Abstract
Background
Tuberculosis (TB) is associated with anti-tumour necrosis factor (TNF) therapy but whether it is drug-specific remains a concern. Our objective was to describe cases of tuberculosis associated with anti-TNF therapy, identify risk factors and estimate the incidence.
Methods
An incidence study with the French population as reference and a case-control analysis. We collected, for 3 years, cases of TB among French patients receiving anti-TNF therapy, whatever the indication, with two controls treated with anti-TNF agents per case.
Results
We collected 69 cases of TB in patients treated for rheumatoid arthritis (n=40), spondylarthropathies (n=18), inflammatory colitis (n=9), psoriasis (n=1) and Behçet’s disease (n=1) treated with infliximab (n=36), adalimumab (n=28) and etanercept (n=5). None of the cases had received correct chemoprophylaxis treatment. The sex and age-adjusted incidence rate of TB was 116.7 per 100,000 patient-years. The SIR was 12.2 (95% confidence interval 9.7–15.5) and was higher for therapy with infliximab and adalimumab than for that with etanercept: 18.6 (13.4–25.8) and 29.3 (20.2–42.4) versus 1.8 (0.7–4.3), respectively. In the case-control analysis, the exposure to infliximab or adalimumab versus etanercept was an independent risk factor for TB: odds ratio=13.3 (2.6–69.0) and 17.1 (3.6–80.6), respectively. Other risk factors were age, the first year of anti-TNF treatment, and being born in an endemic area.
Conclusions
The risk of TB is higher for patients receiving monoclonal-antibody than soluble-receptor anti-TNF therapy. The increased risk with early anti-TNF treatment and the absence of correct chemoprophylaxis treatment favours the reactivation of latent TB.
Keywords: Adult; Aged; Antibodies, Monoclonal; adverse effects; therapeutic use; Arthritis, Rheumatoid; drug therapy; Behcet Syndrome; drug therapy; Case-Control Studies; Colitis; drug therapy; Female; France; Humans; Immunoglobulin G; adverse effects; therapeutic use; Male; Middle Aged; Prospective Studies; Receptors, Tumor Necrosis Factor; therapeutic use; Registries; Risk Factors; Spondylarthritis; drug therapy; Treatment Outcome; Tuberculosis; chemically induced; drug therapy; epidemiology; Tumor Necrosis Factor-alpha; immunology
Keywords: anti-TNF-alpha, tuberculosis, safety, rheumatoid arthritis, inflammatory chronic diseases
INTRODUCTION
Treatment with tumor necrosis factor (TNF) antagonists has been recognized as a risk factor of active tuberculosis (TB) in patients with immune-mediated inflammatory diseases, including rheumatoid arthritis (RA), ankylosing spondylitis (AS), Crohn’s disease (CD), psoriatic arthritis, and psoriasis.(1–7) Most cases of TB develop soon after treatment initiation and correspond to reactivation of a latent TB infection.(4, 5) Scientific organizations and health authorities worldwide have proposed recommendations for screening patients with latent TB infection and treating such patients before initiating anti-TNF treatment, and the effectiveness of these recommendations has been demonstrated.(8)
All three available TNF antagonists have been associated with increased incidence of TB. However, a possible difference between infliximab and etanercept in incidence of TB is suspected from the spontaneous reporting of TB cases to the US Food and Drug Administration (5) before September 2002, when no recommendations of screening for latent TB existed. In one study, the incidence of TB was 28 per 100,000 patient-years for patients receiving etanercept versus 54 per 100,000 for patients receiving infliximab.(9) Likewise, four registries found small differences in incidence of TB between RA patients receiving infliximab or etanercept, although not statistically significant, perhaps because in each of these registries, the number of TB cases was low (2 to 13), these registries being not powered enough to demonstrate very rare events or investigate a difference in risk depending on anti-TNF agent.(1, 2, 6, 7) No specific epidemiologic data are available for adalimumab, but results of phase-3 and -4 clinical studies suggested that use of this drug may increase the risk of TB to a level close to that of infliximab (10).
Thus, we used another strategy to investigate incidence of TB by setting up a national registry to collect all cases of TB occurring during 3 years in French patients receiving anti-TNF therapy, whatever the indication for use. The aim of this large prospective study was to describe cases of TB associated with anti-TNF therapy and their outcome; identify the risk factors of TB occurrence in patients receiving anti-TNF therapy, particularly the anti-TNF agent; and estimate the global and drug-specific incidence of TB after the establishment and widespread knowledge by clinicians of recommendations for TB prophylaxis before anti-TNF therapy.
PATIENTS AND METHODS
The French RATIO (Research Axed on Tolerance of bIOtherapies) registry was designed by a multidisciplinary group to prospectively collect all cases of TB occurring from February 1, 2004, to January 1, 2007, in patients who were receiving anti-TNF therapy.(11) This registry involved clinicians from all concerned medical specialties, the French agency of drugs Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS), and its network of 31 regional pharmaco-vigilance centres.
We conducted an incidence study and a case-control analysis to investigate the risk of newly diagnosed TB associated with the use of anti-TNF agents.
Setting
French guidelines (12) elicited in 2002, recommended anti-TB chemoprophylaxis before all anti-TNF therapy for patients presenting at least one of the following: tuberculin skin test (TST) diameter ≥ 10 mm (revised to ≥ 5 mm in July 2005), abnormal chest X-ray results with calcifications > 1 cm, previous untreated exposure to TB or episode of TB. Correct chemoprophylaxis was defined as a 9-month treatment with isoniazide or a 3-month treatment with 2 anti tuberculosis drugs including rifampicine (12).
Identification and validation of tuberculosis cases
To enhance exhaustiveness of the collection of cases of TB, different sources were used.
All cases reported to the 31 French pharmaco-vigilance regional centres were collected (reporting of severe drug-related adverse events is mandatory for clinicians in France) but also cases pointed out directly to the companies commercializing anti-TNF.
In addition, physicians from all the different French hospital centres implied either in the prescription of TNF blockers (i.e. rheumatology, internal medicine, gastroenterology and dermatology departments) and/or in the management of TB (i.e. infectious diseases centres, intensive care units, chest medicine units) were directly required to report each newly diagnosed case. A direct mail reminder 4 times a year and several communications at congresses or in specialized press encouraged them to report cases.
We obtained also cases from the Institut National de Veille Sanitaire (InVS), the French national public health surveillance system that monitors TB in France, with a mandatory clinician- and biologist-based notification system.
A detailed standardized case report form was completed for each reported case of TB (whatever the source of notification), and additional documents were collected (hospitalization summary, histological and microbiological results, and other data if necessary) to enter the validation process.
Validation of cases
Included in the RATIO registry were all cases (from all sources) with a validated diagnosis of TB according to the 10th revision of the International Classification of Diseases (A15–A19). An expert committee involving 3 experts of TB (SB, DS, OL) validated cases by consensus on the basis of the detailed standardized case report form and the additional documents.
Assessment of exposure to anti-TNF therapy
Infliximab has been available since 1999, etanercept since 2002 and adalimumab since 2004. For all, it is mandatory in France that the first prescription be from a hospital physician. For patients who received more than one anti-TNF agent, we considered three ways of addressing the exposure: first anti-TNF agent, last anti-TNF agent, and any use of the anti-TNF agent.
Incidence study
Incidence rate of TB
We estimated the annual incidence rate of TB in patients treated with anti-TNF therapy, adjusted for age and sex, with the French population used as reference. The numerator consisted in the validated cases of TB from the RATIO registry. For the denominator, we estimated the number of patient-years of receipt of anti-TNF agents in France during the 3-year period of the study (2004, 2005 and 2006) from different sources. For this purpose, we obtained the total number of doses of each anti-TNF agent sold in France for the 3 years study, both according to the national French agency of drugs (AFSSAPS), and to the 3 pharmaceutical firms (Abbott, Schering-Plough and Wyeth). Each firm provided its estimation of the number of patient-years for the 3 anti-TNF agents on the basis of the number of doses sold and the mean dosage used in each indication. These data were very consistent. We used the mean of these different estimates to derive the estimation of the number of patients receiving each anti-TNF agent that we used in the main analysis.
In addition, for sensitivity analysis, we obtained independent data from the Régime Social des Indépendants (RSI), the French health insurance fund for self-employed workers, which involves 3 million enrolees (i.e., 5% of the French population representative of the whole population) and provides claims and demographic data for use of etanercept and adalimumab.
Standardized Incidence Ratios
To assess the distribution of underlying diseases, sex and age of treated patients, we used surveys of samples of prescribing clinicians, hospital pharmacies, and nonhospital pharmacies performed by the firms, and the RSI files for the patients receiving etanercept and adalimumab.
On the basis of these data and the French annual incidence rate of TB by 10-year age and sex class from 2002 to 2006 (from the Institut National de Veille Sanitaire) (13), we estimated the standardized incidence ratio (14) to compare the TB risk associated with anti-TNF treatment with that in the French population.
Risk of TB in patients receiving anti-TNF therapy
A case-control study was performed with cases from the RATIO registry.
Cases
Cases were all validated cases of TB in the registry with a labelling indication for use of anti-TNF treatment (i.e. RA, spondylarthropathy [SpA; AS or psoriatic arthritis], UC or CD, or psoriasis).
Controls
Tuberculosis-free patients receiving anti-TNF treatment in a labelling indication were included from centres involved in the RATIO registry in a global pool of controls. From that pool, we randomly selected patients for a database of controls reflecting the proportion of patients receiving each of the three anti-TNF drug in France. Two controls per case were randomly matched by sex and underlying inflammatory disease from this database of controls. We also used a second sample of controls randomly selected from the same database of controls, with the same matching criteria (second matching). In addition, we used a third sample of controls (1 control per case), adding the time from onset of anti-TNF treatment as matching criteria (third matching).
Statistical analysis
The number of cases of TB in France during the study period determined the sample size. A descriptive analysis was performed for the whole sample.
Annual incidence rate of tuberculosis
The SIR was calculated for anti-TNF agent use as a whole and for agents used individually. The 95% confidence intervals (CIs) for each SIR were calculated, and the deviation of the SIR from 1.00 was tested assuming a Poisson distribution for the observed cases because of the small number of cases.
Case-control study
We identified the risk factors for TB by both univariate and multivariate analysis (conditional logistic regression model). The predefined factors potentially predictive of occurrence of TB considered were age, being born in a TB endemic area (according to the 2008 WHO Report WHO/HTM/TB/2008.393), duration of the underlying inflammatory disease, TST diameter at screening, chest x-ray results indicating TB sequellae at screening, last anti-TNF agent received, time from onset of anti-TNF treatment, use of a potentially immunosuppressive disease-modifying anti-rheumatic drug during the last year, use of steroids during the last year, and use of > 10 mg/day or boluses of steroids during the last year.
Factors included in the stepwise multivariate model were those associated with the status (case or control) in univariate analysis with a significance level of p<0.20. We used the bootstrap method to examine the stability of our final model (1000 replicated bootstrap samples to find candidate variables which were more often retained). A p<0.05 was considered statistically significant.
Subgroup analyses
We performed subgroup analyses for the more frequent underlying inflammatory diseases: RA and SpA
Sensitivity analyses
To better assess the drug-specific risk, we performed sensitivity analyses for the SIR and the OR measuring the risk of adalimumab or infliximab versus etanercept, varying the definition of exposure (all anti-TNF agents, by anti-TNF agent, and for patients receiving only one anti-TNF agent, receiving anti-TNF therapy during at least 6 weeks, and currently receiving anti-TNF therapy), the different denominator estimates (denominator estimates from each of the 3 pharmaceutical firms and from the RSI), and the different criteria used for matching the controls with cases (second and third matching).
The main analyses were independently performed by two statisticians. Statistical analysis involved use of SAS release 9.1 (SAS Inst., Cary, NC).
Compliance with research ethics standards
This study authorized by the ethic committee of AP-HP, GHU Nord (Institutional Review Board of Paris North Hospitals, Paris 7 University, AP-HP; authorization number 162-08). The registry was reported at clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00224562).
RESULTS
Description of the TB cases
We collected data on 75 cases of TB, and 69 cases were validated. Characteristics of the 69 patients with a validated TB diagnosis are in Table 1. In addition, one patient had diabetes, 3 a past history of cancer, none was HIV positive, and 1 had a chronic obstructive pulmonary disease. For the 58 patients receiving only one anti-TNF agent, 34 (58.6%) had received infliximab, 23 (39.7%) adalimumab and 1 (1.7%) etanercept. Two cases (and 4 controls) had also received anakinra.
Table 1.
Characteristics of the cases of tuberculosis (TB)
| TB cases (n=69) | |
|---|---|
| Age (years) | 58.5 ± 15.4 (61.0) |
| Sex (female) | 43 (62.3%) |
| Born in a tuberculosis endemic area | 14 (20.3%) |
| Underlying inflammatory disease | |
| Rheumatoid arthritis | 40 (58.0%) |
| Spondylarthopathy | 18 (26.1%) |
| Ankylosing spondylitis | 15 |
| Psoriatic arthritis | 3 |
| Crohn’s disease or ulcerative colitis | 9 (13.0%) |
| Psoriasis | 1 (1.4%) |
| Behçet’s disease | 1 (1.4%) |
| Duration of the underlying inflammatory disease (years) | 11.2 ± 8.8 (8.3) |
| Anti-TNF treatment | |
| Only one anti-TNF agent received | 58 (84.1%) |
| Two anti-TNF agents received successively | 10 (14.5%) |
| Three Two anti-TNF agents received successively | 1 (1.4%) |
| Last anti-TNF agent received | |
| Adalimumab | 28 (40.6%) |
| Etanercept | 5 (7.2%) |
| Infliximab | 36 (52.2%) |
| Ever used Adalimumab | 30 (43.5%) |
| Ever used Etanercept | 10 (14.5%) |
| Ever used Infliximab | 41 (59.4%) |
| Time since first anti-TNF treatment start§ (months) | 15.9 ± 14.4 (12.0) |
| Time since last anti-TNF treatment start§ (months) | 13.3 ± 12.4 (9.9) |
| Immunosuppressive DMARDs*during the last year | 53 (76.8%) |
| Steroids during the last year | 43 (62.3%) |
| Steroids > 10 mg/day or boluses | 20 (29.0) |
Continuous variables are in mean ± SD (median).
Categorized variables are in no, (%).
Time from onset of first/last anti-TNF treatment to first symptoms of TB, time from onset of last anti-TNF treatment.
immunosuppressive disease-modifying anti-rheumatic drugs (DMARDs): at least one among cellcept, chlorambucil, ciclosporin, cychlophosphamid, mercaptopurine, mycophenolate mofetil, methotrexate, leflunomide, azathioprine, abatacept, anakinra, cortolizumab, CTLA4 Ig, rituximab.
Risk factors of TB, before anti-TNF therapy, were available in 51 patients. 34 (66.7%) showed at least one risk factor (TST diameter ≥ 5 mm, chest x-ray results showing signs of a history of TB, history of an untreated TB or of exposure to TB, or being born in an endemic area): the TST diameter, available in 45 patients, was > 10 mm for 4 patients (9%), 5 to 10 mm for 11 (24%), < 5 mm for 30 (67%). For 6 of 57 patients (10.5%) (not done in 12), the chest x-ray findings suggested a history of TB. In addition, 10 patients had a history of exposure to TB, 1 a personal history of TB, and 3 a history of primary TB infection, and 14 were born in an endemic area. According to French guidelines for TB chemoprophylaxis before anti-TNF initiation, with a TST diameter cut-off of 5 or 10 mm, 26 of 46 (56.5%) or 17 of 43 (39.5%) of the patients should have received chemoprophylaxis, respectively. None of the patients had received correct chemoprophylaxis according to the French recommendations(12)(3 had received incomplete chemoprophylaxis, 2 with TST diameter > 10 mm and 1 without TST before anti TNF treatment), and TB occurred 15 to 34 months later).
Clinical features were restricted to pleuro-pulmonary disease and/or mediastinal adenitis in 27 (39%) patients, and extrapulmonary in 42 (61%): disseminated diseases in 28 (41%), non mediastinal adenitis in 7 (10%), and others in 7 (10%; i.e. 1 spondylodiscitis, 2 meningoencephalitis, 1 sub cutaneous, 1 laryngeal, and 2 colic tuberculosis). The diagnosis of TB was by microbiological evidence (direct Ziehl staining showing acid-fast bacilli and/or Mycobacterium tuberculosis isolation) in 47 patients, histological evidence in 14 patients, clinical suspicion and quantiferon positive test result in 1 patient and clinical suspicion associated with response to anti-TB treatment in 7 patients.
Outcome
Three patients required hospitalization in an intensive care unit. After a median follow-up of 22.9 months, 2 patients died (one with disseminated TB died before diagnosis; another died from a cause unrelated to TB), 45 were considered cured, 5 were still receiving treatment, and in 5 TB relapsed (after a median delay of 9.4 months) (outcome was missing for 12 cases). None of the 5 patients had restarted anti-TNF therapy before relapse.
At TB diagnosis, anti-TNF therapy was not stopped in 2 patients (treated with infliximab). Moreover, anti-TNF therapy was restarted in 8 patients (etanercept in 3, infliximab in 5) after a median anti-TB treatment duration of 5.8 months (range 5 days to 10 months). No recurrence of TB was observed in these 10 patients (median follow-up 17.7 months, range 8.2 to 32.2 months).
Time occurrence of TB with anti-TNF therapy
The median time to occurrence of TB since start of anti-TNF treatment was 12.0 months. The median time since start of the last anti-TNF was 9.9 months. In 4 patients, TB had occurred despite discontinuation of anti-TNF therapy 7.1 to 13.9 months before. In these 4 patients, the anti-TNF was infliximab in 4 and adalimumab in 1.
As indicated in Figure 1, the incidence of TB was higher during the first year of anti-TNF treatment and differed depending on type of treatment: monoclonal antibodies (infliximab or adalimumab) or soluble receptor (etanercept).
Figure 1.
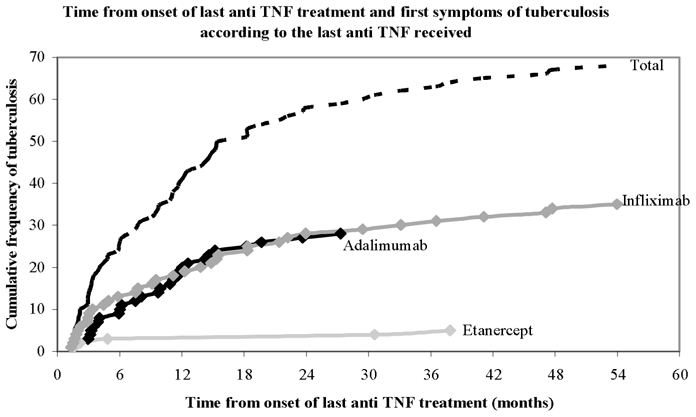
Cumulative incidence of TB as a function of anti-TNF treatment duration as a whole and by anti-TNF agent.
Incidence and risk of TB for patients receiving anti-TNF therapy versus the general population
The main analysis relied on a total number of 57,711 patient-years of use of anti-TNF therapy during the 2004–2006 period, with 18% receiving adalimumab, 51% etanercept and 31% infliximab as the denominator of the incidence rate. The annual incidence rate of TB adjusted for age and sex for patients receiving anti-TNF therapy, with the French population as a reference, was 116.7 (95% CI 10.6–222.9 per 100,000 patient-years). The SIR was 12.2 (9.7–15.5; p<0.0001).
The risk of TB for patients receiving anti-TNF therapy as compared to the French population differed depending on anti-TNF agent used. The annual adjusted-incidence rate of TB was 9.3 (0.0–29.4) per 100,000 for patients receiving etanercept, 187.5 (0.1–374.8) per 100,000 for infliximab, 215.0 (0.0–521.7) per 100,000 for adalimumab but 8.7 per 100,000 for the general French population.(13) The SIR was 1.8 (0.7–4.3; p=0.20) for patients receiving etanercept, 18.6 (13.4–25.8; p<0.0001) for infliximab and 29.3 (20.3–42.4; p<0.0001) for adalimumab. Because the incidence rates and SIRs were close for adalimumab and infliximab and their mechanisms of action are similar, we pooled data for adalimumab and infliximab for subgroup and sensitivity analyses. Still, a difference between monoclonal-antibody and soluble-receptor anti-TNF therapy was observed in the main analysis and in the different sensitivity analyses (Figure 2), even when we separately used the different estimates from independent sources, which gave very consistent adjusted incidence rates and SIRs (suppl Figure 1).
Figure 2. Analysis of the estimation of the SIR for the risk of TB, whatever the anti-TNF agent, and according to the anti-TNF agent received and the underlying inflammatory disease.
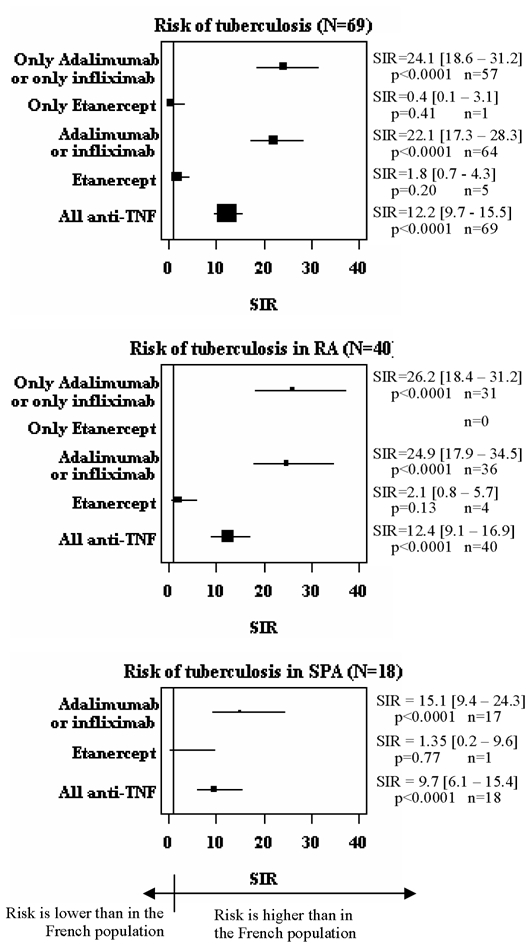
n is the number of cases involved in the calculation (numerator of the incidence rate).The plot size relates to the number of patients treated involved in the calculation (denominator of the incidence rate).
Risk factors of TB for patients receiving anti-TNF therapy
The case-control study involved 68 cases (as described in the methods, the patient treated for Behcet’s disease was not included in this case-control study) and 136 controls (from a pool of controls whose anti-TNF treatments reflected that of all users nationally, i.e. 18% receiving adalimumab, 51% etanercept and 31% infliximab). The results of the univariate case-control analysis are given in Table 2. On multivariate analysis, the following factors were predictive of TB: age, being born in an endemic area, time from onset of anti-TNF treatment, and therapy with adalimumab or infliximab versus etanercept.
Table 2.
Risk factors of TB for patients receiving anti-TNF therapy (univariate analysis)
| TB cases (n=68) |
Controls (n=136) |
OR [95% CI] |
p value |
|
|---|---|---|---|---|
| Age (for a 10 year-increase) | 58.3 (15.5) | 52.6 (16.3) | 1.35 [1.08 – 1.68] | 0.008 |
| Born in a tuberculosis endemic area | ||||
| No | 54 (79.4%) | 128 (94.1%) | 1 | 0.005 |
| Yes | 14 (20.6%) | 8 (5.9%) | 3.50 [1.47 – 8.34] | |
| Duration of the underlying inflammatory disease (years) | 11.4 (8.8) | 13.6 (11.1) | 0.98 [0.95 – 1.01] | 0.11 |
| Tuberculin skin test diameter at screening | ||||
| < 5 mm | 30 (66.7%) | 80 (77.7%) | 1 | 0.38 |
| 5 to 10 mm | 11 (24.4%) | 13 (12.6%) | 2.04 [0.74 – 5.60] | |
| >10 mm | 4 (8.9%) | 10 (9.7%) | 1.47 [0.37 – 5.73] | |
| Chest x-ray findings in favor of TB sequellae at screening | ||||
| No | 50 (90.9%) | 90 (96.8%) | 1 | 0.07 |
| Yes | 5 (9.1%) | 3 (3.2%) | 4.59 [0.88 – 23.84] | |
| Last anti-TNF agent received | ||||
| Etanercept | 5 (7.4%) | 64 (47.1%) | 1 | < 0.001 |
| Adalimumab | 28 (41.2%) | 22 (16.2%) | 21.03 [4.86 – 91.12] | |
| Infliximab | 35 (51.5%) | 50 (36.8%) | 17.55 [3.83 – 80.53] | |
| Last anti-TNF agent received | ||||
| Etanercept | 5 (7.4%) | 64 (47.1%) | 1 | < 0.001 |
| Adalimumab or infliximab | 63 (92.6%) | 72 (52.9%) | 19.67 [4.70 – 81.27] | |
| Time from onset of first anti-TNF treatment¥ (months) | ||||
| ≥ 1 year | 33 (48.5%) | 103 (75.7%) | 1 | < 0.001 |
| < 1 year | 35 (51.5%) | 33 (24.3%) | 3.43 [1.73 – 6.81] | |
| DMARDs during the last year | ||||
| None or nonimmunosuppressiv | 1 | 0.52 | ||
| Immunosuppressive* | 1.24 [0.65 ;2.36] | |||
| Steroids during the last year | ||||
| No | 26 (38.2%) | 66 (48.5%) | 1 | 0.13 |
| Yes | 42 (61.8%) | 70 (51.5%) | 1.67 [0.86 – 3.26] | |
| Steroids > 10 mg/day or boluses during the last year | ||||
| No | 48 (70.6%) | 112 (82.4%) | 1 | 0.05 |
| Yes | 20 (29.4%) | 24 (17.6%) | 2.08 [1.00 – 4.34] |
Continuous variables are in mean (SD).
Categorized variables are in no, (%).
OR: odds ratio
Time from onset of last anti-TNF treatment and first symptoms of TB for the cases, time from onset of last anti-TNF treatment and last news for controls
Immunosuppressive disease-modifying anti-rheumatic drugs (DMARDs): at least one among cellcept, chlorambucil, ciclosporin, cychlophosphamid, mercaptopurine, mycophenolate mofetil, methotrexate, leflunomide, azathioprine, abatacept, anakinra, cortolizumab, CTLA4 Ig, rituximab.
Results of the main multivariate analysis are presented in table 3. Risk of TB was higher with infliximab or adalimumab than with etanercept. (OR=13.3 (95%CI 2.6–69.0; p=0.002) and 17.1 (95%CI 3.6–80.6; p=0.0003), respectively. As for the SIRs, we pooled data for adalimumab and infliximab for the subgroup and sensitivity analyses, for more power. The drug-specific OR were also similar with the second and third matching and in subgroup and sensitivity analysis (Figure 3).
Table 3.
Risk factors of TB for patients receiving anti-TNF therapy (multivariate analysis)
| TB cases and controls (68 cases and 136 controls) | ||
|---|---|---|
| OR [95% CI] | p value | |
| Age (for a 10 year-increase) | 1.69 [1.20 – 2.38] | 0.003 |
| Born in a tuberculosis endemic area | ||
| No | 1 | |
| Yes | 10.35 [2.40 – 44.55] | 0.002 |
| Last anti-TNF agent received | ||
| Etanercept | 1 | |
| Adalimumab | 17.08 [3.62 – 80.59] | < 0.001 |
| Infliximab | 13.29 [2.56 – 69.04] | 0.002 |
| Time from onset of first anti-TNF treatment¥ (months) | ||
| ≥ 1 year | 1 | |
| < 1 year | 5.05 [1.91 – 13.38] | 0.001 |
OR: odds ratio
Time from onset of last anti-TNF treatment and first symptoms of tuberculosis for cases; time from onset of last anti-TNF treatment and last news for controls
Figure 3. Sensitivity analysis of the results of the case-control analysis.
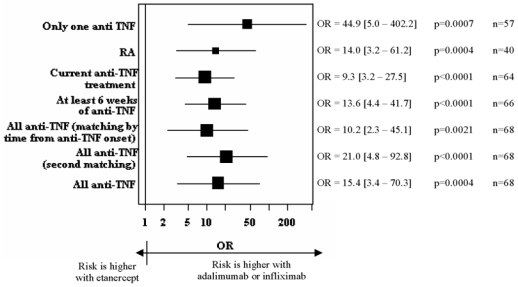
odds ratios (OR) from multivariate analysis for the risk of being treated with adalimumab or infliximab rather than with etanercept. n is the number of cases involved in the calculation. The plot size relates to the number of patients treated involved in the calculation.
DISCUSSION
Our analysis of 69 cases of TB collected over 3 years in the RATIO registry clearly demonstrated a difference in risk of TB between patients receiving monoclonal-antibody than soluble-receptor anti-TNF therapy. We observed a 7- to 17-fold difference in risk depending on the 2 types of anti-TNF agents used, from two different methodological approaches to assess the risk of TB: a comparison of incidence rate with the French general population and a case-control study with controls receiving anti-TNF therapy for the same underlying inflammatory diseases. The risk of TB was higher during the first year of anti-TNF treatment, which favoured the reactivation of latent TB. Moreover, none of the 69 cases had been treated with correct chemoprophylaxis against TB before anti-TNF treatment was initiated.
After the FDA alert of the risk of TB associated with the use of infliximab, numerous countries set up registries to investigate the safety of anti-TNF agents. All registries of biologics but ours are cohort studies involving only a part of the population of focus and thus are not powered enough to demonstrate very rare events or investigate a difference in risk depending on anti-TNF agent used, nor are the randomized controlled trials.(15) In the cohort of the US national data bank for rheumatic diseases (NDB), the rate of TB was not increased in anti-TNF-naïve RA patients (6.2 (1.6–34.4) per 100,000 patient-years) but was increased in RA patients receiving infliximab (52.5 (14.3–134.4) per 100,000 patient-years)(7). However in this study, the rate was calculated from only 4 cases of TB among 6,460 patients receiving infliximab. In the same study, no case of TB was observed for 2,327 patients receiving etanercept. In the British registry, the incidence of TB was 1.5 per 1000 patient-years with infliximab and 0.5 per 1000 patient-years with etanercept, but the number of cases was 7 and 2, respectively.(2) In the Swedish registry, the incidence rates were 1.5 per 1000 patient-years with infliximab and 0.8 per 1000 patient-years with etanercept, 9 cases treated with infliximab alone and 4 with etanercept alone.(1) Finally, among Korean patients, 2 cases of TB were observed among 90 patients receiving infliximab and no case was observed among 103 patients receiving etanercept.(6) The difference of risk between the types of anti-TNF agents was only suggested in these registries and is clearly demonstrated in the RATIO registry. In our registry, patients were included when the adverse event occurred, not when the therapy began. Thus, our population of focus was the whole French population, and this allowed us to collect in 3 years many more cases of TB than that collected by all the other registries. Furthermore, as far as we know, ours is the only biologic registry collecting safety data for patients receiving anti-TNF therapy, for any indication.
Contrary to the standard pharmacovigilance notifications, all the files of TB patients included in the RATIO registry were reviewed and validated by an expert committee, which is a main quality issue, especially for the diagnosis of TB, which may be difficult.
As always, incidence rates might be somewhat under- or overestimated because of an imprecise numerator or denominator. Concerning the numerator, passive surveillance data may underestimate the incidence of adverse events, and thus the annual rate of TB in the RATIO registry might be toward the low end of the true incidence rate.(16, 17) The denominator of the incidence rate was estimated only. The pharmaceutical firms may have used different methodologies for evaluating the denominator. However, because each firm evaluated the number of patient-years during the period for each anti-TNF agent, the difference in risk between agents we observed cannot be explained by the different methodologies used for the different agents. Furthermore, in the sensitivity analysis, we used the different estimates from independent sources separately, which gave very consistent adjusted incidence rates and SIRs (supplementary Figure 1). In addition, our estimates for adalimumab and etanercept did not differ from data obtained from the RSI, which has claims data for outpatients receiving etanercept or adalimumab among 3 million RSI enrolees -- 5% of the French population. The consistency of results of sensitivity analyses from these independent sources is a strong argument for their validity. Furthermore, the difference in risk between agents we found (7- to 17-fold difference) is unlikely to be explained by a lack of precision in the denominator estimate.
Although this national survey design has some limitations (especially the lack of precision in the denominator), it is probably the only way (or at least the most powerful way) to investigate difference in risk with use of anti-TNF agents.
The mechanism by which TNF antagonists reactivate latent TB is not fully understood. In animal models, TNF plays a central role in mediating mycobacterial infections, and soluble TNF but especially membrane TNF, expressed by activated macrophages and T lymphocytes, is essential in protecting against TB infection.(18) In a murine model of chronic TB, neutralization of TNF by antibody but not the TNF-receptor fusion molecule exacerbates chronic TB because of better penetration of antibodies into the granuloma.(19) Humans show higher avidity and better stability in membrane TNF with anti-TNF monoclonal antibodies,(20) which leads in some studies to more efficient apoptosis.(21–23) Even if in other studies, infliximab and etanercept showed no clear difference in inducing apoptosis in vivo(24) the difference in reverse signalling due to a difference in membrane TNF targeting may have other consequences. For example, anti-TB-specific T cells show a clear difference in effector function. Two in vitro studies provided the same results: adjunct infliximab or adalimumab treatment at therapeutic concentrations to specific human anti-TB T cells was much more efficient in decreasing proliferation and interferon gamma secretion by these T cells than adjunct etanercept treatment at therapeutic concentrations.(20, 25) As well, inhibition of interleukin 1 released by lipopolysaccharide-stimulated monocytes is greater with monoclonal-antibody anti-TNF treatment than with soluble-receptor treatment.(26) Finally, monoclonal anti-TNF therapy alleviates Treg impairment seen in RA by inducing apoptosis of membrane TNF-positive nonefficient Treg, thus allowing re-expansion of efficient regulatory T cells (Treg).(27) Thus, our epidemiological results can be supported by differences in action of the two types of anti-TNF agents on specific effector T-cells and on Treg.
Two-thirds of our cases of TB occurred in patients with negative TST results at screening. However, if we strictly follow the national recommendation for prophylactic treatment in France, 56.5% of the patients with a TST result of less than 5 mm and 39.5 % with a result of less than 10 mm should have received an anti-TB prophylactic treatment. Concerning the others, either the screening was not adequate or new TB infection developed, not reactivating latent TB. Our study suggests that taking into account whether patients were born in an endemic area could improve the screening of latent TB. However this information may have been less precisely reported in the control group. Other ways to improve the screening of latent TB, such as use of specific in vitro blood tests assessing the presence of specific anti-TB T-cells (Quantiferon TB or T-spot-TB), should be evaluated.
Our study also confirmed that, as emphasized by others,(8) anti-TB chemoprophylactic treatment is efficient for preventing TB. None of our patients with TB had received a correct prophylactic antibiotic against TB. The efficacy of screening and anti-TB chemoprophylaxis may explain why the median time from the beginning of anti-TNF therapy to TB onset is much higher (12 and 10 months for the first and the last anti-TNF, respectively) in our study than in the first study describing TB in patients treated with infliximab (14 weeks),(9) when no screening for TB was recommended. However, probably we observed both features of TB infection in our study: reactivation of latent TB infection (corresponding to the high risk during the first year of anti-TNF treatment as shown in Figure 1 and in the case-control results) and new infection occurring during follow-up, which will never be avoided with pre-treatment screening.
In conclusion, our study provides clear evidence of a higher risk of TB with monoclonal-antibody anti-TNF therapy than with soluble-receptor therapy. These differences can be supported by differences of action of the 2 types of agents on membrane TNF leading to a differential effect on effector T cells and on Treg. These different mechanisms of action could also explain a better efficacy of monoclonal-antibody therapy in CD, in other granulomatous diseases such as sarcoidosis(28) and in uveitis.(29)
Supplementary Material
Acknowledgments
The authors thank all the clinicians who had reported at least one case in the RATIO Registry: Abitbol (Paris), Allanore (Paris), André (Clermont-Ferrand), Ardizzone (Mulhouse), Bergman (Paris), Azais (Poitiers), Bachelez (Paris), Bardet (Orléans), Beau (Poitiers), Bergman (Paris), Belmatoug (Clichy), Berthelot (Nantes), Blasco (Barbois), Bonnet (Limoges), Bouhnik (Clichy), Bourgarit (Paris), Bouvard (Angers), Bressot (Chalon sur Saone), Briançon (Aix les bains), Brocq (Nice), Cadiot (Reims), Castela (Nice), Visanica (Metz), Combert (La Rochelle), Couret (Valence), Cuillerier (Dreux), Dalle (Lyon), Dasilva (Elbeuf), Debandt (Aulnay sous-bois), Debourdeau (Lyon), Depernet (Langers), Dereure (Montpellier), Descamps (Paris), Duriez (Saint-Brieuc), Fach (Bergerac), Fain (Bondy), Fautrel (Paris), Filippi (Nice), Fior (Bondy), Flourie (Lyon), Fulpin (Marseille), Gaborit (Orange), Gaudin (Grenoble), Gendre (Paris), Ghringhelli (Bordeaux), Gillet (Nancy), Goupille (Tours), Grados (Amiens), Grosclaude (Uriage), Gueit (Rouen), Guillaume (Colmar), Guyot (Roubaix), Hersebach (Rennes), Hoen (Besançon), Houvennagel (Lomme), Beguinot (Reims), Jang-Guyro (Briançon), Jardin (Privas), Justum (Caen), Laharie (Bordeaux), Lambotte (Le kremlin-Bicêtre), Lecompte (Nancy), Leparc (Boulogne-Billancourt), Lequen (Pau), L’hirondel (Caen), Liné (Les Soissons), Lioté (Paris), Lucht (Saint Etienne), Maillefert (Dijon), Marguerie (Berck), Maqub (Arles), Marteau (Paris), Martin (Saint-Brieuc), Mehadaoui (Evreux), Melac-Ducamp (Nevers), Meyer (Paris), Miceli (Le kremlin-Bicêtre), Michelet (Rennes), Morel (Montpellier), Nocent (Bayonne), Novel (Dijon), Pallot Prades (Saint-Etienne), Piroth (Dijon), Perdriger (Rennes), Pertuiset (Pontoise), Petitou (Bigorre), Pouplin (Rouen), Puechal (Le Mans), Pujol (Clermont Ferrand), Rouidi (Dreux), Sacchi (Mantes-La Jolie), Saindenberg (Clichy), Salmon (Paris), Schaeverbeke (Bordeaux) Solau-Gervais (Lille), Sordet (Strasbourg), Sprunk (Bourg en Bresse), Taillan (Monaco), Thao Pham (Marseille), Thevenot (Laon), Thorel (Lorient), Ulmann (Marseille), Dr Vernhes (Libourne), Wendling (Besançon), Zarnitsky (Le Havre), Zabraniecki (Toulouse), Zeller (Paris).
The authors are also grateful to G.R. Auleley, J Deligne and C Blum-Boisgard from the RSI for providing data to validate the denominator estimate of the incidence rate we used, D Che and D Antoine from the Institut National de Veille Sanitaire for providing the French annual incidence rate of tuberculosis by age and sex, and the AFSSAPS and the regional pharmacovigilance centres for their contribution to the exhaustiveness of the RATIO registry.
The authors thank C Roy and G Baron for the statistical analysis, and N Nicolas, S Makhlouf and A Djemoui for their help in collecting and preparing the validation of the cases.
The RATIO was supported by a research grant from INSERM (Réseau de recherche clinique 2003 and 2006) and by an unrestricted grant from Abbott, Schering Plough and Wyeth.
Role of the funding source
The pharmaceutical companies (Abbott, Schering Plough and Wyeth) had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Allanore received consulting and/or talk honorarium from Wyeth (less than 10,000 $), Goupille received consulting and/or talk honorarium from Abbott, Schering Plough and Wyeth (less than 10,000 $ each), Lemann received consulting and/or talk honorarium from Abbott, Schering Plough and UCB (less than 10,000 $ each), Mariette received consulting and/or talk honorarium from Abbott, Schering Plough, UCB and Wyeth (less than 10,000 $ each).
References
- 1.Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Coster L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005;52(7):1986–92. doi: 10.1002/art.21137. [DOI] [PubMed] [Google Scholar]
- 2.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368–76. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 3.Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003;48(11):3013–22. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48(8):2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 5.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 6.Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007;34(4):706–11. [PubMed] [Google Scholar]
- 7.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50(2):372–9. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 8.Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52(6):1766–72. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- 9.Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39(8):1254–5. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- 10.Burmester GR, Mariette X, Montecucco C, Monteagudo-Sáez I, Malaise M, Tzioufas AG, et al. Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the Research in Active Rheumatoid Arthritis (ReAct) trial. Ann Rheum Dis. 2007;66(6):732–9. doi: 10.1136/ard.2006.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tubach F, Salmon-Ceron D, Ravaud P, Mariette X. The RATIO observatory: French registry of opportunistic infections, severe bacterial infections, and lymphomas complicating anti-TnFalpha therapy. Joint Bone Spine. 2005;25:25. doi: 10.1016/j.jbspin.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Mariette X, Salmon Dag R. French guidelines for diagnosis and treating latent and active tuberculosis in patients with RA treated with TNF blockers. Ann Rheum Dis. 2003;62:791. doi: 10.1136/ard.62.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoine D, Che D. Les cas de tuberculose maladie déclarés en France en 2006. Bulletin Epidémiologique Hebdomadaire. 2008. (available on http://www.invs.sante.fr/BEh)
- 14.Kelly AM, Richards D, Kerr L, Grant J, O’Donovan P, Basire K, et al. Failed validation of a clinical decision rule for the use of radiography in acute ankle injury. N Z Med J. 1994;107(982):294–5. [PubMed] [Google Scholar]
- 15.Hyrich KL. Assuming the safety of biologic therapies in rheumatoid arthritis: the challenge of study design. J Rheumatol. 2005;32(Suppl 72):48–50. [PubMed] [Google Scholar]
- 16.Goldman SA. Limitations and strengths of spontaneous reports data. Clin Ther. 1998;20(Suppl C):C40–4. doi: 10.1016/s0149-2918(98)80007-6. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal S, Chen RT. Reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health. 1995;85:1706–9. doi: 10.2105/ajph.85.12.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olleros ML, Guler R, Corazza N, Vesin D, Eugster HP, Marchal G, et al. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette-Guerin infection in the absence of secreted TNF and lymphotoxin-alpha. J Immunol. 2002;168(7):3394–401. doi: 10.4049/jimmunol.168.7.3394. [DOI] [PubMed] [Google Scholar]
- 19.Plessner HL, Lin PL, Kohno T, Louie JS, Kirschner D, Chan J, et al. Neutralization of tumor necrosis factor (TNF) by antibody but not TNF receptor fusion molecule exacerbates chronic murine tuberculosis. The Journal of infectious diseases. 2007;195(11):1643–50. doi: 10.1086/517519. [DOI] [PubMed] [Google Scholar]
- 20.Hamdi H, Mariette X, Godot V, Weldingh K, Hamid AM, Prejean MV, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006;8(4):R114. doi: 10.1186/ar1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology. 2001;121(5):1145–57. doi: 10.1053/gast.2001.28702. [DOI] [PubMed] [Google Scholar]
- 22.Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, Uchino A, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58(5):1248–57. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 23.Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology. 2003;124(7):1774–85. doi: 10.1016/s0016-5085(03)00382-2. [DOI] [PubMed] [Google Scholar]
- 24.Catrina AI, Trollmo C, af Klint E, Engstrom M, Lampa J, Hermansson Y, et al. Evidence that anti-tumor necrosis factor therapy with both etanercept and infliximab induces apoptosis in macrophages, but not lymphocytes, in rheumatoid arthritis joints: extended report. Arthritis Rheum. 2005;52(1):61–72. doi: 10.1002/art.20764. [DOI] [PubMed] [Google Scholar]
- 25.Saliu OY, Sofer C, Stein DS, Schwander SK, Wallis RS. Tumor-necrosis-factor blockers: differential effects on mycobacterial immunity. The Journal of infectious diseases. 2006;194(4):486–92. doi: 10.1086/505430. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, van Dongen H, Scherer HU, Huizinga TW, Toes RE. Suppressor activity among CD4+,CD25++ T cells is discriminated by membrane-bound tumor necrosis factor alpha. Arthritis Rheum. 2008;58(6):1609–18. doi: 10.1002/art.23460. [DOI] [PubMed] [Google Scholar]
- 27.Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflammatory bowel diseases. 2007;13(11):1323–32. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 28.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 29.Imrie FR, Dick AD. Biologics in the treatment of uveitis. Current opinion in ophthalmology. 2007;18(6):481–6. doi: 10.1097/ICU.0b013e3282f03d42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


