Summary
Objective
Prior exposure to intrapartum/neonatal nevirapine (NVP) is associated with compromised virologic treatment outcomes once non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy (ART) is initiated. We examined the longer-term clinical outcomes in a programmatic setting.
Methods
We compared post-12 month mortality and clinical treatment failure (defined by WHO clinical and immunologic criteria) among women with and without prior NVP exposure in Lusaka, Zambia.
Results
Between April 2004 and July 2006, 6,740 women initiated an NNRTI-containing regimen. At 12 months, 78% remained active and were included in this analysis. 12% reported prior NVP exposure, whose time from exposure to ART initiation was: <6 months for 11%, 6–12 months for 13%, >12 months for 37%, unknown for 39%. Overall, women with prior NVP exposure trended toward increased survival (adjusted hazard ratio [AHR]: 0.53; 95% confidence interval [CI]: 0.27–1.06, p=0.07) and toward increased hazard of clinical treatment failure (AHR: 1.18; 95%CI: 0.95–1.47, p=0.14), particularly those with exposure for <6 months (AHR: 1.52; 95%CI: 0.94–2.45, p=0.09).
Conclusions
Prior NVP exposure appeared to increase risk for clinical treatment failure after 12 months of follow-up, but this finding did not reach statistical significance. With growing evidence linking recent NVP exposure to virologic failure, optimized monitoring algorithms should be considered for women with starting NNRTI-based ART. The association between prior NVP exposure and improved survival has not been previously shown and may be a result of residual confounding around health-seeking behaviors.
Keywords: HIV, nevirapine, mother-to-child transmission of HIV, clinical outcomes, Zambia, Africa, anti-retroviral therapy
INTRODUCTION
Peripartum maternal and infant nevirapine (Guay et al. 1999, Jackson et al. 2003) has become a cornerstone for the prevention of mother-to-child HIV transmission (PMTCT) in resource-constrained settings, when used alone or in combination with short-course zidovudine (World Health Organization 2006). Use of the “single-dose” nevirapine (NVP) regimen, however, can result in selection for resistance to non-nucleotide reverse transcriptase inhibitor (NNRTI) drugs, alone or in combination with other drugs. When evaluated by standard population sequencing assays, 15%–69% of women have detectable NNRTI resistance in the weeks following NVP ingestion (Arrive et al. 2007). This proportion rises when more sensitive assays such as oligonucleotide ligation assay and LigAmp assays are used (Flys et al. 2005, Troyer et al. 2008, Chi et al. 2009b)
Recent studies have demonstrated the deleterious impact of prior NVP exposure on virologic responses to NNRTI-based antiretroviral therapy (ART), particularly when NVP is used in the months prior to treatment initiation (Lockman et al. 2007, Stringer et al. 2010). However, there are few data examining the impact of prior NVP exposure on longer-term clinical outcomes. To better understand the clinical impact of prior NVP exposure after 12 months of follow-up, we conducted a follow-up analysis of HIV-infected women initiating NNRTI-based ART in the Lusaka, Zambia HIV care and treatment program (Chi et al. 2007b).
METHODS
In this analysis, we describe the clinical outcomes of women initiating ART in Lusaka, Zambia after 12 months of follow-up. The inclusion and exclusion criteria have been described elsewhere (Chi et al. 2007b). Briefly, women enrolled into the public sector program were asked about previous antiretroviral drug use for PMTCT (Stringer et al. 2003, Chi et al. 2007a). Women who were nulliparous at time of enrollment or who reported the diagnosis of HIV after their last pregnancy were classified as NVP-unexposed. All women included in this analysis received care at the standard of the Zambian National Guidelines for ART care, which are adapted from the World Health Organization (WHO) (Stringer et al. 2006). Those eligible for therapy are prescribed a NNRTI - NVP or efavirenz - along with either zidovudine and lamivudine or stavudine and lamuvidine (Mwango et al. 2009). All patient information was entered into a standardized medical record system, including date of next appointment and report of death (Fusco et al. 2005). The source population was the cohort of women initiating NNRTI-based ART between April 2004 and July 2006, as described in our previous report (Chi et al. 2007b).
We compared women previously exposed to NVP to those who were unexposed, stratifying the exposure according to length of time between last NVP ingestion and ART initiation using established conventions in the medical literature (Lockman et al. 2007). Our outcomes of interest were mortality, clinical treatment failure, and a composite of the two, observed after 12 months of follow-up (“post-12 month”). Excluded were those who died, formally withdrew from the program, or were over one month late for a scheduled appointment at 12 months and never returned for care. Clinical treatment failure was defined as (1) worsening WHO clinical staging after 3 months of ART initiation; (2) CD4 count drop of below 95% of pre ART initiation level after at least 3 months on ART; and/or (3) switch to second-line therapy.
In our survival analysis, we considered patient outcomes that occurred at 12 months or more of follow-up. Log-rank tests were used to evaluate differences in mortality, clinical treatment failure, and a combination of both outcomes using Kaplan-Meier analysis. Multivariate Cox proportional hazards models were used to describe hazard for our outcomes of interest. Demographic and medical characteristics known to be risk factors for mortality were included in these models (Stringer et al. 2006). Adherence over the first 12 months was measured by the medication possession ratio, a pharmacy refill-based metric previously shown to predict mortality (Chi et al. 2009a, Goldman et al. 2008). All analyses were performed using SAS version 9.13 (SAS Institute, Cary, North Carolina). Use of these routinely collected data was approved by the ethical review committees at the University of Zambia (Lusaka, Zambia) and University of Alabama at Birmingham (Birmingham, Alabama, USA).
RESULTS
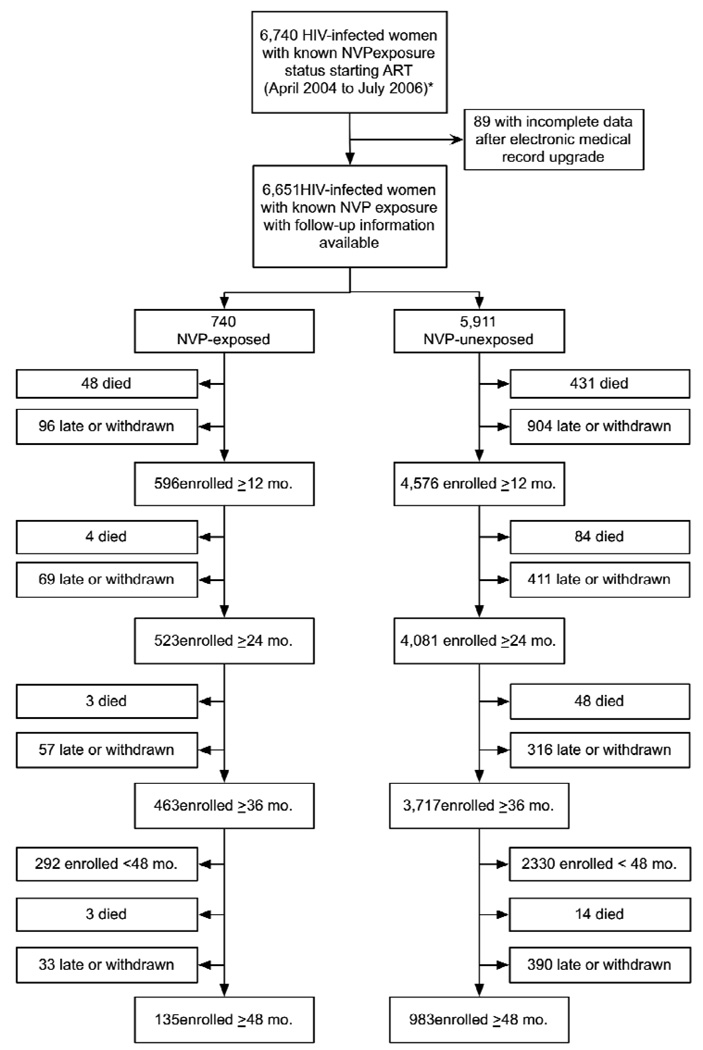
Between April 2004 and July 2006, 6,740 women initiated NNRTI-containing ART with a previously known NVP exposure status (Chi et al. 2007b). Of these, medical information was available for 6,651 (99%); the remaining 89 (1%) had incomplete or missing data due to recent upgrades to our electronic medical record. At 12 months post-ART initiation, 5,172 (78%) remained active in the program and were thus included in the analysis. NVP-exposed women were somewhat better retained in care than unexposed women (81% vs. 77%, p= 0.04). The other patients had either died (n= 479, 7%), withdrew from the program (n= 312, 5%), or were more than a month late since their last visit and never returned to care (n= 688, 10%). The full cohort profile is shown in Figure 1.
Figure 1.
Cohort profile of HIV-infected women with known nevirapine exposure status starting initiating non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy from April 2004 to July 2006 enrolled for at least 12 months
* Source population represents the cohort for our previous analysis of early clinical outcomes
Of the 5,172 women included in this analysis, 596 (12%) reported prior NVP exposure for PMTCT and 4,576 (88%) reported no prior exposure. Differences between these groups were consistent with our previous report (Chi et al. 2007b). At the time of ART initiation, women with previous NVP exposure were younger (median age 30.1 vs. 34.6 years, p< 0.001), had higher baseline CD4+ cell counts (mean 170.1 vs. 144.7 cells/µL, p< 0.001), were more likely to be in WHO clinical stage I or II (42% vs. 31%; p< 0.001), and had higher baseline hemoglobin levels (mean 10.7 g/dL vs. 10.3 g/dL; p< 0.001) than NVP-unexposed women. Parity and gravidity were similar between the two comparison groups (data not shown). Adherence to ART over the first 12 months, as measured by mean medication possession ratio, was statistically different among women with prior NVP exposure (87.9%) compared to those with no previous exposure (89.9%; p< 0.01). Median follow-up did not differ among the NVP-exposed and -unexposed groups in the post-12 month observation period (29.2 vs. 29.3 months, p = 0.52). Of the women with previous NVP exposure, 67 (11%) reported an interval of less than 6 months before ART initiation, 75 (13%) reported a 6 to 12-month interval, and 220 (37%) waited for more than 12 months before ART initiation. For the remaining 234 (39%), timing of NVP exposure was unknown.
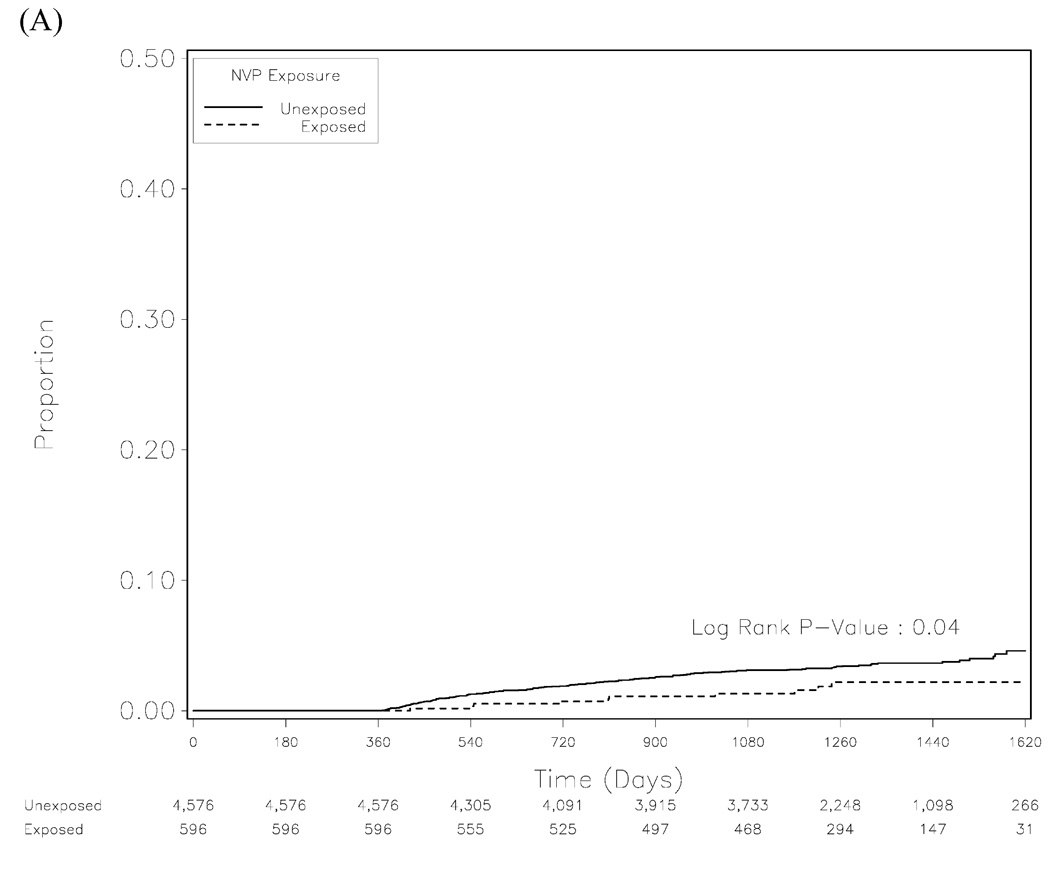
In Kaplan-Meier analysis, the unadjusted risk for mortality appeared lower among NVP-exposed women than in those who were unexposed (p= 0.04; Figure 2A). In adjusted Cox proportional hazards model, NVP exposure was not associated with increased mortality; in fact, previous use of NVP trended towards being protective (adjusted hazard ratio [AHR]: 0.53; 95% confidence interval [CI]: 0.27–1.06, p= 0.07). We then stratified the NVP-exposed arm according to time from exposure to ART initiation. When compared to women with no previous NVP exposure, those with timing intervals of less than 6 months, 6–12 months, and greater than 12 months appeared to have similar hazard in adjusted analyses (Table 1).
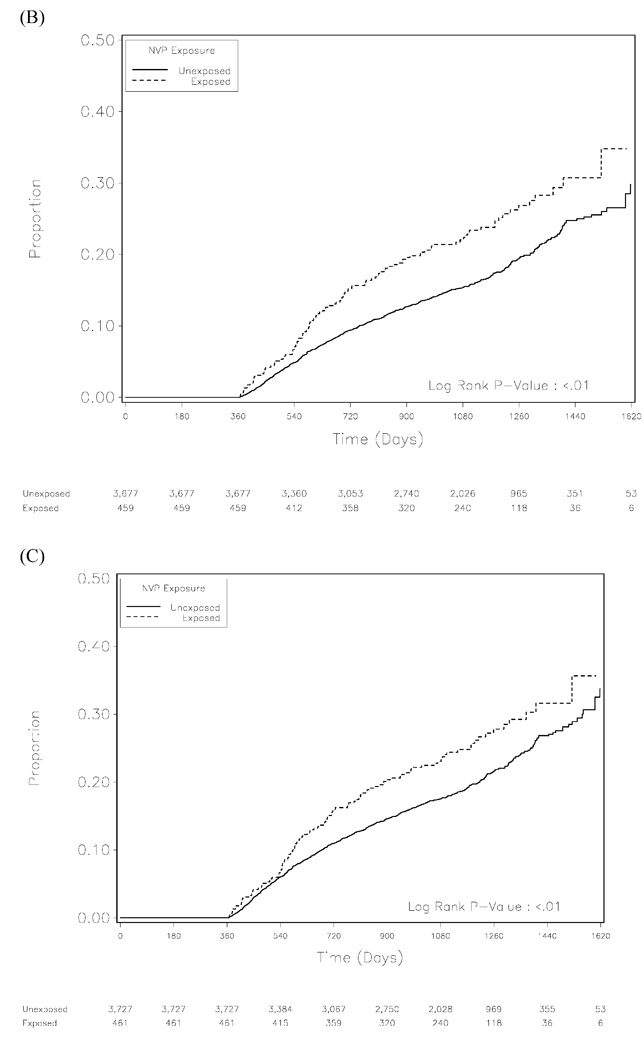
Figure 2.
Kaplan-Meier analysis for mortality (A), clinical treatment failure (B), and a combination of the two outcomes (C).
Table 1.
Adjusted hazard ratios (with 95% confidence intervals) for mortality, clinical treatment failure, and combination outcome of the two, according to nevirapine exposure prior to initiation of non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy
| Crude hazard ratio (95% CI) |
Adjusted hazard ratio (95% CI) |
|
|---|---|---|
| Mortality | ||
| No exposure | Ref | Ref |
| Previous NVP exposure: < 6 months | 0.90 (0.22 – 3.64) | 0.95 (0.23 – 3.88) |
| Previous NVP exposure: 6 to 12 months | 0.41 (0.06 – 2.93) | 0.53 (0.07 – 3.84) |
| Previous NVP exposure: > 12 months | 0.28 (0.07 – 1.14) | 0.33 (0.08 – 1.34) |
| Previous NVP exposure: no timing information | 0.64 (0.26 – 1.57) | 0.58 (0.21 – 1.59) |
| Clinical treatment failure | ||
| No exposure | Ref | Ref |
| Previous NVP exposure: < 6 months | 2.14 (1.36 – 3.38) | 1.52 (0.94 – 2.45) |
| Previous NVP exposure: 6 to 12 months | 1.33 (0.78 – 2.26) | 1.06 (0.61 – 1.84) |
| Previous NVP exposure: > 12 months | 1.12 (0.78 – 1.62) | 0.95 (0.65 – 1.40) |
| Previous NVP exposure: no timing information | 1.58 (1.17 – 2.12) | 1.33 (0.96 – 1.82) |
| Clinical treatment failure or death | ||
| No exposure | Ref | Ref |
| Previous NVP exposure: < 6 months | 1.96 (1.26 – 3.06) | 1.43 (0.90 – 2.28) |
| Previous NVP exposure: 6 to 12 months | 1.24 (0.74 – 2.06) | 1.05 (0.62 – 1.79) |
| Previous NVP exposure: > 12 months | 0.98 (0.68 – 1.42) | 0.85 (0.59 – 1.25) |
| Previous NVP exposure: no timing information | 1.46 (1.10 – 1.95) | 1.23 (0.90 – 1.68) |
All analyses adjusted for age, body mass index, CD4 count, World Health Organization clinical stage, adherence, and nucleotide reverse transcriptase inhibitor backbone. NVP = nevirapine.
When we examined the impact of NVP exposure on clinical treatment failure in Kaplan-Meier analysis, women with previous exposure appeared to have a higher risk (p= 0.01; Figure 2B). Although the hazard for NVP exposure was suggestive of slightly elevated risk in adjusted analysis (AHR: 1.18; 95%CI: 0.95–1.47, p= 0.14), this trended towards greatest statistical significance among those with exposure for less than 6 months (AHR: 1.52; 95%CI: 0.94–2.45, p= 0.09) and those with unknown NVP exposure timing (AHR: 1.33; 95%CI: 0.96–1.82, p= 0.08). The results for our composite outcome analysis closely followed those of clinical treatment failure (Figure 2C, Table 1).
DISCUSSION
In our previous report (Chi et al. 2007b), we found that NVP exposure was not associated with increased risk for mortality (AHR= 1.2, 95%CI= 0.8–1.8) or clinical treatment failure (AHR= 1.1, 95%CI= 0.8–1.5) early in the course of ART. When the analysis was limited to women with available timing information, those with exposure less than 6 months prior to ART initiation had similar risks for death (adjusted HR= 1.0, 95%CI= 0.3–3.1) but trended towards higher rates for treatment failure (adjusted HR= 1.6, 95%CI= 0.9–2.7). An important limitation of our early outcomes analysis was the relatively short patient follow-up; median time was less than 8 months. Here, we build upon our previous work, in hopes of better understanding the consequences of NVP exposure on extended ART outcomes. To minimize the impact of early mortality - as well as the overlap between our two studies - we considered only those outcomes that occurred after 12 months of follow-up. In this analysis, we found that NVP exposure was associated with increased survival after 12 months in both crude and adjusted models. Despite the longer follow-up, the increased hazard for clinical treatment failure was remarkably similar to our earlier work and once again did not reach statistical significance (Chi et al. 2007b).
There is growing consensus regarding the negative impact of previous NVP exposure on later ART outcomes (Jourdain et al. 2004, Lockman et al. 2007, Coovadia et al. 2009, Stringer et al. 2010). The interval between NVP exposure and ART initiation has emerged as an important determinant for later virologic outcomes, with more recent NVP exposure associated with higher risk for virologic failure. In Botswana, high rates of virologic failure at six months were observed among women who initiated ART within six months of NVP exposure, when compared to those without exposure (41.7% vs. 0%; p < 0.001). When the time between NVP exposure and ART initiation was greater than six months, these outcomes were comparable to those without previous NVP exposure (Lockman et al. 2007). Investigators from the multi-center NNRTI Response Study found similar stratification of risk based on time between NVP exposure and ART initiation. Women ingesting NVP within 6 months of ART initiation had higher risk for virologic failure at 12 months (adjusted odds ratio= 2.16; 95%CI = 1.34, 3.49). Those with NVP exposure between 6–12 months also had elevated risk, but it did not reach statistical significance (adjusted odds ratio= 1.47; 95%CI = 0.82, 2.65) (Stringer et al. 2010).
Among women with prior NVP exposure, the risk of virologic treatment failure appears greatest early in the course of ART and this diminishes over time. For example, long-term follow-up of the Thai PHPT-2 cohort demonstrated large differences in virologic failure between NVP-exposed and -unexposed women at 48 months (41% vs. 23%; p= 0.02), but most events occurred within the first 24 months on ART (Jourdain et al. 2009). Although the cumulative difference among NVP-exposed and -unexposed women at 60 months was significant in the Mashi cohort, when limited to post-12 month virologic failure, these differences appeared marginal (5.9% vs. 4.8%). This remained consistent in the “high risk” subset of women with NVP exposure less than 6 months prior to treatment initiation (4.2% vs. 2.8%) (Lockman et al. 2009).
Because poor clinical and immunologic outcomes are known to lag behind virologic ones, we anticipated a greater risk for clinical treatment failure and mortality after 12 months of therapy. That these hazards of clinical treatment failure did not change appreciably between our two analyses was somewhat unexpected. This finding may be attributed to the clinical and immunologic criteria used to define treatment failure in this analysis, adapted from guidelines set forth by the WHO. Several studies have demonstrated their poor performance of such algorithms in predicting virologic outcomes (Reynolds et al. 2009, Bisson et al. 2008, Badri et al. 2008). However, we elected to use this as an outcome measure because, in settings with limited to no capacity for virologic monitoring, such criteria serve as the foundation for clinical decision-making.
We were surprised to find that NVP exposure was associated with increased survival in our analyses. Similar observations have not been made in clinical trials or analogous cohorts. When we compared baseline medical and demographic characteristics, women reporting previous NVP exposure were younger and appeared healthier by immunological and clinical criteria. Although we attempted to adjust for these factors in multivariable analysis, it is possible that residual confounding contributed to the increased survival associated with NVP exposure. It is possible that prior NVP use serves as an indicator of increased health-seeking behavior, which in turn could result in improved clinical outcomes. Given the programmatic nature of our data collection system, such parameters are unavailable for inclusion in multivariable models. Severity of HIV disease between women who previously conceived and those who did not may also be different. Since pregnancy is an obvious prerequisite for inclusion in the NVP-exposed group (and not the NVP-unexposed group), this may have contributed to the differences in treatment outcomes. In our comparisons of baseline characteristics, however, the median parity and gravidity did not differ significantly.
Strengths of this analysis are the length of follow-up and the “real world” perspective provided by this programmatic database. Limitations include the lack of virologic outcomes among our cohort members. Although such testing is available in our setting, its use is restricted for reasons of cost. The sample size had limited capacity to detect subtle differences in treatment outcomes, which was exacerbated by our timing-of-exposure stratifications. The large proportion of women without timing information (39%) and the limited number of participants in each subsequent category may explain lack of statistical significance in many key outcomes. Although this cohort had extended follow-up - with a median of nearly 42 months since ART initiation - it is possible that longer periods are needed to characterize poor clinical or immunologic outcomes associated with prior NVP exposure.
Although not statistically significant, our results appear to support the growing literature surrounding prior NVP exposure and treatment outcomes. Novel strategies are needed to identify and initiate treatment among ART-eligible women during pregnancy, given the clear benefits to both mother and infant (Killam et al. 2010). Use of protease inhibitor-based regimens should also be considered as first-line ART for women who report recent NVP exposure (Lockman 2009). These results also emphasize the need for better performing non-virologic algorithms to predict treatment failure. Where possible, routine virologic monitoring should be considered, particularly when a known risk factor for treatment failure - such as prior NVP exposure - is identified.
Acknowledgements
We acknowledge the Zambian Ministry of Health for consistent and high-level support of operations research in the context of HIV program expansion. The findings and conclusions included herein are solely the responsibility of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention. This work was supported by the President’s Emergency Plan for AIDS Relief, through a multi-country grant to the Elizabeth Glaser Pediatric AIDS Foundation, from the U.S. Department of Health and Human Services and Centers for Disease Control and Prevention’s Global AIDS Program, the National Institutes of Health and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation.
REFERENCES
- Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- Badri M, Lawn SD, Wood R. Utility of CD4 cell counts for early prediction of virological failure during antiretroviral therapy in a resource-limited setting. BMC Infect Dis. 2008;8:89. doi: 10.1186/1471-2334-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson GP, Gross R, Bellamy S, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Cantrell RA, Zulu I, et al. Adherence to first-line antiretroviral therapy affects nonvirologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol. 2009a;38:746–756. doi: 10.1093/ije/dyp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Chintu N, Lee A, Stringer EM, Sinkala M, Stringer JS. Expanded Services for the Prevention of Mother-to-Child HIV Transmission: Field Acceptability of a Pilot Program in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2007a;45:125–127. doi: 10.1097/QAI.0b013e318050d28f. [DOI] [PubMed] [Google Scholar]
- Chi BH, Ellis GM, Chintu N, et al. Intrapartum tenofovir and emtricitabine reduces lowconcentration drug resistance selected by single-dose nevirapine for perinatal HIV prevention. AIDS Res Hum Retroviruses. 2009b;25:1099–1106. doi: 10.1089/aid.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007b;21:957–964. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flys T, Nissley DV, Claasen CW, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192:24–29. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- Fusco H, Hubschman T, Mweeta V, et al. Electronic patient tracking supports rapid expansion of HIV care and treatment in resource-constrained settings. 3rd IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro, Brazil. 2005. Abstract MoPe11 2C37. [Google Scholar]
- Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1031–1035. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. 4-year clinical and therapeutic consequences of intrapartum single-dose nevirapine for the prevention of perinatal HIV in women who subsequently intiated a nevirapine-based ART. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. Abstract 954. [Google Scholar]
- Killam WP, Tambatamba BC, Chintu N, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: a stepped-wedge evaluation. AIDS. 2010;24:85–91. doi: 10.1097/QAD.0b013e32833298be. [DOI] [PubMed] [Google Scholar]
- Lockman S. Lopinavir/ritonavir + tenofovir/emtricitabine is superior to nevirapine + tenofovir/emtricitabine for women with prior exposure to single-dose nevirapine: A5208 ("OCTANE"). 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. Abstract 94LB. [Google Scholar]
- Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- Lockman S, Smeaton L, Ogwu A, et al. Long-term maternal and pediatric virologic outcomes on nevirapine-based HAART following receipt of peripartum single-dose nevirapine (sdNVP) or placebo Botswana. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. Abstract 955. [Google Scholar]
- Mwango A, Giganti M, Mulenga L, et al. First-line tenofovir ART in Zambia. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]
- Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;23:697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer EM, Sinkala M, Stringer JS, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003;17:1377–1382. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JS, Mcconnell MS, Kiarie J, et al. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country prospective cohort study. PLoS Med. 2010;7:e1000233. doi: 10.1371/journal.pmed.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- Troyer RM, Lalonde MS, Fraundorf E, et al. A radiolabeled oligonucleotide ligation assay demonstrates the high frequency of nevirapine resistance mutations in HIV type 1 quasispecies of NVP-treated and untreated mother-infant pairs from Uganda. AIDS Res Hum Retroviruses. 2008;24:235–250. doi: 10.1089/aid.2007.0138. [DOI] [PubMed] [Google Scholar]
- WHO. Geneva: WHO; Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings: towards universal access. 2006