significant group-by-time effects for fatigue, sleep disturbance, or functional status. Unemployed individuals showed greater benefit from the EASE intervention, reporting less pain and symptom interference.
Conclusion
Potential explanations include high variability and/or floor effect for fatigue; incorrect timing of measures; insufficient amount or dose of the intervention; and confounding effects of gender. Future research should consider screening for symptom severity and tailoring interventions.
Keywords: Cancer-related fatigue, sleep disturbance, psycho-educational intervention, randomized controlled trial, telephone counseling, symptom cluster, actigraphy
Introduction
Individuals with cancer often deal with multiple complex symptoms during cancer treatment that can interfere with functioning in usual roles and activities. However, research has typically focused on the alleviation of single symptoms such as pain, fatigue, sleep disturbance, and depression (1, 2). Research is needed to examine the efficacy of intervention for multiple symptoms and determine whether intervention intended for one symptom will influence the severity of other symptoms.
In the research presented here, four symptoms were chosen for consideration. Two symptoms – fatigue and sleep disturbance – were targeted for intervention; the other two, pain and depression, were selected for observation. Cancer-related fatigue (CRF) was a focus of this investigation because a previous clinical trial demonstrated that training in energy conservation strategies significantly reduced fatigue (3). That study also showed that sleep disturbance was a significant problem during cancer treatment. Because sleep disturbance can increase fatigue, it was chosen for intervention in the current research. Two related symptoms, pain and depression, were chosen for evaluation in this research (but not targeted for intervention) because of a growing body of evidence linking them to cancer fatigue (4-7). The study was designed to extend past research systematically and incrementally by examining the effect of an intervention on the target symptoms (fatigue and sleep disturbance) and exploring how the related symptoms (pain and depression) might be influenced by the intervention. Thus, the aim of the research was to test the efficacy of an “Energy and Sleep Enhancement” (EASE) intervention on the primary outcomes of fatigue, sleep disturbance, and functional status and the secondary outcomes of pain and depression.
Past research has demonstrated that some symptoms influence other symptoms and have a negative effect on one or more symptom outcomes (1, 2). Given and colleagues provided evidence that specific symptoms (fatigue, sleep disturbance, and pain) had different effects on outcomes when one, two, or all three symptoms were present. In one study, individuals who reported both pain and fatigue reported more symptoms overall than those who reported either symptom or neither symptom (8). In a separate analysis, pain, fatigue, and sleep disturbance were examined as predictors of functioning (9). Compared with no pain, fatigue, or sleep disturbance, individuals who had one, two, or all three symptoms had incrementally greater risk of impaired functioning during cancer therapy. Testing a mediation model, Beck and colleagues (10) determined that pain influenced fatigue directly and also indirectly through its effect on sleep; this finding suggested that the use of better pain management to improve sleep could also decrease fatigue. The findings of these studies suggested that fatigue, sleep disturbance, and pain could be studied as a symptom cluster because these symptoms tended to co-occur and they influenced one another.
Research has suggested that depression is related to the other symptoms of interest (in this context, “depression” refers to “depressive symptoms,” not a clinical diagnosis) (7, 11-16). Some studies have demonstrated that depressive symptoms were influenced by other symptoms and changes in functioning. In a longitudinal study of outpatients with cancer, Williamson (17) demonstrated that as pain increased and restricted activity, depressive symptoms also increased. Another study of fatigue and depression in cancer patients undergoing treatment demonstrated a similar result: there was a direct relationship between fatigue and depression as well as an indirect relationship between the two symptoms through the influence of fatigue on functional status (18). As fatigue increased and functioning in usual activities decreased, depressive symptoms increased. Because depressive symptoms have been associated with pain, fatigue, and functional status, it makes sense to examine these symptoms in the context of a symptom reduction intervention.
Only a few symptom management studies have examined multiple symptoms (19-21). A comprehensive coping strategy intervention targeted to reduce pain, fatigue, nausea, and depression in breast cancer patients during autologous bone marrow transplant was associated with reduced nausea and fatigue seven days after transplant in comparison to a usual care group; pain, anxiety, and depression were not affected by the intervention (19). A structured symptom assessment for advanced lung cancer patients (21), conducted by research nurses and shared with clinic nurses, demonstrated a significant reduction in symptom distress after six sessions over a six month period when compared with usual care (21). Finally, a cognitive behavioral intervention reduced symptoms during chemotherapy when compared to usual care (20). Patients with higher symptom severity prior to treatment who received the intervention had lower symptom severity at weeks 10 and 20 than a usual care control group.
A few psycho-educational intervention studies aimed to reduce fatigue incorporated interventions to manage sleep disturbances. The results have been mixed. Yates demonstrated better fatigue outcomes one week after completion of the intervention; however, the changes were not sustained over the next two cycles of treatment (22). Ream and colleagues documented better fatigue outcomes after three cycles of chemotherapy; however, outcomes for the earlier cycles were not reported (23). Berger and colleagues (24) evaluated the efficacy of a sleep management intervention to reduce fatigue and improve sleep quality; the intervention group had better sleep quality but not lower fatigue. The results to date do not provide conclusive guidance about the best strategies for management of fatigue and sleep disturbances.
The study reported here extended a beneficial energy conservation and activity management (ECAM) intervention for fatigue to include intervention for sleep disturbance (3). In addition to the main analysis, the ECAM study showed that, after chemotherapy treatment, 89% of participants reported fatigue; 71% reported sleep disturbance; 30% pain; and, 28% depression. Sixty-eight percent of those with fatigue also reported sleep disturbance; 30% pain; and, 28% depression. These results provide an indication that other symptoms in addition to fatigue have been problematic during chemotherapy and should be addressed in symptom research.
Methods
Study Design
The primary aim of this study was to test the efficacy of an “Energy and Sleep Enhancement” (EASE) intervention during cancer chemotherapy. The primary outcomes were fatigue, sleep disturbance, and functional status. The secondary outcomes were pain and depression. This randomized clinical trial compared the EASE intervention with an intervention controlling for time and attention that consisted of information about nutrition and a healthy diet. The study was conducted at four clinical sites: two university health science centers, a community cancer center, and a comprehensive cancer center. Individuals were eligible if they were 18 years of age or older and were beginning a new chemotherapy (CTX) regimen with at least one CTX drug administered intravenously in a cyclic manner (on any schedule) for breast, lung, colorectal, prostate, gynecologic, bladder, or testicular cancer or lymphoma. Any prior treatment other than surgery was completed at least one month previously and the individual could receive concurrent radiation. Participants had to be able to read and write English. Individuals were excluded if their treatment plan included marrow or stem cell transplantation, interleukins, interferons, or tumor necrosis factor; had a chronic fatigue disorder; were being treated for diagnosed sleep disorder (such as narcolepsy or sleep apnea); were enrolled in another study that involved a psychoeducational intervention; had a communication impairment; had overt evidence of psychiatric disorder; or initiated treatment for anemia or depression during the previous three weeks.
The Institutional Review Board (IRB) for each study site approved the research protocol in conformity to federal regulations. Potential participants were approached by telephone or in the clinic and the study was explained. All study participants provided written informed consent. The IRB granted a waiver to retain de-identified demographic information including age, gender, ethnicity, and race from individuals who refused to participate in the study for comparison with study participants.
At each of the recruitment sites, breast cancer patients were the largest cancer population and the group most easily accrued into research because of clinic logistics. So participants in this research were stratified by diagnosis (breast cancer v. non-breast cancer) at each site to ensure equivalency of the experimental and control groups on this factor. Participants were then randomly assigned to receive either the EASE intervention or the nutrition (control) intervention. Random assignments were generated by the statistician and placed in sealed envelopes that were numbered and selected sequentially for each stratification group.
Procedures
Baseline questionnaires measuring subjective symptoms and functional status were completed on Day 1 of the CTX cycle prior to receiving treatment and on Day 4 after the first CTX which coincided with a known time of high fatigue. Also at baseline, an objective measure of sleep disturbance (actigraphy) was obtained along with a companion sleep and symptom diary. Follow-up data points were Days 43-46 or 57-60 depending on the length of the chemotherapy cycle. At both measurement points, patients completed questionnaires and wore the actigraph on the non-dominant wrist on Day 1 and removed it 72 hours later (which was Day 4, the equivalent of three 24-hour periods).
Variables
Demographic and clinical information
Demographic and clinical information was obtained by questionnaire (age, gender, race, ethnicity, marital status, education, employment). Clinical data was abstracted from the medical records (diagnosis, stage, co-morbidities, previous treatment, and current treatment).
Fatigue
Fatigue was measured with the General Fatigue Scale (GFS), a 7-item Likert-type scale, (1 = no fatigue, distress, or impact to 10 = greatest possible fatigue, distress, or impact) (25). The measure was scored by averaging the responses to the items. Cronbach alpha reliability coefficient for this sample was 0.92.
Fatigue was also measured with the fatigue subscale of the Profile of Mood States (POMS) questionnaire. The POMS was developed to assess transient distinct mood states. Originally designed as a 65-item scale (26), the current short version consisted of six subscales with a total of 30 items (27). The fatigue symptom subscale (POMS-F) consisted of five adjectives measuring subjective fatigue (such as weary, tired, etc.) that were rated on a 5-point scale with “0” indicating “not at all” and “4” indicating “extremely.” Items were summed to form a subscale score that ranges from 0-20. This scale is well recognized as a sensitive, valid, and reliable measure of the sensation of fatigue with considerable evidence of validity and reliability (27, 28). The Cronbach alpha coefficient for this sample was 0.94.
To document subjective sleep disturbance, including insomnia, the Pittsburgh Sleep Quality Index (PSQI) was used. The PSQI is a subjective, self-rated, paper-and-pencil questionnaire consisting of 19 items. Responses to the 19 items are grouped into seven component scores that are weighted equally on a 0-3 scale. The seven components of the PSQI are sleep quality, latency, duration, habitual efficiency, disturbances, medication use, and daytime dysfunction. The components are summed to produce a global PSQI score that can range from 0 - 21. A higher score indicates more severe complaints and worse sleep quality (29). Internal consistency reliability and construct validity have been supported in cancer populations (30, 31). In this sample, the Cronbach alpha reliability for the scale was 0.75.
Objective sleep disturbance data were also obtained via continuous non-invasive monitoring using wrist actigraphy, an objective record of movement over time in the form of activity counts (32-34). Actigraphy is a valid, reliable measure of sleep that correlates with polysomnography at approximately 90% agreement. It is a sensitive measure of sleep-wake and activity-rest patterns as well as circadian activity rhythms.
The actigraph measure used in this research was the Octagonal Basic Motionlogger® Actigraph from Ambulatory Monitoring, Inc. (Ardsley, NY). It was an unobtrusive instrument resembling a wrist watch that could be worn in the usual environment, both at home and place of employment. Instructions about actigraph use included pushing a small marker on the side a) when putting on the actigraph for the first time, b) when turning out the light to go to sleep, and c) when getting out of bed in the morning. These markers were used to discriminate day from night when performing the analysis.
Several sleep/wake parameters were examined in this research: total time in bed (indicated by the event markers on the actigraph; total sleep time after sleep onset (in minutes), number of awakenings from sleep onset to morning lights on (indicated by an event marker), minutes awake (WASO-M), and percent time asleep after sleep onset. Data collected continuously over 72 hours were uploaded to a personal computer using the Micro-Mini® Motionlogger Actigraph Interface Connector and analyzed using the Action 4 analysis program (Copyright © 1988-2001; Ambulatory Monitoring, Inc.). Actigraphy data for up to 72 hours were analyzed in one-minute epochs.
Participants also completed an adapted Morin Sleep Diary to provide confirmation of “lights off” and “lights on” for comparison with the event marker on the actigraph, as well as information about naps, medications and environmental factors that aid in the interpretation of the actigraph data. The diary has been used in numerous sleep studies (35) in both healthy people and those with cancer (36-39). A test of reliability of actigraphy scoring indicated 83.4% agreement between two coders. The intraclass correlation coefficients for each sleep parameter ranged from 0.83 to 0.99.
Pain
Pain severity was measured by the intensity subscale of the Brief Pain Inventory (BPI) (40). Patients reported pain severity (worst, least, average and current pain) using a 0-10 scale for each item (0 = no pain to 10 = pain as bad as you can imagine). The four pain items were averaged to yield a pain intensity score ranging from 0-10. The scale is widely used; validity and reliability in cancer treatment have been established (40-42). Cronbach's alpha for the BPI intensity scale was 0.88 in this sample.
Depressive
Depressive symptoms were measured by the depressive symptom subscale of the Profile of Mood States (POMS-D) which consisted of five adjectives describing depression (such as sad, discouraged, gloomy, etc.) rated on a 5-point scale with “0” indicating “not at all” and “4” indicating “extremely.” Items were summed to form a score that ranged from 0-20. Internal consistency as measured by Cronbach alpha was 0.90 in this sample.
Other Side Effects
The Side Effect Checklist (SCL) is a measure of side effect severity based on a measure used in our previous research on coping with cancer treatment. Severity of side effects were rated using a 5-point Likert-type scale (0 = not at all severe to 5 = extremely severe). Summed side effect severity scores have been correlated with outcome measures such as mood and other quality-of-life domains in cancer patients (43, 44). This instrument has acceptable test-retest reliability (r = 0.84) and face validity as well as clinical validity (43-45). Cronbach alpha in this study was 0.87.
Functional status
Functional status was assessed by three measures. Limitation of functioning was measured by adapting the interference items from the Brief Pain Inventory (40) to apply to “symptoms” rather than “pain” only. Respondents were asked to describe how symptoms have interfered with general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life. Each item was rated on a 0-10 scale with the words “did not interfere” at 0 and “completely interfered” at 10. Internal consistency reliability for this sample was 0.91.
The SF-12 provided a second measure of functional status. The SF-12 is a shorter version of the 36-item Short-Form Health Survey (46, 47) that was developed for the Medical Outcomes Study (48). The scale includes physical and mental components of quality of life (SF12-P and SF12-M, respectively). Items for each component were recoded (as needed), summed, and transformed to a 0-100 scale with higher scores indicating better physical or mental functioning. The scale is widely used in cancer populations and norms have been established (49).
Eastern Cooperative Oncology Group (ECOG) Performance Status is a simple rating of ability to function in usual activities. It has been widely used by clinicians to evaluate participants in drug clinical trials. It has been adapted here for patient self-report (50). Participants are asked to select from five statements the one that best described their current activity level: a) I have normal activity without symptoms, b) I have some symptoms, but I do not need to spend any extra time resting during the day, c) I need some time to rest (e.g., in bed), but it amounts to less than half of my normal daytime, d) I need to rest (e.g., in bed) for more than half of my normal daytime, and e) I am unable to get out of bed. This item was scored on a 0-4 scale.
Interventions
Participants in each intervention group received three telephone sessions with a specially trained oncology nurse. The intervention occurred during the second, third, and fourth week after the first CTX treatment. Written intervention materials included a handbook specific to the EASE or control group. The intervention was delivered using an interactive approach that built on the individual's existing knowledge of energy conservation strategies, sleep management, and their unique responses to symptoms. A specific protocol and script were used; however, the nurse was trained to customize the protocol to the needs of the participant.
The tenets of the Common Sense Model (CSM model) (51-53) provided the basis for the EASE intervention. This model is appropriate for the study of multiple symptoms; it proposes three stages of symptom management: representation, coping, and appraisal. In the representation stage, the individual gathers information about the symptom's identity, cause, and pattern to form a mental image of the symptom. In the coping stage, the individual identifies and implements self-care strategies to manage the symptom. During the appraisal stage, the individual evaluates the effectiveness of the strategies and adjusts either the coping methods or symptom representation based on the experience of symptom management (54).
In this study, a research nurse provided symptom management based on the tenets of the Common Sense Model. Information was provided to assist with the formation of an accurate representation of the symptoms of fatigue and sleep disturbance. In the first telephone intervention session conducted approximately one week after the first chemotherapy treatment, the research nurse provided information to each participant about the characteristics of the two symptoms, typical causes of each (such as specific drugs, emotional distress, being over- or under-active), and patterns of symptoms (most severe immediately after treatment, tapering off after 5-7 days. The nurse engaged the participant in a discussion of his/her experience of fatigue and sleep disturbance including likely causes and the patterns during the first week after chemotherapy. EASE group participants also received a handbook that included the information about symptoms and examples of energy conservation and sleep management strategies. Between sessions 1 and 2, participants completed a daily diary (concerning symptoms and sleep patterns) and a priority list of usual activities.
In the second intervention session during the second week after chemotherapy, the nurse used information from the daily diary and priority list to guide the participant to formulate and implement a plan of energy conservation to manage valued activities and a plan for sleep enhancement to manage sleep disturbance (coping phase). The plan included suggested strategies to manage each of the symptoms. Energy conservation strategies for fatigue included decision making about delegating activities and responsibilities; pacing oneself; setting priorities; and engaging in demanding activities at times of peak energy. Sleep enhancement strategies included establishing an optimal environment for sleep; learning and using relaxation techniques to induce sleep at the beginning of the night and after nighttime awakenings; restricting sleep to the same number of hours each night and minimizing nap-taking; and engaging in regular exercise during daytime hours. The participant was directed to use the plan during the next week. In the third session (appraisal stage), the individual evaluated and revised the plan.
The control intervention was designed to control for the amount of time and attention received by the experimental group. The intervention focused on information about nutrition and a healthy diet. This content was chosen because patients with cancer are interested in this topic and because it is of minor relevance to fatigue during aggressive cancer treatment (55). Information on maintaining a healthy diet was discussed in the first session including a description of the food pyramid and healthy food choices. The participant kept a 24-hour dietary record as homework in preparation for the second session. This was discussed during session 2 for adjustments to their diet if needed, as well as a discussion about vitamins. The third session consisted of information about minerals and fiber, as well as an evaluation of the helpfulness of the information provided. Therapeutic nutritional information or information on symptom management was not included in the control intervention. The three control sessions were equivalent to the EASE intervention in terms of the amount of time spent with the individual.
The research nurses at Fox Chase Cancer Center conducted all of the interventions by telephone in order to protect the integrity of the intervention and minimize differences in delivery. Each nurse received eight hours of training in the conduct of the EASE intervention. Training included didactic presentations as well as demonstration and role play. In addition, ongoing bi-monthly supervision was provided by one of the investigators. Nurse adherence to the EASE and control interventions was examined using a checklist of the components of each intervention. This checklist was used to evaluate 20% of the audio-recorded interventions (every fifth case) during the first two years of the study to determine adherence to the telephone protocol. Participant use of the EASE strategies was measured with a brief checklist of intervention behaviors. Individual items were summarized as the number of fatigue and sleep management strategies that were used.
Statistical Analysis
Non-parametric Chi-square analysis and independent t-tests were used as appropriate to examine differences between participants and non-participants, baseline equivalence between study groups (EASE versus control) and between participants who completed all study activities and those who did not. The SAS Mixed Procedure (PROC MIXED) restricted maximum likelihood (REML) method was used to examine the primary hypothesis (56) because the study involved repeated measures that were correlated and there were changes in variability due to attrition. For all analyses, an “intent-to-treat” analysis was conducted in which all available data for participants were included under the missing at random assumption of the mixed-model analysis and all participants were evaluated as randomized regardless of whether they had completed all three intervention sessions.
Results
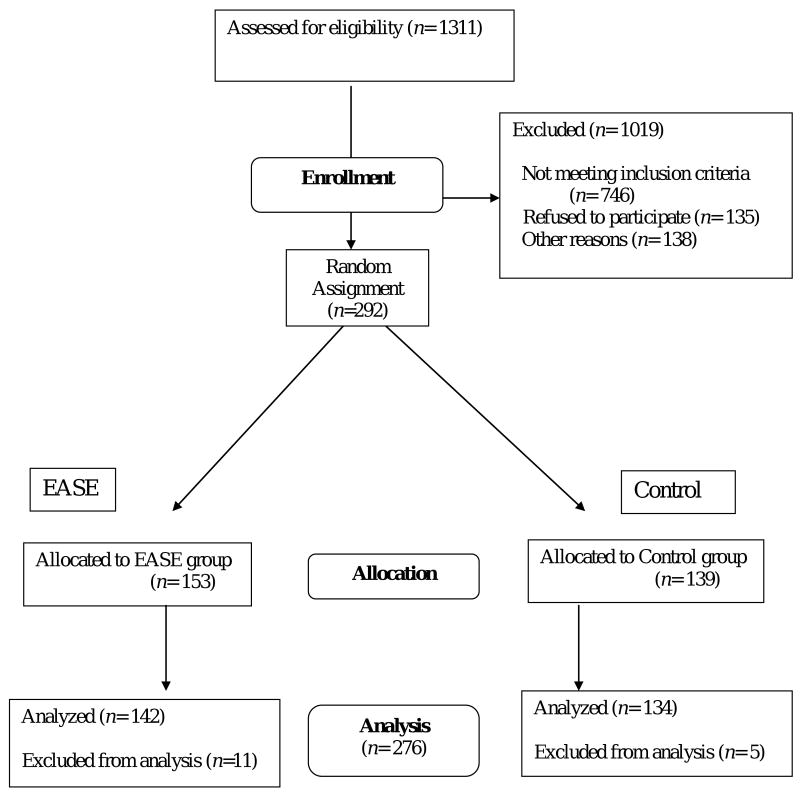
One thousand three-hundred eleven (1,311) individuals were assessed for eligibility. Of these, 746 did not meet all inclusion criteria and an additional 273 declined enrollment (Figure 1). Common reasons for not enrolling included lack of interest (n = 107), poor timing (n = 28), inability to be contacted (n = 59), and initiation of cancer treatment prior to consent (n = 33). Two hundred ninety-two (292) individuals were enrolled in the study between February 2, 2004 and August 31, 2007 and randomized to receive the EASE or control intervention. Sixteen participants were excluded from the analysis, leaving 276 analyzed cases. Reasons for exclusion included severity of illness (n = 4), loss to follow-up (n = 10), and change of treatment (n = 2).
Figure 1.
Eligibility, enrollment, and follow-up of study participants
A comparison between participants and non-participants using Chi-square and t-tests showed that participants (n = 276) and non-participants (n = 135) differed significantly with regard to age, gender, and cancer diagnosis. Non-participants were older and more likely to be male than participants. Breast cancer, and thus, the number of females, was over-represented in the participant group while lung cancer was under-represented.
The final sample (n = 276) was primarily female (83%), Caucasian (90%), married (70%), college educated (42%), and was treated with chemotherapy alone (95%). The most common diagnoses were breast (55%), lung (17%), lymphoma (8%), and ovarian (6%) cancers. The mean age was 53.97 (standard deviation [SD] 12.02).
Considering the primary outcome variables, patient-reported fatigue (GFS) was moderately elevated at baseline and remained elevated at follow-up in both the EASE and control groups (Table 1a). Similarly, patient-reported sleep disturbance (PSQI) at baseline and follow-up was well above the accepted cut-off score of 5 in both groups indicating moderate levels of sleep disturbance. In contrast to patient-reported perception of disrupted sleep, actigraphy readings indicated that total sleep time was almost eight hours and sleep percent was over 85% for both groups at both time points, which is in the normal range (Table 1b). Physical functioning (SF-12 PCS) for EASE and control groups at baseline and follow-up was diminished and similar to norms for a sample with serious medical illness (46) as would be expected in this sample of cancer patients undergoing treatment. Mental functioning (SF-12 MCS) was in normal range for both groups at both time points.
Table 1.
| Table 1a. Means and Standard Deviations for Patient-Reported Outcomes | |||
|---|---|---|---|
| Mean (SD) | |||
| Measure | Pretreatment Baseline | Day 4 Baseline | Follow-Up |
| Manipulation Check | |||
| CLB | |||
| EASE | 10.03 (3.78) | 13.57 (3.1) | |
| Control | 9.5 (3.62) | 12.17 (3.09) | |
| Primary Patient-Reported Outcomes | |||
| GFS | |||
| EASE | 5.19 (2.14) | 4.89 (1.92) | |
| Control | 5.12 (2.05) | 4.82 (2.03) | |
| POMS-F | |||
| EASE | 3.01 (1.13) | 2.85 (1.01) | |
| Control | 3 (1.03) | 2.96 (1.12) | |
| PSQI | |||
| EASE | 8.01 (3.96) | 7.96 (3.59) | |
| Control | 7.83 (4.37) | 8.24 (3.83) | |
| SF12-M | |||
| EASE | 48.95 (10.29) | 49.56 (9.64) | |
| Control | 49.65 (11.11) | 49.8 (9.6) | |
| SF12-P | |||
| EASE | 40.3 (11.21) | 37.2 (8.97) | |
| Control | 41.78 (11.37) | 37.95 (9.59) | |
| Secondary Patient-Reported Outcomes | |||
| BPI | |||
| EASE | 1.99 (2.16) | 2.27 (2.26) | |
| Control | 1.7 (2.14) | 2.15 (2.25) | |
| POMS-D | |||
| EASE | 1.81 (0.94) | 1.63 (0.78) | |
| Control | 1.95 (0.90) | 1.52 (0.66) | |
| SCL | |||
| EASE | 0.42 (0.33) | 0.65 (0.35) | |
| Control | 0.42 (0.34) | 0.7 (0.42) | |
| ECOG-PS | |||
| EASE | 2.03 (0.96) | 2.81 (0.81) | |
| Control | 1.91 (0.94) | 2.96 (0.72) | |
| SXINT | |||
| EASE | 2.5 (2.34) | 3.98 (2.56) | 3.76 (2.4) |
| Control | 2.5 (2.34) | 4.12 (2.57) | 3.85 (2.38) |
| Table 1b. Means and Standard Deviations of Actigraphy Parameters Averaged Over Study Days | |||
| Mean (SD) | |||
| Measure | Day 1-3 Baseline | Follow-Up | |
| Total time in bed (min) | |||
| EASE | 543.6 (82.71) | 537.82 (93.16) | |
| Control | 531.73 (81.42) | 519.32 (99.47) | |
| Total sleep time (min) | |||
| EASE | 465.59 (103.92) | 466.53 (118.95) | |
| Control | 465.02 (79.11) | 461.97 (100.35) | |
| Sleep percent after onset | |||
| EASE | 86.52 (11.55) | 87.4 (13.08) | |
| Control | 88.35 (8.28) | 89.48 (8.92) | |
| Awakenings | |||
| EASE | 10.47 (5.74) | 9.98 (5.46) | |
| Control | 9.34 (5.41) | 8.17 (5.01) | |
| Wake after sleep onset (WASO-M) | |||
| EASE | 69.1 (53.85) | 63.63 (63.56) | |
| Control | 61.3 (44.87) | 53.6 (47.09) | |
GFS = General Fatigue Scale; POMS-F = Profile of Mood States Fatigue Scale; PSQI = Pittsburgh Sleep Quality Index; SF12-M = Short Form 12 Mental Score; SF12-P = Short Form 12 Physical Score; ECOG-PS = Eastern Cooperative Oncology Performance Status; SXINT = Symptom Interference; CLB = Checklist of Behaviors; BPI = Brief Pain Inventory; POMS-D = Profile of Mood States Depression Scale; SCL = Symptom Checklist; EASE = Energy and Sleep Enhancement intervention.
Because this was a randomized clinical intervention trial, baseline equivalence of the intervention groups was examined for the following clinical and demographic variables: cancer diagnosis, clinical stage, gender, ethnic background, marital status, education, age, and employment status (Table 2). The study groups differed only by employment status X2 = 4.00, P = 0.05. Because employment status could influence the need for and motivation to engage in fatigue reduction and/or sleep disturbance reduction behaviors, it was included as an independent variable in the main analyses.
Table 2.
Differences in Demographic and Clinical Characteristics of Study Groupsa
| EASE Group | Control Group | ||
|---|---|---|---|
| Characteristic | P | ||
| n (%) | n (%) | ||
| Age (yrs) (n = 276) | 0.42 | ||
| Mean (SD) | 54.4 (11.8) | 53.5 (12.3) | |
| Gender (n = 276) | 0.29 | ||
| ▪ Female | 114 (80.3%) | 114 (85.1%) | |
| ▪ Male | 28 (19.7%) | 20 (14.9%) | |
| Ethnicity (n = 264) | 0.66 | ||
| ▪ Non-Hispanic | 132 (96.4%) | 121 (95.3%) | |
| ▪ Hispanic | 5 (3.6%) | 6 (4.7%) | |
| Race (n = 272) | 0.47 | ||
| ▪ American Indian/Alaska Native | 3 (2.1%) | 1 (0.8%) | |
| ▪ Asian | 1 (0.7%) | 2 (1.5%) | |
| ▪ Black or African American | 7 (5.0%) | 11 (8.3%) | |
| ▪ White or Caucasian | 127 (90.7%) | 115 (87.1%) | |
| ▪ More than 1 race | 1 (0.7%) | 3 (2.3%) | |
| ▪ Unknown | 1 (0.7%) | 0 | |
| Marital Status (n = 272) | 0.39 | ||
| ▪ Single | 9 (6.4%) | 13 (9.8%) | |
| ▪ Separated or Divorced | 24 (17.1%) | 17 (12.9%) | |
| ▪ Widowed | 7 (5.0%) | 11 (8.3%) | |
| ▪ Married | 100 (71.4%) | 91 (68.9%) | |
| Education (n = 271) | 0.38 | ||
| ▪ 8th Grade or less | 1 (0.1%) | 3 (2.3%) | |
| ▪ Some high school | 3 (2.1%) | 8 (6.1%) | |
| ▪ High school graduate or GED | 31 (22.1%) | 24 (18.3%) | |
| ▪ Technical school graduate | 6 (4.3%) | 8 (6.1%) | |
| ▪ Some college | 41 (29.3%) | 32 (24.4%) | |
| ▪ College graduate | 58 (41.4%) | 56 (42.7%) | |
| Currently Employed (n = 272) | 0.05a | ||
| ▪ Yes | 69 (49.3%) | 81 (61.4%) | |
| ▪ No | 71 (50.7%) | 51 (38.6%) | |
| Study Site (n = 276) | 0.79 | ||
| ▪ Fox Chase Cancer Center | 75 (52.8%) | 77 (57.5%) | |
| ▪ University of Utah | 47 (33.1%) | 41 (30.6%) | |
| ▪ University of Cincinnati | 15 (10.6%) | 11 (8.2%) | |
| ▪ Christiana Medical Center | 5 (3.5%) | 5 (3.7%) | |
| Diagnosis (n = 276) | 0.2 | ||
| ▪ Breast | 73 (51.4%) | 79 (59.0%) | |
| ▪ Lung | 21 (14.8%) | 26 (19.4%) | |
| ▪ Colorectal | 8 (5.6%) | 5 (3.7%) | |
| ▪ Prostate | 2 (1.4%) | 1 (0.1%) | |
| ▪ Gynecologic | 19 (13.4%) | 13 (9.7%) | |
| ▪ Testicular | 0 | 1 (0.1%) | |
| ▪ Lymphoma | 14 (9.9%) | 9 (6.7%) | |
| ▪ Bladder | 5 (3.5%) | 0 | |
| Known Clinical Stage (n = 246) | 0.65 | ||
| ▪ 1 | 25 (19.7%) | 19 (16.0%) | |
| ▪ 2 | 47 (37.0%) | 43 (36.1%) | |
| ▪ 3 | 31 (24.4%) | 37 (31.1%) | |
| ▪ 4 | 24 (18.9%) | 20 (16.8%) | |
Sample size varied by reporting of specific information.
Of the 276 participants, 60 had some missing data. Baseline equivalence between complete and incomplete cases on overall symptom burden (SCL), fatigue (GFS), and functional status (SF-12, symptom interference) was conducted using independent samples t-test. Results show that the incomplete cases had worse health at baseline prior to treatment than those who completed the study: SCL (t(270) = 3.212, P = 0.001 (two-tailed)), SF-12 physical (t(271) = 2.346, P = 0.02 (two-tailed)), SF-12 mental (t(83.70) = 2.034, P = 0.045 (two-tailed)), and symptom interference (t(83.93) = 2.756, P = 0.007 (two-tailed)). Complete and incomplete cases did not differ on fatigue or sleep disturbance measures. Chi-square analysis showed no difference in the number of complete cases between the two intervention groups X2 = 0.551, P = 0.458. These findings suggest that in both groups, participants who were in poorer health at baseline were more likely to have incomplete data.
One hundred fifty-three participants were allocated to the EASE group and 139 to the control group (Figure 1). Seventy-five percent of the EASE participants and 83% of the control group received all three sessions of the intervention. The total amount of intervention time for each group was similar (EASE = 69 minutes; control = 72 minutes). A manipulation check was conducted to determine whether the EASE and control groups differed in the use of intervention strategies. At baseline and follow-up participants in both study groups completed a behavioral checklist (CLB); 10 items referred to energy conservation strategies (delegation, planning, pacing, etc.), and nine items described sleep promotion strategies (using relaxation strategies, avoiding caffeine before bedtime, establishing set sleep and wake times, etc.). A repeated-measures analysis of variance (ANOVA) revealed that the EASE group used significantly more intervention strategies over time compared with the control group (Tables 3a). This finding indicates that the EASE intervention influenced participants as predicted: individuals who were taught behavioral strategies to manage fatigue and sleep disturbance reported using more than the control group who were not taught these strategies.
Table 3.
| Table 3a. F Tests and P-Values for Patient-Reported Outcomes F test (Repeated measures design with maximum likelihood estimates) | |||||||
|---|---|---|---|---|---|---|---|
| Measure | df | Study Group | Time | Working Status | Study Group by Time | Study Group by Working Status | Study Group by Time by Working Status |
| Manipulation Check | |||||||
| CLB | 1/268 | 6.38 a | 163.38 b | 0.00 | 4.91 a | 0.00 | 0.14 |
| Primary Patient-reported Outcomes | |||||||
| GFS | 1/262 | 0.22 | 2.91 | 0.13 | 0.02 | 0.06 | 0.81 |
| POMS-F | 1/250 | 0.26 | 0.95 | 0.56 | 0.47 | 1.29 | 0.11 |
| PSQI | 1/265 | 0.05 | 0.11 | 0.30 | 0.58 | 0.17 | 0.00 |
| SF12-M | 1/268 | 0.62 | 0.00 | 0.24 | 0.00 | 0.49 | 1.94 |
| SF12-P | 1/268 | 0.84 | 28.42 b | 0.18 | 0.18 | 0.70 | 0.74 |
| Secondary Patient-Reported Outcomes | |||||||
| SXINT (T1a-T1b-T2) | 2/268 | 0.01 | 46.20 b | 0.64 | 0.37 | 0.00 | 3.64 a |
| SXINT (T1a-T2) | 1/268 | 0.00 | 46.29 b | 1.80 | 0.86 | 0.53 | 6.87 c |
| SXINT (T1b-T2) | 1/260 | 0.11 | 0.84 | 0.63 | 0.01 | 0.44 | 5.17 a |
| BPI | 1/261 | 0.94 | 4.44 a | 0.65 | 0.32 | 0.09 | 3.95 a |
| SCL | 1/268 | 0.29 | 106.59 b | 1.85 | 0.00 | 0.29 | 0.00 |
| POMS-D | 1/184 | 0.00 | 16.94 b | 0.31 | 1.94 | 0.01 | 6.85 c |
| ECOG | 1/263 | 0.42 | 150.82 b | 4.93 a | 3.26 | 3.35 | 0.07 |
| Table 3b. Tests and P-Values for Actigraphy F test (Repeated measures design with maximum likelihood estimates) | |||||||
| Measure | df | Study Group | Time | Working Status | Study Group by Time | Study Group by Working Status | Study Group by Time by Working Status |
| Total time in bed (min) | 1/251 | 1.21 | 0.55 | 0.64 | 0.32 | 0.13 | 0.31 |
| Total sleep time (min) | 1/251 | 0.00 | 0.22 | 1.38 | 0.17 | 0.96 | 0.09 |
| Sleep percent after onset | 1/251 | 1.71 | 3.52 | 8.47** | 0.07 | 0.80 | 0.04 |
| Number of Awakenings | 1/251 | 2.78 | 4.99* | 0.33 | 1.39 | 0.17 | 1.46 |
| Wake after sleep onset (WASO-M) | 1/251 | 1.50 | 4.76* | 8.12** | 0.12 | 0.80 | 0.05 |
GFS = General Fatigue Scale; POMS-F = Profile of Mood States Fatigue Scale; PSQI = Pittsburgh Sleep Quality Index; SF12-M = Short Form 12 Mental Score; SF12-P = Short Form 12 Physical Score; ECOG = Eastern Cooperative Oncology Performance Status; SXINT = Symptom Interference (T1a = Time 1a; T1b = Time 1b); CLB = Checklist of Behaviors; BPI = Brief Pain Inventory; POMS-D = Profile of Mood States Depression Scale; SCL = Symptom Checklist; EASE - Energy and Sleep Enhancement intervention.
P < 0.05.
P < 0.001.
P < 0.01.
A repeated-measures ANOVA was used to test the efficacy of the EASE intervention. Three hypotheses were tested. In comparison with the control group, it was predicted that the EASE intervention group would report: 1) less fatigue over time, 2) less sleep disturbance over time, and 3) less disruption of functional status over time. Each measure of the primary outcomes (fatigue, sleep disturbance, and functional status) was examined in a separate repeated measure analysis of variance (Table 3a). Actigraphy measures of sleep were analyzed in a similar manner (Table 3b). In each analysis, there was a single between-subjects independent variable -- study group with two levels (EASE or control). In addition, there was a single within-subjects variable -- time (with two occasions of measurement). Current employment was included in the analysis as an additional independent variable because the intervention and control groups differed on this variable. The EASE and control groups did not differ on fatigue (GFS, POMS-F), sleep disturbance (PSQI, actigraph measures), or functional status (SF12-M or SF12-P) over time. There were significant three-way interactions for symptom interference (SXINT), pain (BPI), and depression (POMS-D) indicating that unemployed individuals who received the EASE intervention had less pain and less interference with functioning than employed individuals in the same group. While these differences were statistically significant, the actual differences were small and, therefore, not clinically meaningful.
Because of the overall null result of the study, we conducted a post hoc subset analysis to determine if other factors could have influenced the study results. In separate analyses, gender, cancer diagnosis (breast cancer versus non-breast, lung cancer versus non-lung), and baseline ECOG performance status were added to the linear mixed method analysis as independent variables. These analyses (study group by covariate by time interaction) were used to examine whether the intervention benefited a specific group (such as males or females, breast or other cancers, etc.). Although several of these factors were associated with the primary outcomes, there were no significant interactions between these factors and study group assignment.
Discussion
The study results indicate that the EASE intervention did not improve fatigue, reduce sleep disturbance, or prevent functional decline during chemotherapy. Both intervention and control groups demonstrated an increase in fatigue and decline in physical functioning. There was no difference over time between the intervention and control groups despite the fact that the EASE intervention group reported using more behavioral intervention strategies that had been taught than the control group. A positive outcome in both groups was a decrease in the average number of nighttime awakenings over time. The finding that unemployed individuals benefited more from the EASE intervention than those who were employed raises questions about the burden associated with behavioral interventions for individuals who continue to work during cancer treatment.
The results are puzzling because a previous intervention trial (3) of fatigue management using the energy conservation (ECAM) component of the current intervention demonstrated a modest reduction of fatigue during treatment. This follow-up study built upon that successful intervention by introducing an additional intervention component that was proposed to have a more powerful beneficial effect on fatigue, sleep disturbance, and functional status than was previously observed.
In order to make sense of the study results, we have considered several potential explanations. The first possibility is that differences in the design of the two studies could have influenced the outcome. In fact, there were four important differences between the two studies: type of cancer treatment, complexity of chemotherapy regimens, amount of intervention provided, and timing of the outcome measures.
In the previous ECAM clinical trial, 47% of the sample received chemotherapy, 44% had radiotherapy, and 9% got both treatment modalities concurrently. The two groups that received chemotherapy had more severe fatigue and worse functional status over time than the radiotherapy group. It is possible that the radiotherapy group benefited more from the ECAM intervention and contributed more to improvement of fatigue scores than the chemotherapy groups. Despite the EASE intervention, a significant increase in fatigue and sleep disturbance and a decline in functional status occurred from before to after chemotherapy. It is possible that the intervention was not powerful enough to overcome the negative effect of chemotherapy on symptoms and functioning.
Another difference between the two studies is that the number and complexity of chemotherapy regimens has changed dramatically over the years. In the previous study, most cytotoxic chemotherapy was given on a 21- or 28-day schedule with drugs given on the first day by bolus or infusion followed by a three- or four-week recovery period. In the EASE study, chemotherapy regimens were more variable, intensive, and complex. The FOLFOX regimen for colorectal cancer involved a 48-hour infusion every 14 days; sometimes the biologic, bevacizumab, was also given. Dose-dense chemotherapy for breast cancer consisted of four 14-day cycles of doxorubicin and cyclophosphamide followed by four 14-day cycles of paclitaxel; in some cases, trastuzumab was given with paclitaxel. For participants who received this regimen, the final data collection point coincided with the first cycle of paclitaxel. Some chemotherapy regimens for lung cancer were given weekly; others were given every week for three weeks followed by one week of recovery. All of these treatment variations could have increased the intensity of treatment-related symptoms such that an intervention focused on only two symptoms was no longer powerful enough to be effective. In fact, our measure of other treatment side effects demonstrated a dramatic increase in the intensity of symptoms over time as well as wider variability in the number and intensity of symptoms reported by study participants. It is also possible that the variability of chemotherapy regimens resulted in different patterns of fatigue and sleep disturbance for each regimen.
A third difference between the two studies was the amount of behavioral intervention provided. In both trials, the intervention was conducted by telephone in three separate sessions. The previous intervention was focused on providing information about fatigue and teaching energy conservation skills. In the current trial, information was provided about both fatigue and sleep disturbance; both energy conservation and sleep modification strategies were taught. It is possible that the amount of time spent in the intervention (intervention dose) for the EASE condition was insufficient for individuals to develop skill in using both energy conservation and sleep improvement strategies as well as incorporate these strategies into their daily lives.
Another potential explanation for the lack of intervention effectiveness could be the number of intervention sessions received by participants (dose). Only three intervention sessions were conducted in contrast to other intervention trials for multiple symptoms (19-21). In those studies, more sessions appeared to equate with more consistent benefit from the intervention. A meta-analysis has also suggested that 8 to 10 sessions of psycho-educational intervention are needed to achieve maximal behavior change (57).
A fourth difference in the two studies was the timing of the outcome measures. In the previous ECAM trial, measurements were taken prior to the first chemotherapy cycle and three days after the second and third chemotherapy treatments. Fatigue scores measured prior to the initiation of chemotherapy were lower than the two measurements taken after the next two chemotherapy sessions. Reasoning that baseline scores for fatigue and sleep disturbance due to chemotherapy should be evaluated before and after treatment, baseline measures of the primary outcomes in the current study occurred before treatment and four days after chemotherapy treatment. Then changes in the primary outcomes (if present) would be more clearly attributed to the EASE intervention. Despite the improved study design, we did not observe the expected decrease in fatigue and sleep disturbance or improvement of functional status for the EASE group; indicating that the intervention did not have the predicted effect. Given the complexity and variability of the chemotherapy regimens in this study, it is possible that the timing of our measures did not capture symptom changes due to the intervention because individuals receiving weekly or biweekly therapy may have an up-and-down pattern of symptoms corresponding to each dose of CTX (58).
Another potential explanation for the result is the possibility that a large number of participants had low fatigue and/or sleep disturbance or symptoms that were not severe enough to demonstrate improvement due to the EASE intervention. Three days after the first chemotherapy treatment, 29% of the EASE sample reported fatigue scores less than 4 on a 1 to 10 scale indicating that almost one-third of study participants had a low level of fatigue. Likewise, 50% of participants rated their usual sleep quality as “fairly good” or “very good” prior to intervention; also actigraph results demonstrated that two-thirds of the sample had sleep efficiency ratings (the ratio of time asleep and total time in bed) of 85% or greater three days after chemotherapy which is considered normal. This suggests the presence of a “floor” effect that could have influenced study results; it also argues against the assumption that everyone who gets chemotherapy will have a high level of fatigue or sleep disturbance requiring intervention.
Future Directions
Several lessons learned form the basis for recommendations for future symptom management studies. We noted that the timing of measures was not optimal in the context of the complex treatment regimens that were used in the EASE study. There are at least two ways to approach this problem. First, it may be possible to limit variability by limiting study eligibility to a few diagnoses and treatment regimens. However, this may still leave considerable variability. Using the example of breast cancer treatment, one study noted sixteen regimens at least four of which were commonly used (24). It is also likely that new regimens will continue to be introduced during the course of a symptom management trial. A second approach would be more frequent monitoring of symptoms. However, this approach also has deficiencies with regard to patient burden and adherence. It is critical to identify efficient and simple methods for symptom monitoring such as automated systems (59-63).
Another issue that needs exploration is the amount or “dose” of symptom intervention. Intervention efficacy could be related to the dose of intervention (number and frequency of intervention sessions), yet little is known about the dose of intervention needed to alleviate multiple symptoms. While most behavioral intervention studies have described the “intended” time or amount of intervention, few studies have examined the “actual” amount of intervention delivered. It is not clear whether an optimal dose of intervention is a fixed amount or a variable dose that is adapted to symptom severity. Future intervention trials would benefit from an exploration and possibly tailoring the intervention dose. In addition, trials involving a tailored intervention targeting symptoms as part of a symptom cluster would be beneficial.
Attention must also be paid to symptom severity as a potential eligibility criterion for symptom management trials. A run-in period could be used to monitor symptoms so a minimum level of symptom severity can be documented for eligibility to participate. Finally, it may be necessary to compare a symptom intervention with a usual care control group.
Despite the lack of positive findings from this clinical trial, it is essential to continue to examine behavioral interventions for symptom management during chemotherapy. There are several reasons for this recommendation. First, many individuals with multiple symptoms during chemotherapy could benefit from effective behavioral interventions. “Behavioral” interventions are identified because symptom management focuses primarily on patient behavior. Although medication may be prescribed for a symptom, symptom management focuses on the use of drugs and behavioral strategies for optimal benefit. Second, the results of previous research suggest that benefit can be derived from symptom management conducted over time by skilled nurses (19-21). Further research could inform nurses of the most effective management methods to control symptoms.
Acknowledgments
The authors would like to thank the following persons for their contributions to this research: Mary Alice Tinari, RN, of Fox Chase Cancer Center, for her work in recruitment and interventions, Karen Lindau for her assistance with recruitment, and Jia-Wen Guo and Bharathi Vedula for their work on data entry and management at the University of Utah's College of Nursing.
This study was supported by the National Institute of Nursing Research (R01NRO4573).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barsevick AM. The concept of symptom cluster. Semin Oncol Nurs. 2007;23(2):89–98. doi: 10.1016/j.soncn.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum Online. 2007;34(5):971–980. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 3.Barsevick AM, Dudley W, Beck S, et al. A randomized clinical trial of energy conservation for cancer-related fatigue. Cancer. 2004;100:1302–1310. doi: 10.1002/cncr.20111. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 5.Gaston-Johansson F, Fall-Dickson JM, Bakos AB, Kennedy MJ. Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Pract. 1999;7(5):240–247. doi: 10.1046/j.1523-5394.1999.75008.x. [DOI] [PubMed] [Google Scholar]
- 6.Glover J, Dibble SL, Dodd MJ, Miaskowski C. Mood states of oncology outpatients: does pain make a difference? J Pain Symptom Manage. 1995;10(2):120–128. doi: 10.1016/0885-3924(94)00073-t. [DOI] [PubMed] [Google Scholar]
- 7.Smets EM, Visser MR, Willems-Groot AF, et al. Fatigue and radiotherapy: (B) experience in patients 9 months following treatment. Br J Cancer. 1998;78(7):907–912. doi: 10.1038/bjc.1998.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Given CW, Given B, Azzouz F, Kozachik S, Stommel M. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. J Pain Symptom Manage. 2001;21(6):456–466. doi: 10.1016/s0885-3924(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 9.Given BA, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res. 2001;50(4):222–232. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer: using a mediation model to test a symptom cluster. Oncol Nurs Forum. 2005;32(3):E48–E55. doi: 10.1188/04.ONF.E48-E55. [DOI] [PubMed] [Google Scholar]
- 11.Passik SD, Kirsh K, Theobald D, et al. Use of a depression screening tool and a fluoxetine-based algorithm to improve the recognition and treatment of depression in cancer patients: a demonstartion project. J Pain Symptom Manage. 2002;24(3):318–327. doi: 10.1016/s0885-3924(02)00493-1. [DOI] [PubMed] [Google Scholar]
- 12.Passik SD. Impediments and solutions to improving the management of cancer-related fatigue. J Natl Cancer Inst Monogr. 2004;(32):136. doi: 10.1093/jncimonographs/lgh030. [DOI] [PubMed] [Google Scholar]
- 13.Passik SD, Breitbart WS. Depression in patients with pancreatic carcinoma. Diagnostic and treatment issues. Cancer. 1996;78(3 Suppl):615–626. doi: 10.1002/(SICI)1097-0142(19960801)78:3<615::AID-CNCR42>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract. 2000;14(4):275–290. discussion 291-298. [PubMed] [Google Scholar]
- 15.Stone P, Hardy J, Broadley K, et al. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79(9-10):1479–1486. doi: 10.1038/sj.bjc.6690236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone P, Richards M, A'Hern R, Hardy J. Fatigue in patients with cancers of the breast or prostate undergoing radical radiotherapy. J Pain Symptom Manage. 2001;22(6):1007–1015. doi: 10.1016/s0885-3924(01)00361-x. [DOI] [PubMed] [Google Scholar]
- 17.Williamson GM, Schulz R. Activity restriction mediates the association between pain and depressed affect: a study of younger and older adult cancer patients. Psychol Aging. 1995;10(3):369–378. doi: 10.1037//0882-7974.10.3.369. [DOI] [PubMed] [Google Scholar]
- 18.Barsevick AM, Dudley WN, Beck SL. Cancer-related fatigue, depressive symptoms, and functional status: a mediation model. Nurs Res. 2006;55(5):366–372. doi: 10.1097/00006199-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Gaston-Johansson F, Fall-Dickson JM, Nanda J, et al. The effectiveness of the comprehensive coping strategy program on clinical outcomes in breast cancer autologous bone marrow transplantation. Cancer Nurs. 2000;23(4):277–285. doi: 10.1097/00002820-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Given CW, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 21.Sarna L. Effectiveness of structured nursing assessment of symptom distress in advanced lung cancer. Oncol Nurs Forum. 1998;25(6):1041–1048. [PubMed] [Google Scholar]
- 22.Yates P, Aranda S, Hargraves M, et al. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2005;23(25):6027–6036. doi: 10.1200/JCO.2005.01.271. [DOI] [PubMed] [Google Scholar]
- 23.Ream E, Richardson A, Alexander-Dann C, et al. Supportive intervention for fatigue in patients undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage. 2006;31(2):148–161. doi: 10.1016/j.jpainsymman.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18(6):634–646. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 25.Meek PM, Nail LM, Jones LS. Internal consistency reliability and construct validity of a new measure of cancer treatment related fatigue: the general fatigue scale. Oncol Nurs Forum. 1997;24(2):334–335. [Google Scholar]
- 26.McNair DM, Lorr M, Droppleman LF. Profile of Mood States manual. 2nd. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 27.McNair DM, Lorr M, Droppleman LF. Profile of Mood states (revised) San Diego: Educational Industrial and Testing Service; 1992. [Google Scholar]
- 28.Meek PM, Nail LM, Barsevick A, et al. Psychometric testing of fatigue instruments for use with cancer patients. Nurs Res. 2000;49(4):181–190. doi: 10.1097/00006199-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1 Spec):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 31.Beck S, Schwartz A, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27(2):140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Berger AM, Wielgus KK, Young-McCaughan S, et al. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36(2):191–199. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown AC, Smolensky MH, D'Alonzo GE, Redman DP. Actigraphy: a means of assessing circadian patterns in human activity. Chronobiol Int. 1990;7(2):125–133. doi: 10.3109/07420529009056964. [DOI] [PubMed] [Google Scholar]
- 34.Hauri P, Wisbey J. Actigraphy and insomnia: a closer look. Part 2. Sleep. 1994:408–410. [Google Scholar]
- 35.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30(10):1317–1324. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14(3):201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger AM, Farr LA, Kuhn BR, et al. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33(4):398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo HH, Chiu MJ, Liao WC, Hwang SL. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc. 2006;105(1):64–69. doi: 10.1016/S0929-6646(09)60110-8. [DOI] [PubMed] [Google Scholar]
- 39.Levin RD, Daehler MA, Grutsch JF, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. 2005;93(11):1202–1208. doi: 10.1038/sj.bjc.6602859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 41.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in pain research and therapy. Vol. 12. New York: Raven Press; 1989. pp. 391–402. [Google Scholar]
- 42.Cleeland CS. Research in cancer pain. What we know and what we need to know. Cancer. 1991;67(3 Suppl):823–827. doi: 10.1002/1097-0142(19910201)67:3+<823::aid-cncr2820671412>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Nail LM, Jones LS, Greene D, Schipper DL, Jensen R. Use and perceived efficacy of self-care activities in patients receiving chemotherapy. Oncol Nurs Forum. 1991;18(5):883–887. [PubMed] [Google Scholar]
- 44.Greene D, Nail LM, Fieler VK, Dudgeon D, Jones LS. A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Pract. 1994;2(1):57–62. [PubMed] [Google Scholar]
- 45.King KB, Nail LM, Kreamer K, Strohl RA, Johnson JE. Patients' descriptions of the experience of receiving radiation therapy. Oncol Nurs Forum. 1985;12(4):55–61. [PubMed] [Google Scholar]
- 46.Ware J, Jr, Kosinski M, Keller S. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliabilty and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 48.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 49.Thome B, Hallberg IR. Quality of life in older people with cancer -- a gender perspective. Eur J Cancer Care. 2004;13(5):454–463. doi: 10.1111/j.1365-2354.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 50.Dajczman E. Does NSCLC patient-rated performance status predict survival more accurately than physician ratings?. Journal of Clinical Oncology; 2007 ASCO Annual Meeting; 2007. p. 9022. [Google Scholar]
- 51.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–216. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 52.Brownlee S, Leventhal H, Leventhal EA. Regulation, self-regulation, and construction of the self in the maintenance of physical health. In: Boekaerts M, Zeidner M, Pintrich PR, editors. Handbook of self-regulation. San Diego: Academic Press; 2000. pp. 369–416. [Google Scholar]
- 53.Diefenbach MA, Leventhal H. The common-sense model of illness representation: theoretical and practical considerations. J Social Distress Homeless. 1996;5(1):11–38. [Google Scholar]
- 54.Barsevick AM, Whitmer K, Walker L. In their own words: using the common sense model to analyze patient descriptions of cancer-related fatigue. Oncol Nurs Forum. 2001;28(9):1363–1369. [PubMed] [Google Scholar]
- 55.Brown J, Byers T, Thompson K, et al. Nutrition during and after cancer treatment: a guide for informed choices by cancer survivors. CA Cancer J Clin. 2001;51(3):153–187. doi: 10.3322/canjclin.51.3.153. quiz 189-192. [DOI] [PubMed] [Google Scholar]
- 56.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for mixed models. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 57.Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: a meta-analysis of randomized experiments. Health Psychol. 1995;14(2):101–108. doi: 10.1037//0278-6133.14.2.101. [DOI] [PubMed] [Google Scholar]
- 58.Erickson J, Berger AM. Sleep-wake disturbances. In: Brown C, editor. A guide to oncology symptom management. Pittsburgh: Oncology Nursing Society; in press. [Google Scholar]
- 59.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31(1):85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 60.Mooney KH, Beck SL, Friedman RH, Farzanfar R. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract. 2002;10(3):147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 61.Brown CG, Beck SL, Peterson DE, et al. Patterns of sore mouth in outpatients with cancer receiving chemotherapy. Support Care Cancer. 2009;17(4):413–428. doi: 10.1007/s00520-008-0509-y. [DOI] [PubMed] [Google Scholar]
- 62.Carpenter JS, Rawl S, Porter J, et al. Oncology outpatient and provider responses to a computerized symptom assessment system. Oncol Nurs Forum. 2008;35(4):661–669. doi: 10.1188/08/ONF.661-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stepanski EJ, Walker MS, Schwartzberg LS, et al. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]