Summary
The signal transducers of the Transforming Growth Factor β (TGFβ)/Bone Morphogenetic Protein (BMP), the Smads, promote the expression of a subset of miRNAs by facilitating the cleavage reaction by Drosha. The mechanism that limits Smad-mediated processing to a selective group of miRNAs remained hitherto unexplored. In this study, we expand the number of TGFβ/BMP-regulated miRNAs (T/B-miRs) to 20. Interestingly, a majority of T/B-miRs contain a consensus sequence (R-SBE) within the stem region of the primary transcripts of T/B-miRs (pri-T/B-miRs). Here, we demonstrate that Smads directly bind the R-SBE. Mutation of the R-SBE abrogates TGFβ/BMP-induced recruitment of Smads, Drosha, and DGCR8 to pri-T/B-miRs, and impairs their processing, while introduction of R-SBE to unregulated pri-miRNAs is sufficient to recruit Smads and allow regulation by TGFβ/BMP. Thus, Smads are multifunctional proteins which modulate gene expression transcriptionally through DNA binding, and post-transcriptionally by pri-miRNA binding and regulation of miRNA processing.
Introduction
miRNAs are noncoding single-stranded (ss) RNA molecules of ~21–25 nucleotides (nt) in length. miRNAs regulate gene expression by targeting mRNAs in a sequence-specific manner and triggering translational repression or mRNA degradation (Kim et al., 2009). The sequence of many miRNAs is conserved between distantly related organisms, and microRNAs function in numerous biological processes (Niwa and Slack, 2007). Moreover, aberrant miRNA expression is associated with developmental abnormalities and human diseases, including cardiovascular disorders and cancer (Slack and Weidhaas, 2006; Small et al., 2010). Thus, it is essential to understand the mechanisms that regulate miRNA expression.
miRNAs are first transcribed by RNA polymerase II as long primary transcripts, termed pri-miRNAs, containing a 5' cap and poly(A) tail. Pri-miRNAs are processed in the nucleus by the RNase III enzyme Drosha, releasing a ~65–70 nt hairpin shaped precursor miRNA (pre-miRNA). The Pre-miRNA is then exported to the cytoplasm and undergoes a second processing step by the RNase III Dicer, resulting in a ~22 nt mature miRNA-miRNA* duplex. The mature miRNA is then incorporated into the RNA-induced silencing complex (RISC) where it mediates silencing of target genes (Carthew and Sontheimer, 2009; Kim et al., 2009).
The biogenesis of miRNA is regulated at multiple steps in response to physiological stimuli, and the mechanisms involved are beginning to be outlined (Kim et al., 2009). The genomic regions encoding miRNAs display the defining features of the promoters of protein coding genes, including specific histone modifications, CpG islands, TATA box, transcription initiator elements, and transcription factor binding sites (Ozsolak et al., 2008). This similarity suggests that miRNA expression may be regulated transcriptionally. Indeed, several transcription factors have been identified to control miRNA expression (Davis and Hata, 2009).
Drosha processing of pri-miRNA takes place concurrently with or shortly after transcription (Morlando et al., 2008). Drosha associates with at least 20 distinct polypeptides, including DGCR8 (also known as Pasha) and RNA helicases p68 or p72, to form a large “microprocessor” complex (Gregory et al., 2004). A typical metazoan pri-miRNA consists of a 33-bp stem, a terminal loop, and ssRNA flanking segments. Drosha transiently interacts with the pri-miRNA stem, cleaving at ~11 bp from the ssRNA-dsRNA junction to generate the pre-miRNA (Han et al., 2006). DGCR8, which can directly interact with pri-miRNA, assists this process by correctly positioning Drosha on the pri-miRNA (Han et al., 2006). The exact role of p68 or p72 in the Drosha microprocessor complex is less clear, but a gene deletion study in mouse indicated that p68 and p72 are required for the biogenesis of a subset of miRNAs (Fukuda et al., 2007). Other proteins may also interact with Drosha or the pri-miRNA, with varying degrees of specificity. For instance, Lin28 and nuclear ribonucleoprotein (hnRNP) A1 have been shown to bind to the terminal loop region of pri-let-7 and pri-miR-18a, respectively, and alter cleavage by the Drosha microprocessor complex (Guil and Caceres, 2007; Michlewski et al., 2008; Viswanathan et al., 2008). The precise number or identity of miRNAs that are regulated at the level of the first cleavage step is unclear. However, the inefficiency of processing of some pri-miRNAs by the Drosha/DGCR8 complex suggests that auxiliary factors are critical for the processing of a number of pri-miRNAs.
We have previously shown that TGFβs and BMPs, two subgroups of factors within the TGFβ family, mediate the rapid, post-transcriptional, induction of miR-21 and miR-199a in human primary pulmonary smooth muscle cells (PASMCs). We observed that R-Smads, the transducers of TGFβ and BMP signals, translocate to the nucleus in response to ligand stimulation, associate with the large Drosha/DGCR8/p68 microprocessor complex and facilitate the cleavage of pri-miRNA to pre-miRNA by Drosha. However, the molecular mechanism that selects miR-21 and miR-199a for regulation by the TGFβ-Smad pathway remained unclear.
In this study, we identified an expanded set of miRNAs which are regulated post-transcriptionally by TGFβ and BMP signaling (T/B-miRs). Surprisingly, the stem region of primary transcripts of T/B-miRs contains a conserved sequence similar to the Smad binding element (SBE) found in the promoters of TGFβ/BMP regulated genes (Massague et al., 2005). We demonstrate that Smads directly associate with R-SBE through the amino-terminus MH1 domain. Mutations in the R-SBE abolish the TGFβ/BMP-mediated induction of pre-miRNA synthesis and impair pri-miRNA binding to Drosha and DGCR8 in vivo. Altogether, these results suggest that sequence-specific association of Smads to pri-miRNAs provides a platform for Drosha and DGCR8 docking and mediates more efficient cleavage by Drosha. Versatile nucleic acid recognition by Smad proteins provides a mechanism of selection and regulation of a set of pri-miRNAs by the TGFβ/BMP signaling pathway.
Results
Identification of miRNAs regulated by TGFβ signaling pathway
To identify miRNAs regulated by R-Smads similarly to miR-21 and miR-199a (Davis et al., 2008), we performed a miRNA microarray profiling analysis (Applied Biosystems) of PASMCs stimulated with BMP4 or TGFβ for 24 hr. Unsupervised hierarchical clustering analysis was performed on miRNAs significantly altered by TGF-β or BMP4 treatment (>1.6 fold), this analysis revealed 4 major clusters (Fig. 1A). Approximately 5% of the miRNAs analyzed (20 out of 377), including miR-21 were induced more than 1.6-fold by both BMP4 and TGFβ (Fig. 1A, Cluster 1). In contrast to the large number of miRNAs upregulated by BMP4 or TGFβ, few miRNAs were repressed by these growth factors (Fig. 1A, Cluster 4). As we previously identified miR-21 and miR-199a as increased by both TGFβ and BMP4, we focused on miRNAs in Cluster 1 (T/B-miRs). Analysis of over-represented motifs within the sequences of T/B-miRs revealed the presence of a consensus sequence (5'-CAGAC-3') in the stem region on average 10 bp from the terminal loop (Fig. 1B). Interestingly, the consensus sequence is similar to the Smad binding element (SBE) found in the promoter region of TGFβ target genes, such as plasminogen activator inhibitor type-1 (PAI-1), TGFβ1, α2(I) collagen, and germline Igα constant region (Dennler et al., 1998; Massague et al., 2005) and thus we call it RNA SBE (R-SBE). While 17 out of 20 T/B-miRs contain R-SBE, none of the unregulated miRNAs contained R-SBE (0 out of 38). Thus, R-SBE is a signature sequence found among most T/B-miRs.
Fig.1. miRNA array analysis of TGFβ or BMP regulated miRNAs in PASMC.
A. Unsupervised hierarchical clustering of the fold induction of microRNAs following 24H TGFβ or BMP4 treatment compared to mock treated PASMC was performed using Gene Pattern, and displayed as a heatmap where red corresponds to induction of mature miRNA by growth factor treatment and green corresponds to reduction relative to the mock treated control. B. Sequence logo representing the conserved motif present in miRNAs in Cluster 1 is indicated. The overall height of each stack indicates the sequence conservation at that position (measured in bits). The X-axis represents the relative location of the conserved nt along the mature miRNA, for clarity, only 5bp flanking the conserved CAGAC sequence are displayed.
R-SBE is critical for the TGFβ/BMP-dependent pri- to pre-miRNA processing
To test if an R-SBE is essential for ligand-dependent recruitment of Smad and/or pri- to pre-miRNA processing, 2 bp mutations were introduced in the R-SBE of human pri-miR-21 (Fig. 2A, R-SBE M1–M3). We also generated mutants targeted to the terminal loop region (Loop mut), and a sequence upstream of the R-SBE in the stem region (5' mut) (Fig. 2A). Predicted foldings of pre-miR-21 mutants are not dramatically altered from the wild type pre-miR-21 (Fig. S1A and S1B). BMP-dependent processing of these mutants was examined in mouse C3H10T1/2 cells. As PCR primers are designed specifically for human pri- or pre-miR-21 sequence, they detect only the exogenous pri- or pre-miR-21 transcripts, thus allowing an in vivo measure of miRNA processing (Fig. 2B). Levels of the vector derived pri-miR-21 (WT or mutants) were unaltered by BMP treatment (Fig. 2B, black bars). As expected, addition of BMP4 enhanced the processing of WT pre-miR-21 (Fig. 2B WT). However, induction of pre-miR-21 was abolished when the R-SBE sequence was mutated (Fig. 2B, hatched bars). Importantly, pre-miRNA induction was abolished in the M3 mutant which contains a mutated RSBE sequence but conserved double-stranded (ds) stem structure (Fig. 2B, see also Fig. S1B). Unlike the R-SBE mutants, mutations in the terminal loop region or in the sequence adjacent to the R-SBE did not significantly alter the BMP-dependent cropping of pri-miRNA (Fig. 2B, “Loop mut” and “5' mut”). Altogether, these results demonstrate that the R-SBE sequence is critical for BMP/TGFβ-dependent induction of pri-miRNA processing of T/B-miRs (Fig. 2B).
Fig.2. The R-SBE sequence is essential for Smad-mediated regulation of Drosha processing.
A. Schematic diagram of pre-miR-21 wild type and mutant sequences. Red and blue characters indicate the mature miRNA sequence and the R-SBE sequence found in pre-miR-21, respectively. Yellow highlighted characters indicate mutations introduced. B. Mouse C3H10T1/2 cells were transfected with human pri-miR-21 expression constructs, followed by treatment with or without 3 nM BMP4 for 2 hr and subjected to qRT-PCR analysis using primers to detect exogenous pri-miR-21 or pre-miR-21, normalized to GAPDH. Fold induction by BMP relative to mock treated cells is displayed. Induction of WT, 5' mut, and Loop mut by BMP4 is statistically significant. (*P<0.05, n=3) C. Cos7 cells were transfected with pri-miR-21 expression constructs along with Flag-Smad1, Flag-Drosha or Flag-DGCR8 expression constructs, and stimulated with BMP4 for 2 hr. RNA-IP assay using anti-Flag antibody was performed, followed by PCR amplification of exogenous pri-miR-21. Relative enrichment above a non-specific control IgG antibody IP control is shown. Expression of pri- or pre-miR-21 prior to IP was examined by qRT-PCR analysis using indicated primers, normalized to GAPDH (Input). (*P<0.05, n=3) Error bars represent s.e.m. See also supplemental Fig. S1.
Consistent with the result of in vivo processing (Fig. 2B), RNA-IP analysis indicated that Smad1 was recruited to wild type (WT) pri-miR-21, as well as “Loop mut” or “5' mut” (Fig. 2C, Smad IP) following BMP4 treatment. However, both the basal and the BMP4-induced recruitment of Smad1 to the R-SBE mutants (M1–M3) was significantly decreased (Fig. 2C). Thus, the R-SBE is required for ligand-induced recruitment of Smad proteins to R-SBE miRNAs. Similar to Smad1, BMP4 stimulation enhanced recruitment of both Drosha and DGCR8 to WT or Loop mut, but not to the R-SBE mutants (Fig. 2C, Drosha IP and DGCR8 IP). Thus, recruitment of Smad to the R-SBE is essential for the ligand-induced recruitment of the Drosha/DGCR8 microprocessor complex and subsequent cropping of the pri-miRNAs. In contrast, the terminal loop and the 5' region sequences are not critical for the ability of TGFβ or BMP to induce miRNA processing and recruitment of Smad1, Drosha, and DGCR8, but do somewhat affect the basal levels of these measurements, suggesting that alterations in RNA secondary structure can alter basal recruitment of the Drosha/DGCR8 complex (Fig. S1C).
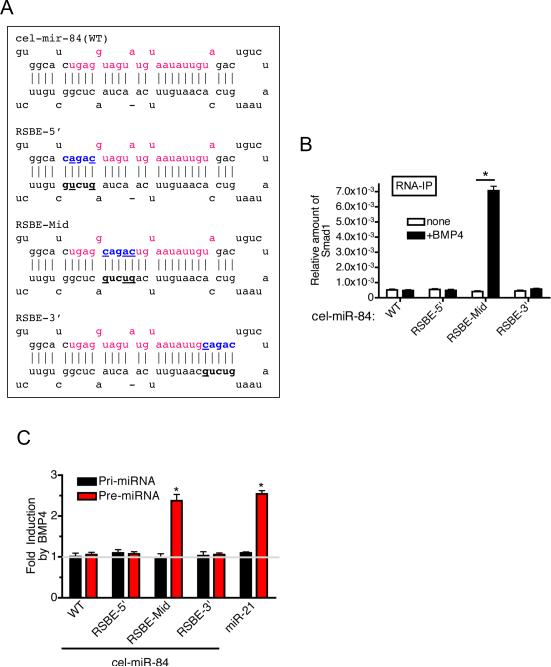
Introduction of an R-SBE is sufficient to enable BMP-mediated maturation of pri-miRNA
We examined if the introduction of an R-SBE sequence (5'-CAGAC-3') to an otherwise unregulated pri-miRNA is sufficient to confer the ability to bind to Smad, and hence TGFβ/BMP-mediated processing. An R-SBE was inserted in three locations in the stem region of a ~150 bp C. elegans pri-miR-84 construct (pri-cel-miR-84), which does not contain an R-SBE-like sequence (Fig. 3A). RNA secondary structure predictions confirmed that these mutations do not dramatically perturb the structure of pre-cel-miR-84 (Fig. S2A and S2B). BMP4-induced association of Smad1 with pri-miR-84 was examined by RNA-IP analysis in vivo (Fig. 3B). As expected, wild type pri-cel-miR-84 (WT) showed no association with Smad1 either in vitro (Fig. S3C) nor in vivo (Fig. 3B, WT). Similarly, pri-miRNAs containing the R-SBE inserted into the extreme 5' or 3' regions of the mature cel-miR-84 sequence (RSBE-5' and RSBE-3', respectively, Fig. 3A and S3B) showed no association with Smad1 in vivo (Fig. 3B). Interestingly, introduction of R-SBE the within the middle of the mature cel-miR-84 sequence, similar to the location of the R-SBE most commonly observed in T/B-miRs (Fig. 3A) confered Smad1 association upon BMP4 treatment in vivo (Fig. 3B). Furthermore, RSBE-mid pre-miRNA was increased ~2.4-fold upon BMP4 treatment, similar to the level of induction of endogenous pre-miR-21 (2.5-fold) (Fig. 3C). BMP-induced expression of mature miRNA was confirmed using a sensor construct containing complementary binding sites for mature cel-miR-84(RSBE-Mid) at the 3'-UTR of a luciferase reporter gene (Fig. S2D). These results indicate that the presence of an R-SBE within the mid region of the mature miRNA sequence is sufficient to bestow BMP-dependent Smad recruitment, as well as processing of pri-miRNA.
Fig.3. Introduction of R-SBE is sufficient for the TGFβ-regulated processing of pri-miRNA.
A. Schematic of pre-miRNA sequence of cel-miR-84 wild type and mutants. Red and blue characters indicate mature cel-miR-84 sequence and the location of the introduced R-SBE sequence, respectively. B. RNA-IP assay was performed in Cos7 cells transfected with Flag-Smad1 and different pri-cel-miR-84 constructs. Cells were treated with BMP4 for 2 hr followed by immunoprecipitaion of RNA fragments with anti-Flag antibody, and PCR amplification of pri-cel-miR-84. Fold enrichment of Flag IP relative to IgG control is presented (*P<0.01, n=3). C. Cos7 cells were transfected with pri-cel-miR-84 constructs or pri-miR-21 expression construct (positive control), followed by treatment with or without 3 nM BMP4 for 2 hr and subjected to qRT-PCR analysis using indicated primers, normalized to GAPDH. (*P<0.01, n=3). Error bars represent s.e.m. See also supplemental Fig. S2.
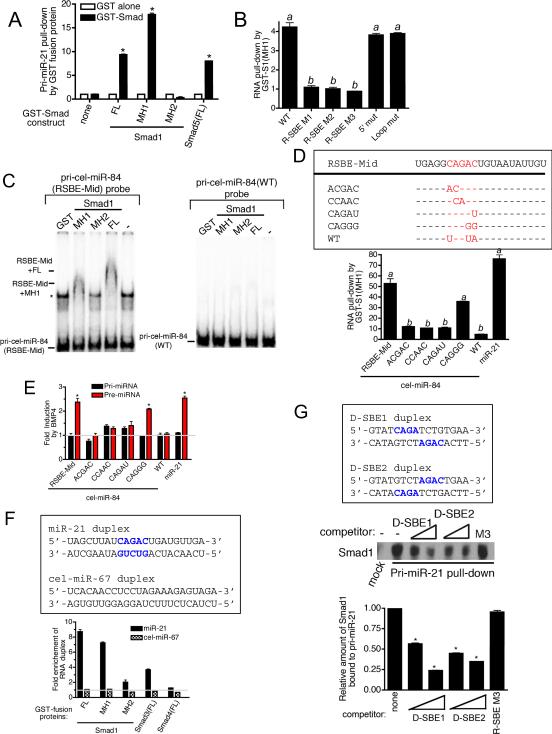
Smad MH1 domain binds to R-SBE
The sequence requirement for Smad-mediated processing, and the similarity of the R-SBE to the sequence recognized by Smads in DNA suggested that the Smads may directly associate with pri-miRNAs. To examine this possibility, we partially purified recombinant GST-Smad fusion proteins (Fig. S3A), conjugated them to glutathione S-sepharose beads, and used them to pull down in vitro transcribed ~150 nt pri-miR-21. The pri-miR-21 transcripts co-precipitating with GST-Smad fusion proteins were quantitated by qRT-PCR analysis (Fig. 4A). Full-length Smad5 [Smad5(FL)] and Smad1 [Smad1(FL)] were able to pull-down ~9-fold more pri-miR-21 compared to GST protein alone (Fig. 4A). The MH1 domain of Smad1, which is responsible for binding to DNA, precipitated ~18-fold more pri-miR-21 than GST protein alone (Fig. 4A). However, the carboxyl terminus MH2 domain of Smad1, did not bind pri-miR-21 (Fig. 4A). Thus, the MH1 domain of R-Smads directly interacts with pri-miR-21 in vitro. To determine the role of R-SBE, GST-Smad1(MH1) fusion protein was used to pull down in vitro transcribed pri-miR-21 transcripts, WT or mutants (see Fig. 2A). All three R-SBE mutants (R-SBE M1-M3) exhibited reduced binding to Smad1(MH1) compared to WT pri-miR-21 (Fig. 4B). Conversely, the Loop and the 5' mutants bound Smad1(MH1) as efficiently as WT pri-miR-21 (Fig. 4B), indicating that the R-SBE is specifically required for R-Smad binding. Specific and direct binding of Smad1 with R-SBE was confirmed by the electrophoretic mobility shift assay (EMSA) using cel-miR-84 (RSBE-Mid or WT) as a probe (Fig. 4C). Consistent with the in vivo Smad1 binding result (Fig. 3B), pri-cel-miR-84(RSBE-Mid), but not pri-cel-miR-84(WT) was able to bind to both full length Smad1 (FL), and the MH1 domain (Fig. 4C). Furthermore, an excess of unlabeled pri-cel-miR-84(RSBE-Mid) competed for the binding of Smad1(MH1) to RSBE-Mid probe, while a probe containing a mutated R-SBE, pri-cel-miR-84(RSBEmut), failed to compete (Fig. S3D). Consistently, the RSBE-Mid pri-miRNA was found to associate with Smad1(MH1) in the GST pull down assay (Fig. 4D, bottom panel). With one exception, substitutions of the nucleotides within the R-SBE of primary transcripts of RSBE-Mid (see Fig. 4D, top panel) dramatically reduced in vitro Smad1(MH1) binding (Fig. 4D, bottom panel). Although the predicted secondary structures of the mutants are not significantly altered (Fig. S2B), in vivo processing assays indicated that mutation of the R-SBE nucleotides abrogated BMP-dependent induction of pre-miRNA (Fig. 4E). Interestingly, the introduction of CAGGG resulted in a reduced but significant binding to Smad1(MH1) and BMP-dependent processing (Fig. 4D and 4E, CAGGG).
Fig.4. Direct association of Smad MH1 domain and the R-SBE.
A. In vitro transcribed wild type pri-miR-21 was mixed with indicated recombinant, sepharose bead-immobilized GST-fusion proteins. Associated RNA was eluted, and subjected to qRT-PCR analysis to detect pri-miR-21. The relative amount of pri-miR-21 pulled down with GST-Smad fusion proteins, normalized to the amount pulled down with GST alone is presented (*P<0.01, n=3). B. In vitro transcribed pri-miR-21 constructs were mixed with recombinant GST-Smad1(MH1) or GST alone and the relative amount of pri-miR-21 transcripts pulled down with GST-Smad1(MH1) fusion protein was normalized to the amount pulled down with GST alone and presented. Values labeled with the same letter do not differ significantly from one another (P<0.05, n=3). C. EMSAs were performed with 1 nM of radiolabeled pri-cel-miR-84(RSBE-Mid) (left panel) or the wild type (WT) pri-cel-miR-84 (right panel) probe and recombinant GST-Smad1(FL, MH1 or MH2) proteins. Shifted bands as a result of specific binding to Smad1 are indicated. Asterisk indicates a non-specific band. Experiment was performed 3 times and a representative blot shown. D. Schematic diagram of mature miRNA sequence of wild type cel-miR-84, cel-miR-84 introduced with R-SBE (RSBE-Mid) and mutants (top panel). In vitro transcribed pri-cel-miR-84(RSBE-Mid), pri-miR-21 (positive controls), wild type cel-miR-84 (negative control) or R-SBE mutants were mixed with recombinant GST-Smad1(MH1) or GST alone and the relative amount of pri-miRNAs pulled down with GST-Smad1(MH1) fusion protein was normalized to the amount pulled down with GST alone and presented (bottom panel). Values labeled with the same letter do not differ significantly from one another (P<0.05, n=3). E. C3H10T1/2 cells were transfected with pri-cel-miR-84 constructs (WT or mutants) or pri-miR-21 expression construct (control), followed by treatment with or without 3 nM BMP4 for 2 hr and subjected to qRT-PCR analysis using indicated primers, normalized to GAPDH. (*P<0.01, n=3). F. Synthetic RNA duplexes (miR-21 or cel-miR-67) were mixed with recombinant GST alone or indicated GST-Smad fusion proteins. Fold enrichment of RNA duplex pulled down with GST-Smad fusion proteins over GST protein alone is presented. G. 3- or 30-fold molar excess of synthetic DNA duplex; SBE1 or SBE2, or 30-fold molar excess of in vitro transcribed pri-miR-21 R-SBE mutant 3 (R-SBE-M3) were added during Smad1 pull-down assay using in vitro transcribed pri-miR-21(WT) conjugated to agarose beads. The amount of Smad1 associated with pri-miR-21 in the presence of competitors was examined by immunoblot analysis with anti-Smad1 antibody (top panel). The relative amount of Smad1 bound to pri-miR-21 was quantitated by densitometry and presented (bottom panel). Error bars represent s.e.m. See also supplemental Fig. S3.
The R-SBE-dependent interaction between R-Smad and pri-miRNAs was further analyzed using pri-miR-21 transcripts, either wild type or mutants (5' Loop or R-SBE M3), that were immobilized to agarose beads and used to precipitate associated proteins from BMP4-treated nuclear extracts. The association of Smad1, p68, and Drosha with pri-miR-21 was examined by immunoblotting. WT, “5' mut”, or “Loop mut” associated with Smad1 and Drosha at a similar level, while the R-SBE M3 showed reduced interaction (Fig. S3F). Unlike Smad1 or Drosha, association of p68 with pri-miR-21 did not require the R-SBE (Fig. S3A). Furthermore, while the association of immobilized pri-miR-21 (WT) with Smad1 could be competed with an excess of in vitro transcribed pri-miR-21, WT or “Loop mut”, R-SBE M3 did not compete for binding to Smad1 (Fig. S3G). The mutation of the terminal loop region exhibited no effect on Smad-pri-miRNA association (Fig. S3G), however, the presence of the loop could still play a structural role in facilitating the interaction with R-Smads. Thus, we examined the direct association of bacterially-expressed and partially purified GST-Smad(MH1) with a synthetic 22 bp RNA duplex with sequence matching miR-21 (Fig. 4F, top panel). As a control, RNA duplex with C. elegans miR-67 (cel-miR-67) sequence, which does not contain a R-SBE, was used (Fig. 4F, top panel). GST-fused Smad1(FL) and Smad3(FL) were found to specifically bind to the miR-21 duplex, while Smad4(FL) did not interact with either miR-21 or control duplexes (Fig. 4F). This is consistent with the previous observation that R-Smads but not Smad4 are essential for the regulation of pri-miRNA processing (Davis et al., 2008). The MH1 domain of Smad1 was sufficient for specific interaction with miR-21 duplex, similar to the previous report of Smad-DNA association (Shi et al., 1998) (Fig. 4F, MH1). Furthermore, deletion of the MH1 domain of Smad1 dramatically reducted miR-21 binding (Fig. 4F, MH2). Altogether, these results indicate that the MH1 domain of R-Smads recognizes and binds the R-SBE in the context of a short dsRNA stem. EMSA analysis further confirms that a double stranded R-SBE is required as a single-stranded RNA complimentary to the SBE (ss-R-SBE) or single stranded mature miR-21 (ss-miR-21) failed to compete for Smad binding (Fig. S3D and S3E). Although the MH1 domain is sufficient for the recognition and binding to R-SBE containing pri-miRNAs, the MH1 domain of Smad1 alone was unable to promote pri-miRNA processing in vivo of either pri-miR-21 or pri-cel-miR-84(RSBE-Mid) (Fig. S3H). This observation suggests that other regions of R-Smad, such as the MH2 domain, which interacts with p68, are essential for the regulation of the pri-miRNA processing (Davis et al., 2008).
To gain further insight into the interaction of Smads with R-SBE, we examined whether a DNA duplex containing SBE sequence could compete with pri-miR-21 for binding R-Smad. Binding of Smad1 to pri-miR-21 was examined in the presence of 3- or 30-fold molar excess of competitors consisting of 16 bp DNA oligonucleotides with SBE sequence (D-SBE1 or D-SBE2), which has been shown to bind to two molecules of the MH1 domain of R-Smad in vitro (Shi et al., 1998). As a negative control, we used in vitro transcribed pri-miR-21 R-SBE M3 (see Fig. 2A), which does not bind R-Smads (see Fig. 4B and Fig. S3F and S3G). Unlike R-SBE M3, DNA duplexes containing SBE competed with pri-miR-21 for Smad1 binding (Fig. 4G). Similarly, binding of Smad1(MH1) to cel-miR-84(RSBE-Mid) or pri-miR-21 was competed by D-SBE2 in EMSA (Fig. S3D and S3E). Altogether, these results suggest that R-Smad binds to D-SBE and R-SBE with a similar affinity and that a similar region of the R-Smad MH1 domain is responsible for the binding to both R-SBE and D-SBE.
Post-transcriptional regulation by TGFβ/BMP4 of miRNAs containing R-SBE
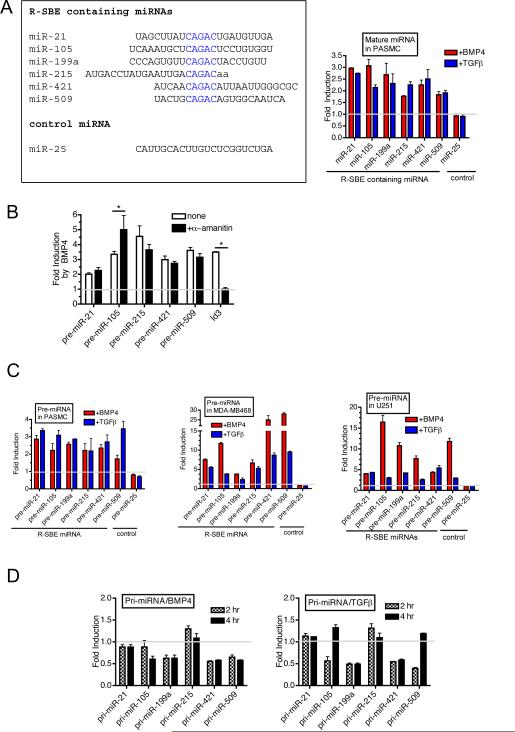
Next, we analyzed the expression of four miRNAs (miR-105, 215, 421, and 509) that contain an R-SBE (5'-CAGAC-3') (R-SBE miRNAs), in parallel with previously identified R-SBE miRNAs; miR-21 and miR-199a following TGFβ and BMP4 stimulation (Fig. 5A). miR-25, which does not contain an R-SBE and is not regulated by TGFβ or BMP4, was included as a negative control (Fig. 5A). qRT-PCR analysis confirmed that all six R-SBE miRNAs were rapidly induced ~2–3-fold within 6 hr of TGFβ or BMP4 treatment in PASMCs (Fig. 5A). Induction of R-SBE miRNAs by BMP4 was conserved in the presence of an inhibitor of RNA polymerase II α-amanitin, supporting the post-transcriptional regulation of these R-SBE miRNAs by BMP4 (Fig. 5B). Importantly, induction of a transcriptional target of BMP4, Id3 was abolished, confirming the effectiveness of transcriptional inhibition by α-amanitin (Shepherd et al., 2008) (Fig. 5B). R-SBE pre-miRNAs were induced upon TGFβ or BMP4 treatment within 2 hr in PASMC (Fig. 5C, PASMC) and in the human breast carcinoma cell line MDA-MB-468, which has a deletion in the gene encoding Smad4, an essential cofactor for transcriptional regulation by R-Smads (Fig. 5C, MDA-MB-468). These results suggest that the induction of RSBE miRNAs is Smad4-independent and likely to occur post-transcriptionally (Davis et al., 2008). A time course study indicated that induction of pre-miRNAs in PASMC is generally rapid, with a significant increase observed as early as 2 hr after BMP4 or TGFβ stimulation (Fig. S4A). It has been demonstrated that, similar to the Smads, p53 associates with p68 and Drosha to facilitate the Drosha processing of a sub-set of miRNAs, which are different from R-SBE miRNAs (Suzuki et al., 2009). In MDA-MB-468 cells, which express a mutant form of p53(R273H), induction of pre-miRNA expression of R-SBE miRNAs by BMP4/TGFβ treatment (average ~ 14-fold induction) is stronger than that of PASMCs (average ~ 2-fold induction), which express wild type p53 (Fig. 5C). Similar to MDA-MB-468 cells, U251 glioma cells, which also express p53(R273H) but express wild type Smad4, strongly induced R-SBE pri-miRNA processing (average ~ 9-fold induction) (Fig. 5C U251). Together with the observations that downregulation of p53 by siRNA in PASMC potentiated the BMP4-mediated induction of R-SBE pre-miRNAs (Fig. S4B), and overexpression of p53(WT) in MDA-MB-468 cells antagonized the induction of R-SBE pre-miRNA expression by BMP4 treatment (Fig. S4C), these results suggest that the TGFβ/BMP-dependent regulation of Drosha processing of R-SBE containing pri-miRNAs is affected by the cellular p53 status. These results also raise the interesting possibility that other p68-associating proteins, such as ERα (Fukuda et al., 2007), could affect the Drosha processing of T/B-miRs.
Fig.5. Identification of miRNAs regulated by the TGFβ signaling pathway post-transcriptionally.
A. Sequence alignment of analyzed miRNAs containing R-SBE (left panel). Levels of expression of mature miRNAs, normalized to U6, were examined in PASMCs treated with 3nM BMP4 or 400pM TGFβ1 for 6 hr (right panel). miR-25 does not contain R-SBE and is not regulated by BMP4 or TGFβ1. Fold induction after treatment relative to mock treated PASMC is presented. B. PASMCs were pre-treated with RNA pol II inhibitor α-amanitin for 5 hr followed by treatment with or without 3 nM BMP4 for 2 hr and subjected to qRT-PCR analysis using primers to detect pre-miRNAs or Id3, normalized to GAPDH. Fold change relative to untreated cells is presented. (*P<0.05, compared to no treatment, n=3). C. Human PASMCs, MDA-MB-468 cells, or U251 cells were treated with 3 nM BMP4 or 400 pM TGFβ1 for 2 hr and subjected to qRT-PCR using indicated primers, normalized to GAPDH. The fold induction relative to mock treated sample is presented. D. Time-course expression of indicated pri-miRs, normalized to GAPDH, was examined by qRT-PCR in PASMCs stimulated with 3 nM BMP4 (left panel) or 400 pM TGFβ1 (right panel) for 2 or 4 hr. Fold induction compared to untreated samples are presented. Error bars represent s.e.m. See also supplemental Fig. 4 and supplemental table 5.
We next examined the expression of the primary transcripts of R-SBE miRNAs (R-SBE pri-miRNAs). R-SBE pri-miRNAs were not significantly increased after 2 or 4 hr of TGFβ or BMP4 treatment, prior to the induction of mature miRNA at 6 hr. (Fig. 5D). Rather, the majority of the R-SBE pri-miRNA levels were decreased upon TGFβ or BMP4 stimulation (Fig. 5D), suggesting that a rapid induction of processing from primary to precursor miRNA causes a transient reduction of pri-miRNAs. Altogether, these results confirm that the TGFβ/BMP4 pathway post-transcriptionally regulates the R-SBE miRNAs examined (Davis et al., 2008).
R-Smads facilitate recruitment of Drosha to R-SBE-containing pri-miRNAs
RNA-immunoprecipitation (RNA-IP) indicated that BMP4 strongly induces the recruitment of R-Smads and Drosha to the primary transcripts of R-SBE miRNAs (Fig. 6A). No association of these proteins with the transcripts of the TMEM49 gene, which is encoded upstream of miR-21, was detected (Fig. 6A, TM). Furthermore, BMP4 treatment did not alter recruitment of R-Smads or Drosha to transcripts of miRNAs that are not regulated by BMP/TGFβ signaling, such as miR-214 and miR-222 (Fig. 6A), establishing a correlation between the presence of an R-SBE in endogenously expressed primary miRNA transcripts and the ability to recruit R-Smads and Drosha in response to TGFβ family ligands. To examine whether R-Smads are specifically required for recruitment of Drosha to pri-miRNAs of R-SBE miRNAs, we knocked down endogenous BMP-specific R-Smads [Smad1 and Smad5; Smad8 is not expressed in PASMC (Fig. S5B)] by siRNA (Fig. S5A). Knockdown of Smad1/5 abolished induction of a transcriptional target of BMP4; Id3 gene (Fig. S5B), however, it did not significantly affect the level of expression of R-SBE pri-miRNAs, confirming that transcription of the R-SBE miRNA genes does not depend on R-Smads (Fig. 6B, Pri-miRNA). Despite little change in the pri-miRNA levels, Smad1/5 knockdown resulted in a significant reduction of pre-miRNA induction concomitant with a loss of Drosha recruitment to the transcripts of R-SBE miRNAs (Fig. 6B, Pre-miRNA and Drosha IP). Thus, R-Smads facilitate the recruitment of Drosha to R-SBE containing pri-miRNAs.
Fig.6. Smad is essential for recruitment of Drosha.
A. RNA-IP assay was performed in PASMCs treated with BMP4 for 2 hr followed by immunoprecipitaion of RNA fragments with anti-Smad1/5 antibody, anti-Drosha antibody, or non-specific IgG (IgG), and PCR amplification was performed to detect indicated pri-miRNAs. Fold induction of binding relative to untreated PASMC is presented. Primers for miR-214 and -222 serve as negative controls as they are not regulated by TGFβ or BMP. A primer set recognizing the TMEM49 coding region (TM) was used as an additional negative control. (*P<0.001, n=3) B. PASMCs were transfected with non-targeting control siRNA (si-Control) or mixture of siRNAs for Smad1 and Smad5 (si-Smads). Twenty-four hr after transfection, cells were treated with 3 nM BMP4 for 2 hr, and subjected to RNA-IP analysis to examine recruitment of Drosha to indicated pri-miRNAs or TM using anti-Drosha antibody. The relative enrichment over IgG control IP is shown. qRT-PCR analysis of pri-miRNAs and pre-miRNAs, normalized to GAPDH, prior to immunoprecipitation is shown (input). As a control for R-smad knockdown, the level of Id3, normalized to GAPDH was monitored. (*P<0.001, n=3). Error bars represent s.e.m. See also supplemental Fig. S5.
Discussion
In this study we demonstrated that direct association of Smad proteins with a SBE-like sequence in mature miRNAs acts as a molecular tag for the preferential association of Drosha and DGCR8 with a set of pri-miRNAs, facilitating their processing by Drosha upon TGFβ or BMP4 stimulation.
Regulation of Drosha processing by DNA binding proteins
The MH1 domain of R-Smad recognizes a similar sequence in the interaction with double stranded RNA and DNA with a comparable affinity. Although transcription of R-SBE containing miRNAs in this study is not significantly regulated by Smads, it is intriguing to speculate that the expression of other R-SBE-containing miRNAs might be regulated by Smads both transcriptionally and post-transcriptionally in light of the finding that transcription and Drosha processing of some miRNA genes are closely coupled (Morlando et al., 2008). Interestingly, all of the R-SBE-containing miRNAs contain potential SBE sites within 5Kb upstream of the pre-miRNA site (Table S1). A potential difference between DNA-binding and RNA-binding of R-Smad is the requirement for Co-Smad; Smad4. Although R-Smad alone is able to bind to the SBE sequence in vitro, in vivo, it usually to binds DNA as a heteromeric complex with Smad4. We showed previously that Smad4 is not required for the regulation of pri-miRNA processing by R-Smads (Davis et al., 2008). As R-Smads are imported to the nucleus with or without Smad4 upon ligand stimulation, we speculate that R-Smad uncoupled with Smad4 could be preferentially recruited to RNA, while R-Smad/Smad4 complexes could be recruited to DNA and regulate transcription. Furthermore, as the MH1 domain of R-Smads is responsible for binding to both DNA and RNA, it is possible that R-SBE-containing pri-miRNAs might interefere with the Smad-mediated transcriptional responses by sequestering Smads from D-SBEs.
Recently, p53 and estrogen receptor α (ERα) were demonstrated to regulate processing by the Drosha microprocessor complex through interaction with p68/p72 (Suzuki et al., 2009). Similar to the Smads, p53 increases the expression of a subset of miRNAs by facilitating the processing of pri-miRNAs by the Drosha complex through interaction with RNA helicase p68 (Suzuki et al., 2009). Conversely, ERα bound to estradiol (E2) inhibits the pri- to pre- processing of a subset of miRNAs by promoting the dissociation of the Drosha microprocessor complex from pri-miRNAs (Yamagata et al., 2009). It appears that a subset of miRNAs distinct from T/B-miRs are regulated by p53 or ERα (Suzuki et al., 2009). We observed that cell lines in which p53 is mutated, such as MDA-MB-468 or U251, exhibit a greater degree of induction of pre-T/B-miRs upon ligand stimulation (Fig. 5C). It is possible that R-Smad and p53 compete for binding to p68 or other components of the microprocessor complex and hence exhibit opposing effects on the maturation of miRNAs regulated by these proteins.
It is yet unexplored what determines the selective regulation of specific miRNA processing by p53 or ERα. Some degree of specificity may be provided by the RNA helicases (p68 or p72) with which regulatory proteins interact as p68 and p72 seem to preferentially participate in the synthesis of a partially overlapping but distinct set of miRNAs (Fukuda et al., 2007). An alternative possibility is that p53 and ERα recognize and bind to a specific RNA sequence or structure in pri-miRNAs distinct from the R-SBE. It is of note that p53 is known to bind both DNA and RNA (Miller et al., 2000), thus the p53 nucleic acid binding activity might play dual functions both in transcription and maturation of pri-miRNA, similar to Smads. In addition to Smad and p53, other transcription factors known to bind both DNA and RNA include TFIIIA, Stat1, and WT1 (Cassiday and Maher, 2002). The RNA helicases p68 and p72 have been shown to interact with several additional DNA binding proteins, including MyoD, Runx2, androgen receptor, and β-catenin (Davis and Hata, 2009). Thus, it is intriguing to speculate that some of these DNA binding proteins might have dual roles and also participate in the pri-miRNA processing as a part of the Drosha microprocessor complex in the nucleus.
Variants of the R-SBE sequence
An analysis of miRNA sequences encoded in the human genome revealed that approximately 2% (15 out of 706) of the identified human miRNAs contain an R-SBE (5'-CAGAC-3', see Table 1). All the R-SBE miRNAs analyzed with the notable exception of miR-214 (see below), were induced by TGFβ or BMP4 in PASMC. Thus, we propose that an R-SBE in the mature miRNA sequence is a molecular signature for the miRNAs whose biosynthesis is controlled by the TGFβ-Smad signaling pathway. While the majority of R-SBE sequences analyzed (5/7) occur on the 5' arm of the double-stranded stem, miR-421 and miR-600 are encoded on the 3' arm, suggesting that Smad-mediated processing is independent of the strand within the hairpin. Interestingly, we have found that miR-23a and -23b, which contain a 5'-CAGGG-3' sequence, are also regulated post-transcriptionally by TGFβ and BMP4 through Smads at the first processing step (Fig. 1A). Of the miRNAs detected in the miRNA microarray and containing a 5'-CAGGG-3' sequence, 9 out of 10 are induced by TGFβ and/or BMP4 (Table 1). Additionally, pri-cel-miR-84 containing an internal CAGGG sequence in the stem binds to Smad1 and is processed in a BMP4 dependent manner in vivo (see Fig. 4C and 4D), thus, we speculate that 5'-CAGGG-3' could also serve as a Smad binding sequence. Nearly all nucleic acid residues in the R-SBE form base pairing in the stem region of pre-miRNAs. Interestingly, the second adenine residue of miR-21 does not form a base pairing and generates a single nucleotide “bulge”. In the case of miR-214, which contains a 5'-CAGAC-3' sequence but is not induced by TGFβ or BMP4, the first cytosine residue of the R-SBE is part of a 3 nt single-stranded “bubble” region. Therefore, we speculate that R-Smads may be able to associate with R-SBE containing a single nucleotide bulge but not with an R-SBE in a bubble region.
Table 1. List of CAGAC or CAGGG – containing miRNAs in the human genome.
miRNAs indicated in red were found to be induced by TGFβ and/or BMP4 in PASMCs. miRNAs indicated in green are regulated by neither TGFβ nor BMP4. miRNA in white are either not present on the miRNA array or not expressed in PASMC. miRNAs indicated in bold characters are investigated in this study. The percentage at the bottom of the table indicates the number of detected miRNAs containing the indicated sequence that are regulated by either TGFβ or BMP4.
| CAGAC | CAGGG | CAGGG (continued) |
|---|---|---|
| hsa-miR-21 | hsa-miR-23a | hsa-miR-630 |
| hsa-miR-105 | hsa-miR-23b | hsa-miR-648 |
| hsa-miR-199a-5p | hsa-miR-103 | hsa-miR-659 |
| hsa-miR-214 | hsa-miR-107 | hsa-miR-671-3p |
| hsa-miR-215 | hsa-miR-140-3p | hsa-miR-770-5p |
| hsa-miR-300 | hsa-miR-188-3p | hsa-miR-877 |
| hsa-miR-421 | hsa-miR-220c | hsa-miR-933 |
| hsa-miR-509-5p | hsa-miR-331-5p | hsa-miR-940 |
| hsa-miR-600 | hsa-miR-345 | hsa-miR-1205 |
| hsa-miR-631 | hsa-miR-487a | hsa-miR-1207-5p |
| hsa-miR-1208 | hsa-miR-487b | hsa-miR-1266 |
| hsa-miR-1284 | hsa-miR-498 | hsa-miR-1290 |
| hsa-miR-1292 | hsa-miR-513a-5p | hsa-miR-1321 |
| hsa-miR-1324 | hsa-miR-612 | hsa-miR-1909 |
| hsa-miR-623 | hsa-miR-1915 | |
| 89% | 90% |
Recruitment of Drosha and DGCR8 to R-SBE-bound Smads
We showed that association of Smads with R-SBE is essential for the ligand-induced recruitment of Drosha and DGCR8. Thus, we propose that when Smad binds to pri-miRNAs, it may provide an optimal landing site for DGCR8 and/or Drosha, and thereby promote the more efficient cleavage of specific pri-miRNAs. Alternatively, Smad may recruit or activate auxiliary factors, such as p68, which then facilitate the recruitment of Drosha/DGCR8 to specific pri-miRNAs. Unlike DGCR8, which contains two dsRNA-binding domains (dsRBD) and binds directly to pri-miRNAs, Drosha has one dsRBD and binds weakly to pri-miRNAs. The observation that the in vitro pri-miRNA processing reaction with purified Drosha is inefficient and inaccurate also points to the requirement of accessory factor(s) for the efficient cleavage of pri-miRNA by Drosha (Han et al., 2004; Han et al., 2006). We and others have previously reported that the MH2 domain of R-Smads interacts with the p68 RNA helicase (Warner et al., 2004). As p68 contains an independent RNA binding ability, it is plausible that p68 functions as an RNA binding cofactor for Smads which stabilizes Smad-R-SBE association, similar to the Smad-D-SBE association which requires various DNA binding cofactors (Massague et al., 2005).
Role of Smad-R-SBE association and the mechanism of regulation
A typical metazoan pri-miRNA consists of a 33-bp stem in which the mature miRNA is encoded ~11 bp from the dsRNA-ssRNA junction, as well as a terminal loop and ssRNA flanking sequence (Han et al., 2006). It was demonstrated that DGCR8 associates with pri-miRNAs and serves as a molecular ruler to measure the distance from the dsRNA-ssRNA junction where it positions Drosha (Han et al., 2006). We found that the R-SBE is located within the mature miRNA; 4~12 bp away from the Drosha cleavage site, and ~ 9bp away from the 5'-end of the mature miRNA. A study of chimeric pri-cel-miR-84 in which the R-SBE sequence was introduced in the different regions of the stem indicates that the position of the R-SBE within the mature miRNA is critical for the ligand-mediated regulation of processing (Fig. 4C). We hypothesize that a role of the Smad-R-SBE interaction may involve promoting the correct positioning of the Drosha/DGCR8 complex on the stem-loop structure, thus the location of the R-SBE within the pri-miRNA may be crucial for providing a correct positioning of other components of the processing machinery. Interestingly, in some cases the Drosha/DGR8 complex aberrantly recognizes the terminal loop of a pri-miRNA, resulting in an abortive processing site ~11bp from the terminal loop (Han et al., 2006). As the R-SBE is located on average 10 bp from the terminal loop, we speculate that the association of Smad with R-SBE may mask the abortive processing site and promote a productive processing reaction to give rise to the mature miRNA sequence.
Other Drosha-interacting proteins
Similar to our study on the regulation of Drosha processing by Smad proteins, it has been reported that hnRNP A1 post-transcriptionally facilitates the processing of pri-miR-18a by Drosha. hnRNP A1 binds directly to the terminal loop region of pre-miR-18a and other pre-miRNAs that contain the hnRNP A1 binding sequence. hnRNP A1 binding promotes the structural rearrangement of the miRNA stem and promotes miRNA cropping by Drosha. (Guil and Caceres, 2007; Michlewski et al., 2008). The crystal structure of the Smad-MH1 domain in complex with DNA indicates that Smad binding alters the local conformation of DNA (Shi et al., 1998). Thus, it is plausible that, similar to hnRNP A1, the association of Smads with the R-SBE may alter the local conformation of the pri-miRNA, thereby facilitating association of Drosha, DGCR8 and possibly other auxiliary factors.
In conclusion, this study uncovers the unique nature of R-Smads as multifunctional proteins which modulate mRNA expression through DNA binding, and post-transcriptionally modulate miRNA expression through RNA binding. It also provides a molecular basis for the specific regulation of a set of miRNAs by the TGFβ signaling pathway and a role of Smad proteins as accessory factors for the Drosha/DGCR8 microprocessor. Our study and others suggest that phylogenetically conserved sequences in the stems or terminal loops of pri-miRNAs may affect miRNA regulation, likely as platforms for the recruitment of accessory factors, such as Smads and hnRNP A1, which then promote efficient processing by Drosha. A conserved sequence may also serve as a mechanism to coordinate expression of a group of miRNAs in response to a growth factor signal or other physiological stimulus.
Experimental Procedures
Cell culture
Human primary pulmonary artery smooth muscle cells (PASMCs) were purchased from Lonza (#CC-2581) and maintained in Sm-GM2 media (Lonza) containing 5 % fetal bovine serum (FBS, Sigma). Cos7, U251, MDA-MB468, and C3H10T1/2 cells (American Type Culture Correction) were maintained in Dulbecco's Modified Eagle media (DMEM) supplemented with 10% FBS (Sigma). Cells were maintained at 37°C in the presence of 5% CO2.
Growth factor stimulation and transfections
Recombinant human TGFβ1 (#240-B-002) and BMP4 (#314-BP-010) were purchased from R&D Systems. All growth factor stimulations were performed under starvation conditions (0.2% FBS). All plasmid transfections were performed using Fugene6 (Roche). siRNA transfections were performed using RNAimax (Invitrogen). See supplemental information for more information.
Immunoblot and Antibodies
Immunoblot was performed as previously described (Davis et al., 2008) using Anti-Flag epitope tag (M2, Sigma), anti-p68 (clone PAb204, Upstate), anti-GAPDH (2E3-2E10, Abnova), anti-Smad1/5 (Calbiochem), anti-DGCR8 (#10996-1-AP, ProteinTech Group) and anti-Drosha (#07-717, Upstate) antibodies. See supplemental information for more information.
MiRNA microarray analysis and Identification of conserved sequence
Human microRNA array A v2.0 (Applied Biosystems) was used to quantitate microRNA levels of 377 human microRNAs according to the manufacturer's directions. Total RNA (850 ng) was isolated from PASMC treated with vehicle, recombinant human BMP4 or TGFβ1 (R&D Systems) for 24 hr. Data processing and cluster analysis of microRNAs altered at least +/− 1.6 fold by growth factor treatment was performed using GenePattern (http://genepattern.broadinstitute.org/) (Reich et al., 2006). Sequence motif detection was performed using Improbizer program (http://www.cse.ucsc.edu/~kent/improbizer/improbizer.html) on cluster 1 miRNAs. See also supplemental information.
Real-time RT-PCR analysis, RNA-Immunoprecipitation assay, and primers
Real-time RT-PCR analysis and RNA-IP were performed as previously described (Davis et al., 2008). See supplementary information for details.
Pri-miR-21 or C. elegans pri-miR-84 Expression construct
156 bp wild type or mutant pri-miR-21 sequence or 152 bp wild type or mutant pri-cel-miR-84 containing ~40nt flanking each pre-miRNA was amplified by PCR and cloned into pcDNA3.1(+). See supplementary information section for details.
Electrophoretic mobility shift assay (EMSA)
EMSA was conducted using in vitro transcribed, [32P]-UTP-labeled pri-cel-miR-84 (WT or RSBE-Mid) or WT pri-miR-21 probe. Pri-miRNA probe was mixed with recombinant Smad1(FL, MH1, or MH2) protein with or without competitor RNA or DNA as indicated. Binding reactions were conducted under a condition as described previously with minor modifications (Piskounova et al., 2008). See supplemental information for further details.
In vitro RNA synthesis, RNA affinity purification and GST-SMAD-RNA pull down assay
RNA synthesis was performed by in vitro transcription reaction using MAXIscript kit (ambion). In vitro transcribed RNAs were covalently linked to adipic acid dihydrazide agarose beads as previously described (Guil and Caceres, 2007). GST-Smad fusion proteins were expressed in E.coli, and partially purified by glutathione S-sepharose beads. See supplementary information for detailed procedures.
Statistical Analysis
The results presented are average of at least three experiments each performed in triplicate with standard errors. Statistical analyses were performed by analysis of variance, followed by Tukey's multiple comparison test or by Student's t test as appropriate, using Prism 4 (GraphPAD Software Inc.). P values of <0.05 were considered significant and are indicated with asterisks.
Supplementary Material
Acknowledgements
We thank all members of the Hata and Lagna Labs for helpful suggestions and critical discussion. We also thank Drs. N.V. Kim, and S. Kato for sharing reagents. This work was supported by grants from the National Institute of Health: HD042149 and HL082854 to A.H. and HL086572 to G. L. and from the American Heart Association: 0940095N to A.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiday LA, Maher LJ., 3rd Having it both ways: transcription factors that bind DNA and RNA. Nucleic Acids Res. 2002;30:4118–4126. doi: 10.1093/nar/gkf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hata A. Regulation of MicroRNA Biogenesis: A miRiad of mechanisms. Cell Commun Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHAmediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type1 gene. Embo J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H, Nakamura T, et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat Cell Biol. 2007;9:604–611. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Guil S, Caceres JF. The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat Struct Mol Biol. 2007;14:591–596. doi: 10.1038/nsmb1250. [DOI] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Michlewski G, Guil S, Semple CA, Caceres JF. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell. 2008;32:383–393. doi: 10.1016/j.molcel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, Suthiphongchai T, Zambetti GP, Ewen ME. p53 binds selectively to the 5' untranslated region of cdk4, an RNA element necessary and sufficient for transforming growth factor beta- and p53-mediated translational inhibition of cdk4. Mol Cell Biol. 2000;20:8420–8431. doi: 10.1128/mcb.20.22.8420-8431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol. 2008;15:902–909. doi: 10.1038/nsmb.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Slack FJ. The evolution of animal microRNA function. Curr Opin Genet Dev. 2007;17:145–150. doi: 10.1016/j.gde.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Ozsolak F, Poling LL, Wang Z, Liu H, Liu XS, Roeder RG, Zhang X, Song JS, Fisher DE. Chromatin structure analyses identify miRNA promoters. Genes Dev. 2008;22:3172–3183. doi: 10.1101/gad.1706508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, Gregory RI. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008;283:21310–21314. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- Shepherd TG, Theriault BL, Nachtigal MW. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene. 2008;414:95–105. doi: 10.1016/j.gene.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang Y-F, Jayaraman L, Yang H, Massagué J, Pavletich N. Crystal structure of a Smad MH1 domain bound to DNA: Insights on DNA-binding in TGF-ß signaling. Cell. 1998;94:585–594. doi: 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- Slack FJ, Weidhaas JB. MicroRNAs as a potential magic bullet in cancer. Future Oncol. 2006;2:73–82. doi: 10.2217/14796694.2.1.73. [DOI] [PubMed] [Google Scholar]
- Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121:1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DR, Bhattacherjee V, Yin X, Singh S, Mukhopadhyay P, Pisano MM, Greene RM. Functional interaction between Smad, CREB binding protein, and p68 RNA helicase. Biochem Biophys Res Commun. 2004;324:70–76. doi: 10.1016/j.bbrc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Fujiyama S, Ito S, Ueda T, Murata T, Naitou M, Takeyama K, Minami Y, O'Malley BW, Kato S. Maturation of MicroRNA Is Hormonally Regulated by a Nuclear Receptor. Mol Cell. 2009;36:340–347. doi: 10.1016/j.molcel.2009.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.