Abstract
Lung involvement is the leading cause of death in systemic sclerosis (SSc), however lung transplantation (LT) for systemic disease remains controversial. Our objective was to comprehensively evaluate post-LT outcomes for SSc compared to idiopathic pulmonary fibrosis (IPF).
We retrospectively evaluated bilateral LT recipients (LTR) with SSc or IPF at our center between 1/1/2003 and 12/31/2007. The primary endpoint was all-cause mortality at 1 year post-LT. Secondary endpoints included assessments of acute rejection (AR), pulmonary function, infection, and chronic rejection.
14 patients with SSc and 38 patients with IPF underwent LT. Apart from a younger SSc cohort (53.2 vs. 58.8 years, p=0.02), the two groups were well matched. One year all-cause mortality was no different between SSc (6.6%) and IPF (13.1%) groups, after adjusting for age (p=0.62). Rates of AR ≥ 2 were significantly increased for the SSc compared to the IPF group (HR 2.91; p=0.007). Other endpoints including chronic rejection, infection, and pulmonary function showed no differences.
SSc LTR experience similar survival 1 year post-LT when compared to IPF. AR rates may be significantly higher in the SSc group. Longer follow-up is necessary to determine the effects of gastrointestinal dysfunction and AR on late allograft function in SSc LTR.
Keywords: lung transplantation, systemic sclerosis
Introduction
Pulmonary arterial hypertension (PAH) and pulmonary fibrosis (PF) are presently the leading causes of SSc related death 1. LT for respiratory insufficiency secondary to systemic disease remains controversial. However, the 2006 International Society of Heart & Lung Transplantation (ISHLT) report deemed LT to be an option for patients with connective tissue disease (CTD), provided the systemic disease was otherwise quiescent 2. However, this report found the available data to be insufficient to support specific pre-LT guidelines for patients with CTD. Although prior studies of SSc and LT have addressed survival 1, 3, 4, they may not focus on other important post-LT parameters such as rejection, infection, and pulmonary function.
The purpose of this manuscript is to comprehensively report our single-center experience of SSc post-bilateral LT with a focus on rejection, infection, and pulmonary function, as well as survival. We use idiopathic pulmonary fibrosis (IPF) as a comparator group, given its similar spectrum of lung disease and prognosis.
Study Design and Methods
Study Design
We retrospectively assessed all LT recipients (LTR) who underwent LT between 1/1/2003 and 12/31/2007 at the University of California, Los Angeles (UCLA). Appropriate consent was obtained in accordance with our institutional review board. We identified all LTR with SSc (limited or diffuse) based on the American College of Rheumatology criteria per UCLA rheumatology. During this interval, a total of 243 LTs were performed, of which 15 (6.1%) were done for SSc-associated PF and/or PAH. All LTs for SSc were bilateral, with the exception of one patient who underwent single LT. We also identified all LTR with IPF who underwent bilateral LT during this period (38 of 243, 15.6%). The diagnosis of IPF was established using explant tissue histopathology, defined as usual interstitial pneumonia (UIP) in the appropriate clinical context 6. SSc explant tissue displayed a mixed histopathology [UIP (n=9); NSIP (nonspecific interstitial pneumonia) with fibrosis (n=3); NSIP without fibrosis (n=1); features of both UIP and NSIP with fibrosis (n=2)].
Patient Selection
Patients with SSc and IPF referred for LT were evaluated in accordance with the general principles established by guidelines for the selection of LT candidates 2. Potential SSc LTR were assessed for the extent of gastroesophageal reflux (GER) and esophageal/gastrointestinal dysmotility (collectively, GI dysfunction) by evaluation with dual pH probe study, barium esophagram, nuclear medicine quantitative gastric emptying study, esophageal manometry, and/or upper endoscopy. SSc LT candidates with objective evidence for GI dysfunction, unable to be subjectively controlled by aggressive medical therapy (combination of high-dose proton pump inhibitor +/− H2 blocker, high-dose pro-motility agent, and lifestyle modification counseling), were excluded for LT. Importantly, the DeMeester score 7, 8 (based on dual pH probe study) in isolation, was neither an inclusion nor exclusion criterion. In addition, potential SSc LTR were excluded if there was evidence for any one of the following while receiving aggressive medical therapy: symptomatic esophageal stricture or upper gastrointestinal ulcer, esophageal atonia/achalasia, or abnormal gastric emptying (<25% clearance at 90 minutes post-ingestion). Other inclusion and exclusion criteria are outlined in Table 3.
Table 3.
| Pre-transplant Spirometric parameter | SSc Mean (SD) | IPF Mean (SD) | p-value |
|---|---|---|---|
| FEV1 | 1.5 (0.6) | 1.7 (0.8) | 0.26 |
| FEV1 % predicted | 47.3 (19.8) | 51.4 (16.9) | 0.45 |
| FVC | 1.7 (0.7) | 1.9 (1.0) | 0.48 |
| FVC % predicted | 42.2 (17.3) | 49.3 (18.3) | 0.20 |
| FEV1/FVC | 86.3 (6.5) | 92.7 (15.7) | 0.04* |
| DLCO | 6.7 (1.9) | 7.3 (3.6) | 0.66 |
| DLCO % predicted | 27.9 (9.0) | 27.5 (11.4) | 0.91 |
p-value is statistically significant by t-test orWilcoxon rank sum test.
Single Versus Bilateral LT
Preferred bilateral and mandatory single LT are performed for ages ≤60 and ages ≥65, respectively. Either single or bilateral LT is possible for ages 61–64 and the following factors determine this decision: obstructive coronary artery disease, degree of PAH, suppurative lung disease, prior thoracic surgery, and body habitus. Our program performs all bilateral LT on cardiopulmonary bypass.
Post-LT Immunosuppression and Surveillance Protocols
LTR received perioperative immunosuppresion including methylprednisolone, tacrolimus, and mycophenolate mofetil, and prophylactic antimicrobials as previously described 9. Induction chemotherapy was provided with either basiliximab or rabbit antithymocyte globulin. Surveillance bronchoscopy (including transbronchial biopsies (TBB) and bronchoalveolar lavage (BAL)) was performed to assess for acute rejection (AR) and possible infection at 1, 3, 6, and 12 months, post-LT. Additional bronchoscopic assessments and/or lower respiratory tract sampling (eg sputum) were done when clinically indicated and were included in our data collection. Spirometric testing was obtained at surveillance bronchoscopy time points and included percent reference and absolute values for FEV1 (forced expiratory volume, 1 second), FVC (forced vital capacity), and FEV1/FVC ratio 10.
SSc-Specific Post-LT Prophylaxis
High-dose proton pump inhibitors (eg pantoprazole 80mg orally twice per day) and pro-motility agents were (re)initiated for SSc LTR immediately post-LT. An ACE inhibitor was initiated as tolerated by systemic blood pressure as prophylaxis for renal crisis. The head of the bed was kept ≥ 45 degrees for GER prophylaxis. Radial arterial catheters were avoided given the high prevalence of Raynaud’sphenomenon in SSc.
Study Definitions
PAH was defined by right heart catheterization (RHC) 11. An evaluation for other potential causes of PAH 11 was negative in all SSc and IPF cases.
Acute rejection (AR) was histologically defined using ISHLT criteria 12 and treated as previously described 9. An average AR score was calculated by adding the sum of AR grades of each rejection episode during the first year post-LT 13 and dividing by the number of bronchoscopic assessments. Lymphocytic bronchiolitis (LB) was either present or absent.
Chronic rejection clinically manifests as bronchiolitis obliterans syndrome (BOS) and was assessed based on existing ISHLT criteria 14. BOS was diagnosed after exclusion of AR, infection, increased body mass index, and/or anastomotic complications.
Primary graft dysfunction (PGD) was graded post-LT, as previously defined 15. A lung allocation score (LAS) score was calculated for each patient and used to determine priority for LT (www.unos.org/resources).
Infection was defined as any microbiologic isolate based on either expectorated sputum or BAL fluid (BALF). All BALF was routinely sent for microbiologic testing including (acid fast) bacteria, viral, and fungal organisms.
Baseline LTR Demographics and Time Dependent Variables
The baseline demographic and perioperative data included age, gender, renal function, donor/recipient cytomegalovirus (CMV) status, transthoracic echocardiographic (TTE) estimation of right ventricular systolic pressure (RVSP), RHC-based hemodynamic variables, LAS, cardiopulmonary bypass time, donor ischemia time, and PGD at 72 hours post LT. The variables analyzed between groups over the first year post-LT included spirometry, AR, LB, and BOS.
Statistical Analysis
Data were analyzed using SAS vs.9.1 (SAS Institute Inc., Cary, NC, USA) and Stata vs.8 (StataCorp LP, College Station, TX, USA). For purposes of this manuscript, the single LT SSc patient data (n=1) was only included in the survival analysis and was intentionally excluded for all other analyses, in an effort to provide homogeneous populations for study comparison. Groups were compared using Student’s t-test (or Wicoxon rank-sum tests) for continuous variables, Chi-square test for categorical variables, and Fisher’s exact test for small sample sizes. Descriptive statistics are presented as means ± standard deviations (SD) or medians (25th, 75th percentiles). To test for differences within groups over time, paired t-tests and Wilcoxon signed rank tests were performed. As this was an exploratory analysis, there was no adjustment for multiplicity. Significance was prespecified at p≤0.05. In addition, survival analysis was employed to assess time-to-event outcomes. The Cox proportional hazards model was used to assess differences in time to death post-LT between groups, adjusting for age. The time to first AR ≥ 1, or AR ≥ 2, was compared between groups. Kaplan-Meier curves are provided for the survival analysis and time-to-event analysis.
Results
A total of 243 LT were performed between 1/1/2003 and 12/21/2007. 14 and 38 bilateral LT were performed in patients with SSc and IPF, respectively. Among SSc LTR, the indication for LT was isolated PAH in 2 patients (14%), PF in 6 patients (43%), and combined PAH/PF in 6 patients (43%); 16 (42%) of 38 IPF patients had associated PAH. The pre-LT median (25th, 75th percentiles) PA pressure by RHC was similar between the SSc and IPF groups [(29; 23,45) vs. (23.5; 20,32.5), p=0.11, respectively]. There were no other significant differences in pre-LT hemodynamic parameters between groups (Table 1). The median DeMeester score in our SSc cohort was 42.6, suggesting pathologic GER despite aggressive medical therapy (Table 1). A DeMeester score was not routinely performed in the IPF group.
TABLE 1.
| IPF (n=38) | SSc (n=15) | p-value | |
|---|---|---|---|
| Baseline Demographic | |||
| Female Gender, n (%) | 9 (24%) | 4 (29%) | 0.73 |
| Limited Disease, n (%) | - | 11 (79%) | - |
| Age, years | 58.8 (53.6–62.3) | 53.2 (42.6–58) | 0.02* |
| Patient followup days | 788 (550–950) | 632 (442–1033) | 0.99 |
| DeMeester Score | - | 42.6 (32.3–52.7) | - |
| Mean RA pretransplant | 6.03 (4.0–8.0) | 7.07 (2.0–10) | 0.90 |
| Mean PAP pretransplant | 23.5 (20–32.5) | 29 (23–45) | 0.11 |
| CO pretransplant | 5.31 (4.47–6.0) | 4.83 (4.2–5.6) | 0.30 |
| Echo RVSP | 51 (37–62) | 61 (40–82) | 0.43 |
| Lung Allocation Score | 42.5 (36.7–45.3) | 42 (35.9–46.7) | 0.98 |
| # Bronchoscopies | 5 (4–6) | 6 (4–7.5) | 0.19 |
| T72 PGD 2–3 | 8 (21%) | 3 (20%) | 1 |
| T72 PGD 3 | 4 (11%) | 2 (13%) | 1 |
| Donor Ischemia, minutes | 334+/−60 | 354+/−68 | 0.34 |
| CPB time, minutes | 199+/−38 | 217+/−55 | 0.26 |
| CMV status | - | - | 0.81 |
| Lung Function (12 mo) | |||
| FEV1 (% predicted) | 77 (69.5–85.5) | 74.5 (65.5–99.5) | 1 |
| FVC (% predicted) | 73 (63.5–80) | 68.5 (58–99) | 0.85 |
| FEV1/FVC (%) | 80 (73–86) | 83 (76–84.5) | 0.93 |
| Renal Function | |||
| Creatinine Clearance (pre transplant) | - | 92.2 (64.8–118) | - |
| Creatinine Clearance (12 mo) | - | 63.2 (55.5–78.5) | - |
| Survival | |||
| # Deaths at 12 months | 5 | 1 | 0.53 |
p value is statistically significant by t-test, Wilcoxon rank sum test, Chi-square test, or Fisher’s exact test.
All values are median (25th–75th percentile) unless otherwise stated.
Patient follow up days (mean ± SD).
PAP (mean pulmonary artery pressure); RA (right atrial pressure); CO (cardiac output); RVSP (right ventricular systolic pressure); T72 PGD 2–3 (primary graft dysfunction grade 2 or 3 at 72 hours post lung transplant); CPB (cardiopulmonary bypass); CMV (cytomegalovirus) status includes the four potential combinations of donor and recipient CMV status
Baseline characteristics
Demographics were similar between the groups (Table 1). However, the mean age of LTR with SSc was younger (53.2 vs. 58.8 years, p=0.02), and 11 of 14 (79%) had limited disease. Median (25th, 75th percentile) follow up (days) was 632 (442, 1033) and 788 (550, 950) for patients with SSc and IPF, respectively (p=0.99). The pre-LT creatinine clearance [CrCl (mL/min)] for patients with SSc was within normal limits [92.2] (Table 1). There were no differences between groups in the rates of pre-LT HLA mismatching or the type of induction therapy (data not shown). Pre-LT spirometry was similar between both SSc and IPF (Table 3) with the sole exception of FEV1/FVC ratio which was statistically decreased in the SSc group. It should be noted that SSc serologic data, specifically with regards to autoantibodies (i.e. anti-centromere, anti-topoisomerase, etc.), was not uniformly performed in our SSc cohort.
Survival Analysis
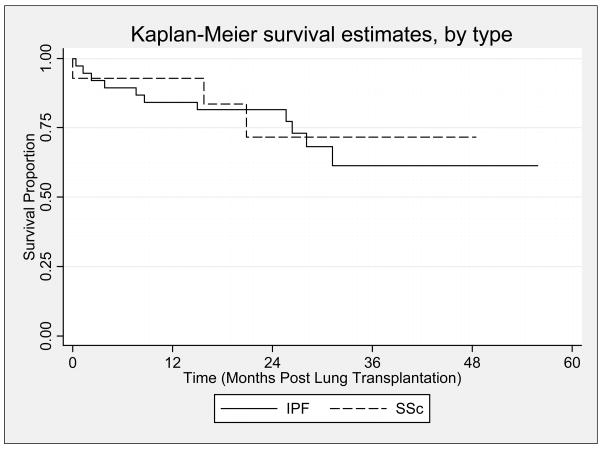
During the first year post-LT, 1 (6.6%) SSc and 5 (13.1%) IPF LTR died (p=0.62). During a median follow up of 632 days (range 0 to 1474 days) (SSc) and 788 days (IPF), there were 3 deaths in patients with SSc (21.4%) and 11 deaths in patients with IPF (28.9%), (p=0.69, Figure 4). In the SSc group, causes of death included complications of BOS (n=1), scleroderma renal crisis (n=1), and complications of induction anesthesia in the setting of severe PAH (n=1). In comparison, causes of death for IPF included complications of BOS (n=3), sepsis/pneumonia (n=4), community acquired adenoviral infection (n=1), perforated bowel (n=1), air embolus (n=1), and complications of thorocosternotomy wound dehiscence (n=1). Among those who died during the study period, the median days followed was 481 (range 1 to 612) in the SSc group compared to 233 (range 38 to 390) in the IPF group, (p=0.64).
Figure 4.
Kaplan-Meier plot displaying the survival estimates for the idiopathic pulmonary fibrosis group [indicated by a solid line (—)] vs. the systemic sclerosis group [indicated by a dashed line (---)]. There was no significant difference in overall survival between groups (log-rank test: p=0.69).
Infection
During the first year post-LT, there were no significant differences between mean (SD) number of BALF [(5.7±1.9) vs. (4.8±2.3); p=0.19] or sputum [(3.3±3.5) vs. (4.0±4.8); p=0.65] assessments made between LTR with SSc and IPF, respectively. In addition, no differences were observed between the median number of bacterial, viral (including CMV)], fungal, or AFB isolates (Table 2).
Table 2.
| IPF | SSc | ||
|---|---|---|---|
| Infections (12 mo)† | n = 37 | n = 13 | p-value |
| Bacteria | 0.17 (0–0.5) | 0.2 (0–0.5) | 0.54 |
| Fungus | 0 (0–0.25) | 0 (0–0.25) | 0.39 |
| Virus | 0 (0–0) | 0 (0–0) | 0.91 |
| AFB | 0 (0–0) | 0 (0–0.13) | 0.22 |
| Rejection (TBBx 12 mo) | |||
| Any Acute Rejection (AR) | 38% | 69% | 0.06 |
| Never Rejection, n (%) | 62.2% | 31% | 0.05* |
| Average Acute Rejection Score | 0 (0–0.4) | 0.5 (0–0.67) | 0.05* |
| # of LB, n (%) | 24 (65%) | 11(85%) | 0.29 |
| AR ≥ 2, n (%) | 22% | 62% | 0.01* |
p value is statistically significant by t-test, Wilcoxon rank sum test, Chi-square test, or Fisher’s exact test; all values are median (25th–75th percentile) unless otherwise stated.
AFB (acid fast bacilli); TBBx (transbronchial biopsy); LB (lymphocytic bronchitis)
accounts for bronchoalveolar lavage and expectorated sputum sampling
Acute Rejection
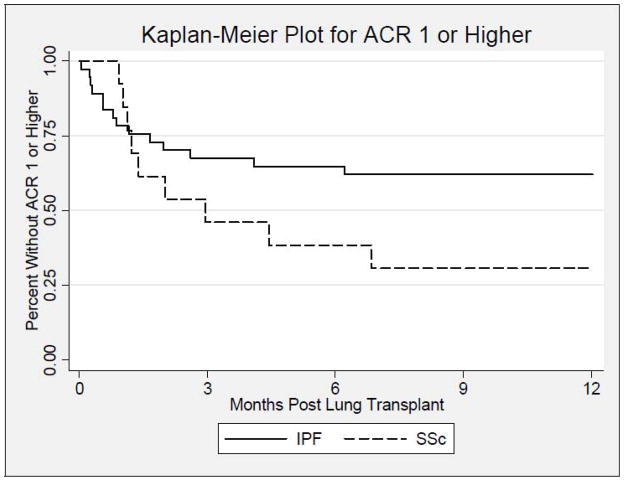
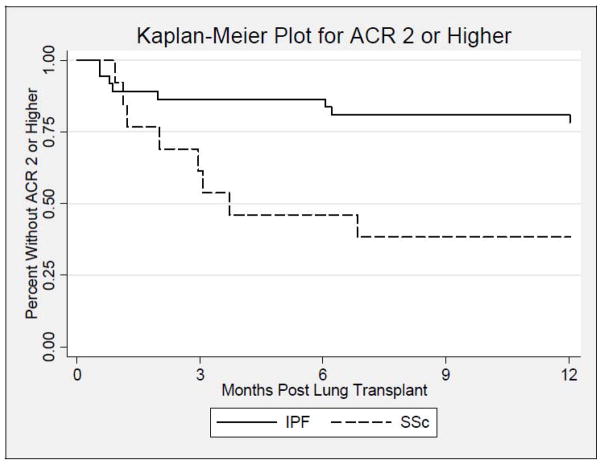
During the first year post-LT, the proportion of patients who developed AR ≥ 1 was higher in the SSc group (9/13; 69%) compared to the IPF group (14/37; 38%) and trended toward statistical significance (p=0.06, Figure 1). In addition, the proportion of patients that developed AR scores ≥ 2 was significantly higher in the SSc group (8/13; 62%) compared to the IPF group (8/37; 22%) (HR 2.91; p=0.007, Figure 2). After adjusting for age, this finding remained significant (p=0.049). The median (25th, 75th percentile) time (days) to first episode of AR score ≥ 2 was 75.0 (35.5, 103.0) in SSc (n=8) vs. 43.0 (20.5,186.5) in IPF (n=8); p=0.49. The average AR score during the first year post-LT was significantly higher in the SSc group (0.46±0.44) compared to (0.22±0.36) in the IPF group; (p=0.048, Table 2). However, the proportion of SSc and IPF LTR with LB [(11/13;85%) vs. (24/37;65%); p=0.29], and the median number of bronchoscopic assessments during the first year post-LT were similar between both groups, [4.5(3.0,6.0) vs. 4.0(2.0,5.0); p=0.78]. The number of SSc and IPF patients who developed AR grade 1, 2, or 3 were 1 (8%), 3 (23%), and 5 (38%), compared to 6 (16%), 4 (11%), 4 (11%), respectively (Table 2).
Figure 1.
Kaplan-Meier plot displaying the estimated time to first acute rejection ≥1 for the idiopathic pulmonary fibrosis group [indicated by a solid line (—)] vs. the systemic sclerosis group [indicated by a dashed line (---)], during the first year after lung transplantation. There is no significant difference between groups (log-rank test: p=0.06).
Figure 2.
Kaplan-Meier plot displaying the estimated time to first acute rejection ≥2 for the idiopathic pulmonary fibrosis group [indicated by a solid line (—)] vs. the systemic sclerosis group [indicated by a dashed line (---)], during the first year after lung transplantation. There is a significant difference between groups (log-rank test: p=0.007).
Pulmonary Function
At 1 year post-LT, there were no differences in median percent reference values for FEV1 [74.5 vs. 77.0; p=NS], FVC [68.5 vs. 73.0; p=NS], or FEV1/FVC ratio [83.0 vs. 80.0; p=NS], when comparing SSc to IPF, respectively(Table 1). In addition, no differences were found between groups when comparing the change in these same spirometric parameters between 3 and 6 months, 6 and 12 months, and 3 and 12 months, post-LT (data not shown).
Chronic Rejection (BOS)
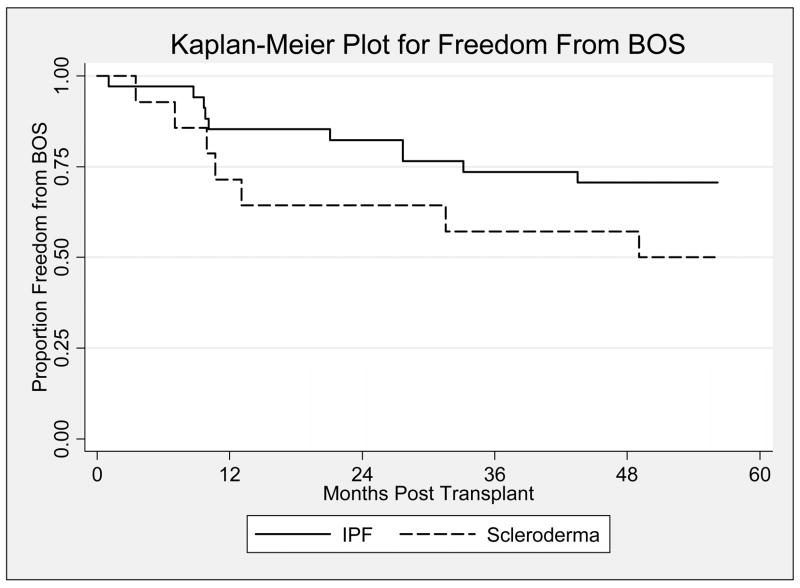
The median time (days) to onset of BOS (1492 vs. 1322) and freedom from BOS at 1 (70% vs. 88%), 2 (63% vs. 84%), and 3 (52% vs. 60%) years post-LT were similar between the SSc and IPF groups, respectively; Mantel-Cox p=0.29 (Figure 3).
Figure 3.
Kaplan-Meier plot displaying freedom from chronic rejection (BOS) post lung transplantation for the idiopathic pulmonary fibrosis group [indicated by a solid line (—)] vs. the systemic sclerosis group [indicated by a dashed line (---)]. For the first five years after lung transplantation, there is no significant difference between the two groups (Log-rank; p=0.14).
Discussion
Our report marks the largest cohort of SSc patients who underwent bilateral LT at a single center. The primary endpoint of all-cause mortality at 1 year post-LT was similar between the SSc (n=1; 6.6%) and IPF (n=5; 13.1%) groups. In addition, during a median follow up of 632 days (range 0 to 1474 days) for the SSc group and 788 days (range 12 to 1700 days) for the IPF group, the post-LT survival (78.6% vs. 71.1%) was no different. AR ≥ 2 was significantly increased in the SSc group (62%) compared to the IPF group (22%), over the first year post-LT. Nonetheless, the freedom from BOS was similar in both groups, and there were no differences in pulmonary function or rates of infection.
Similar to prior reports, we chose IPF as a comparator group given the similar spectrum of lung disease to SSc. In addition, IPF uniquely represents the most common indication for LT at our center. At baseline, the two groups showed no significant differences with the exception of a statistically lower mean age at time of LT in the SSc group (53.2 vs. 58.8). While there was no predefined selection for PAH or limited SSc in our cohort, 86% of the SSc patients had PAH as an element of their disease and 79% had limited disease. We suspect limited disease was over-represented given the inherent early mortality of individuals with diffuse disease 16.
The largest retrospective case series to date reviewed 47 SSc LTs in over 23 U.S. centers. The overall 1-year survival was 68%, however the results must be interpreted in the context of variable transplant experience, and lack of standardized selection criteria 3. Recently, Schachna and coworkers reported survival rates post-LT in SSc (n=29) compared to either IPF (n=70) or IPAH (n=38), at two large university centers 1. The majority of LTs performed in all 3 cohorts were single LT and the 2-year cumulative survival was >60% and similar across all 3 groups. Importantly, these authors focused on pre-LT clinical parameters, demographics, overall survival, and less so, on other accepted predictors of post-LT survival including AR, infection, and BOS 17.
In our study, 1-year post-LT survival tended to be greater among SSc patients with a survival rate of 93.4%, compared with 86.9% for IPF. In addition, over a median follow-up period of approximately 2 years, the cumulative survival rate was similar between SSc (80%) and IPF (71.1%). Of the deaths that occurred in our SSc group (n=3), one patient died within 24 hours of LT due to complications of general anesthesia in the setting of severe PAH and right ventricular dysfunction. After this unexpected death, we instituted a policy to perform RHC just prior to anesthesia induction in patients with significant pre-LT PAH to optimize pulmonary hemodynamics. The second patient died after 481 days due to renal crisis, while the third patient died after 635 days secondary to complications of BOS. Although there was no difference in mortality comparing non-PAH with PAH patients or limited versus diffuse SSc patients (data not shown), future study with larger numbers should incorporate comparison of these groups.
LT for CTD remains controversial due to the unpredictable capacity for systemic organ involvement and potential for disease recurrence. One of the SSc deaths was in fact due to renal crisis, a well known complication of SSc. To date, there has been no report to support recurrence of systemic sclerosis in the lung allograft.
We observed a statistically increased proportion of patients with SSc developing AR ≥ 2 compared to the IPF group (p=0.007). With the exception of age, other known predictors of post-LT morbidity (i.e. recipient PAH, donor ischemia time, infection, and PGD) were similar between groups and likely do not explain this finding. We hypothesize that the inherent predisposition for GI dysfunction (particularly esophageal dysmotility) may contribute to the increased AR seen in our SSc cohort. However, the absence of a systematic assessment for GER in the IPF (pre and post-LT) and SSc (post-LT) groups makes it difficult to draw any definitive conclusion regarding the association of GI dysfunction and post-LT alloreactivity. To test this hypothesis in subsequent study, researchers may consider an assessment of BALF pepsin 18 and/or bile acids, incorporation of impedance testing to evaluate for non-acid reflux 19, the contribution of the actual surgical intervention 20, and the effect of azithromycin use 21. Nevertheless, it is reassuring that, despite the evidence of widespread GER in our SSc patients (median DeMeester score 42.6), patient survival was not affected for a median follow-up of approximately 2 years.
The frequency and severity of AR post-LT is a reproducible risk factor for subsequent BOS. In turn, BOS is the single most important determinant of late mortality (> 1year) post-LT 22. Although we found an increased rate of AR in our SSc cohort, freedom from BOS was no different at 1 (70% vs. 88%), 2 (63% vs. 84%), and 3 (52% vs. 60%) years post-LT, when compared to IPF. The proportion of IPF LTR with freedom from BOS is consistent with the reported ISHLT registry data of 75% at 2.5 years 22. Importantly, severe PGD at 72 hours post-LT is another accepted risk factor for subsequent BOS 23 and, apart from showing no difference between groups, the 13% PGD rate in SSc is consistent with a recent large registry report 24. Interestingly, freedom from BOS for SSc (compared to IPF) appeared decreased at all time points, but was not statistically significant. Longer follow up is necessary to draw any conclusions regarding freedom from BOS for SSc LTR.
Based on our experience and that of other investigators 4, 3, 1, 25, and in concert with accepted guidelines for the selection of LT candidates 26, we have suggested a UCLA algorithm for the assessment of patients with systemic sclerosis for LT (Table 4). We recommend the pre-LT evaluation be a coordinated, multidisciplinary effort to avoid post-LT morbidity and mortality related to the potential for extrapulmonary disease in the setting of underlying SSc. The evaluation for GI dysfunction is particularly important given the association between GER and chronic rejection (BOS) post-LT 27, justifying the increasing consideration of early anti-reflux surgery 28, 29, 25, 30. Importantly, none of our SSc patients have undergone anti-reflux surgery to date.
Table 4.
UCLA Lung Transplant Program algorithm in consideration of bilateral lung transplantation (LT) for individuals with underlying systemic sclerosis
General
|
| Extrapulmonary Disease |
Gastrointestinalb
|
Skin/Musculoskeletal
|
Renal
|
Cardiacd
|
Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–55.
we recommend the following tests be performed for all potential SSc LT recipients: barium esophagram, nuclear medicine quantitative gastric emptying study, esophageal manometry, and upper endoscopy; consider the following tests: dual pH probe study +/− impedance testing
combination of high-dose proton pump inhibitor +/− H2 blocker, high-dose pro-motility agent, and antireflux measures including lifestyle modification counseling
consider cardiac magnetic resonance imaging
absence of clinical congestive heart failure, and preservation of liver function testing (including synthetic function)
the relatively poorer prognosis of SSc PAH compared to idiopathic PAH might warrant earlier referral and active listing for LT
Our study has limitations, especially those pertaining to a retrospective analysis including inherent selection bias and the potential for missing data. We recognize our limited sample size and available longitudinal follow-up, rendering it difficult to make a definitive conclusion regarding survival. In addition, we did not systematically collect SSc serologic autoantibody data and as such, were unable to report this baseline demographic. To our knowledge, this manuscript represents the largest single center experience of bilateral LTs for SSc published to date; moreover, we have conducted an additional 6 bilateral LT for SSc since January 1, 2008. This is the first study to comprehensively report the post-bilateral LT complications at a single center in patients with SSc including acute and chronic rejection. Importantly, all SSc LTs to date (with one exception) at our institutution have been bilateral, a direct consequence of our center’s donor protocol which is largely driven by recipient age. These limitations not withstanding, we demonstrate that cumulative survival of SSc at 1 and 2 years was similar to those with IPF, with an overall survival of 80% during a median follow up of 632 days. However, there may be an increased risk of AR in SSc LTR, and whether this translates to an increased risk of future BOS requires further study.
References
- 1.Schachna L, Medsger TA, Jr, Dauber JH, et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 2006;54:3954–61. doi: 10.1002/art.22264. [DOI] [PubMed] [Google Scholar]
- 2.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Massad MG, Powell CR, Kpodonu J, et al. Outcomes of lung transplantation in patients with scleroderma. World J Surg. 2005;29:1510–5. doi: 10.1007/s00268-005-0017-x. [DOI] [PubMed] [Google Scholar]
- 4.Levine SM, Anzueto A, Peters JI, Calhoon JH, Jenkinson SG, Bryan CL. Single lung transplantation in patients with systemic disease. Chest. 1994;105:837–41. doi: 10.1378/chest.105.3.837. [DOI] [PubMed] [Google Scholar]
- 5.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 7.Johnsson F, Joelsson B, Isberg PE. Ambulatory 24 hour intraesophageal pH-monitoring in the diagnosis of gastroesophageal reflux disease. Gut. 1987;28:1145–50. doi: 10.1136/gut.28.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindlbeck NE, Heinrich C, Konig A, Dendorfer A, Pace F, Muller-Lissner SA. Optimal thresholds, sensitivity, and specificity of long-term pH-metry for the detection of gastroesophageal reflux disease. Gastroenterology. 1987;93:85–90. doi: 10.1016/0016-5085(87)90318-0. [DOI] [PubMed] [Google Scholar]
- 9.Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9:1903–11. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 11.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult lung and heart-lung transplant report--2005. J Heart Lung Transplant. 2005;24:956–67. doi: 10.1016/j.healun.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Girgis RE, Tu I, Berry GJ, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200–8. [PubMed] [Google Scholar]
- 14.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 15.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen S, Ullman S, Shen GQ, Wiik A, Halberg P. Influence of clinical features, serum antinuclear antibodies, and lung function on survival of patients with systemic sclerosis. J Rheumatol. 2001;28:2454–9. [PubMed] [Google Scholar]
- 17.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–21. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 18.Stovold R, Forrest IA, Corris PA, et al. Pepsin, a biomarker of gastric aspiration in lung allografts: a putative association with rejection. Am J Respir Crit Care Med. 2007;175:1298–303. doi: 10.1164/rccm.200610-1485OC. [DOI] [PubMed] [Google Scholar]
- 19.Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med. 2009;179:408–13. doi: 10.1164/rccm.200808-1359OC. [DOI] [PubMed] [Google Scholar]
- 20.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689–93. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 21.von Rosensteil NA, Adam D. Macrolide antibacterials. Drug interactions of clinical significance. Drug Saf. 1995;13:105–22. doi: 10.2165/00002018-199513020-00005. [DOI] [PubMed] [Google Scholar]
- 22.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation:twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–95. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Huang HJ, Yusen RD, Meyers BF, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8:2454–62. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009 doi: 10.1111/j.1399-0012.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 25.Gasper WJ, Sweet MP, Golden JA, et al. Lung transplantation in patients with connective tissue disorders and esophageal dysmotility. Dis Esophagus. 2008;21:650–5. doi: 10.1111/j.1442-2050.2008.00828.x. [DOI] [PubMed] [Google Scholar]
- 26.Maurer JR, Frost AE, Estenne M, Higenbottam T, Glanville AR. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. J Heart Lung Transplant. 1998;17:703–9. [PubMed] [Google Scholar]
- 27.Hadjiliadis D, Duane Davis R, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17:363–8. doi: 10.1034/j.1399-0012.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 28.Cantu E, 3rd, Appel JZ, 3rd, Hartwig MG, et al. J Maxwell Chamberlain Memorial Paper. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142–51. doi: 10.1016/j.athoracsur.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Patti MG, Gasper WJ, Fisichella PM, Nipomnick I, Palazzo F. Gastroesophageal reflux disease and connective tissue disorders: pathophysiology and implications for treatment. J Gastrointest Surg. 2008;12:1900–6. doi: 10.1007/s11605-008-0674-9. [DOI] [PubMed] [Google Scholar]
- 30.Shitrit D, Amital A, Peled N, et al. Lung transplantation in patients with scleroderma: case series, review of the literature, and criteria for transplantation. Clin Transplant. 2009;23:178–83. doi: 10.1111/j.1399-0012.2009.00958.x. [DOI] [PubMed] [Google Scholar]