Abstract
Epidemiological and experimental studies suggest a positive correlation between chronic respiratory inflammatory disease and the ability to cope with adverse stress. Interactions between neuroendocrine and immune systems are believed to provide insight toward the biological mechanisms of action. The utility of an experimental murine model was employed to investigate the immunological consequences of stress-controllability and ovalbumin-induced airway inflammation. Pre-conditioned uncontrollable stress exacerbated OVA-induced lung histopathological changes that were typical of Th2-predominant inflammatory response along respiratory tissues. Importantly, mice given the ability to exert control over aversive stress attenuated inflammatory responses and reduced lung pathology. This model represents a means of investigating the neuro-immune axis in defining mechanisms of stress and respiratory disease.
Keywords: stress, controllability, asthma, immunity
1. Introduction
The relationship between perceived and uncontrollable stress events and propensity of disease has been an accepted idea for decades, but uncovering the mechanistic links has only recently become a focus of epidemiological and experimental research. Meta-analysis studies on the role of psychosocial factors and disease have begun to quantify measures that support such associations {Steptoe et al., 2007}. In particular, a majority of data has been collected on populations with atopic disorders (e.g. allergic rhitnitis, dermatitis and asthma), which suggest that psychosocial interventions in conjunction with typical pharmacologic treatments can facilitate useful treatments in combat of atopic diseases {Chida et al., 2008}. Furthermore, researchers have demonstrated a positive correlation between mental disorders and the incidence of disease {Hartmann et al, 2010, Li, C et al., 2010, Giardino, ND, et al., 2010}.
Stress disorders continue to escalate each year and are believed to contribute to the increase in mal-adaptive behavioral and physiological responses, resulting in pathological conditions along the respiratory airways including asthma, chronic obstructive pulmonary disease and respiratory infection (Sandberg et al. 2000, Chen, Miller 2007, Wright 2005). In a study by Jones et al., a positive correlation was found between posttraumatic stress disorder (PTSD) and COPD {Jones RC, 2009}. A recent study also documented an increase risk for contracting SARS among healthcares was related to chronic stress among healthcare workers {McAlonan GM, 2007}. Furthermore, there is a growing appreciation for the association between stress and asthma as reported by in the National Survey of Children Health (NSCH), which documented psychosocial stressors as important risk factors {Subramanian, SV, 2009). On the basis of emerging epidemiological evidence in support of stress and health status, further experimental data is needed to reveal the underlying mechanisms relating stress and disease pathogenesis.
Immune status is believed to be a major physiological focal point in explaining the interaction between stress and disease. In this regard, it has previously been shown that stress-induced alterations in neuropeptide activity have a profound impact on immune-mediated inflammatory processes {}. Mediators of immune function play a key role in both the protective and pathogenic responses along the respiratory airways. Notably, altered CD4+ T cell responses including, T helper cell-type 2 (Th2) mediated by the increased secretion of IL-4, IL-5, and IL-13 (Larche, et al. 2003, Wills-Karp 1999), IFN-γ production by Th1 cells, IL-10 and/or TGF-β by CD4+ T regulatory (Tregs) cells and IL-17 by Th17 subsets can all participate in both the progress and limitation of respiratory disease (Wakashin et al. 2008, Maddox, Schwartz 2002). Overactive Th2 cellular activation is known to exacerbate allergic airway disease {Robinson, 1992, 1993 and Mazzarella et al., 2000}, and has been implicated in stress-induced immunopathoglogical responses. Likewise, studies have demonstrated an association between stress-induced impaired Th1 cellular responses and increased susceptibility to viral and bacterial pneumonias {Gonzalez et al., 2008, Mays, JW et al., 2010}. Also, recent experimental studies have documented the significant role of Th17 as well as T regulatory cell activation in modulation of chronic inflammatory conditions {Wakasin, 2008, Fujiwara, 2007, Komiyam, 2006, Nakae, 2003}, and their role in neuro-immune regulation of respiratory disease. Therefore, the immunoregulatory mechanisms responsible for the pathogenesis of respiratory disease are believed to involve complex interactions among multiple cellular immune populations.
The interaction between stress and disease has led to the emerging field of psychoneuroimmunology research (PNIR)”, aimed toward understanding the relationship between central nervous and immune system functioning. In this regard, animal models of stress are useful to study neuro-immune interactions and disease. Previous studies have established unpredictable stressors as valid instruments for producing fear, depression and anxiety all indicative of mal-adaptive physiological and behavioral responses (Anisman, Matheson 2005) (Mikics, Baranyi et al. 2008). (Chourbaji et al. 2005b, Chourbaji et al. 2005a). (Rybnikova et al. 2007). While these studies in mice have focused on behavioral psychopathologies, few have documented alterations in disease susceptibility. For example, exposure to aversive shock increased tumor progression (Yee et al. 2008, Vegas et al. 2009) and viral infection (Ashcraft, Hunzeker & Bonneau 2008). Importantly, experimental models have demonstrated stress-associated influences on CD4+ T cell responses and respiratory disease (Marshall, Agarwal 2000, Marshall 2004a, Marshall 2004b). Utilizing a murine model of stress-controllability and allergen-induced pulmonary inflammation, we implement for the first time, a model of allergic airway inflammation a model of learned helpless behavior in mice to demonstrate that the extent of perceived stress and the ability to exert control translates into a concomitant diminished Th2-dominant inflammatory response.
2. Methods
Mice
The Institutional Animal Care and Use Committee approved all protocols in compliance with the NIH guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85-23, revised 1996). Adult (6-8 weeks of age) female Balbc/J mice (Jackson Laboratories, PA) were used in all studies. Mice were fed ad libitum and maintained in a 12 hr light-dark cycle under specific pathogen-free conditions. Mice were housed 4 per cage under conditions of optimal temperature and humidity. All animals were allowed to acclimate to the surrounding environment two weeks prior to experimentation.
Tail shock apparatus and stress paradigm
Following acclimation to the colony environment, mice were randomly assigned to the following experimental conditions: escapable stress, inescapable stress and home cage environment. Mice in the escapable condition were able to extinguish tail shock by turning a wheel placed in reach of its front legs. When the mouse terminated the shock it also terminated the tail shock for a parallel mouse in the inescapable condition, allowing both subjects to receive shocks for the same duration. Selected mice were also placed in the shock apparatus, but were not exposed to shock (sham). An additional group of mice remained in the home cage environment and were handled similarly to escapable, inescapable, and sham shock conditions, but were never exposed to the shock apparatus.
Mice were exposed to escapable and inescapable conditions on days 8, 10 and 12 of the experimental protocol (Figure 1). On experimental stress days, mice were individually transported to shock apparatus (Med Associates, St. Albans, VT) housed in a separate location. Mice were placed in their respective plexiglass boxes divided by an opaque sound-proof wall to inhibit visual and audible responses. The electrical probe that delivered the electrical shock was secured to the tail, ¾ from the end, including the sham-treated mice. Aversive shocks were administered for exactly 15 minutes on the day of the stress paradigm between the hours of 2:00 PM to 3:00 PM. MED-PC® Version IV software (Med associates, INC.) controlled the administration and timing of a 0.2 mA current over a random 30s intervals lasting 15 minutes. The computer software recorded duration of shock and wheel turns to determine the number of wheel and latency frequency between shock and wheel turn. Each chamber was thoroughly cleansed between trials.
Figure 1. Stress paradigm and allergen sensitization and challenge.
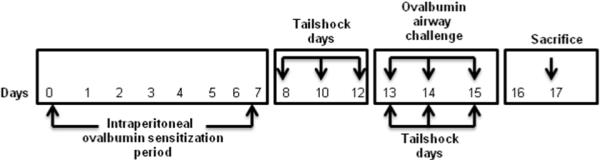
Prior to stress exposure, mice were sensitized with OVA and aluminum hydroxide adjuvant on day 0 and day 7. Groups of mice were exposed to escapable or inescapable stress on days 8, 10, 12, 13, 14 and 15. Escapable, inescapable and non-stressed groups (Home cage and Sham) of mice were exposed to either OVA or PBS by intranasal route on days 13, 14 and 15. On day 17, mice were euthanized.
Allergen sensitization and challenge
On day 1 and 7, mice were administered 25mg ovalbumim i.p. (Sigma-Aldrich, St. Louis, Missouri) with aluminum hydroxide gel (13 mg/ml) adjuvant (Sigma-Aldrich) diluted in the PBS (Mediatech Inc., Herndon, VA). On day 13, 14, 15 mice were anesthetized with isoflurane and challenged intranasally with 20 ul OVA (10ug/ml) in PBS or PBS alone. On day 17 mice were sacrificed after anaesthetizing with xylazine + ketamine.
Cell isolation
Bronchiolalveolar lavage fluid (BALF) containing cells was aspirated by perfusion of 2 ml of wash media using a blunt 25 guage needle. The volume of the fluid retrieved was recorded and stored for further analysis.
Single cell suspensions from lung tissue were prepared as previously described (Jones et al. 2002). Briefly lungs were infused with sterile PBS to eliminate contaminating RBCs. Lung tissue was separated into single lobes, pooled, minced and digested for 1 hr in digestion medium containing 300 units/ml collagenase type II (Worthington, Lakewood, NJ.) and 0.01% DNAse (Sigma-Aldrich, St. Louis, MO). After digestion lung supernatants were passed through a nylon mesh filter (LabPak, Depew, NY.) into sterile 15 ml conical tubes and washed 2X with RPMI (Hyclone, Logan, UT.) wash media supplemented with 1% antibiotic cocktail. Lymphocytes were prepared by ficoll-hypaque (Lympholyte M, Cedarlane Laboratories ltd., Ontario, CA.) centrifugation. Contaminating RBCs were removed using ammonium chloride potassium (ACK) lysis buffer. The total viable cell count was determined with a haemocytometer (Hausser Scientific, Horsham, PA).
Histopathology and immunohistochemistry
Dissected lungs were perfused by instillation of 2 ml of 2% paraformaldehyde (Sigma Chemical, Co., St. Louis, MO) intratracheally followed by fixation in 2% paraformaldehyde at 4°C for 4 hours and overnight incubation in 30% solution of sucrose at 4°C. On the next day each individual lobes of the lung were frozen using OCT (Optimum Cutting Temperature) embedding medium (Sakkura Finetechnical, Torance, CA). Thin tissue sections (10μm) were prepared on SuperFrost/plus microscope slides (Fisher Scientific) using Ultrapro 5000 cryostat (vibratome, St. Louis, MO). The tissue sections were stained with Hematoxylin and Eosin (H&E) (FisherBrand). Additional tissue sections were stained using Periodic Acid Schiff’s Base Staining (PAS) for further confirmation. Analysis was performed using Image Pro software (Olympus, Centervalley, PA). The Images were captured on Olympus AX70 fluorescent microscope (Olympus, Centervalley, PA).
Cytokine detection in bronchiolar lavage
Enzyme-Linked Immunosorbent Assays (ELISAs) were performed using OptEIA sets for mouse interferon-gamma (IFN-γ), interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-10 (IL-10) from BD Biosciences (Franklin Lakes, NJ) and interleukin-13 (IL-13) from eBiosciences (San Diego, CA). Briefly flat bottom 96 micro-well plates were coated with 100μl (per well) capture antibody (OptEIA anti-mouse monoclonal antibodies for different cytokines IL-4, IL-5, IL-10, IL-13, and IFN-γ diluted 1:250 in coating buffer (0.1M carbonate or 0.1M phosphate). The plate was sealed and incubated overnight at 4° C. The plate was then washed 5 times with ≥ 200μl/well wash buffer (PBS with 0.05% Tween-20). After the last wash the plate was inverted and blotted on a paper towel to remove residual buffer. This wash procedure was used throughout the rest of the protocol unless otherwise noted. After washing, the plate was blocked with ≥ 200μl/well assay diluent (PBS with 10% FBS) and incubated overnight at 4°C. The plate was washed, and then samples (with suitable dilutions) and standards (OptEIA serially diluted recombinant mouse IL-4, IL-5, IL-10, IL-13, IFN) were pipetted into the appropriate wells of the plate (100μl/well of sample and standard). The plate was sealed and incubated overnight at 4°C. After wash procedure, 100μl/well Detection Antibody (OptEIA biotinylated anti-mouse IL-4, IL-5, IL-10, IL-13, IFN-γ monoclonal antibody) diluted 1:250 in assay diluent was added, and the plate was sealed and incubated for an hour at RT (room temperature). After washing, 100μl/well Enzyme Reagent (OptEIA avidin-horseradish peroxide conjugate) diluted 1:250 in assay diluent was added, and the plate was sealed and incubated for 30 minutes at RT. For the final plate wash, plates were rinsed with wash buffer 7 times, letting the buffer sit in the wells for ≥30 seconds per rinse. 100μl/well TMB (Tetramethylbenzidine, BD Pharmingen) was added and the plate was incubated for approximately 20 minutes (normally) unsealed at RT in dark. 50μl/well stop solution (0.25M HCL) was added to halt the reaction and the absorbance (at 450 nm) of the plate was read on an ELISA reader using the computer program Gen5 (Bio-Tek Instruments).
Flow cytometry
Two and three color flow cytometric staining was performed using fluorescently-labeled antibodies conjugated to the following flourochromes: FITC (Fluroscein isothiocyanate), R-PE (R-phycoerythrin) and cy-chrome. One million lymphocytes out of lung single cell suspensions were washed with washing buffer (2% FBS in 1X PBS with 2mM EDTA) and incubated with an optimal concentration of anti-FcBlock Antibody (clone 2.4G2) (BD Pharmingen, SanDiego, CA) to block non-specific binding to FcRs for 30 min. Multicoloured staining was performed by incubating cells at 4°C with compatible combinations of pre-tittered concentrations of following antibodies: anti-GR1+FITC, anti-CD8+FITC, anti-CD3+PE, anti-CD4+PE-Cy7, anti-B220+FITC, anti-MHC II+PE, anti-CD19+PE-Cy5, anti-CD11c+Alexa Flour 488, anti-F/480+PEcy7. Unstained lymphocytes served as negative controls. Antibodies were obtained from BD Pharmingen, San Deigo, CA. Gating of lymphocytes was identified by forward-scatter/ side-scatter profile. Data acquisition was done on FC500 flow cytometer (Beckman Coulter, Miami, FL). Further analysis was done on CXP software (Beckman Coulter).
Statistical Analysis
Statistical analysis was performed using GraphPad prism Version 4.0 for Windows (GraphPad Software, San Diego, USA). For multi-experimental group analysis, data were subjected to analysis of variance (univariant ANOVA) followed Post Hoc tests (Tukey) for group differences. For analysis of two-group differences, student’s t test was employed, followed by Post Hoc analysis. All data are expressed as the mean ± SEMs. The two-tailed level of significance was set up at p ≤ 0.05.
3. Results
Induction of airway inflammation diminishes avoidance response to aversive tailshock and attenuates weight gain
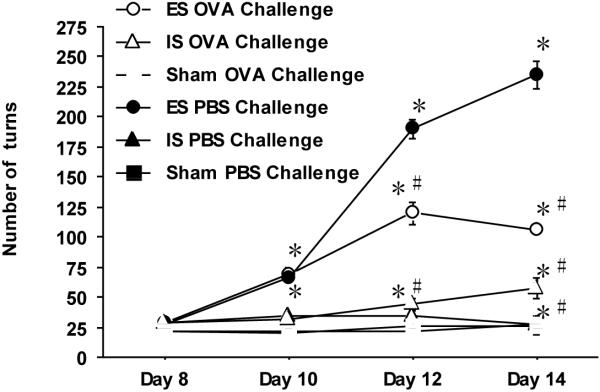
Prior to experimentation, mice exposed to the stress paradigm were tested to assure equivalent wheel turn performance. OVA sensitized mice were subjected to four sessions (days 8,10,12, and 14) of escapable or inescapable tail shock. Sham-shocked and home cage groups of mice served as non-shocked controls. On days 12 and 14, the stress paradigm was preceded by an intranasal OVA or PBS challenge. The wheel turn frequency was recorded as a measure of learned avoidance proficiency. Figure 2 illustrates a similar number of wheel turn responses to aversive shock on the first day (Day 8) between all mice regardless of their priming status (OVA vs PBS). On the second stress day (Day 10), higher numbers (p ≤ 0.05) of wheel turn responses were observed among OVA and PBS-primed escapable mice as compared to OVA and PBS-primed inescapable and sham-treated mice. By the third stress exposure (Day 12), escapable mice increased their wheel response by more than 300% (p ≤ 0.05) compared to their inescapable mates and sham-treated mice. Acute OVA challenge restrained this response by half among the escapable PBS counterparts (p ≤ 0.05). Both escapable groups were far more active than their inescapable counterparts. No significant difference in wheel turn response was observed between OVA and PBS-challenged inescapable-treated and sham-treated mice. On day 14, PBS-treated escapable mice continued to increase their wheel turn responses while those of the other three groups reached a plateau or began to decline. These results confirm the progressive learning ability of mice to escape aversive stimuli. Interestingly, OVA priming was not itself a discriminating factor but the subsequent acute airway challenge did reduce response the wheel turn responses in both escapable and inescapable groups as compared to their PBS experimental counterparts.
Figure 2. Provocation of airway hypersensitivity diminishes avoidance response to aversive tailshock.
The total number of wheel turns during each session of tailshock was recorded for each subject and plotted as a function of stress days during the duration of the stress paradigm. Data represents mean (n=15) ± std. error in the number of wheel turns at a given time point. (*) and (#) designates significant p ≤ 0.05) difference between each experimental group at each time point. OVA; ovalbumin-treated, PBS; Phosphate buffered saline-treated, ES; escapable stress and IS; inescapable stress, and Sham.
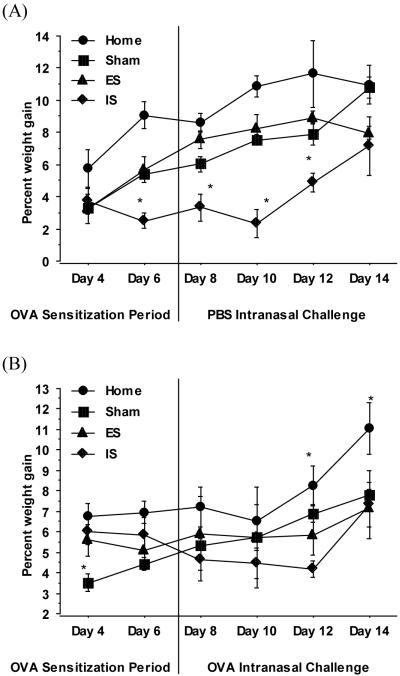
Upon initiation of the stress paradigm, altered wheel turn proficiency given uncontrolled aversive stress impacted growth rate. Though more variable, those in the escapable stress group maintained their growth rates closer to the non-stressed groups. Following the acute intranasal OVA challenge, animals in the inescapable stress group accelerated their rate of gain. Whether this was lean mass or perhaps inflammation mediated fluid retention is undetermined (Figure 3A and 3B).
Figure 3. Kinetics of body weight changes in response to OVA-challenge of mice exposed escapable and inescapable stress.
Body weight was determined at selected time point for the duration of the stress paradigm. The percent change in body weight from the initial body weight was calculated and plotted as a function of respective days among PBS (A) and OVA-challenged (B) mice exposed to escapable (ES), inescapable stress (IS) or no stress conditions designated as Home and Sham. Data represents mean (n=15) ± std. error. (*) and (#) indicates significant (p ≤ 0.05) difference between experimental groups.
Inescapable exacerbates OVA-induced airway inflammatory responses
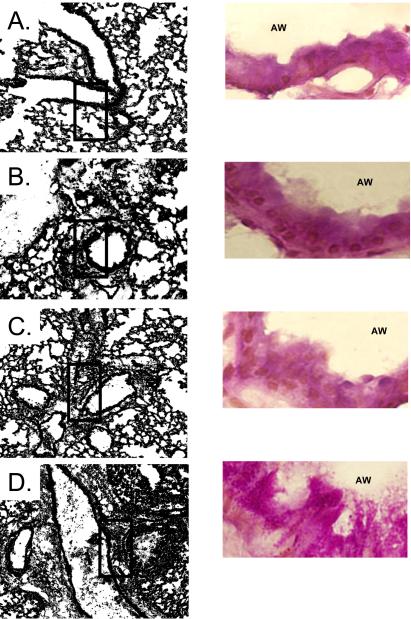
Gross histopathological changes in response to OVA airway challenge were compared between non-stressed, escapable and inescapable stressed mice. A qualitative increase in cellular infiltrate surrounding bronchiolar airways was observed in the lungs of inescapable mice as compared to escapable OVA-treated counterparts, sham-treated, and home caged mice (Figure 4A). Bronchiolar hyperplasia of the epithelium surrounding both small and large airways was significantly (p ≤ 0.05) greater in OVA challenged mice exposed to inescapable stress as compared to escapable and non-stressed mice (Figure 5). No differences were observed between inescapable, escapable and non-stressed PBS-challenged mice (data not shown). As controls, PBS sensitized mice challenged with OVA did not demonstrate histological changes along the respiratory airways (data not shown).
Figure 4. Inescapable stress exacerbates OVA-induced airway inflammatory responses.
Qualitative differences in gross histopathology were determined within lung parenchyma and surrounding airways and vessels. Results in representative of n=5 mice/experimental condition; A denotes No stress (Home) + OVA challenge, B denotes no stress (Sham), C denotes, escapable stress + OVA challenge and D denotes inescapable stress + OVA challenge.
Figure 5. Bronchiole-associated airway hyperplasia in response to Inescapable stress.
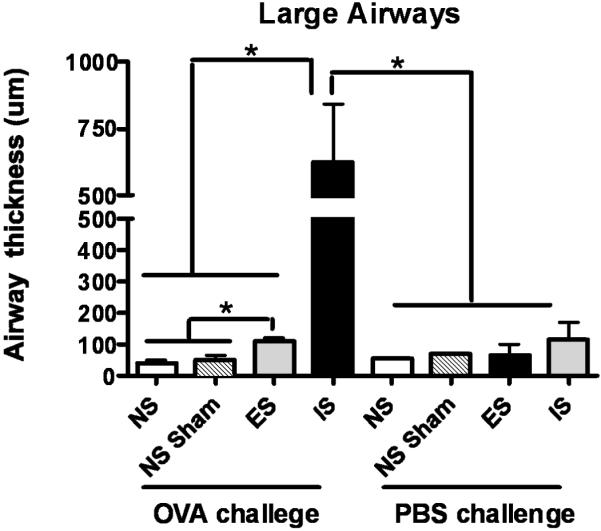
Lung tissue sections were stained with Periodic Acid and Schiff’s Base to determine airway hyperplasia and mucous formation. Results in representative of n=5 mice/experimental condition. Quantitative determination of airway thickness was calculated by mean difference between the thickness of inner endothelium border to that of the mean diameter of the outer endothelium borders of representative small and large airways. Data represents mean (n=5) ± std. error. Asterisk (*) indicates significant (p ≤ 0.05) difference between NS; no stress, NS Sham; sham-treated, ES; escapable and IS; inescapable groups.
Inescapable stress corresponds with a distinct leukocyte and eosinophil accumulation in lung
In response to antigenic provocation, mobilization of leukocytes and eosinophils is a hallmark of the inflammatory cascade in asthma. The number and phenotype of pulmonary immune cells was determined. OVA challenge increased the total number of lung parenchyma cells (p ≤ 0.05) in all groups compared to their PBS challenged counterparts. PBS challenged inescapable-stressed mice demonstrated a significantly (p ≤ 0.05) higher number of total lung cells than PBS non-stressed mice. Inescapable OVA-challenged mice demonstrated a significant (p ≤ 0.05) increase in total lung cells as compared to both escapable and non-stressed counterparts. In contrast, cell numbers in escapable-OVA-challenged mice were not different from non-stressed-OVA-challenged (Figure 6).
Figure 6. Total cell numbers in lung as determined by trypan blue staining.
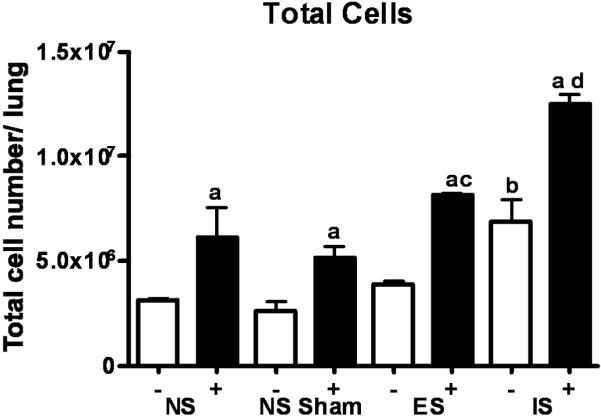
Data represents mean (n=3) independent experiments ± std. error. (*) denotes significant (p ≤ 0.05) difference in total cell number between PBS and OVA-treated mice between ES; escapable, IS; inescapable, Sham and NS groups.
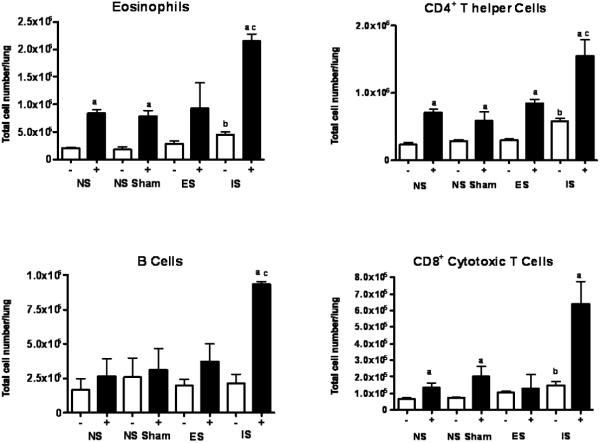
Although stress alone did not alter eosinophil counts in any group, nasal OVA exposure resulted in a 3-4-fold increase (p ≤ 0.05) in non-stressed animals (Figure 7). Importantly, an interaction emerged when stress and OVA were combined; eosinophil counts rose further (p ≤ 0.05) with the highest cell counts in the inescapable stress group. CD4+ T lymphocytes presented a similar pattern to eosinophils except that they were higher in inescapable stress group (Figure 7). However much like the eosinophils, CD4+ cells were progressively higher when animals were exposed to intranasal antigen and once again, the highest numbers observed were in the inescapable group. B and CD8+ T lymphocytes (Figure 7) were unresponsive to stress alone but increased dramatically in the inescapable group when OVA challenged (p ≤ 0.001).
Figure 7. Inescapable stress corresponds with a distinct leukocyte and eosinophil accumulation in lung.
The phenotype and distribution of leukocytes and eosinophils in the lung was determined by flow cytometry. Data represents mean (n=3) independent experiments ± std. error. (*) denotes significant (p ≤ 0.05) difference in total cell number between between NS; no stress, NS Sham; sham-treated, ES; escapable and IS; inescapable groups.
Inescapable stress enhances the preferential activation of lung Th2 cytokine responsiveness
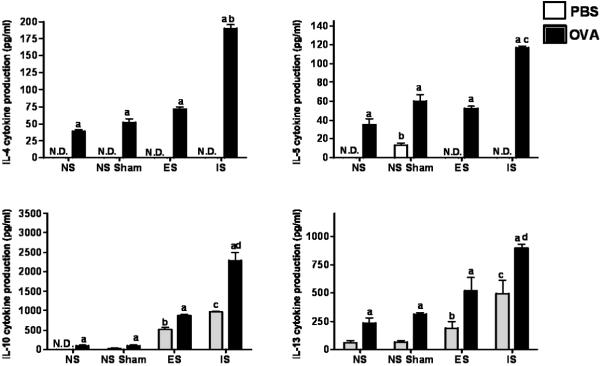
Since Th2 cytokine responses are consistent with allergic airway inflammatory responses, IL-4, IL-5, IL-10, IL-13 and IFN-γ were determined in bronchiolar lavage (Figure 8). OVA challenge of non-stressed mice produced increases in IL-4, IL-5, IL-10, IL-13 (p ≤ 0.05). Both forms of stress increased of IL-10 and IL-13 in the PBS-challenged mice compared to their non-stressed counterparts (p ≤ 0.05). IL-4, IL-5, IL-10, and IL-13 increased stepwise in OVA-challenged mice from non-stressed, to escapable stress, to inescapable stress (p ≤ 0.05). IFN-γ was undetectable regardless of the treatment (data not shown).
Figure 8. BALF cytokine production in responses to OVA-challenge under conditions of escapable and inescapable stress.
IL-4, IL-5 IL-10, IL-13, IFN-γ cytokine production was determined in the BALF of mice (n=8/group) by ELISA. (*) indicates significant (p ≤ 0.05) differences in the mean ± std. error between PBS and OVA-treated mice within each experimental condition and differences between ovalbumin (OVA)-treated mice between all experimental conditions, PBS and OVA-treated mice between ES; escapable, IS; inescapable, NS Sham and NS groups. N.D. represents cytokine concentration not detected in BALF fluid within the limitations of ELISA assay. IFN-γ was not detected in BALF fluids.
Characterization of APC phenotypes in lungs of OVA-challenged mice exposed to escapable and inescapable stress
Antigen presentation of environmental antigens plays an important role in the regulation of CD4+ T cells. DC and macrophage populations were designated as CD11c+, F4/80− MHC IIhi and F4/80+, CD11c− and MHCIIhi, respectively. A subpopulation of CD11c+, F4/80+ and MHClo was also measured in the lung parenchyma given stress and OVA challenge. CD11c+ F4/80+ MHC IIlo cells were the major subtype found in the lung followed by F4/80+ MHC IIhi macrophages and CD11+ F4/80− MHC IIhi DCs, respectively. OVA challenge increased CD11c+ F4/80+ MHC IIlo and DCs (p ≤ 0.05) in all groups regardless of the stress. In the case of CD11c+ F4/80+ MHC IIlo cells, the increase was progressive with the highest numbers observed in the inescapable stress group (p ≤ 0.05). Only inescapable stress led to significant (p ≤ 0.05) increases in DCs and macrophages (Figure 9).
Figure 9. Characterization of APC phenotypes in lung of OVA-challenged mice exposed to escapable and inescapable stress.
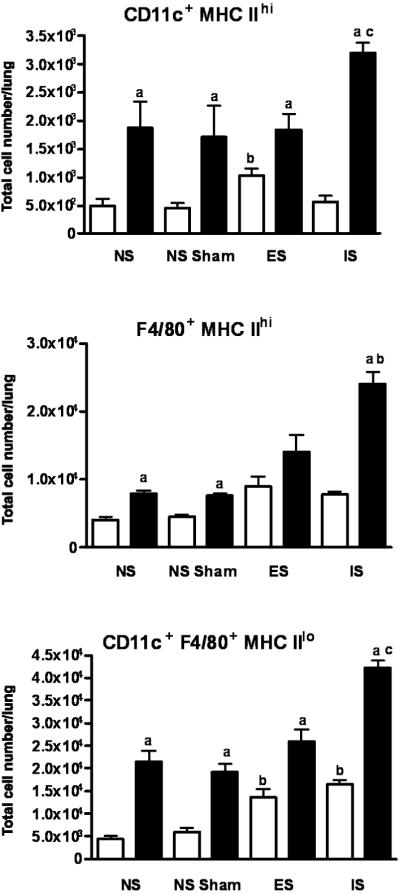
The phenotype and distribution of CD11c+ F4/80− MHC II, F4/80+ CD11c− MHC II+ and CD11c+ F4/80+ MHC II+ cells in the lung was determined by flow cytometry. Data represents mean (n=3) independent experiments ± std. error. (*) denotes significant (p ≤ 0.05) difference between PBS and OVA-treated mice within each experimental group, PBS and OVA-treated mice between ES; escapable, IS; inescapable, NS Sham and NS groups.
4. Discussion
Anecdotal evidence suggests that behavioral disposition is linked to disease severity (Marshall 2004b, Rafinski 1975). Individuals that cope with stress well and exhibit a sense of control are thought to have healthy outcomes {Pascoe et al., 2009, Hankonen, N et al., 2009} An understanding of stress-induced alterations in immune function may provide important insight into the role of behavior in the pathogenesis of inflammatory diseases. The fear-conditioning model of learned helplessness provides an opportunity to evaluate differences in the immune response during controllable and uncontrollable stress in an experimental setting (Chourbaji et al. 2005a, Belda et al. 2008). This is the initial use of the model to study the interaction between stress and the immune-mediated inflammatory responses along the respiratory tract in mice. The purpose of the current study was to establish a murine model of stress-induced exacerbation of airway inflammatory disease and test the hypothesis that given the ability to extinguish aversive stimuli can translate into a protective outcome.
Stress induced alterations in brain function can modify peripheral homeostasis (Wright 2005) and facilitates disease susceptibility. Notable behavioral examples are stress mediated changes in temperament, physical activity and appetite (Khazan 2009). Body mass is a known characteristic of health status that can be influenced by stress and illness (de Ferranti, Mozaffarian 2008, Coccurello, D’Amato & Moles 2009). Activation of the hypothalamic pituitary adrenal axis (HPA) is a key component of the stress response that orchestrates a host of physiologic adaptations (Valentino, Van Bockstaele 2008). In the current study, stress suppressed weight gain and those affected most were those with the least control.
The behavioral response to aversive stress was verified by recording the frequency of the wheel turn response by mice subjected to escapable and inescapable tail shock before and after OVA-induced ALH. Consistent with previous reports, mice having the capacity to extinguish aversive stimuli acquire over time, greater escape proficiency (Mikics, Baranyi & Haller 2008, Baratta et al. 2007). Likewise, despite the proven ability to escape, mice unable to extinguish stress develop a helpless behavior, reflected by a much lower wheel turn frequency. In addition, acute exposure to the sensitized antigen dramatically depressed high wheel turn frequency in mice that had successfully learned to escape. Despite the onset of the acute illness, mice in control of their stress still responded significantly better than their helpless counterparts. Although the helpless mice clearly developed a more sever illness, their failure to respond was predetermined by their prior behavioral disposition. This later observation supports the thesis that one’s ability to cope through control of environment can be an important determinant of the response to disease.
The study hypothesized that the inability to control stress would translate into more severe inflammatory responses following allergen airway challenge. Consistent with numerous studies, qualitative differences in lung histopathology was noted in the lungs of OVA challenged mice as compared to their PBS counterparts. Importantly, uncontrollable stress resulted in significantly more severe inflammatory changes in lung airway morphology. Airways were narrowed and goblet cell hyperplasia was evident, both known features of asthma pathogenesis. These results are consistent with clinical findings that link stress and pulmonary airway disease among asthmatics (Chen, Miller 2007).
Experimental models provide an excellent tool in defining the relationships between psychological stress, neuroendocrine activity and immune function (Quarcoo, Pavlovic & Joachim 2009, Joachim et al. 2006). Immune responses are clearly important participants in the inflammation associated with airway pathogenesis. Increased parenchymal cell numbers confirmed the histopathological evidence of the inflammatory infiltration and the numbers were clearly higher in mice with less control of their environment. In many cases, inflammatory responses along the respiratory tract that facilitate disease pathogenesis are primarily related to preferential Th2 activation. In the current study, cytokine production provided clear confirmation of a prominent Th2 cellular response in this model. In the lungs, CD4+ T cells were the major lymphocyte population with corresponding increases in airway IL-4 and IL-13 and a lack of IFN-γ cytokine production following OVA-challenge. As hypothesized, mice in control of their stress were able dampen this Th2-driven inflammatory disease by limiting CD4+ T cell pulmonary infiltration and Th2-associated cytokine production. In contrast, B cells and eosinophils which produce damaging IgE and histamine were highest in the OVA-challenged helpless mice. Consistent with this observation IL-5 which mediators their functions was elevated in these same animals (Ying, S Durham, SR, 1995). There is mounting evidence that Th2-dominant responses combine with a general imbalance in immune parameters to determine disease severity. In addition to examining CD4+ Th2-driven responses, the current study indicates the induction of CD8+ T cells. Previous studies suggest that CD8+ T cells may serve as protective regulators of inflammatory responses through cell fratricide (Su et al. 2004). In support of this concept, other studies of chronic respiratory infection demonstrated that depletion of CD8+ T cells corresponded with enhanced CD4+ T cell-associated inflammatory responses (Jones et al. 2002). Furthermore, the current studies reveal significant IL-10 cytokine production in response to inescapable stress of OVA-treated mice. Interestingly, IL-10 cytokine production was the greatest among all cytokines evaluated. Recently, studies have emphasized the role of IL-10 secretion by CD4+ Tregulatory cells as participants in limiting the severity of asthmatic attacks (Lewkowich et al. 2005, Nguyen, Vanichsarn & Nadeau 2009). Thus, current data indicates the complexity in the regulation of lymphocyte-derived cytokine responses mediated by stress controllability.
Antigen presenting cells (APCs) are a vital part of the induction and maintenance of CD4+ and CD8+ T cells responses and have been implicated in regulation of airway disease (Hutchison et al. 2009, Willart, Lambrecht 2009). In addition, studies indicate that functional responses by APCs can be predicted by the diversity of APC phenotypes (Randolph, Jakubzick & Qu 2008, Fadilah, Cheong 2007). To date, few studies have investigated the impact of stress exposure on the distribution and phenotype of APC in an experimental model of airway inflammation. As a potential link between the cell-mediated responses and stress controllability demonstrated above, APC phenotypes were determined in lung under conditions of OVA-challenge and stress. Inescapable stress and OVA-challenge resulted in the preferential increase in a population of CD11c+ F4/80+ MHC II+ phenotype, followed by F4/80+ CD11c− MHC II+ (macrophages) and CD11c+ F4/80− MHC II+ (dendritic cells) cells. Since CD11c+ F4/80+ MHC II+ cells were the major phenotype increased in the lungs given inescapable stress, this suggests a preference in the heterogeneity of maturing APCs with in the lung during airway challenge. Thus, distinct APC sub-types may impact the preferential induction of CD4+ T cell responses that dictate asthma pathogenesis. To date, studies have described the complexity of roles for pulmonary APCs in respiratory pathogenesis (Burchell, Strickland & Stumbles 2009, Kool et al. 2009). Yet, the impact of such phenotypes in response to stress remains unknown. Future studies, which define this critical interaction promise to improve our understanding the induction and regulation inflammatory reactions that participate in respiratory pathogenesis. Also, future studies which aim at defining further the interaction between the control of stress, the neuro-immune axis and the evolution of disease will facilitate comprehensive interventions against respiratory and other inflammatory diseases.
Acknowledgments
We thank Dr. Jerry Simecka (University of North Texas Health Science Center, Fort Worth, Texas) for support of this project. This project was also supported by grant #P20MD001633 from the National Center On Minority Health And Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center On Minority Health And Health Disparities or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akana SF. Feeding and stress interact through the serotonin 2C receptor in developing mice. Physiology & Behavior. 2008;94(4):569–579. doi: 10.1016/j.physbeh.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience and biobehavioral reviews. 2005;29(4-5):525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Anson K, Ponsford J. Coping and emotional adjustment following traumatic brain injury. The Journal of head trauma rehabilitation. 2006;21(3):248–259. doi: 10.1097/00001199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Archea C, Yen IH, Chen H, Eisner MD, Katz PP, Masharani U, Yelin EH, Earnest G, Blanc PD. Negative life events and quality of life in adults with asthma. Thorax. 2007;62(2):139–146. doi: 10.1136/thx.2006.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS & neurological disorders drug targets. 2006;5(5):485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Ashcraft KA, Hunzeker J, Bonneau RH. Psychological stress impairs the local CD8+ T cell response to mucosal HSV-1 infection and allows for increased pathogenicity via a glucocorticoid receptor-mediated mechanism. Psychoneuroendocrinology. 2008;33(7):951–963. doi: 10.1016/j.psyneuen.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146(4):1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belda X, Rotllant D, Fuentes S, Delgado R, Nadal R, Armario A. Exposure to severe stressors causes long-lasting dysregulation of resting and stress-induced activation of the hypothalamic-pituitary-adrenal axis. Annals of the New York Academy of Sciences. 2008;1148:165–173. doi: 10.1196/annals.1410.038. [DOI] [PubMed] [Google Scholar]
- Bender BG, Lerner JA, Kollasch E. Mood and memory changes in asthmatic children receiving corticosteroids. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(6):720–725. doi: 10.1097/00004583-198811000-00010. [DOI] [PubMed] [Google Scholar]
- Burchell JT, Strickland DH, Stumbles PA. The role of dendritic cells and regulatory T cells in the regulation of allergic asthma. Pharmacology & therapeutics. 2009 doi: 10.1016/j.pharmthera.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Busse WW, Kiecolt-Glaser JK, Coe C, Martin RJ, Weiss ST, Parker SR. NHLBI Workshop summary. Stress and asthma. Am J Respir Crit Care Med. 1995;151(1):249–52. doi: 10.1164/ajrccm.151.1.7812562. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21(8):993–9. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. 2008 Jan;70(1):102–16. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain research.Brain research protocols. 2005a;16(1-3):70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis-Segura C, Spanagel R, Gass P. Social and structural housing conditions influence the development of a depressive-like phenotype in the learned helplessness paradigm in male mice. Behavioural brain research. 2005b;164(1):100–106. doi: 10.1016/j.bbr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Coccurello R, D’Amato FR, Moles A. Chronic social stress, hedonism and vulnerability to obesity: lessons from rodents. Neuroscience and biobehavioral reviews. 2009;33(4):537–550. doi: 10.1016/j.neubiorev.2008.05.018. [DOI] [PubMed] [Google Scholar]
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clinical chemistry. 2008;54(6):945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- Fadilah SA, Cheong SK. Dendritic cell immunobiology and potential roles in immunotherapy. The Malaysian journal of pathology. 2007;29(1):1–18. [PubMed] [Google Scholar]
- Galil N. Depression and asthma in children. Curr Opin Pediatr. 2000;12(4):331–5. doi: 10.1097/00008480-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Giardino ND, Curtis JL, Abelson JL, King AP, Pamp B, Liberzon I, Martinez FJ. The Impact of Panic Disorder on Interoception and Dyspnea Reports in Chronic Obstructive Pulmonary Disease. Biol Psychol. 2010 Feb 19; doi: 10.1016/j.biopsycho.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Gonzales XF, Deshmukh A, Pulse M, Johnson K, Jones HP. Stress-induced differences in primary and secondary resistance against bacterial sepsis corresponds with diverse corticotropin releasing hormone receptor expression by pulmonary CD11c+ MHC II+ and CD11c− MHC II+ APCs. Brain, behavior, and immunity. 2008;22(4):552–564. doi: 10.1016/j.bbi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RG, F AN., Jr Mechanisms of acute allergic reactions. Artif Organs. 1984;8(3):311–7. doi: 10.1111/j.1525-1594.1984.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Hankonen N, Absetz P, Haukkala A, Uutela A. Socioeconomic status and psychosocial mechanisms of lifestyle change in a type 2 diabetes prevention trial. Ann Behav Med. 2009 Oct;38(2):160–5. doi: 10.1007/s12160-009-9144-1. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Bäzner E, Wild B, Eisler I, Herzog W. Effects of Interventions Involving the Family in the Treatment of Adult Patients with Chronic Physical Diseases: A Meta-Analysis. Psychother Psychosom. 2010 Feb 20;79(3):136–148. doi: 10.1159/000286958. [DOI] [PubMed] [Google Scholar]
- Holt PG, Upham JW. The role of dendritic cells in asthma. Current opinion in allergy and clinical immunology. 2004;4(1):39–44. doi: 10.1097/00130832-200402000-00009. [DOI] [PubMed] [Google Scholar]
- Hutchison S, Choo-Kang BS, Gibson VB, Bundick RV, Leishman AJ, Brewer JM, McInnes IB, Garside P. An investigation of the impact of the location and timing of antigen-specific T cell division on airways inflammation. Clinical and experimental immunology. 2009;155(1):107–116. doi: 10.1111/j.1365-2249.2008.03800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim G. An assessment of social support in people with inflammatory bowel disease. Gastroenterology nursing: the official journal of the Society of Gastroenterology Nurses and Associates. 2002;25(6):246–252. doi: 10.1097/00001610-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Sagach V, Quarcoo D, Dinh T, Arck PC, Klapp BF. Upregulation of tumor necrosis factor-alpha by stress and substance p in a murine model of allergic airway inflammation. Neuroimmunomodulation. 2006;13(1):43–50. doi: 10.1159/000094394. [DOI] [PubMed] [Google Scholar]
- Johansson I, Hildingh C, Wenneberg S, Fridlund B, Ahlstrom G. Theoretical model of coping among relatives of patients in intensive care units: a simultaneous concept analysis. Journal of advanced nursing. 2006;56(5):463–471. doi: 10.1111/j.1365-2648.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- Jones HP, Tabor L, Sun X, Woolard MD, Simecka JW. Depletion of CD8+ T cells exacerbates CD4+ Th cell-associated inflammatory lesions during murine mycoplasma respiratory disease. Journal of immunology (Baltimore, Md.: 1950) 2002;168(7):3493–3501. doi: 10.4049/jimmunol.168.7.3493. [DOI] [PubMed] [Google Scholar]
- Kang DH, Kim CJ, Suh Y. Sex differences in immune responses and immune reactivity to stress in adolescents. Biol Res Nurs. 2004;5(4):243–54. doi: 10.1177/1099800403262749. [DOI] [PubMed] [Google Scholar]
- Khazan I. Psychophysiological stress assessment using biofeedback. Journal of visualized experiments: JoVE. 2009;29:10.3791/1443. doi: 10.3791/1443. pii: 1443. doi, no. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, Coyle A, Clausen BE, Hoogsteden HC, Lambrecht BN, Hammad H. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. Journal of immunology (Baltimore, Md.: 1950) 2009;183(2):1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. The Journal of allergy and clinical immunology. 2003;111(3):450–63. doi: 10.1067/mai.2003.169. quiz 464. [DOI] [PubMed] [Google Scholar]
- Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. The Journal of experimental medicine. 2005;202(11):1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ford ES, Zhao G, Balluz LS, Berry JT, Mokdad AH. Undertreatment Of Mental Health Problems In Adults With Diagnosed Diabetes And Serious Psychological Distress: The Behavioral Risk Factor Surveillance System, 2007. Diabetes Care. 2010 Feb 25; doi: 10.2337/dc09-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. American journal of respiratory and critical care medicine. 2002;165(8):1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Maddox L, Schwartz DA. The pathophysiology of asthma. Annual Review of Medicine. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- Marshall GD. Internal and external environmental influences in allergic diseases. J Am Osteopath Assoc. 2004a;104(5 Suppl 5):S1–6. [PubMed] [Google Scholar]
- Marshall GD. Neuroendocrine mechanisms of immune dysregulation: applications to allergy and asthma. Ann Allergy Asthma Immunol. 2004b;93(2 Suppl 1):S11–7. doi: 10.1016/s1081-1206(10)61482-2. [DOI] [PubMed] [Google Scholar]
- Marshall GD, Jr, Agarwal SK. Stress, immune regulation, and immunity: applications for asthma. Allergy and Asthma Proceedings: The Official Journal of Regional and State Allergy Societies. 2000;21(4):241–246. doi: 10.2500/108854100778248917. [DOI] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, Padgett DA, Sheridan JF. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J Immunol. 2010 Feb 15;184(4):2014–25. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikics E, Baranyi J, Haller J. Rats exposed to traumatic stress bury unfamiliar objects--a novel measure of hyper-vigilance in PTSD models? Physiology & Behavior. 2008;94(3):341–348. doi: 10.1016/j.physbeh.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Vanichsarn C, Nadeau KC. Impaired IL-10-dependent Induction of Tolerogenic Dendritic Cells by CD4+CD25hiCD127lo/- Natural Regulatory T Cells In Human Allergic Asthma. American journal of respiratory and critical care medicine. 2009 Nov 1;180(9):823–33. doi: 10.1164/rccm.200905-0761OC. 2009. [DOI] [PubMed] [Google Scholar]
- Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009 Jul;135(4):531–54. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarcoo D, Pavlovic S, Joachim RA. Stress and airway reactivity in a murine model of allergic airway inflammation. Neuroimmunomodulation. 2009;16(5):318–324. doi: 10.1159/000216189. [DOI] [PubMed] [Google Scholar]
- Rafinski T. [Mental aspects of allergic diseases] Pol Tyg Lek. 1975;30(5):217–9. [PubMed] [Google Scholar]
- Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Current opinion in immunology. 2008;20(1):52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybnikova E, Mironova V, Pivina S, Tulkova E, Ordyan N, Vataeva L, Vershinina E, Abritalin E, Kolchev A, Nalivaeva N, Turner AJ, Samoilov M. Antidepressant-like effects of mild hypoxia preconditioning in the learned helplessness model in rats. Neuroscience letters. 2007;417(3):234–239. doi: 10.1016/j.neulet.2007.02.048. [DOI] [PubMed] [Google Scholar]
- Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H. Stress increases the risk of asthmatic attacks in children. Duodecim; laaketieteellinen aikakauskirja. 2000;116(20):2305–2306. [PubMed] [Google Scholar]
- Scarinci IC, McDonald-Haile J, Bradley LA, Richter JE. Altered pain perception and psychosocial features among women with gastrointestinal disorders and history of abuse: a preliminary model. The American Journal of Medicine. 1994;97(2):108–118. doi: 10.1016/0002-9343(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Su MW, Pyarajan S, Chang JH, Yu CL, Jin YJ, Stierhof YD, Walden P, Burakoff SJ. Fratricide of CD8+ cytotoxic T lymphocytes is dependent on cellular activation and perforin-mediated killing. European journal of immunology. 2004;34(9):2459–2470. doi: 10.1002/eji.200425096. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Marmot M, Wardle J. Positive affect and psychosocial processes related to health. Br J Psychol. 2008 May;99(Pt 2):211–27. doi: 10.1111/j.2044-8295.2008.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European journal of pharmacology. 2008;583(2-3):194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas O, Garmendia L, Arregi A, Beitia G, Azpiroz A. Effects of antalarmin and nadolol on the relationship between social stress and pulmonary metastasis development in male OF1 mice. Behavioural brain research. 2009 doi: 10.1016/j.bbr.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Verstraelen S, Bloemen K, Nelissen I, Witters H, Schoeters G, Van Den Heuvel R. Cell types involved in allergic asthma and their use in in vitro models to assess respiratory sensitization. Toxicology in vitro: an international journal published in association with BIBRA. 2008;22(6):1419–1431. doi: 10.1016/j.tiv.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. American journal of respiratory and critical care medicine. 2008;178(10):1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2009;39(1):12–19. doi: 10.1111/j.1365-2222.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annual Review of Immunology. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biological psychology. 2008;78(1):20–28. doi: 10.1016/j.biopsycho.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Wright K. Examining racial and ethnic disparities and predictors of medication use among California’s African-American, Latino, and White children with asthma. Journal of National Black Nurses’ Association: JNBNA. 2007;18(2):1–15. [PubMed] [Google Scholar]
- Wright RJ. Stress and atopic disorders. The Journal of allergy and clinical immunology. 2005;116(6):1301–1306. doi: 10.1016/j.jaci.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, Wand M, Perkins D, Weiss ST, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113(6):1051–7. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Yee JR, Cavigelli SA, Delgado B, McClintock MK. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosomatic medicine. 2008;70(9):1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]