Abstract
Aldosterone effects are mediated by the mineralocorticoid receptor (MR), a transcription factor highly expressed in the distal nephron. Given that MR expression level constitutes a key element controlling hormone responsiveness, there is much interest in elucidating the molecular mechanisms governing MR expression. To investigate whether hyper- or hypotonicity could affect MR abundance, we established by targeted oncogenesis a novel immortalized cortical collecting duct (CCD) cell line and examined the impact of osmotic stress on MR expression. KC3AC1 cells form domes, exhibit a high transepithelial resistance, express 11β-hydroxysteroid dehydrogenase 2 and functional endogenous MR, which mediates aldosterone-stimulated Na+ reabsorption through the epithelial sodium channel activation. MR expression is tightly regulated by osmotic stress. Hypertonic conditions induce expression of tonicity-responsive enhancer binding protein, an osmoregulatory transcription factor capable of binding tonicity-responsive enhancer response elements located in MR regulatory sequences. Surprisingly, hypertonicity leads to a severe reduction in MR transcript and protein levels. This is accompanied by a concomitant tonicity-induced expression of Tis11b, a mRNA-destabilizing protein that, by binding to the AU-rich sequences of the 3′-untranslated region of MR mRNA, may favor hypertonicity-dependent degradation of labile MR transcripts. In sharp contrast, hypotonicity causes a strong increase in MR transcript and protein levels. Collectively, we demonstrate for the first time that optimal adaptation of CCD cells to changes in extracellular fluid composition is accompanied by drastic modification in MR abundance via transcriptional and posttranscriptional mechanisms. Osmotic stress-regulated MR expression may represent an important molecular determinant for cell-specific MR action, most notably in renal failure, hypertension, or mineralocorticoid resistance.
Osmotic stress regulates mineralocorticoid receptor expression in cortical collecting duct cells.
The regulation of sodium balance and water homeostasis is ensured by the steroid hormone aldosterone, which is the main mineralocorticoid in humans (1). In polarized epithelial tissues such as the kidney, aldosterone actions are mediated by the mineralocorticoid receptor (MR), a ligand-dependent transcription factor belonging to the nuclear receptor superfamily. Indeed, aldosterone, by binding to the MR, directly stimulates expression of ionic transporters, such as the epithelial Na+ channel (ENaC) and the Na+,K+-ATPase. MR also induces early expression of other target genes encoding important mediators of the mineralocorticoid response that stimulate ENaC-mediated Na+ reabsorption in the kidney: serum and glucocorticoid-regulated kinase 1, ubiquitin-specific protease Usp2–45, KS-WNK1 kinase (with no lysine K), and glucocorticoid-induced leucine zipper transcription factor (2).
Several lines of evidence suggest that a key step in the mineralocorticoid response is the regulation of the MR expression level. Indeed, MR knockout mice die shortly after birth of renal sodium loss (3), whereas AQP2-Cre-MR mice, with targeted MR inactivation in the cortical collecting duct (CCD), present with sodium wasting when subjected to a low-salt diet (4). Moreover, RNA interference inhibition of MR prevents the development of cold-induced hypertension and renal damage in mice (5) and induces a syndrome resembling autosomal dominant pseudohypoaldosteronism type 1 in a transgenic rat model (6). In humans, pseudohypoaldosteronism type 1 patients carrying a heterozygous MR mutation that leads to MR haploinsufficiency (7) present with typical features of aldosterone resistance. However, increased MR expression also causes deleterious effects in mice and humans. Indeed, MR-overexpressing mice develop kidney dysfunction and mild cardiomyopathy associated with arrhythmias (8), whereas conditional cardiac MR overexpression leads to life-threatening arrhythmias (9). It is interesting that Quinkler et al. (10) demonstrated a 5-fold increase in renal MR expression in patients with renal failure and heavy proteinuria, linking MR activation to kidney disease.
In the kidney, MR is expressed mostly in the distal convoluted tubules and in the CCD of the distal nephron, with higher expression levels in the renal cortex compared with that observed in the medulla (11, 12). However, little information is available on the molecular mechanisms regulating MR expression in the nephron segments exposed to extreme fluctuations of extracellular fluid composition, given the ability of the kidney to dilute urine below plasma osmolality or to concentrate the urine above plasma osmolality. Indeed, water excretion relies on the corticopapillary gradient, which is tightly regulated most notably by hormones (13).
In the renal medulla, where MR expression is low, the driving force for water reabsorption is provided by elevated urea and NaCl concentrations, which generate high osmolarity levels (1200 mosmol/kg). Thus, inner medullar renal cells must accumulate intracellular osmolytes to reduce intracellular ionic strength. This adaptation, which represents a protective mechanism from hypertonic stress in mammalian cells, is mediated by the tonicity-responsive enhancer binding protein (TonEBP or NFAT5/OREBP), a transcription factor belonging to the Rel family (14, 15). TonEBP activity is up-regulated by hypertonicity through combined enhanced TonEBP transcription and increased TonEBP nuclear localization. This leads to increased transcription of sodium-chloride-betaine cotransporter, sodium-myo-inositol cotransporter, aldose reductase, and taurine transporter, which mediate intracellular accumulation of betaine, myoinositol, sorbitol, and taurine, respectively (16). In the renal cortex, where MR expression is high, the tubular lumen is rather hypotonic reaching 50 mosmol/kg because the apical membrane of epithelial cells is impermeable to water leading to sustained osmotic differences.
To the best of our knowledge, no study has ever reported whether hypertonicity or hypotonicity influences MR expression. Here, we investigated MR expression under osmotic stress in a new immortalized and differentiated CCD-derived cell line that we established by a targeted oncogenesis strategy in mice. We demonstrate that fluctuations in extracellular fluid composition both in vitro and in vivo are accompanied by drastic modification in renal MR abundance via transcriptional and posttranscriptional mechanisms. Elucidating such underlying mechanisms is clearly of major importance in the design of new pharmacological strategies for modulating cell-specific MR action.
Results
Expression of functional MR in the immortalized KC3AC1 cell line
The KC3AC1 cell line has been established from microdissected CCDs of a transgenic mouse by means of a targeted oncogenesis strategy in which the P1 proximal promoter of the human MR gene drove the expression of the simian virus 40 (SV40) large T Antigen (TAg) in all aldosterone target tissues (17). When grown on collagen I-coated petri dish, KC3AC1 cells form islets of epithelial-like cells and rapidly develop numerous domes at confluence (Fig. 1A). Positive nuclear immunostaining of TAg further confirmed that KC3AC1 cells are immortalized (Fig. 1A, inset). Electron microscopic analyses revealed that these cells grow as a monolayer of epithelial cells, when cultivated on filters, and exhibit desmosomes and apical microvilli (see inset), two typical features of polarized cells (Fig. 1B). The presence of functional MR in KC3AC1 cells was assessed by Scatchard analysis. Specific aldosterone binding sites were detected in cytosolic fractions of KC3AC1 cells with a dissociation constant (Kd) compatible with the affinity of aldosterone for MR (∼1.3 ± 0.3 nm) and the estimated MR concentration was at approximately 30 fmol/mg protein (Fig. 1C). Competition studies, using 100-fold excess of unlabeled aldosterone and the mineralocorticoid antagonist, spironolactone, enabled identification of these specific [3H]aldosterone binding sites as MR (Fig. 1D). To further substantiate MR protein identification in KC3AC1 cells, MR immunocytochemistry and Western blot analyses were performed with a novel polyclonal anti-MR antibody raised against the first 18 amino acids of human MR (hMR), which are conserved in the mouse and rat species. Purified 39N antibody allowed the immunodetection of MR protein in the cytoplasm of KC3AC1 cells cultured in serum-starved medium (Fig. 1E) and in the nucleus of cells after 30 min incubation with 10 nm aldosterone (Fig. 1F). Direct Western blotting revealed a band at a molecular mass of approximately 130 kDa in KC3AC1 cell lysates (Fig. 1G). As a positive control, we used rabbit M cells, stably overexpressing hMR (18), and as expected, a band was present in M-cell homogenates but absent in lysates of parental RCSV3 cells (19), devoid of MR protein. Taken together, these complementary approaches clearly demonstrated that KC3AC1 cells express functional MR protein.
Fig. 1.
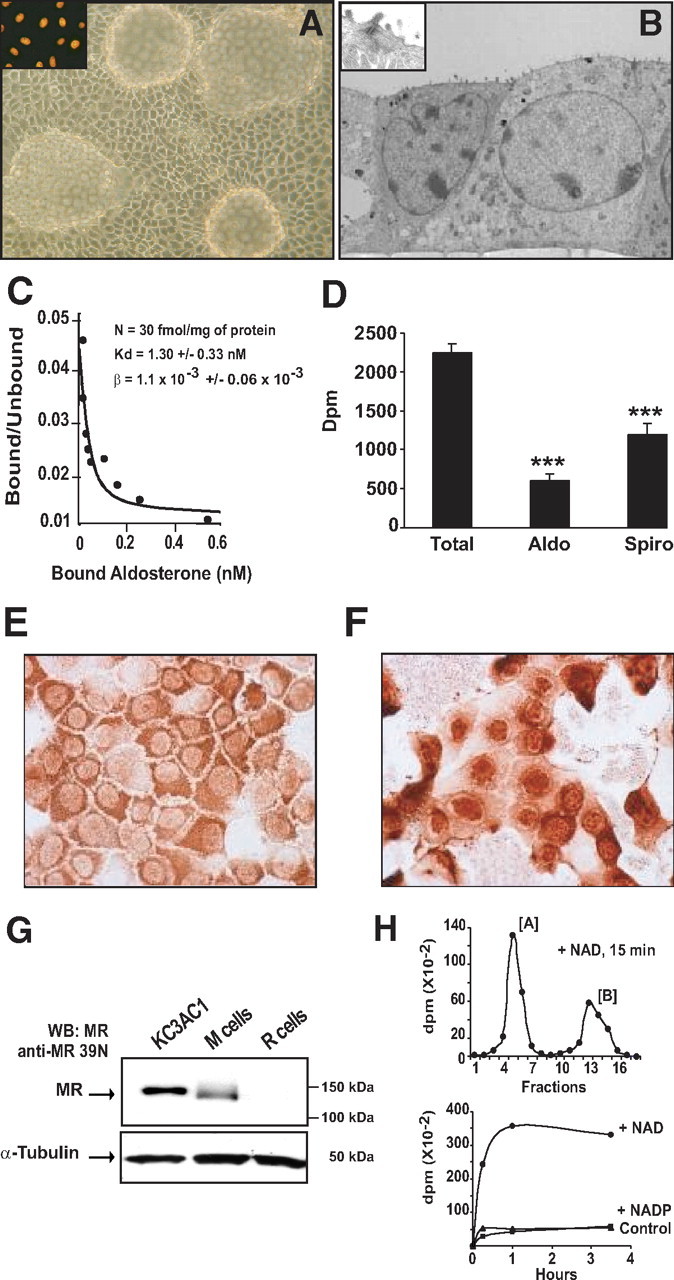
Expression of functional MR in the immortalized KC3AC1 cell line. A, Phase-contrast micrograph of confluent KC3AC1 cells grown on a petri dish coated with collagen I (passage 6). Cells form islets of characteristic epithelial cells and rapidly develop large and numerous domes, suggesting vectorial transepithelial hydroelectrolytic transport (optical lens, ×20). Strong nuclear immunostaining was observed in all cells with a monoclonal anti-TAg antibody, confirming that KC3AC1 cells are immortalized (inset). B, Electron microscopic micrographs of KC3AC1 cells grown on filters forming monolayers of epithelioid cells and presenting microvilli at the apical side of the membrane and a desmosome (see inset). C, Scatchard analysis. Cytosolic fractions of KC3AC1 cells (passage 9) were incubated with increasing concentrations of [3H]aldosterone for 4 h at 4 C. Bound (B) and unbound (U) hormone were separated by the charcoal-dextran technique. U was calculated as
(Continued) the difference between total and bound hormone concentration. The curve was simulated from the best model of interaction (B = nU/(Kd + U) + βU), where n is the maximum number of specific binding sites, Kd is the dissociation constant, and β is the constant of nonspecific binding. Mean values are given with the corresponding intraexperimental sd. Experimental points are means of triplicate determinations. D, whole-cell binding. Cells were incubated in MM 24 h before the experiment. Then, 10 nm [3H]aldosterone with and without a 100-fold excess of unlabeled aldosterone (Aldo) or spironolactone (Spiro) was added to cells. After 1 h of incubation at 37 C, cells were rinsed twice with ice-cold PBS, lysed with cold ethanol, and the radioactivity was counted. Data represent means ± sem of three independent determinations. Statistical significance: ***, P < 0.001. E, F, Immunocytochemical detection of MR in KC3AC1 cells incubated in the absence (C) or presence of 10 nm aldosterone for 30 min (D). Note the presence of cytoplasmic labeling when cells were incubated in MM and nuclear labeling in presence of the ligand. G, Western blot analysis of MR expression in KC3AC1 cells. Twenty to 30 μg of proteins from KC3AC1 cell homogenates were processed for immunoblotting with anti-MR 39N antibody. Note the presence of a band of approximately 130 kDa in KC3AC1 cell lysates. This band was clearly immunodetected in M-cell lysates, which stably overexpress MR, but were absent in the parental RCSV3 cell lysates, which do not express MR. H, HPLC analysis. Cells were incubated for 1 h with 10 nm [3H]corticosterone [B], and time-dependent production of [3H]dehydrocorticosterone [A] was measured by HPLC and the radioactivity of each fraction was counted. Top, Typical chromatogram obtained after HPLC analysis. Compound [B] was recovered between fractions 12 and 16, whereas compound [A] was recovered between fractions 4 and 7. Bottom, Cofactor specificity of 11βHSD2 activity was assessed in permeabilized KC3AC1 cells after incubation with 10 nm [3H]corticosterone in the absence or presence of 1 mm NADP or 1 mm NAD.
We next examined whether the 11β-hydroxysteroid dehydrogenase type 2 enzyme (11βHSD2), one of the mechanisms conferring MR selectivity, was expressed in KC3AC1 cells. Initially, the presence of 11βHSD2 mRNA was demonstrated by RT-PCR analysis (data not shown). Figure 1H (top) presents a typical HPLC chromatogram illustrating the metabolic conversion of [3H]corticosterone, compound [B], to 11-dehydro-[3H]corticosterone, compound [A], after incubation of KC3AC1 lysates with 1 mm NAD (a specific 11βHSD2 cofactor), for 15 min at 37 C. The robust metabolic conversion of compound [B] to [A] is highly suggestive of the presence of the 11βHSD2 enzyme in these cells. We then investigated the cofactor specificity of this enzymatic activity and showed that the metabolic conversion of compound [B] clearly depended on the presence of NAD cofactor, but not on NADP (a specific cofactor for 11βHSD1), thus confirming the expression of 11βHSD2 in these CCD cells (Fig. 1H, bottom).
Aldosterone stimulates Na+ reabsorption in KC3AC1 cells through ENaC
Given that in the CCD, Na+ reabsorption is mainly mediated by the ENaC, we searched for its expression in KC3AC1 cells and showed by RT-PCR (Fig. 2A) and Western blot analyses (Fig. 2B) that α-, β-, and γ-subunits of ENaC are expressed in these cells. When grown on filters with complete medium, KC3AC1 cells developed a high transepithelial resistance (RT ∼3000–5000 Ω/cm2) associated with high short-circuit current values (Isc∼25 μA/cm2) and a transepithelial voltage VT of ∼ +12.5 mV. We further investigated the functional integrity of ENaC. Apical administration of 10−5 m amiloride, a specific inhibitor of this channel, resulted in a strong (>90%) and rapid decrease of Isc (Fig. 2C), consistent with ENaC-dependent Na+ transport in KC3AC1 cells.
Fig. 2.
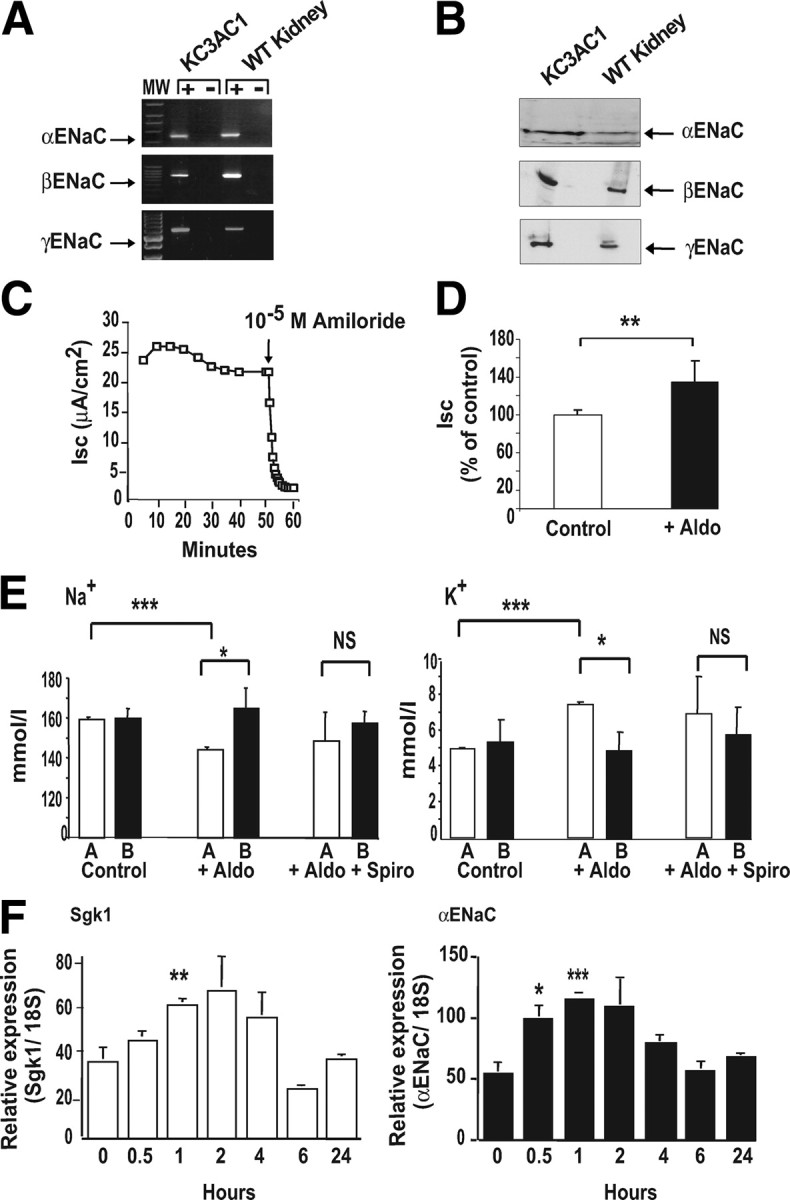
Aldosterone stimulates Na+ reabsorption in KC3AC1 cells through ENaC. A, RT-PCR was performed as described in Materials and Methods and allowed the detection of a 555-bp (aENaC), a 631-bp (βENaC), and a 670-bp (gENaC) amplicon. B, Western blot analysis allowed the detection of the three subunits of ENaC at 90 kDa (αENaC), 92 kDa (βENaC), and 85 kDa (γENaC). C, electrophysiological studies. Cells were grown on Snapwell filters in MM for 24 h. The short-circuit current (Isc, μA/cm2), transepithelial voltage (VT, mV) and transepithelial resistance (RT, Ω/cm2) were measured as described previously (29 ). When 10−5 m amiloride was applied at the apical side of the KC3AC1 cells, the short-circuit current was almost completely abolished within minutes. D, Cells, cultured on Snapwell filters as described above, were incubated for 18 h in the absence (Control) or presence of 10−8 m aldosterone (+Aldo). Each point represents mean ± sem of at least three independent determinations performed in duplicate or in triplicate. Statistical significance: **, P < 0.01. E, Ionic measurements. Cells were seeded on collagen I-coated Transwell filters and were cultured for 5 d in the epithelial medium. Cells were rinsed with PBS then were cultivated in MM for 24 h in the presence of 10−8 m aldosterone (+Aldo) or 10−8 m aldosterone + 10−6 m spironolactone (+Aldo + Spiro). A = apical; B = basolateral. Four hundred microliters of the supernatant was recovered from the medium bathing the apical and basolateral surface of the KC3AC1 cells. Na+ (left) and K+ ions (right) were then measured by the “Centre d’Experimentation Fontionnelle Intégrée” of the IFR Claude Bernard (Paris, France) on an Olympus automate. Data represent means ± sem of three independent determinations. Statistical significance: *, P < 0.05, and ***, P < 0.001. F, qRT-PCR analysis of sgk1, and αENaC mRNA expression in KC3AC1 cells after aldosterone treatment. Highly differentiated KC3AC1 cells were grown on filters for 24 h in MM and subsequently treated for various periods of time (0, 0.5, 1, 2, 4, 6, or 24 h) with 10 nm aldosterone. Results were normalized by the amplification of 18S RNA and are expressed as relative fold induction compared with basal conditions. Data represent means ± sem of at least three independent determinations performed in duplicate. Statistical significance: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
We next investigated whether KC3AC1 cells remained sensitive to aldosterone action. We showed that nanomolar aldosterone concentrations for 18 h significantly increased Isc, indicating an enhanced ionic transport in KC3AC1 cultured on filters (Fig. 2D). Aldosterone exposure also increased Na+ concentration in the basolateral compartment (Fig. 2E, left) and K+ concentration in the apical compartment (Fig. 2E, right). Aldosterone-stimulated Na+ reabsorption and K+ secretion are mediated by MR, because simultaneous administration of spironolactone prevented these ionic transports. Finally, we examined the ability of endogenous MR to act as a ligand-dependent transcription factor. Quantitative RT-PCR (qRT-PCR) analysis showed that aldosterone exposure rapidly induced a 2- to 3-fold increase in the expression of two prototypical aldosterone target genes, the kinase Sgk1 (Fig. 2F, left) and the α-subunit of ENaC (Fig. 2F, right).
Altogether, these results clearly demonstrate that KC3AC1 cells are highly differentiated CCD-derived cells possessing all the necessary molecular components for aldosterone action, most notably endogenous expression of functional MR. Thus, they constitute a suitable cell-based model to further investigate the regulatory mechanisms controlling MR expression.
Analysis of MR transcript and protein stability
We first demonstrated that MR expression depends on cell differentiation status. Indeed, as determined by qRT-PCR, steady-state levels of MR mRNA were higher in dome-forming cells at d 7 than in KC3AC1 cells just before the cells become confluent (d 2) (supplemental Fig. S1, which is published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). We, therefore, decided to analyze MR expression only when KC3AC1 cells were forming domes at d 7.
We studied the stability of MR transcripts by qRT-PCR in KC3AC1 cells. As illustrated in Fig. 3A, we showed that MR mRNA rapidly declined after actinomycin D exposure with a calculated half-life time (t1/2) of approximately 3 h. We further examined MR translation product by performing Western blot analysis of whole KC3AC1 cell protein extracts. Figure 3B reveals that, as expected, a 50% decrease in MR protein expression was observed after 24 h of exposure to actinomycin D. We also examined the half-life of MR protein by submitting KC3AC1 to cycloheximide, an inhibitor of protein synthesis. As demonstrated in Fig. 3C, MR protein levels also declined rapidly with an estimated half-life of 12 h.
Fig. 3.
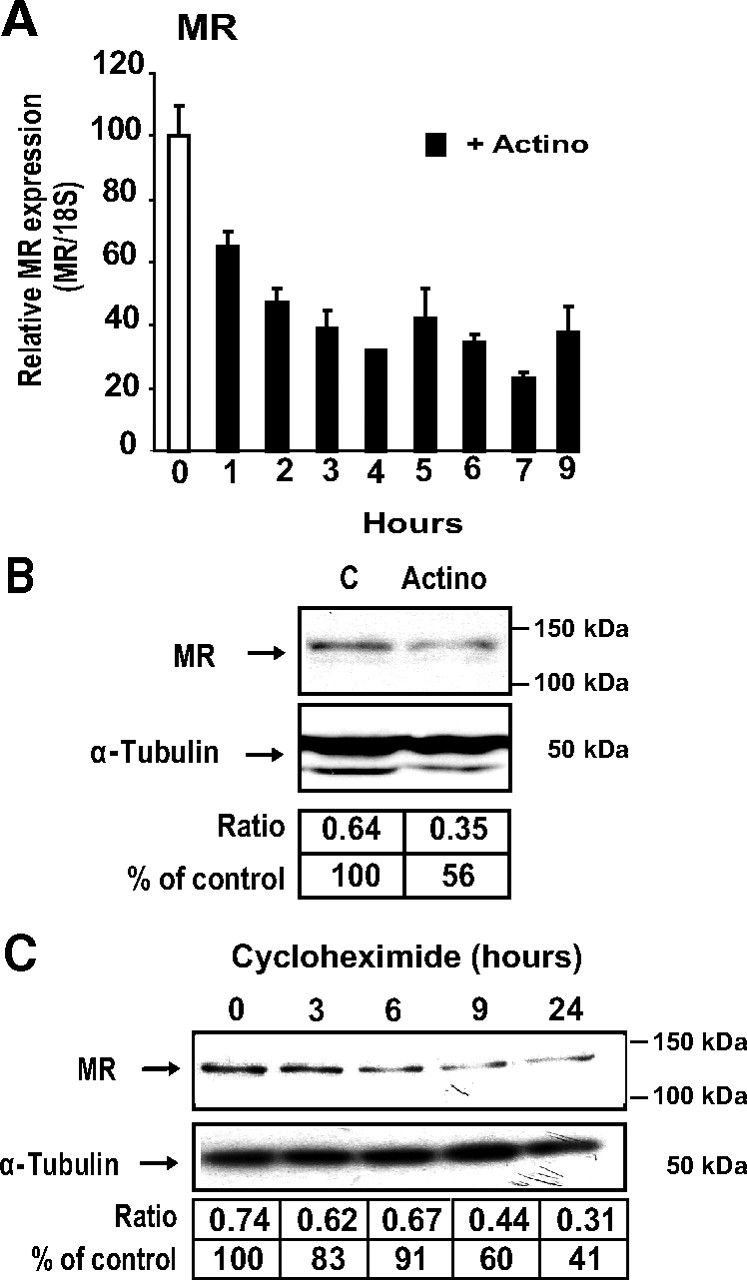
Analysis of MR transcript and protein stability. A, Analysis of MR transcript by qRT-PCR. Differentiated KC3AC1 cells were incubated for various periods of time in the absence or in the presence of 0.4 μm actinomycin D. Total RNA was extracted and processed for qRT-PCR. Results were normalized by the amplification of 18S RNA and were expressed as relative to the control. B and C, Western blot analysis of MR expression in KC3AC1 cells. B, Differentiated KC3AC1 cells were incubated for 18 h in the absence or in presence of 0.4 μm actinomycin D. C, Differentiated KC3AC1 cells were incubated for various periods of time in the absence or in the presence of cycloheximide (5 μg/ml). Twenty micrograms of protein from KC3AC1 cell homogenates were processed for immunoblotting with anti-MR 39N antibody (1/2000). α-Tubulin was used as loading control. MR was normalized to α-tubulin protein levels after digitalization on a gel scanner by use of QuantityOne software (Bio-Rad, Marnes-la-Coquette, France). Results are presented as ratio MR/α-tubulin and as percentage of control.
Hypertonic stress inhibits MR expression
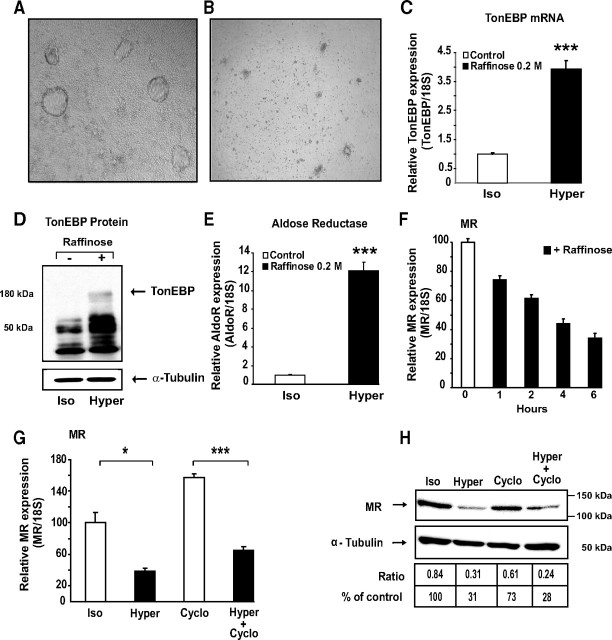
Given the major role played by MR expression level in determining mineralocorticoid responses, we were interested in examining whether variations in extracellular osmolarity, known to occur in the tubular segments of the distal nephron, could affect renal MR expression. We first examined whether hypertonicity could influence endogenous MR expression. Hypertonic stress exerted a dramatic effect on cell morphology, the domes rapidly disappearing after exposition to raffinose for 6 or 24 h (Fig. 4, A and B). TonEBP (TonE Binding Protein) plays a pivotal role in protecting renal cells from hypertonic stress. This osmoregulatory transcription factor binds tonicity response elements (TonEs), located in the promoter sequences of target genes such as Aldose Reductase gene (AldoR). We therefore examined whether TonEBP was expressed in KC3AC1 cells. With use of qRT-PCR, we showed a 5-fold increase of basal TonEBP transcript levels when cells were subjected to a hypertonic stress mimicked by raffinose exposure (Fig. 4C). Western blot analyses performed with an anti-TonEBP polyclonal antibody confirmed that TonEBP expression is clearly induced under hypertonic conditions (Fig. 4D). As expected, TonEBP activated transcription of AldoR, whose expression increased by 17-fold after raffinose exposure (Fig. 4E).
Fig. 4.
Hypertonic stress inhibits MR expression. A, B, Phase-contrast micrographs of a monolayer of differentiated KC3AC1 cells incubated in the absence (A) or in presence of 0.2 m raffinose (B) for 6 h. Note the absence of domes in raffinose-treated cells. C, E, Hypertonicity stimulates TonEBP and aldose reductase expression. Differentiated KC3AC1 cells were incubated in the absence or the presence of 0.2 m raffinose for 6 h. mRNA expression was measured by qRT-PCR. Results are expressed as attomoles/femtomoles of 18S are mean ± sem of 30–36 independent determinations. Statistical significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001. D, Western blot analysis of TonEBP in KC3AC1 cell lysates exposed for 18 h to raffinose. Fifteen micrograms of protein from KC3AC1 cell homogenates were processed for immunoblotting with anti-TonEBP SHN50 antibody. Note the presence of a specific band of approximately 180 kDa in KC3AC1 cell lysates exposed to raffinose. F, G, Analysis of MR transcript by qRT-PCR. F, Differentiated KC3AC1 cells were incubated for various periods of time in the absence or presence of 0.2 m raffinose. G, Differentiated KC3AC1 cells were incubated for 6 h in the absence (Iso) or in the presence of 0.2 m raffinose (Hyper) and/or cycloheximide (Cyclo, 5 μg/ml). Total RNA was extracted and processed for qRT-PCR as described in Fig. 3. Data represent means ± sem of at least three independent determinations performed in duplicate. Statistical significance: *, P < 0.05 and ***, P < 0.001. H, Western blot analysis of MR expression in KC3AC1 cells. Differentiated KC3AC1 cells were incubated for 18 h in the absence (Iso) or in the presence of 0.2 m raffinose (Hyper) and/or cycloheximide (5 μg/ml). Western blot analyses were performed as described in Fig. 3.
Characterization of the 5′-flanking region of the human MR gene allowed us to identify six TonEs in the promoter region of hMR, referred to as T1 to T6 (supplemental Fig. S2A). They all share sequence motifs similar to the consensus sequence TGGAAANNYNY, suggesting that TonEBP might be involved in the regulation of MR expression in renal cells. EMSA showed that raffinose-induced TonEBP specifically binds the T1 sequence (supplemental Fig. S2B) with high affinity (Kd ∼ 0.45 nm; supplemental Fig. S3). Transient transfection assays using wild-type and mutant T1-driven luciferase reporter constructs, together with dominant-negative TonEBP, further demonstrated that TonEBP functions as a hypertonicity-induced transcription factor via binding to functional hMR TonE elements in KC3AC1 cells (supplemental Fig. S2, C and D).
Unexpectedly, raffinose exposure led to a time-dependent decrease of MR mRNA steady-state levels as measured by qRT-PCR, suggesting that hypertonic stress may inhibit MR gene transcription and/or accelerate MR transcript degradation (Fig. 4F). This effect seems specific to MR because hypertonicity did not affect β-actin or glucocorticoid receptor expression (data not shown). To further elucidate the molecular mechanisms involved in this raffinose-induced repression of MR expression, KC3AC1 cells were further incubated in the absence or presence of cycloheximide and subjected to raffinose exposure. Cycloheximide treatment alone induced a significant 1.5-fold increase in MR mRNA levels, suggesting a critical role of putative destabilizing proteins in regulating MR expression. It is interesting that hypertonic stress, even in the presence of cycloheximide, was still able to significantly reduce MR transcripts, consistent with repression of MR gene transcription independently of the neosynthesized proteins (Fig. 4G). This was also true at the protein level, because a 70% decrease in MR expression was observed after 18-h raffinose exposure in the absence or in the presence of cycloheximide (Fig. 4H). Taken together, our results are consistent with the hypothesis that extracellular hypertonicity inhibits MR expression both by altering MR gene transcription and by controlling MR mRNA decay.
Identification of Tis11b as a direct tonicity-induced target gene
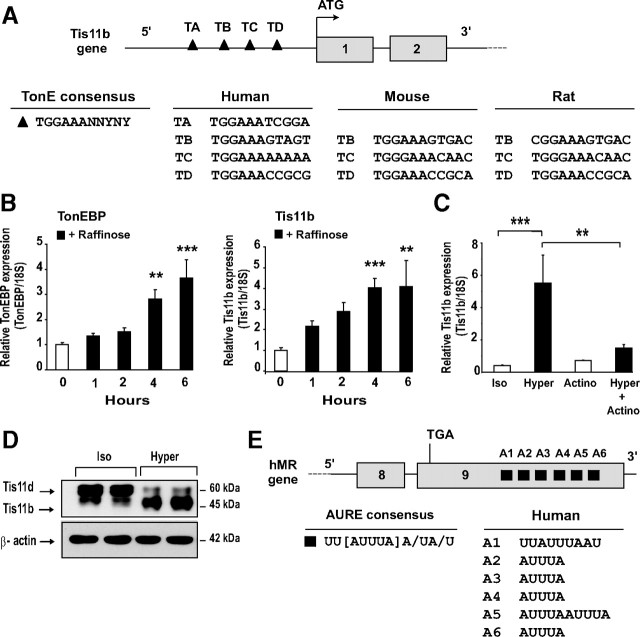
Among the proteins that control mRNA stability, we hypothesized that Tis11b (tetradecanoyl phorbol acetate-inducible-sequence 11b) might play a role in the control of MR mRNA decay. This protein belongs to a family of RNA-binding proteins (comprising also TIS11/TTP/tristetraprolin and Tis11d) that share characteristic tandem CCCH-type zinc-finger domain. All members of the family are involved in the regulation of several short-lived cytokine mRNAs and appear to share similar AU-rich elements (AURE)-binding and mRNA-destabilizing activities (20, 21). Of particular importance, in silico analysis of the human Tis11b promoter revealed the presence of 4 TonE elements, referred to as TA to TD, suggesting that TonEBP might also be involved in the regulation of Tis11b expression in renal cells. We noticed that the TonE elements TB to TD were highly conserved among human, mouse, and rat species (Fig. 5A). As expected, when KC3AC1 cells were exposed for 6 h under a hypertonic stress, a significant time-dependent increase in TonEBP expression was observed (Kruskal-Wallis test, P < 0.0001; post test: Dunn’s multiple comparison test, **, P < 0.01 and ***, P < 0.001). This was accompanied by a concomitant 4-fold increase (Kruskal-Wallis test, P < 0.0001; post test: Dunn’s multiple comparison test, **, P < 0.01 and ***, P < 0.001) in Tis11b mRNA levels (Fig. 5B). Moreover, actinomycin D almost completely abolished the increase of Tis11b mRNA induced by raffinose, suggesting a direct transcriptional control of tonicity-induced Tis11b expression (Fig. 5C). Western blot analysis, using a polyclonal antibody that recognizes both Tis11d and Tis11b isoforms, confirmed at the protein level that Tis11b expression was increased after an 18-h raffinose exposure, whereas Tis11d expression was clearly reduced (Fig. 5D). Tis11b was shown to interact with AU-rich elements (AUREs), thus promoting rapid degradation of AURE-harboring mRNAs. These regulatory motifs consist of pentamers of AUUUA, of nonamers of UUAUUUA(U/A)(U/A) or U-rich elements, which are generally located in the 3′-untranslated region (3′-UTR) of labile transcripts such as cytokines, growth factors, and proto-oncogenes (22). Interestingly enough, in silico analysis revealed that MR mRNA harbors several AUREs (A1 to A6) in its extended 3′-UTR, most of which are highly conserved between species (Fig. 5E).
Fig. 5.
Identification of Tis11b as a direct tonicity-induced target gene. A, Localization of TonE responsive elements in the Tis11b gene 5′-flanking region. Gray boxes indicate the two exons of the gene. TonE consensus sequence is presented. Four TonE (TA to TD) were located in the promoter, at position −1596 bp, −1167 bp, −833 bp, and −660 bp, respectively, upstream of the translational ATG start site of exon 1. Note that the TonE, TB to TD, are conserved between human, mouse, and rat species. B, Hypertonicity stimulates TonEBP and Tis11b expression. Differentiated KC3AC1 cells were incubated in the absence or the presence of 0.2 m raffinose for various periods of time up to 6 h. mRNA expression was measured by qRT-PCR. Results are expressed as attomoles/femtomoles of 18S are mean ± sem of three independent determinations performed in duplicate. Statistical significance: **, P < 0.01; ***, P < 0.001. C, Transcriptional control of Tis11b expression under hypertonicity. Differentiated KC3AC1 cells were incubated for 6 h in the absence (Iso) or in the presence of 0.2 m raffinose (Hyper) and/or 0.4 μm actinomycin D (Actino). Data represent means ± sem of at least three independent determinations performed in duplicate. Statistical significance: **, P < 0.01 and ***, P < 0.001. D, Western blot analysis of Tis11b in KC3AC1 cell lysates exposed for 18 h to raffinose. Thirty micrograms of protein from KC3AC1 cell homogenates were processed for immunoblotting by use of a polyclonal antibody that recognizes both Tis11d and Tis11b isoforms. E, Localization of AUREs in the 3′-UTR of MR transcript. Gray boxes indicate the last exons 8 and 9 of hMR gene. AURE consensus sequence is presented. Six AUREs (A1 to A6) were identified in the 3′-UTR of MR transcript, at position 599 bases (b), 850 b, 933 b, 972 b, 2041 b, and 2456 b respectively, downstream of the stop codon TGA in exon 9 of human MR gene. Note that AURE A1 to A4 are conserved among human, mouse, and rat species.
Hypotonic stress stimulates MR expression
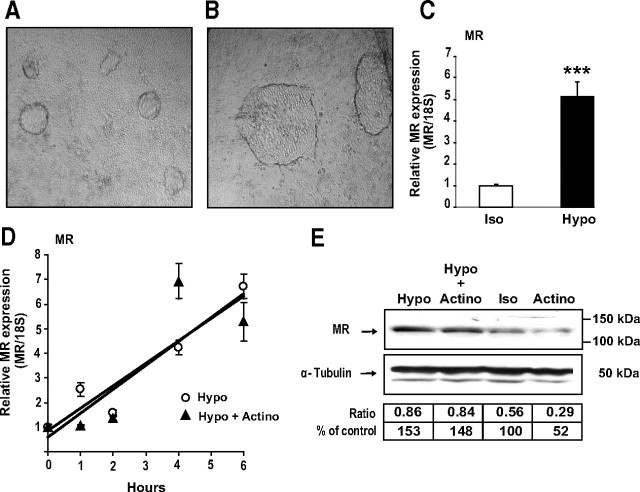
Contrary to the results obtained under hypertonicity, hypotonic conditions induced an increase both in dome formation and size (Fig. 6, A and B) and dramatically enhanced MR expression by 5-fold at messenger RNA level (Fig. 6C). To further clarify the potential underlying molecular mechanisms, KC3AC1 cells were subjected to hypotonic conditions in the absence or presence of actinomycin D. qRT-PCR (Fig. 6D) and Western blot analyses (Fig. 6E) revealed that hypotonic stress increases MR expression both at the mRNA and protein levels, irrespective of actinomycin D. Collectively, these findings strongly suggest that hypotonicity-regulated MR expression is not a consequence of stimulation of MR transcription, but rather of increased mRNA and protein stability.
Fig. 6.
Hypotonic stress stimulates MR expression. A and B, Phase-contrast micrographs of monolayers of differentiated KC3AC1 cells incubated for 6 h with isotonic (A) or a hypotonic medium (B). Note that the domes seemed larger in KC3AC1 cells incubated under hypotonic conditions. C and D, Analysis of MR transcripts by qRT-PCR. C, Differentiated KC3AC1 cells were incubated for 6 h with isotonic (Iso) or a hypotonic medium (Hypo). D, Differentiated KC3AC1 cells were incubated for various periods of time under hypotonic conditions (Hypo) and/or in the presence of 0.4 μm actinomycin D (Actino). Total RNA was extracted and processed for qRT-PCR as described in Fig. 3. Statistical significance: ***, P < 0.001. E, Western blot analysis of MR expression in KC3AC1 cells. Differentiated KC3AC1 cells were incubated for 18 h with isotonic (Iso) or hypotonic medium (Hypo) and/or in the presence of 0.4 μm actinomycin D (Actino). Western blot analyses were performed as described in Fig. 3.
Renal MR expression is altered by water deprivation and water intoxication in mice
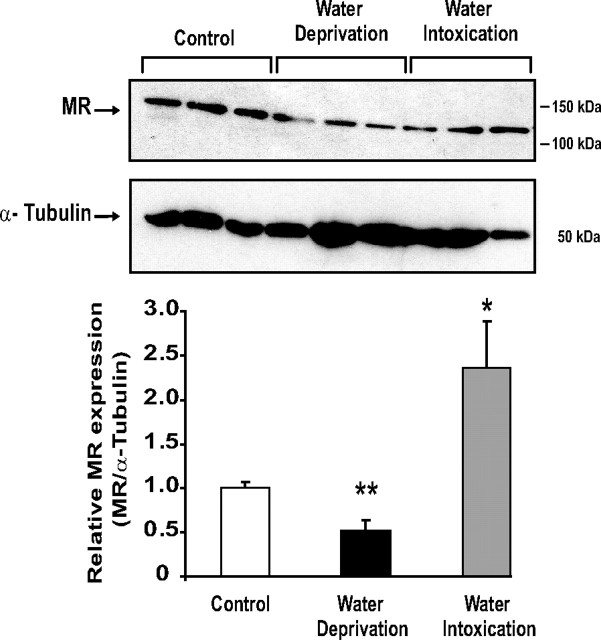
To further address the physiological relevance of our in vitro findings, we subjected male mice to archetypal water imbalance situations (Fig. 7). Water deprivation for 18 h, a condition known to increase urine osmolality and renal medullary sodium concentration (23), induced a significant 50% decrease in MR protein levels in the whole kidney of mice subjected to such hypertonic stress. In contrast, acute water intoxication after oral administration of hypotonic fluid (3% vol/kg body weight) and concomitant ip injection of vasopressin, a method for inducing positive water balance (24), led to a 2-fold increase in renal MR expression. Taken together, these in vivo results support our findings previously obtained with KC3AC1 cells.
Fig. 7.
Renal MR expression is altered by water deprivation and water intoxication in mice. Fifty micrograms of protein from kidney homogenates obtained from the three groups (control, water-deprived, and water-intoxicated mice, three animals per condition) were loaded on 7.5% SDS-PAGE followed by direct immunoblotting with anti-MR 39N antibody (1/4000). α-Tubulin was used as loading control. MR protein levels were normalized to those of α-tubulin after digitalization on a gel scanner by use of QuantityOne software (Bio-Rad). Results are means ± sem of at least three independent determinations and are expressed relative to MR expression measured in the control group, arbitrarily set at 1. Statistical significance: *, P < 0.05 and **, P < 0.01.
Discussion
MR expression level in a target cell dictates aldosterone responsiveness. This assertion is particularly relevant in several animal models and in human diseases, where alteration of renal MR expression leads to severe pathophysiological consequences of impaired or excessive aldosterone responses. This underlines the importance of maintaining appropriate levels of MR expression and the necessity of elucidating the regulatory mechanisms of MR expression. However, such investigations have been hampered by the lack of appropriate cellular models expressing endogenous MR and responsive to aldosterone action. Although several useful human and mouse cell lines have been established from the CCD (19, 25, 26, 27, 28, 29, 30), these models have some limitations (31, 32). For instance, some of them do not maintain endogenous MR expression and/or only respond to pharmacological doses of aldosterone (micromolar concentrations). Others do not form domes or display suboptimal electrophysiological properties (minimal transepithelial resistance, low short-circuit current).
In the present study, we aimed at analyzing MR expression level under physiological conditions in renal cells prone to be exposed to large variations of extracellular fluid composition. This prompted us to establish a novel CCD cell line by means of a targeted oncogenesis strategy in mice, using the SV40 TAg under the control of the P1 promoter of the hMR gene. This strategy has already proven to be an efficient experimental means of deriving several differentiated mineralocorticoid-sensitive cell lines (17, 33, 34).
KC3AC1 cells possess all features of polarized epithelial cells and form domes at confluence, suggesting the occurrence of vectorial ionic transport. We showed that these cells express functional ENaC and 11βHSD2 enzyme. The NAD-dependent 11βHSD2 catalytic activity was calculated at approximately 50 fmol/μg protein·1 h, a very high activity, which was of the same order of magnitude as that estimated in isolated rat CCD (35) and in renal cells stably transfected with 11βHSD2 cDNA (36). Binding assays and immunodetection enabled us to unambiguously demonstrate the presence of functional MR in KC3AC1 cells. More importantly, we established that these cells remain capable of responding to mineralocorticoids, because physiological aldosterone concentrations stimulated Na+ reabsorption associated with induction of SCNN1A (αENaC) and Sgk1 gene expression, in accordance with previous studies (28, 37, 38, 39). Altogether, functional properties of KC3AC1 cells indicate that this cell line represents a valuable model to analyze MR expression and to decipher the mineralocorticoid signaling pathway in the CCD.
MR steady-state level results from equilibrium between its synthesis and degradation. Thus, MR abundance may increase after a rise in the transcription/translation rate or alternatively may decrease because of modifications of mRNA or protein stability. To the best of our knowledge, few data exist on the mechanisms controlling MR expression levels. We previously demonstrated that hMR gene activation is under the control of two alternative promoters (P1 and P2), which give rise to two mRNA isoforms (hMRα and hMRβ) (40), yet questioning the molecular events involved in the control of MR gene transcription (41). Multiple protein variants are generated from one single gene by mechanisms of alternative RNA splicing and alternative translational initiation, offering the possibility of combinatorial patterns of receptor expression (42).
Herein, we provide new information regarding MR expression, both at messenger and protein levels. We demonstrate that MR transcript has a relatively short t1/2 (3 h), suggesting that MR mRNA is an unstable molecule, very likely subjected to strict regulatory mechanisms of mRNA turnover, whereas the MR protein also has a short half-life estimated at 12 h. More importantly, our study brings new insights into the mechanisms modulating MR levels in the CCD under hyper- and hypotonic conditions. In vivo, hypertonicity represents a genuine threat to renal cells exposed to extraordinarily high levels of NaCl and urea, because such osmotic stress causes numerous cellular perturbations (cytoskeletal rearrangement, apoptosis, alteration of DNA replication, transcription, and translation) (43). Renal cells can accommodate hypertonic stress by developing an adapted cellular response that relies on an enhanced TonEBP activity via increased nuclear translocation, transactivation, and abundance (44). This osmoregulatory transcription factor binds TonE elements present in promoter sequences of several osmotically regulated genes, resulting in an enhanced production of intracellular osmolytes, which maintain appropriate intracellular ionic strength. TonEBP also stimulates transcription of the heat shock protein 70 (45) and contributes to urinary concentration by direct stimulation of aquaporin-2 (46) and UT-A urea transporter (47) expression. Several recent studies reported a role of hypertonicity in the expression of genes either involved in tight junction properties, including MUPP1 (multi-PDZ protein-1), ZO1 (Zonula occludens 1), Af6 and claudin-4 (Cldn4) (48, 49) and/or belonging to the mineralocorticoid signaling pathway such as increased expression and activity of Sgk1 (23, 50, 51). This is particularly interesting given the pivotal role played by Sgk1 in regulating Na+ reabsorption in the CCD and controlling cell volume (52).
Using KC3AC1 cells, we showed that TonEBP transcript and protein levels increased dramatically under exposure to 200 mm raffinose or 150 mm NaCl, an effect associated with a 17-fold increase in AldoR gene expression. Identification of several functional TonE elements in the P1 promoter and intron A of the hMR gene suggests to us that activation of TonEBP signaling pathway can directly stimulate MR expression. However, counterintuitively, hypertonicity decreased by more than 50% MR transcript and protein levels after short-term (3 h) and long-term (18 h) exposure, excluding a direct involvement of TonEBP in the down-regulation of MR expression under hypertonic stress. Alternatively, given that a decrease in MR abundance may be the consequence of a decline in MR gene transcription and/or a modification in MR stability, we hypothesize that a putative destabilizing protein, whose activity is regulated by hypertonicity, may participate to the control of MR expression. Indeed, we identified Tis11b as a direct tonicity-induced target gene and demonstrated that it fulfills all the criteria of MR mRNA-destabilizing protein. We showed that Tis11b promoter contains several highly conserved TonE-responsive elements and its expression, at both mRNA and protein levels, increases under raffinose exposure. As previously demonstrated for several short-lived mRNAs, including vascular endothelial growth factor (53) and Star (54), the 3′-UTR of MR mRNA contains multiple highly conserved AUREs to which Tis11b may bind and thus facilitate its degradation. Direct binding of Tis11b to the 3′-UTR of MR and its functional consequences in terms of MR stability remains to be elucidated. Taken together, we propose that hypertonic stress activates the TonEBP signaling pathway in KC3AC1 cells, leading to a parallel increase of the mRNA-destabilizing protein Tis11b expression, which may favor hypertonicity-dependent degradation of labile MR transcripts. We therefore demonstrate for the first time a posttranscriptional regulation of MR mRNA as a pivotal process in the control of MR expression under hypertonic conditions.
At variance with hypertonicity, hypotonic conditions not only preserved the presence of domes, but also seemed to increase their size. It has been proposed that hypotonic stress initially leads to cell swelling followed by normalization of cell volume through a regulatory volume decrease, involving a reduction of intracellular Cl−. This biphasic mechanism was reported to modify gene expression and the activity of some proteins, including ion channels (55). Thus, intracellular Cl− was thought to act as a signal increasing Na+ reabsorption via p38 MAPK kinase and c-Jun N-terminal kinase in A6 epithelial renal cells (56, 57). Recently, hypotonicity was also shown to modify cell architecture associated with actin cytoskeletal remodeling (58).
Here, we demonstrated in KC3AC1 cells that hypotonicity increased MR transcript and protein levels and this effect was not prevented by actinomycin D, suggesting that MR expression level increased without affecting MR gene transcription, but instead through an increase of its mRNA and protein stability. Importantly, this increase of MR levels under hypotonic stress was consistent with recent reports on hypotonic-induced activation of the mineralocorticoid signaling pathway. Indeed, hypotonicity has been shown to increase Na+,K+-ATPase cell surface expression in the renal mpkCCDc14 cell line (59) and Na+ reabsorption in A6 cells via up-regulation in β- and γ-ENaC subunits (60). Sgk1 expression has also been enhanced by hypotonic stress in renal A6 cells (61). This increase in both ENaC and Sgk1 gene expression could be mediated by intracellular Ca2+, as a second messenger in hypotonic stress-induced Na+ transport (62).
Collectively, our results provide clear evidence that osmotic stress (hyper- and hypotonicity) greatly affects the expression of a nuclear receptor both at messenger and protein levels, in accordance with our in vivo findings, indicating that renal MR expression is subjected to tight osmoregulatory control and varies as a function of hydroelectrolytic homeostasis. From a phylogenetic point of view, our results are also reminiscent of variations of MR expression observed in salmon during their acclimation from fresh to salt water. MR expression increases in their gills during de-smoltification (from salt to fresh water) and it is down-regulated after transfer to salt water during smoltification (63).
Finally, it would be important to determine whether MR expression variations under osmotic stress occur in other Na+-transporting epithelia (inner ear, sweat, and salivary glands) and to evaluate whether other types of cellular stress, such as hypoxia, redox status, pH, and ATP depletion, may also contribute to the regulation of MR expression.
Materials and Methods
Establishment of the KC3AC1 CCD cell line
The KC3AC1 cell line was isolated from a 4-wk-old P1-TAg transgenic mouse (founder 3) (34). Microdissection of CCDs was performed as reported earlier. In brief, kidneys were perfused under sterile conditions via the abdominal aorta at room temperature with 4 ml of solution A containing DMEM/HAM’s F-12 medium (1:1); 20 mm HEPES, pH 7.4; 100 U/ml penicillin; 100 μg/ml streptomycin; 2 mm glutamine; 20% fetal calf serum; and 0.25% collagenase (CLS type I, 146 U/mg from Clostridium histolyticum; Worthington Biochemical, Freehold, NJ). Kidneys were removed and cut into slices, which were further incubated for 20 min at 30 C in an aerated 0.07% collagenase solution A. After careful rinsing, tissue was microdissected by hand with the help of thin needles under stereomicroscopic observation in ice-cold medium A devoid of collagenase. CCDs were recognized according to morphological criteria reported earlier (64) and fragments of approximately 0.5 mm were transferred to collagen-coated 12-well plates, and cultured in fresh medium. The latter was removed every 2 d for 2 wk, until the formation of a monolayer of cells, which were selected for their epithelioid morphology and the nuclear immunodetection of SV40 TAg. The KC3AC1 cell line was first cultured until passage 10, and then it was subcloned by limit dilution to obtain a homogeneous cell population, which was routinely cultured at 37 C, in a humidified incubator gassed with 5% CO2, in an epithelial medium composed of DMEM/HAM’s F-12 (1:1); 2 mm glutamine; 50 nm dexamethasone (Sigma Chemical Co., St Louis, MO); 50 nm sodium selenite (Sigma); 5 μg/ml transferrin; 5 μg/ml insulin (Sigma); 10 ng/ml EGF (Tebu, Le Perray en Yvelines, France); 2 nm T3 (Sigma); 100 U/ml penicillin/streptomycin; 20 mm HEPES, pH 7.4; and 5% dextran charcoal-treated serum. All reagents were purchased from Invitrogen (Cergy Pontoise, France), except when stated otherwise.
Cell culture
KC3AC1 cells (passages 6-20) were seeded either on collagen I-coated (Institut Jacques Boy, Reims, France) Transwell/Snapwell filters (Corning Costar, Brumath, France) or on collagen I-coated petri dishes with the epithelial medium. To study corticosteroid actions, the epithelial medium was replaced by a minimal medium (MM) which has the same composition as the epithelial medium, but lacks dexamethasone and dextran charcoal-treated serum.
Hormones and drugs
Aldosterone was purchased from Acros Organics (Noisy Le Grand, France), whereas raffinose, cycloheximide, actinomycin D, spironolactone, and amiloride were purchased from Sigma.
Hypertonic and hypotonic stresses
Hypertonic medium (500 mosmol/kg) was composed of isoosmotic medium (300 mosmol/kg) supplemented with 200 mm raffinose, whereas hypotonic medium (150 mOsm/kg) represented a 1:1 dilution of isoosmotic medium with sterile culture water. Control experiments performed with 50% medium diluted with sterile 150 mm NaCl resulted in no appreciable difference from isoosmotic conditions.
In vivo water deprivation and water intoxication studies
Two-month-old male B6D2 mice, weighing approximately 25 g, were used in this study in accordance with the guidelines for the care and use of laboratory animals. Animals had free access to water and standard chow, with the exception of the water-restricted group, which was subjected to water deprivation for 18 h. For water intoxication, animals were orally fed once with 3% vol/kg of body weight of a 10 mm glucose solution prepared with tap water (approximately 800 μl/mouse), using gavage feeding needles (Phymep, Paris, France). Mice received simultaneously an ip injection of dDAVP (Minirin, Ferring GmbH, Kiel, Germany) at a concentration of 400 ng/kg body weight. After 6 h, animals were killed and kidneys were collected and snap-frozen in liquid nitrogen for subsequent Western blot analysis as described previously (65).
Electron microscopic analyses
KC3AC1 cells were grown on Transwell filters with complete epithelial medium. Electron microscopic analyses were performed as described previously (34).
Hormone binding assays
Whole-cell-specific binding of [3H]aldosterone and [3H]dexamethasone was determined in KC3AC1 cells grown on a collagen I-coated 12-well plate, as described previously (34). Values of binding parameters were determined at equilibrium by Scatchard analysis of [3H]aldosterone and [3H]dexamethasone binding to KC3AC1 cytosolic fractions as described previously (66).
Western blot analyses
Total protein extracts were prepared from KC3AC1 cells cultured on collagen I-coated petri dishes under isotonic, hyper-, or hypotonic medium or from mice kidneys. In brief, cells were lysed in lysis buffer, as described previously (67). For ENaC Western blotting, immunoblots were incubated overnight in 5% milk-Tris buffer saline/0.1% Tween (TBST) before incubation with rabbit anti-α, β, γ ENaC antibodies (1:5000) for 1 h at room temperature. After extensive washes, membranes were incubated with a goat antirabbit peroxidase-conjugated second antibody (1:8000) for 1 h at room temperature. For MR Western blotting, immunoblots were incubated overnight in 5% milk-TBST followed by incubation for 1 h at room temperature with anti-MR 39N (1:2000) and with peroxidase-conjugated goat antirabbit antibody, dilution 1:15000 (Vector Laboratories, Burlingame, CA). Rabbit polyclonal anti-MR antibody (39N) was generated by use of the human MR 1-18 peptide and purified by affinity chromatography (Double X/XP boosting antibody production program; Eurogentec, Seraing, Belgium). For TonEBP Western blotting, blots were incubated at room temperature for 1 h with polyclonal anti-TonEBP (1:2000) followed by incubation with peroxidase-conjugated goat antirabbit antibody, dilution 1:15,000. For Tis11b and Tis11d Western blot blotting, immunoblots were incubated in 5% milk-TBST for 1 h at room temperature. The blot was probed for 2 h in PBS containing 0.1% Tween with antibodies to a C-terminal peptide fragment (amino acids 300–320) of TIS11b (1:1000 dilution) that recognize both TIS11b and its family member TIS11d. The membrane was thoroughly washed with the same buffer (3 × 10 min), and then incubated for 1 h with horseradish peroxidase-labeled goat antirabbit IgG. In all cases, membranes were washed and the antigen-antibody complex was visualized by the ECL+ detection kit (GE Healthcare Europe GmbH, Orsay, France). For loading normalization, membranes were incubated with anti-α-tubulin or anti-β-actin antibody (Sigma).
Immunocytochemical analyses
Immunodetection of the SV40 TAg was performed as described previously (68). For MR immunodetection with 39N antibody (1:500), cells were cultured in Lab-Tek chambers (Nunc, Dutscher, Brumath, France) and were processed as described previously (69).
11βHSD2 catalytic activity
KC3AC1 cells were seeded at 6 × 105 cells per well on collagen I-coated 12-well plates and were cultured for 48 h in epithelial medium before incubation overnight in MM. 11βHSD2 activity was determined as described previously (34).
Electrophysiological studies
The measurement of short-circuit current (Isc, μA/cm2), transepithelial voltage (VT, mV) and transepithelial resistance (RT, Ω/cm2) were performed on KC3AC1 cells grown on collagen I-coated Snapwell filters as described previously (27).
Ions measurements
Cells were seeded on collagen I-coated Transwell filters and were cultured for 5 d in epithelial medium. Thereafter, medium was replaced for 24 h by MM before hormonal stimulation performed in this MM. The next day, K+, Na+, and Cl− ions were measured as described previously (34).
RT-PCR
Total RNA was extracted from cells with TRIZOL reagent (Invitrogen) according to the manufacturer’s recommendations, and RNA was thereafter processed for RT-PCR, as described previously (34).
Quantitative RT-PCR
In brief, 1 μg of total RNA was treated by use of the DNase I Amplification Grade procedure (Invitrogen), and then RNA was reverse-transcribed by use of the high-capacity cDNA RT kit from Applied Biosystems (Courtaboeuf, France). Samples were diluted 10-fold, then 1/20 of the reverse transcription reaction was used for qRT-PCR using the Power SYBR Green PCR Master Mix (Applied Biosystems). The final primer concentration was 300 nm for each primer. Reaction parameters were carried out on an ABI 7300 Sequence Detector (Applied Biosystems) and were as follows: 95 C for 10 min followed by 40 cycles at 95 C for 15 sec and 60 C for 1 min. For preparation of standards, amplicons were purified from agarose gel and subcloned into pGEMT-easy plasmid (Promega, Charbonnières-les-Bains, France), then sequenced to verify the identity of each fragment. Standard curves were generated by use of serial dilutions of linearized standard plasmids, spanning 6 orders of magnitude and yielding correlation coefficients larger than 0.98 and efficiencies of at least 0.95, in all experiments. Standard and sample values were determined in duplicate from three independent experiments. Relative expression within a given sample was calculated as a ratio (amol of specific gene/femtomoles of 18S). Results are mean ± sem and present the relative expression compared with that obtained with control cells, which were arbitrarily set at 1. Table 1 indicates primer sequences of genes analyzed by RT-PCR and by qRT-PCR.
Table 1.
Primer sequences of genes analyzed in RT-PCR and qRT-PCR
| Name | Accession no. | Amplicon size (bp) | Sense primer | Antisense primer | |||
|---|---|---|---|---|---|---|---|
| Primers for RT-PCR | |||||||
| αENaC | AF112185 | 555 | CTAATGATGCTGGACCACACC | AAAGCGTCTGTTCCGTGATGC | |||
| βENaC | AF112186 | 631 | GCCAGTGAAGAAGTACCTGC | CCTGGGTGGCACTGGTGAA | |||
| γENaC | AF112187 | 670 | AAGAATCTGCCGGTTCGAGGC | TACCACTCCTGGATGGCATTG | |||
| Primers for q RT-PCR | |||||||
| 18S | X00686 | 66 | CCCTGCCCTTTGTACACACC | CGATCCGAGGGCCTCACTA | |||
| mMR | M36074 | 153 | ATGGAAACCACACGGTGACCT | AGCCTCATCTCCACACACCAAG | |||
| αENaC | AF112185 | 150 | GGACTGGAAAATCGGCTTCC | TAGAGCAGGCGAGGTGTCG | |||
| Sgk1 | AF205855 | 150 | TCACTTCTCATTCCAGACCGC | ATAGCCCAAGGCACTGGCTA | |||
| TonEBP | AY050663 | 127 | CCTCTCCTCACCGTCGTCATC | CTCCCACCTCCTTCTCATTCTG | |||
| AldoR | BC085310 | 79 | GGCCGTGAAAGTTGCTATTGA | GGTCCCGGGCTGTGAGAT | |||
| Tis11b | NM_007564.3 | 100 | CGACACACCAGATCCTAGTCCTT | TGCATAAAACTTCGCTCAAGTCA | |||
The abbreviations of the genes, their GenBank or National Center for Biotechnology Information accession number, and 5′- to 3′-nucleotide sequences of the sense and antisense primers are presented.
EMSA
Differentiated KC3AC1 cells were starved for 3 h in MM and exposed to hypertonic stimulation. Nuclear protein extraction and gel mobility shift assays using [32P]dCTP-labeled double-strained oligonucleotides (specific activity of approximately 108 cpm/μg of DNA) were performed as described elsewhere (70). Oligonucleotide sequences were as follows:
T1 TonE sense: 5′-AGCTTGAACTAGTGGAAAAATCCAATA-3′ and T1 TonE antisense 5′AGCTTATTGGATTTTTCCACTAGTTCA-3′;
T1M TonE mutant sense 5′-AGCTTGAACTAGTTGAAAAGCCATA-3′ and T1M TonE mutant antisense 5′-AGCTTATTGGCTTTTCAACTAGTTCA;
T2 TonE sense 5′-TAACCGTGCTGGAAAAACCCTTTA-3′ and T2 TonE antisense 5′-TGTTTAAGGGTTTTTCCAGCACG-3′. Bold underlined letters indicate consensus site sequences for tonicity response element.
Transfection assays
For transient transfections, cells were seeded in a six-well plate at 5 × 105 cells/well in complete medium. The next day, cells were transfected in Optimem Medium (Invitrogen) with Lipofectamine 2000 (Invitrogen), according to the manufacturer’s recommendations. After a 6-h incubation period, medium was replaced by isotonic, hypertonic, or hypotonic medium. The next day, cells were harvested and β-galactosidase and luciferase assays were performed as described elsewhere (71). Luciferase activities were normalized to β-galactosidase activities and expressed as a percentage of relative transcriptional activities compared with controls.
Statistical analyses
Data are expressed as the mean ± sem. Mann-Whitney U test was used to determine significant differences between two groups. For multiple comparisons, Kruskal-Wallis test followed by Dunn’s post test was performed with use of the computer software Prism 4 (GraphPad Software, San Diego, CA). Statistical significance is indicated at P values of < 0.05, 0.01, and 0.001.
Acknowledgments
We thank Jacqueline Bauchet and the “Centre d’Exploration Fonctionnelle Intégrée” for ionic measurements (IFR Claude Bernard, Paris), Annick Ganieux (IFR de Bicêtre, Le Kremlin Bicêtre) for plasmid preparation, Françoise Cluzeaud (CRB3, INSERM Unité 773, Medical School Bichat, Paris) for her assistance in electron microscopy, Pr. H. Moo Kwon (University of Maryland, Baltimore, MD) for the generous supplies of the dominant negative-TonEBP plasmid together with the TonEBP antibody, and Dr Martin Schmidlinfrom (University of Basel, Switzerland) for Tis11b antibodies.
NURSA Molecule Pages:
Ligands: Aldosterone | Spironolactone;
Nuclear Receptors: MR.
Footnotes
This work was supported Institut National de la Santé et de la Recherche Médicale, the University Paris-Sud 11, and the Bonus Qualite Recherche 2003 of the University Paris-Diderot. P.K. was a recipient of fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche, France.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 21, 2009
Abbreviations: AURE, AU-rich element; CCD, cortical collecting duct; ENaC, epithelial Na+ channel; SV40, simian virus 40; 11βHSD2, 11β-hydroxysteroid dehydrogenase type 2; hMR, human MR; MM, minimal medium; MR, mineralocorticoid receptor; qRT-PCR, quantitative RT-PCR; TonE, tonicity response element; TAg, T antigen; TonEBP, tonicity-responsive enhancer binding protein; UTR, untranslated region.
References
- 1.Bonvalet JP1998. Regulation of sodium transport by steroid hormones. Kidney Int Suppl 65:S49–S56 [PubMed]
- 2.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombès M2007. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5:e012 [DOI] [PMC free article] [PubMed]
- 3.Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schütz G1998. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA 95:9424–9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, Berger S2007. Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18:1679–1687 [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Bello-Roufai M, Wang X2008. RNAi inhibition of mineralocorticoid receptors prevents the development of cold-induced hypertension. Am J Physiol Heart Circ Physiol 294:H1880–H1887 [DOI] [PubMed]
- 6.Lim HY, van den Brandt J, Fassnacht M, Allolio B, Herold MJ, Reichardt HM2008. Silencing of the mineralocorticoid receptor by ribonucleic acid interference in transgenic rats disrupts endocrine homeostasis. Mol Endocrinol 22:1304–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller DS, Zhang J, Zennaro MC, Vallo-Boado A, Rodriguez- Soriano J, Furu L, Haws R, Metzger D, Botelho B, Karaviti L, Haqq AM, Corey H, Janssens S, Corvol P, Lifton RP2006. Autosomal dominant pseudohypoaldosteronism type 1: mechanisms, evidence for neonatal lethality, and phenotypic expression in adults. J Am Soc Nephrol 17:1429–1436 [DOI] [PubMed] [Google Scholar]
- 8.Le Menuet D, Isnard R, Bichara M, Viengchareun S, Muffat-Joly M, Walker F, Zennaro MC, Lombès M2001. Alteration of cardiac and renal functions in transgenic mice overexpressing human mineralocorticoid receptor. J Biol Chem 276:38911–38920 [DOI] [PubMed] [Google Scholar]
- 9.Ouvrard-Pascaud A, Sainte-Marie Y, Bénitah JP, Perrier R, Soukaseum C, Cat AN, Royer A, Le Quang K, Charpentier F, Demolombe S, Mechta-Grigoriou F, Beggah AT, Maison-Blanche P, Oblin ME, Delcayre C, Fishman GI, Farman N, Escoubet B, Jaisser F2005. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation 111:3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinkler M, Zehnder D, Eardley KS, Lepenies J, Howie AJ, Hughes SV, Cockwell P, Hewison M, Stewart PM2005. Increased expression of mineralocorticoid effector mechanisms in kidney biopsies of patients with heavy proteinuria. Circulation 112:1435–1443 [DOI] [PubMed] [Google Scholar]
- 11.Doucet A, Katz AI1981. Mineralcorticoid receptors along the nephron: [3H]aldosterone binding in rabbit tubules. Am J Physiol 241:F605–F611 [DOI] [PubMed]
- 12.Lombès M, Farman N, Oblin ME, Baulieu EE, Bonvalet JP, Erlanger BF, Gasc JM1990. Immunohistochemical localization of renal mineralocorticoid receptor by using an anti-idiotypic antibody that is an internal image of aldosterone. Proc Natl Acad Sci USA 87:1086–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenton RA, Knepper MA2007. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87:1083–1112 [DOI] [PubMed] [Google Scholar]
- 14.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM1999. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 96:2538–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo SK, Dahl SC, Handler JS, Kwon HM2000. Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am J Physiol Renal Physiol 278:F1006–F1012 [DOI] [PubMed]
- 16.Burg MB, Ferraris JD, Dmitrieva NI2007. Cellular response to hyperosmotic stresses. Physiol Rev 87:1441–1474 [DOI] [PubMed] [Google Scholar]
- 17.Le Menuet D, Viengchareun S, Muffat-Joly M, Zennaro MC, Lombès M2004. Expression and function of the human mineralocorticoid receptor: lessons from transgenic mouse models. Mol Cell Endocrinol 217:127–136 [DOI] [PubMed] [Google Scholar]
- 18.Massaad C, Houard N, Lombès M, Barouki R1999. Modulation of human mineralocorticoid receptor function by protein kinase A. Mol Endocrinol 13:57–65 [DOI] [PubMed] [Google Scholar]
- 19.Vandewalle A, Lelongt B, Geniteau-Legendre M, Baudouin B, Antoine M, Estrade S, Chatelet F, Verroust P, Cassingena R, Ronco P1989. Maintenance of proximal and distal cell functions in SV40-transformed tubular cell lines derived from rabbit kidney cortex. J Cell Physiol 141:203–221 [DOI] [PubMed] [Google Scholar]
- 20.Carballo E, Lai WS, Blackshear PJ2000. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood 95:1891–1899 [PubMed] [Google Scholar]
- 21.Ogilvie RL, Abelson M, Hau HH, Vlasova I, Blackshear PJ, Bohjanen PR2005. Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay. J Immunol 174:953–961 [DOI] [PubMed] [Google Scholar]
- 22.Guhaniyogi J, Brewer G2001. Regulation of mRNA stability in mammalian cells. Gene 265:11–23 [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Grigsby CL, Law CS, Ni X, Nekrep N, Olsen K, Humphreys MH, Gardner DG2009. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J Clin Invest 119:1647–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbalis JG, Drutarosky MD1988. Adaptation to chronic hypoosmolality in rats. Kidney Int 34:351–360 [DOI] [PubMed] [Google Scholar]
- 25.Vandewalle A, Ronco P, Cassingena R1991. Establishment of permanent renal tubule cell lines by infection with the wild-type and a thermosensitive mutant of the simian virus 40. Am J Kidney Dis 17:619–621 [DOI] [PubMed] [Google Scholar]
- 26.Prié D, Friedlander G, Coureau C, Vandewalle A, Cassingéna R, Ronco PM1995. Role of adenosine on glucagon-induced cAMP in a human cortical collecting duct cell line. Kidney Int 47:1310–1318 [DOI] [PubMed] [Google Scholar]
- 27.Blot-Chabaud M, Laplace M, Cluzeaud F, Capurro C, Cassingéna R, Vandewalle A, Farman N, Bonvalet JP1996. Characteristics of a rat cortical collecting duct cell line that maintains high transepithelial resistance. Kidney Int 50:367–376 [DOI] [PubMed] [Google Scholar]
- 28.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A1999. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10:923–934 [DOI] [PubMed] [Google Scholar]
- 29.Djelidi S, Beggah A, Courtois-Coutry N, Fay M, Cluzeaud F, Viengchareun S, Bonvalet JP, Farman N, Blot-Chabaud M2001. Basolateral translocation by vasopressin of the aldosterone-induced pool of latent Na-K-ATPases is accompanied by α1 subunit dephosphorylation: study in a new aldosterone-sensitive rat cortical collecting duct cell line. J Am Soc Nephrol 12:1805–1818 [DOI] [PubMed] [Google Scholar]
- 30.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC2005. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16:878–891 [DOI] [PubMed] [Google Scholar]
- 31.Bens M, Chassin C, Vandewalle A2006. Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells. Pflugers Arch 453:133–146 [DOI] [PubMed] [Google Scholar]
- 32.Chassin C, Bens M, Vandewalle A2007. Transimmortalized proximal tubule and collecting duct cell lines derived from the kidneys of transgenic mice. Cell Biol Toxicol 23:257–266 [DOI] [PubMed] [Google Scholar]
- 33.Zennaro MC, Le Menuet D, Viengchareun S, Walker F, Ricquier D, Lombès M1998. Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action. J Clin Invest 101:1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teixeira M, Viengchareun S, Butlen D, Ferreira C, Cluzeaud F, Blot-Chabaud M, Lombès M, Ferrary E2006. Functional IsK/ KvLQT1 potassium channel in a new corticosteroid-sensitive cell line derived from the inner ear. J Biol Chem 281:10496–10507 [DOI] [PubMed] [Google Scholar]
- 35.Alfaidy N, Blot-Chabaud M, Robic D, Kenouch S, Bourbouze R, Bonvalet JP, Farman N1995. Characteristics and regulation of 11β-hydroxysteroid dehydrogenase of proximal and distal nephron. Biochim Biophys Acta 1243:461–468 [DOI] [PubMed] [Google Scholar]
- 36.Bocchi B, Fagart J, Cluzeaud F, Fay M, Rafestin-Oblin ME, Farman N2003. Glucocorticoid metabolism by 11-β hydroxysteroid dehydrogenase type 2 modulates human mineralocorticoid receptor transactivation activity. J Steroid Biochem Mol Biol 84:239–244 [DOI] [PubMed] [Google Scholar]
- 37.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA1999. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104:R19–R23 [DOI] [PMC free article] [PubMed]
- 38.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D1999. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96:2514–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Náray-Fejes-Tóth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Tóth G1999. sgk is an aldosterone-induced kinase in the renal collecting duct. Effects on epithelial Na+ channels. J Biol Chem 274:16973–16978 [DOI] [PubMed] [Google Scholar]
- 40.Zennaro MC, Keightley MC, Kotelevtsev Y, Conway GS, Soubrier F, Fuller PJ1995. Human mineralocorticoid receptor genomic structure and identification of expressed isoforms. J Biol Chem 270:21016–21020 [DOI] [PubMed] [Google Scholar]
- 41.Le Menuet D, Viengchareun S, Penfornis P, Walker F, Zennaro MC, Lombès M2000. Targeted oncogenesis reveals a distinct tissue-specific utilization of alternative promoters of the human mineralocorticoid receptor gene in transgenic mice. J Biol Chem 275:7878–7886 [DOI] [PubMed] [Google Scholar]
- 42.Pascual-Le Tallec L, Lombès M2005. The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol 19:2211–2221 [DOI] [PubMed] [Google Scholar]
- 43.Ferraris JD, Burg MB2007. Tonicity-regulated gene expression. Methods Enzymol 428:279–296 [DOI] [PubMed] [Google Scholar]
- 44.Kim JA, Jeon US, Kwon MS, Lim SW, Kwon HM2007. Transcriptional activator TonE-binding protein in cellular protection and differentiation. Methods Enzymol 428:253–267 [DOI] [PubMed] [Google Scholar]
- 45.Woo SK, Lee SD, Na KY, Park WK, Kwon HM2002. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol 22:5753–5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Féraille E, Martin PY2006. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17:1521–1531 [DOI] [PubMed] [Google Scholar]
- 47.Nakayama Y, Peng T, Sands JM, Bagnasco SM2000. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J Biol Chem 275:38275–38280 [DOI] [PubMed] [Google Scholar]
- 48.Lanaspa MA, Almeida NE, Andres-Hernando A, Rivard CJ, Capasso JM, Berl T2007. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells. Proc Natl Acad Sci USA 104:13672–13677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanaspa MA, Andres-Hernando A, Rivard CJ, Dai Y, Berl T2008. Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proc Natl Acad Sci USA 105:15797–15802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldegger S, Barth P, Raber G, Lang F1997. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA 94:4440–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell LM, Leong ML, Kim B, Wang E, Park J, Hemmings BA, Firestone GL2000. Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J Biol Chem 275:25262–25272 [DOI] [PubMed] [Google Scholar]
- 52.McCormick JA, Bhalla V, Pao AC, Pearce D2005. SGK1: a rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology (Bethesda) 20:134–139 [DOI] [PubMed] [Google Scholar]
- 53.Cherradi N, Lejczak C, Desroches-Castan A, Feige JJ2006. Antagonistic functions of tetradecanoyl phorbol acetate-inducible-sequence 11b and HuR in the hormonal regulation of vascular endothelial growth factor messenger ribonucleic acid stability by adrenocorticotropin. Mol Endocrinol 20:916–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan H, Cherradi N, Feige JJ, Jefcoate C2009. cAMP-dependent posttranscriptional regulation of steroidogenic acute regulatory (STAR) protein by the zinc finger protein ZFP36L1/TIS11b. Mol Endocrinol 23:497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyazaki H, Shiozaki A, Niisato N, Marunaka Y2007. Physiological significance of hypotonicity-induced regulatory volume decrease: reduction in intracellular Cl− concentration acting as an intracellular signaling. Am J Physiol Renal Physiol 292:F1411–F1417 [DOI] [PubMed]
- 56.Niisato N, Post M, Van Driessche W, Marunaka Y1999. Cell swelling activates stress-activated protein kinases, p38 MAP kinase and JNK, in renal epithelial A6 cells. Biochem Biophys Res Commun 266:547–550 [DOI] [PubMed] [Google Scholar]
- 57.Taruno A, Niisato N, Marunaka Y2007. Hypotonicity stimulates renal epithelial sodium transport by activating JNK via receptor tyrosine kinases. Am J Physiol Renal Physiol 293:F128–F138 [DOI] [PubMed]
- 58.Tamma G, Procino G, Svelto M, Valenti G2007. Hypotonicity causes actin reorganization and recruitment of the actin-binding ERM protein moesin in membrane protrusions in collecting duct principal cells. Am J Physiol Cell Physiol 292:C1476–C1484 [DOI] [PubMed]
- 59.Vinciguerra M, Arnaudeau S, Mordasini D, Rousselot M, Bens M, Vandewalle A, Martin PY, Hasler U, Feraille E2004. Extracellular hypotonicity increases Na,K-ATPase cell surface expression via enhanced Na+ influx in cultured renal collecting duct cells. J Am Soc Nephrol 15:2537–2547 [DOI] [PubMed] [Google Scholar]
- 60.Niisato N, Taruno A, Marunaka Y2007. Involvement of p38 MAPK in hypotonic stress-induced stimulation of β- and γ-ENaC expression in renal epithelium. Biochem Biophys Res Commun 358:819–824 [DOI] [PubMed] [Google Scholar]
- 61.Rozansky DJ, Wang J, Doan N, Purdy T, Faulk T, Bhargava A, Dawson K, Pearce D2002. Hypotonic induction of SGK1 and Na+ transport in A6 cells. Am J Physiol Renal Physiol 283:F105–F113 [DOI] [PubMed]
- 62.Taruno A, Niisato N, Marunaka Y2008. Intracellular calcium plays a role as the second messenger of hypotonic stress in gene regulation of SGK1 and ENaC in renal epithelial A6 cells. Am J Physiol Renal Physiol 294:F177–F186 [DOI] [PubMed]
- 63.Kiilerich P, Kristiansen K, Madsen SS2007. Hormone receptors in gills of smolting Atlantic salmon, Salmo salar: expression of growth hormone, prolactin, mineralocorticoid and glucocorticoid receptors and 11β-hydroxysteroid dehydrogenase type 2. Gen Comp Endocrinol 152:295–303 [DOI] [PubMed] [Google Scholar]
- 64.Morel F, Butlen D1990. Hormonal receptors in the isolated tubule. Methods Enzymol 191:303–325 [DOI] [PubMed] [Google Scholar]
- 65.Martinerie L, Viengchareun S, Delezoide AL, Jaubert F, Sinico M, Prevot S, Boileau P, Meduri G, Lombès M2009. Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology 150:4414–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lombes M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP1992. Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res 71:503–510 [DOI] [PubMed] [Google Scholar]
- 67.Viengchareun S, Bouzinba-Segard H, Laigneau JP, Zennaro MC, Kelly PA, Bado A, Lombès M, Binart N2004. Prolactin potentiates insulin-stimulated leptin expression and release from differentiated brown adipocytes. J Mol Endocrinol 33:679–691 [DOI] [PubMed] [Google Scholar]
- 68.Viengchareun S, Servel N, Fève B, Freemark M, Lombès M, Binart N2008. Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2. PLoS ONE 3:e1535 [DOI] [PMC free article] [PubMed]
- 69.Kamenicky P, Viengchareun S, Blanchard A, Meduri G, Zizzari P, Imbert-Teboul M, Doucet A, Chanson P, Lombès M2008. Epithelial sodium channel is a key mediator of growth hormone-induced sodium retention in acromegaly. Endocrinology 149:3294–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lombès M, Binart N, Oblin ME, Joulin V, Baulieu EE1993. Characterization of the interaction of the human mineralocorticosteroid receptor with hormone response elements. Biochem J 292:577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pascual-Le Tallec L, Kirsh O, Lecomte MC, Viengchareun S, Zennaro MC, Dejean A, Lombès M2003. PIAS1 interacts with the N-terminal domain of mineralocorticoid receptor and represses its transcriptional activity—implication of SUMO-1 modification. Mol Endocrinol 17:2529–2542 [DOI] [PubMed] [Google Scholar]