Abstract
Buprenorphine is approved as pharmacotherapy for opioid dependence in non-pregnant patients in multiple countries, and is currently under investigation for pregnant women in the US and Europe. This research evaluates the disposition of buprenorphine, opiates, cocaine, and metabolites in 5 term placentas from a US cohort. Placenta and matched meconium concentrations were compared, and relationships between maternal buprenorphine dose, placenta concentrations, and neonatal outcomes following controlled administration during gestation were investigated. Buprenorphine and/or metabolites were detected in all placenta specimens and were uniformly distributed across this tissue (CV<27.5%, 4 locations), except for buprenorphine in 3 placentas. In 2 of these, buprenorphine was not detected in some locations and, in the 3rd placenta, was totally absent. Median (range) concentrations were buprenorphine 1.6ng/g (not detected to 3.2), norbuprenorphine 14.9ng/g (6.2 to 24.2), buprenorphine-glucuronide 3ng/g (1.3 to 5.0) and norbuprenorphine-glucuronide 14.7ng/g (11.4 to 25.8). Placenta is a potential alternative matrix for detecting in utero buprenorphine exposure, but at lower concentrations (15–70 fold) than in meconium. Statistically significant correlations were observed for mean maternal daily dose from enrollment to delivery and placenta buprenorphine-glucuronide concentration, and for norbuprenorphine-glucuronide concentrations and time to neonatal abstinence syndrome (NAS) onset and duration, and for norbuprenorphine/norbuprenorphine-glucuronide ratio and maximum NAS score, and newborn length. Analysis of buprenorphine and metabolites in this alternative matrix, an abundant waste product available at the time of delivery, may be valuable for prediction of neonatal outcomes for clinicians treating newborns of buprenorphine-exposed women.
Keywords: buprenorphine, placenta, Neonatal Abstinence Syndrome, in utero drug exposure, opioid-dependence
INTRODUCTION
In 2002, buprenorphine (BUP) was approved in the United States for office-based treatment of opioid dependence under the Drug Addiction Treatment Act of 2000 1. Currently, high-risk populations such as pregnant women in Europe 2, 3, Australia 4, and the US 5 are beginning to receive BUP 4, emphasizing the need for data on the drug’s disposition in the maternal-fetal dyad. In adults, BUP undergoes phase I metabolism (N-dealkylation) by cytochrome P450 3A4 (CYP3A4) to norbuprenorphine (NBUP) primarily in the liver 6. BUP and NBUP phase II metabolism (glucuronidation) to buprenorphine-glucuronide (BUP-Gluc) and norbuprenorphine-glucuronide (NBUP-Gluc), respectively, occurs through the action of uridine diphosphate glucuronosyltransferase (UGT) 1A1 7 and UGT2B7 8. Recently, Rouguieg et al. 9 showed that BUP glucuronidation involves predominantly UGT1A1 and 2B7 isoforms, and for NBUP glucuronidation UGT1A1 and UGT1A3.
The placenta is the interface between maternal and fetal blood, and in humans, is haemochorial, that is the chorion or membrane enclosing the fetus is in direct contact with the mother’s blood. Endogenous and exogenous compounds are exchanged between maternal and fetal circulations via the placenta that also has important endocrine and metabolic functions. Human placenta expresses several CYP genes including the CYP3A family, although placental activity of CYP3A4 has not been demonstrated 10. Deshmukh et al. 11 showed that the enzyme aromatase/CYP19 is the major enzyme catalyzing the biotransformation of BUP to NBUP in term human placenta. Although this enzyme is present early in gestation, it is most active at term, due to increased placental mass and an increase in the number of catalytic proteins 12,13. With regard to phase II metabolism, isoforms of the UGT2 subfamily (UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15 and UGT2B17) have been identified in term human placenta 14, but there are no specific data on BUP and NBUP glucuronidation in placenta.
The human fetal liver possesses a relatively well-developed metabolic capacity for xenobiotics 15, including multiple CYPs, although the variety and concentrations are lower than in adults 16. CYP3A7 is the only fetal CYP form characterized unequivocally at the protein level, and is the major constituent in human fetal liver. The shift between CYP3A7 and CYP3A4 metabolism occurs immediately after birth, and is transcriptionally controlled 17. No data are available on fetal BUP and NBUP glucuronidation, but morphine glucuronides are formed at high rates by UGT2B7 in fetal baboon liver, rivaling adult activity 18.
Drugs cross the placenta mainly by passive diffusion from the mother to the fetus, with the extent of transfer dependent upon the drug’s physicochemical properties. Diffusion is favored if compounds are lipid-soluble, small (<500 Da), and poorly bound to proteins. Thus, BUP-Gluc and NBUP-Gluc diffusion should be slow because of greater polarity and molecular weights >500 Da. Other transfer mechanisms through the placenta involve specific transporters, such as permeability glycoprotein (P-gp), an important efflux transporter for multiple basic compounds from the fetal to maternal circulation 19. However, BUP is not a substrate for this specific transporter 20.
Nanovskaya et al. 21 conducted an in vitro term placenta lobule dual perfusion study with BUP. Placenta was shown to be a depot for BUP with less than 10% of maternal dose transferred to the fetal circuit, and only 5% of perfused BUP metabolized to NBUP during a 4 h perfusion. However, BUP and NBUP glucuronidation by placenta, and metabolite accumulation in this tissue were not evaluated. To our knowledge, no placenta BUP concentrations have been reported in authentic specimens after maternal BUP administration.
BUP treatment during gestation provides a unique model of controlled drug administration to investigate BUP and metabolite disposition in placenta, and correlations between maternal dose and placenta concentrations, and placenta concentrations and neonatal outcomes. The neonatal abstinence syndrome (NAS) develops as a consequence of withdrawal from chronic opioid exposure during gestation, and includes signs and symptoms of dysfunction of the autonomic nervous system, gastrointestinal tract, and respiratory system 22. With appropriate early medication and supportive interventions, NAS can be alleviated without damaging consequences 23. Kacinko et al. 24 and Marquet et al. 25 described correlations between BUP and metabolite concentrations in meconium and neonatal outcomes, and the present research evaluates correlations in an alternative matrix - placenta.
For the first time, the disposition of BUP and metabolites in placenta from pregnant opioid-dependent women receiving BUP pharmacotherapy are reported, in conjunction with maternal BUP doses, matched meconium concentrations and neonatal outcomes. Also, the ability of placenta analysis to identify heroin and cocaine relapse during gestation was investigated by comparing thrice-weekly urine specimen results collected during gestation to drugs in this new alternative matrix.
MATERIALS AND METHODS
Participants
The Center for Addiction and Pregnancy (CAP) at the Johns Hopkins Bayview Medical Center (JHBMC) in Baltimore, MD, recruited participants for a double-dummy, flexible, randomized, stratified, parallel-group controlled study comparing methadone and BUP for opiate addiction treatment during pregnancy 26. Inclusion criteria were 21–40 years old, 16–30 weeks estimated gestational age (EGA) of the fetus by sonogram, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) diagnosis of current opioid dependence, maintenance pharmacotherapy request, recent self-reported opioid use of more than 4 days in the past 7, and an opiate-positive urine specimen. Exclusion criteria were undocumented methadone-positive urine, current DSM-IV alcohol abuse or dependence, self-reported benzodiazepine use more frequent than 7 times a month or once weekly, currently taking another Axis I disorder medication, serious concurrent illness, previous diagnosis of preterm labor, evidence of fetal malformation, and human immunodeficiency virus or sickle-cell trait positive tests 26.
Eligible applicants were stratified at study entry on cocaine use (yes/no), EGA (16–23 weeks and 6 days; 24–30 weeks) and opioid use (3 or less than 3, and greater than 3 times per day). Participants were assigned to one of two treatment groups according to a computerized dynamic balanced randomization procedure 27. Research staff, with no other study involvement, generated the randomized allocation sequence. Additional study detail and primary outcome data were previously published 26.
Maternal BUP dosing
All women received oral methadone for 3–5 days after initially qualifying for the protocol during more extensive screening procedures. Women were randomized and transitioned to study medication with immediate-release morphine in divided daily doses. BUP-maintained women received sublingual BUP HCl (2 mg each, maximum 24 mg/day; manufactured by Reckitt-Benckiser Healthcare UK Limited, Hull, England) and placebo tablets totaling 12 tablets/day, along with 40 mL liquid placebo. Double-blind medication increases or decreases were determined by physicians based on medication compliance, participant request, urine toxicology results, and participant self-report of opioid withdrawal symptoms or craving 26.
Urine drug testing
Urine specimens were assayed 3 times a week for cocaine, opiates, cannabis, and benzodiazepines by immunoassay (Dade Behring Diagnostics, Deerfield, IL) with cutoffs of 300 ng/mL for cocaine and opiates, 200 ng/mL for benzodiazepines, and 100 ng/mL for cannabis 24.
Neonatal outcome measures
Apgar scores at 1 and 5 min, EGA at delivery, birth weight (g), head circumference (cm), length (cm), hospital stay duration from birth until discharge to the research unit (days), and NAS scores, need for treatment and amount of treatment were obtained from medical records. NAS was systematically assessed for 10 days using a 19-item modified Finnegan Scale 22, 26. Number of NAS observations varied from 6 to 8 per day while in the hospital to 2 per day while on the research unit. Time to NAS onset (h) was defined as the time from birth until the first score >4. The score of 4 was selected as the cutoff on the basis of clinical experience and preliminary blinded-condition comparison data from drug-exposed and nondrug-exposed neonates 23. Peak NAS score was defined as the highest score obtained and time-to-peak (h) was calculated from time of birth to peak NAS score. NAS duration (h) was defined as the time from first score >4 to time after which all scores were <5. Treatment for NAS with morphine solution (0.02 mg/drop) was initiated when a neonate received 2 consecutives scores of 9 or greater. Neonates were reduced in medication by one drop per day if every score for 24 h was 8 or below, and they were discharged following 24 h of no medication and NAS scores less than or equal to 8 26.
Placenta analysis
The entire term placenta was collected at delivery and stored at −20°C until analysis. BUP and metabolites, NBUP, BUP-Gluc, and NBUP-Gluc were quantified in placenta by a previously published LCMS procedure 28. Briefly, 2 g of placenta was homogenized in 6 mL 0.1% perchloric acid in water in the presence of deuterated BUP and NBUP. After 15 min centrifugation at 2,057 g, the liquid supernatant was subjected to solid-phase extraction (SPE) with preconditioned Strata-XC cartridges (Phenomenex, Torrence, CA). Reverse-phase separation was achieved with a Synergi Polar column (2.1×75 mm, 4 μm; Phenomenex, Torrence, CA) within 20 min under gradient conditions. Mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. Quantification was achieved in a LCQ Deca XP Plus Ion-Trap Mass Spectrometer (ThermoScientific, San Jose, CA) with positive electrospray ionization by selected ion monitoring of precursor ions m/z 468.4 for BUP; 414.3 for NBUP; 644.3 for BUP-Gluc, and 590.0 for NBUP-Gluc. BUP and NBUP were identified by MS2, monitoring fragments m/z 396.3, 414.0 and 426.2 for BUP, and 340.2, 364.2 and 382.2 for NBUP. Glucuronide conjugates were identified by MS3 by m/z 396.0 and 414.0 for BUP-Gluc, and m/z 340.1 and 382.0 for NBUP-Gluc.
The assay was linear from 1 to 50 ng/g. Intra-, inter-assay, and total imprecision were <14.7%, and analytical recovery was between 96.2 and113.1%. Extraction efficiencies ranged from 40.7 to 66.5%, and process efficiency from 38.8 to 70.5%. No significant matrix effect was detected for any compound. The method was specific (no endogenous or exogenous interferences) and sensitive (LOD 0.8 ng/g).
Cocaine, benzoylecgonine (BE), morphine, codeine, 6-acetylmorphine (6AM), methadone, and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), were quantified in placenta by LCMS 29. Briefly, 5 mL of 0.1% perchloric acid were added to 1 g of human placenta in a blender until complete homogenization. Deuterated internal standard solution was added to the homogenate, followed by centrifugation. The supernatant was subjected to SPE with mixed mode-reversed phase Strata-XC cartridges (Phenomenex, Torrence, CA). Chromatographic separation was performed on a Synergi Polar RP column (75 mm × 2.1 mm, 4 μm; Phenomenex, Torrance, CA) with a gradient of (A) 0.1% formic acid and (B) acetonitrile. Transitions monitored in a LCQ Deca XP Ion-Trap Mass Spectrometer (Thermo Scientific, San Jose, CA) with positive electrospray ionization were as follows: 310.1>265.0, 247.1, 219.2 for methadone, 278.2>249.1, 234.2 for EDDP, 304.1>182.1, 150.1, 82.1 for cocaine, 290.1>168.1, 150.0, 82.1 for BE, 286.2>201.1, 229.0, 268.1 for morphine, 300.2>215.1, 243.0, 282.2 for codeine, and 328.2>211.1, 268.1, 193.1 for 6AM. Calibration curves were linear between 10–2000 ng/g for methadone, and 2.5–500 ng/g for the rest of the analytes. Intra and inter-assay imprecision were <15.0%, and intra- and inter-assay analytical recovery were >85.0% for all analytes. Extraction efficiency was >46.0%, with significant matrix effect, although deuterated internal standards compensated for ion suppression or enhancement.
BUP and metabolite disposition in placenta
BUP and metabolite concentrations were examined in 4 different locations in each of the 5 term placentas from BUP-maintained pregnant women (1 monozygotic set of twins and 4 single births). These locations were numbered from 1 to 4 according to closeness to the umbilical cord: 1 closest (1 cm) to umbilical cord, 2 and 3 intermediate locations (4 and 6 cm), and 4 farthest (10 cm) from the umbilical cord. Placenta specimens included maternal and the fetal portions. The variability of biomarker distribution in the tissue was expressed as concentration CV of each analyte in these 4 locations. To better understand placenta function, plasma from the mother at delivery (n=2) and umbilical cord plasma (mixture of vein and arterial blood, n=6) were analyzed using a previously published method 30.
Statistical analyses
Statistical analyses were performed with SPSS version 13.0. Pearson correlations evaluated relationships between placenta and meconium concentrations, placenta concentrations and maternal BUP dose, and placenta concentrations and neonatal outcomes. Statistical probability (p) <0.05 was considered statistically significant. The Kolmogorov-Smirnov test was employed to evaluate normal distribution of the data. Placenta concentrations of BUP, NBUP, BUP-Gluc, and NBUP-Gluc were mean values of the 4 locations analyzed from each placenta. T-test was employed to compare dosing, time since last dose before delivery, and EGA differences between group with NBUP as predominant metabolite and group with NBUP-Gluc as predominant metabolite in placenta.
RESULTS
Participants
A total of 1,490 women were screened, with 57 preliminarily qualified and consented to participate in the study. Most women did not qualify for continued screening due to entrance into treatment outside the allowed gestational age or lack of qualification for methadone maintenance at CAP treatment entry 26. Finally, 30 women were randomized, 15 to methadone and 15 to BUP, of which 9 BUP-maintained women participated through delivery. Five of 9 term placentas were collected and correctly labeled from 1 monozygotic set of twins (participant A) and 4 single births (participants B, C, D, and E), yielding 5 placentas and 6 infants. Demographic, self-reported drug use history, and BUP dosing are included in Table 1. No participant provided a urine sample that screened positive for cannabis throughout pregnancy. Participant B’s urine specimens were positive for benzodiazepines 22.2% of the time, with her last positive result 43 days before delivery. Cocaine and opiates urine screening results are shown in Table 2.
Table 1.
Demographics and self-reported drug use history for the past 30 days prior to admission, and buprenorphine (BUP) doses for 5 opioid-dependent pregnant women.
| Participant | A | B | C | D | E | Mean±SE |
|---|---|---|---|---|---|---|
| Age (years) | 30 | 32 | 32 | 28 | 31 | 30.6±0.8 |
| Race | AAa | AA | AA | AA | AA | - |
| Years education | 10 | 10 | 12 | 8 | 10 | 10.0±0.6 |
| Employment | No | No | No | No | No | - |
| EGAb | 25 | 20 | 26 | 18 | 26 | 23.0±1.7 |
| Cocaine use | Yes | Yes | Yes | Yes | Yes | - |
| Opioidusec | 1 | 2 | 2 | 1 | 2 | - |
| Alcohol use (days) | 0 | 5 | 0 | 0 | 0 | 1.0±1.0 |
| Smoking (days) | 0 | 30 | 30 | 0 | 30 | 18.0±7.4 |
| # cigarettes/day | 0 | 3 | 20 | 0 | 10 | 6.6±3.8 |
| Days in study at delivery | 92 | 118 | 111 | 151 | 98 | 114.0±10.3 |
| BUP dose at delivery (mg/day) | 18 | 24 | 18 | 18 | 14 | 18.4±1.6 |
| Mean daily BUP dose (mg/day) | 15.9 | 18.3 | 15.9 | 12.3 | 13.6 | 15.2±1.0 |
| Mean 3rd trimester daily BUP dose (mg/day) | 16.9 | 21.8 | 17.1 | 13.3 | 13.7 | 16.6±1.5 |
| Mean last month daily BUP dose (mg/day) | 18 | 24 | 18 | 18 | 14 | 18.4±1.6 |
| Cumulative BUP dose (mg) | 1464 | 2160 | 1760 | 1850 | 1330 | 1712.8±146.5 |
| Cumulative 3rd trimester BUP dose (mg) | 1084 | 1396 | 1330 | 1132 | 1162 | 1220.8±60.3 |
| Cumulative last month BUP dose (mg) | 558 | 766 | 522 | 522 | 434 | 560.4±55.3 |
African-American
Estimated gestational age at admission (weeks)
Opioid use in the past 30 days at admission. 1 ≤ 3/day; 2≥ 4/day
Table 2.
Thrice-weekly urine tests and placenta results from five buprenorphine-maintained pregnant women.
| Participant | Cocaine | Opiates | ||||||
|---|---|---|---|---|---|---|---|---|
| Total %Posa | %Pos 3rd Tb | Days from last posc | Placenta | Total %Pos | %Pos 3rd T | Days from last pos | Placenta | |
| A | 32.5 | 40.7 | 8 | NEGd | 75 | 74.1 | 7 | POSe |
| B | 35.3 | 11.1 | 43 | NEG | 45.1 | 14.8 | 55 | NEG |
| C | 2.1 | 0 | 82 | NEG | 8.5 | 12.5 | 30 | NEG |
| D | 23.8 | 27 | 54 | NEG | 17.5 | 16.2 | 56 | NEG |
| E | 0 | 0 | - | NEG | 5 | 0 | 93 | NEG |
% Positive urine tests from enrollment to birth.
% Positive urine tests in the 3rd trimester.
Days from last positive urine specimen to birth.
Benzoylecgonine, cocaine or morphine, 6-AM, codeine were not detected.
Benzoylecgonine, cocaine or morphine, 6-AM, codeine were > LOQ.
Neonatal outcomes
Three male and 3 female normal birth weight infants were delivered full-term. None required treatment for NAS. Neonatal outcome measures are summarized in Table 3.
Table 3.
Neonatal outcomes for six infants from 5 opioid-dependent pregnant women maintained on buprenorphine.
| Participant | A | B | C | D | E | Mean±SE | |
|---|---|---|---|---|---|---|---|
| Baby 1 | Baby 2 | ||||||
| Gender | M | M | F | F | M | F | |
| Birth weight (g) | 2740 | 2730 | 2890 | 3525 | 3355 | 3560 | 3133.3±159.3 |
| Head circumference (cm) | 33 | 34 | 31 | 35 | 38 | 33 | 34.0±1.0 |
| Length (cm) | 49 | 48 | 48 | 53 | 52 | 50 | 50.0±0.9 |
| Gestational age at delivery (weeks) | 37 | 37 | 37 | 39 | 40 | 40 | 38.3±0.6 |
| 1-min Apgar Score | 8 | 9 | 8 | 8 | 7 | 8 | 8.0±0.3 |
| 5-min Apgar Score | 9 | 9 | 9 | 9 | 9 | 8 | 8.8±0.2 |
| Length of hospital stay (days) | 5 | 5 | 8 | 4 | 4 | 4 | 5.0±0.6 |
| Peak NAS score | 6 | 8 | 9 | 3 | 5 | 7 | 6.3±0.9 |
| Time-to-peak NAS score (h) | 24 | 122 | 167 | 78 | 205 | 45 | 108.5±28.0 |
| Time to NAS onset (h) | 34 | 19 | 29 | - | 205 | 3 | 58.0±37.1 |
| Duration NAS (h) | 110 | 149 | 208 | - | 6.5 | 110 | 116.7±32.9 |
| % NAS scores > 4 | 14 | 24 | 29 | - | 3.6 | 58 | 21.4±8.6 |
Analytical results
BUP and/or metabolites were detected in all 5 term placenta specimens as described in Table 4. BUP was not detected in one placenta (placenta E), and the predominant analyte was NBUP-Gluc in 3 cases (placenta C, D, and E) and NBUP in 2 (placenta A and B). BUP concentrations ranged from none detected to 3.2 ng/g (median 1.6 ng/g), NBUP from 6.2 to 24.2 ng/g (median 14.9 ng/g), BUP-Gluc from 1.3 to 5.0 ng/g (median 3 ng/g) and NBUP-Gluc from 11.4 to 25.8 ng/g (median 14.7 ng/g). Biomarker concentration variability were within 27.5% CV in 4 different areas of each of 5 placentas, except for BUP in subjects C and D, where BUP was not detected in locations 2, 3 or 4. Inter-individual variability was much higher for BUP (n=4, 85.9%; and none detected in participant E), than for NBUP (n=5, 36.3%), BUP-Gluc (n=5, 38.6%), and NBUP-Gluc (n=5, 25.8%). BUP and metabolite concentrations in meconium 24 and in umbilical cord plasma 30 from these subjects were previously reported. Table 5 summarizes BUP and metabolite concentrations in maternal plasma, placenta, umbilical cord plasma, and meconium for all available data.
Table 4.
Buprenorphine (BUP), norbuprenorphine (NBUP), buprenorphine-glucuronide (BUP-Gluc), and norbuprenorphine-glucuronide (NBUP-Gluc) concentrations in 4 different locations of 5 placenta specimens from buprenorphine-maintained women.
| Participant | Analyte | Location 1 (ng/g) | Location 2 (ng/g) | Location 3 (ng/g) | Location 4 (ng/g) | Mean (ng/g) | CV (n=4, %) |
|---|---|---|---|---|---|---|---|
| A | BUP | 2.5 | 2.7 | 3.2 | 3.2 | 2.9 | 13.0 |
| NBUP | 17.3 | 16.6 | 24.2 | 23 | 20.3 | 19.3 | |
| BUP-Gluc | 3.2 | 2.5 | 3.4 | 2.8 | 3.0 | 14.0 | |
| NBUP-Gluc | 16.0 | 14.0 | 15.9 | 16.2 | 15.5 | 6.6 | |
| B | BUP | 3.1 | 2.5 | 2.4 | 2.0 | 2.5 | 17.4 |
| NBUP | 20.1 | 19.1 | 18.1 | 15.3 | 18.1 | 11.4 | |
| BUP-Gluc | 3.8 | 4.2 | 3.1 | 3.2 | 3.6 | 14.8 | |
| NBUP-Gluc | 14.5 | 13.5 | 13.2 | 11.7 | 13.2 | 8.5 | |
| C | BUP | 1.2 | 1.0 | 0.0 | 0.0 | 0.6 | - |
| NBUP | 8.0 | 6.9 | 6.2 | 6.3 | 6.9 | 12.1 | |
| BUP-Gluc | 5.0 | 4.1 | 3.4 | 3.6 | 4.0 | 17.8 | |
| NBUP-Gluc | 16.2 | 12.6 | 11.4 | 11.9 | 13.0 | 16.7 | |
| D | BUP | 2.4 | 0.0 | 2.0 | 0.0 | 1.1 | - |
| NBUP | 20.6 | 12.0 | 21.5 | 14.2 | 17.1 | 27.5 | |
| BUP-Gluc | 1.5 | 1.3 | 2.0 | 1.4 | 1.6 | 17.4 | |
| NBUP-Gluc | 24.0 | 18.7 | 25.8 | 21.6 | 22.5 | 13.6 | |
| E | BUP | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | - |
| NBUP | 13.6 | 11.5 | 14.5 | 14.2 | 13.4 | 10.1 | |
| BUP-Gluc | 1.5 | 1.8 | 1.9 | 2.0 | 1.8 | 11.2 | |
| NBUP-Gluc | 13.0 | 12.2 | 15.0 | 15.5 | 13.9 | 11.1 |
Table 5.
Buprenorphine (BUP), norbuprenorphine (NBUP), buprenorphine-glucuronide (BUP-Gluc), and norbuprenorphine-glucuronide (NBUP-Gluc) concentrations in maternal plasma at delivery, placenta, umbilical cord plasma, and meconium from five buprenorphine-maintained women.
| Participant | Analyte | Maternal plasma (ng/mL) | Placenta (ng/g) | Umbilical cord plasma (ng/mL) | Meconium (ng/g) | ||
|---|---|---|---|---|---|---|---|
| Baby 1 | Baby 2 | Baby 1 | Baby 2 | ||||
| A | BUP | NAa | 2.9 | 0.7 | 0.6 | 59.3 | 59.6 |
| NBUP | NA | 20.3 | 0.8 | 0.9 | 468.4 | 373.4 | |
| BUP-Gluc | NA | 3.0 | 4.4 | 3.8 | 36.7 | 110.0 | |
| NBUP-Gluc | NA | 15.5 | 22.8 | 22.0 | 79.7 | 234.2 | |
| B | BUP | 0.6 | 2.5 | 0.0 | 240.5 | ||
| NBUP | 1.9 | 18.1 | 1.1 | 1228.6 | |||
| BUP-Gluc | 6.5 | 3.6 | 1.6 | 56.3 | |||
| NBUP-Gluc | 23.1 | 13.2 | 34.4 | 651.6 | |||
| C | BUP | NA | 1.1 | 0.3 | 23.9 | ||
| NBUP | NA | 6.9 | 1.3 | 730.6 | |||
| BUP-Gluc | NA | 4.0 | 13.9 | 8.2 | |||
| NBUP-Gluc | NA | 13.0 | 35.8 | 0.0 | |||
| D | BUP | NA | 2.2 | 0.3 | 40.4 | ||
| NBUP | NA | 17.1 | 2.9 | 505.4 | |||
| BUP-Gluc | NA | 1.6 | 2.2 | 51.2 | |||
| NBUP-Gluc | NA | 22.5 | 39.4 | 450.2 | |||
| E | BUP | 0.2 | 0.0 | 0.1 | 165.4 | ||
| NBUP | 2.9 | 13.4 | 1.9 | 858.4 | |||
| BUP-Gluc | 18.2 | 1.8 | 2.0 | 80.5 | |||
| NBUP-Gluc | 42 | 13.9 | 24.2 | 0.0 | |||
Specimen not available
Cocaine and opiates were analyzed in one intermediate location in each placenta. Morphine was 9 ng/g in placenta A; cocaine, BE, codeine, 6AM, methadone, and EDDP were not detected in any specimen.
Correlation of placenta and meconium BUP biomarker concentrations
Pearson correlation coefficients were calculated for placenta and meconium analyte concentrations, total BUP and metabolite concentration (BUP+NBUP+BUP-Gluc+NBUP-Gluc), and BUP/NBUP, BUP/BUP-Gluc, and NBUP/NBUP-Gluc ratios. There were no significant correlations between placenta and meconium concentrations or ratios.
Correlation of maternal BUP dose and BUP and metabolite placenta concentrations
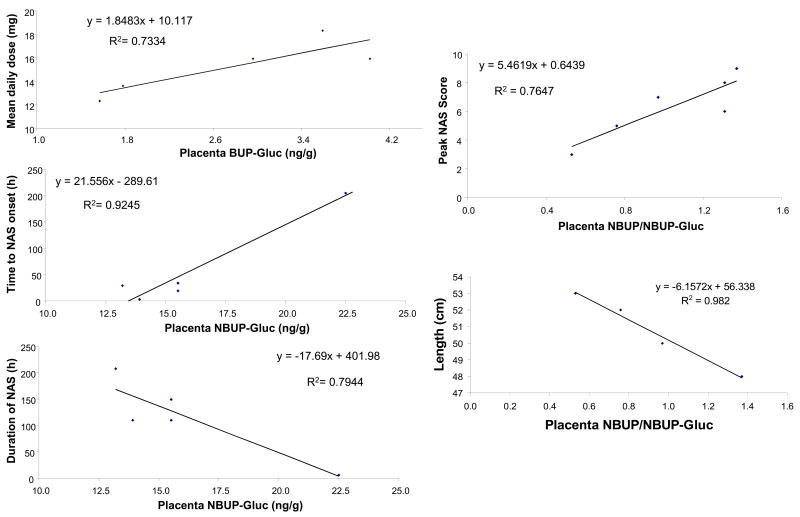
Pearson correlation coefficients were calculated for BUP, NBUP, BUP-Gluc, NBUP-Gluc concentrations, total BUP and metabolite concentration, and BUP/NBUP, BUP/BUP-Gluc, and NBUP/NBUP-Gluc ratios in placenta and days enrolled at delivery, BUP dose at delivery, mean daily dose (from enrollment to delivery), mean 3rd trimester daily dose, mean last month daily dose, cumulative dose (from enrollment to delivery), cumulative 3rd trimester dose, and cumulative last month dose. A statistically significant positive correlation was observed between maternal mean daily BUP dose throughout gestation and placenta BUP-Gluc concentration (p=0.029, r=0.86, n=5). See Figure 1. No correlations were found between any measure of maternal BUP dose and BUP, NBUP or NBUP-Gluc placenta concentrations.
Figure 1.
Statistically significant correlations between buprenorphine-glucuronide (BUP-Gluc) concentrations in placenta and maternal buprenorphine dose, and between norbuprenorphine (NBUP) and norbuprenorphine-glucuronide (NBUP-Gluc) concentrations in placenta and neonatal outcomes.
Correlation BUP and metabolite placenta concentrations and neonatal outcomes
Pearson correlation coefficients were calculated for BUP, NBUP, BUP-Gluc, NBUP-Gluc concentrations, total BUP and metabolite concentration, and BUP/NBUP, BUP/BUP-Gluc, and NBUP/NBUP-Gluc ratios in placenta and birth weight (g), head circumference (cm), length (cm), EGA at delivery (weeks), Apgar scores at 1 and 5 min, length of hospital stay (days), peak NAS score, time to peak NAS score (hours), time to NAS onset (hours), duration of NAS (hours), and % NAS scores >4. Since weight, length, head circumference, and gestational age at delivery can be altered by twin status, these correlation analyses were performed without twin data (n=4).
Placenta NBUP-Gluc concentration correlated positively with time to NAS onset (p=0.009, r=0.96, n=6), and negatively with duration of NAS (p=0.042, r=−0.89, n=6). NBUP/NBUP-Gluc ratio correlated positively with maximum NAS score (p=0.023, r=0.87, n=6), and negatively with newborn length (p=0.009, r=−0.99, n=4). See Figure 1. No correlations were found between BUP or BUP-Gluc and neonatal outcomes.
Comparison between NBUP-Gluc and NBUP as predominant analyte groups
The NBUP-Gluc group showed statistically significant higher EGA than the NBUP group (p=0.001). No statistically significant differences were observed between these groups with regard to maternal dosing and time since last dose before delivery.
DISCUSSION
BUP and/or metabolites were detected in all placenta specimens from BUP-maintained pregnant women, with NBUP or NBUP-Gluc as the predominant analyte, followed by BUP-Gluc, and BUP, only in low concentrations. Placenta specimens with NBUP-Gluc as the predominant analyte were from women with a significantly higher EGA at delivery than those with NBUP as the primary analyte (p=0.001), suggesting that glucuronidation may depend upon fetal or placental maturity. In maternal plasma at delivery and in umbilical cord plasma, the distribution of BUP and metabolites was: NBUP-Gluc>BUP-Gluc>NBUP>BUP. BUP and metabolites may just accumulate in placenta, but most likely metabolism occurs as well. Compared to concentrations in maternal plasma, NBUP concentrations are higher in placenta. Similarly, compared to umbilical cord plasma, thought primarily to reflect fetal blood circulation, NBUP concentrations are higher and NBUP-Gluc concentrations are lower in placenta than in umbilical cord plasma. In accordance with Nanovskaya et al 21, these data suggest that the placenta is capable of phase I metabolism, transforming BUP to NBUP, thus, increasing NBUP concentrations in this tissue. Also these data suggest that the fetus is able to metabolize NBUP to NBUP-Gluc (phase II metabolic capability), because NBUP-Gluc concentrations increase and NBUP concentrations decrease in umbilical cord plasma compared to placenta (Table 5). BUP-Gluc concentrations decrease from maternal plasma to placenta and from placenta to umbilical cord plasma, suggesting reduced diffusion of this analyte from the mother to the fetus through the placenta. BUP-Gluc concentrations in placenta could result from direct deposition from maternal blood, or from phase II BUP metabolism in this tissue.
A statistically significant positive correlation was noted between maternal mean daily BUP dose and placental BUP-Gluc concentration. A possible mechanism for this finding could be saturation of phase I BUP metabolism to NBUP in placenta, as BUP dose increases. This might shift BUP metabolism to phase II glucuronidation, yielding higher placental BUP-Gluc concentrations at higher BUP doses. Another possibility is that BUP-Gluc simply accumulates in placenta from maternal blood, increasing concentrations at higher BUP doses.
No significant differences in BUP and metabolite concentrations in different areas of the placenta were observed, except that BUP, present in the lowest concentrations of the 4 analytes (3.2 ng/g maximum), was not detected in some distant locations from the umbilical cord in some specimens. Placenta specimens collected close to the umbilical cord (location 1) were more likely to yield positive BUP results, suggesting that this is the optimal collection location for BUP detection. However, BUP metabolites exhibited homogeneous distribution in the tissue (CV<27.5%), yielding positive results with similar concentrations in any of the evaluated locations.
Comparing BUP biomarkers in placenta and meconium, there were differences in the predominant analyte detected, and in analyte concentrations. In meconium, NBUP concentrations >373.4 ng/g were found in all specimens, whereas in placenta, the main analytes were NBUP and NBUP-Gluc at concentrations between 6 and 26 ng/g. NBUP-Gluc was not detected in 2 of 6 meconium specimens. BUP, identified in all meconium specimens (8.2–110 ng/g), was absent in one placenta, non-detectable in certain placenta locations from 2 subjects, and the highest concentration was only 3.2 ng/g. The physicochemical properties of the biomarkers, placental diffusion and metabolism, and fetal metabolism may influence differential accumulation in these matrices. The enterohepatic circulation of BUP contributes to its accumulation in meconium. Cone et al. reported that free BUP and NBUP were detected in higher concentrations in adult feces than the glucuronides 31.
Cocaine and opiates were detected in both meconium specimens from subject A twins 24, while subject A placenta was only positive for morphine. Meconium specimens contained 122 and 124 ng/g m-OH-benzoylecgonine with cocaine and BE concentrations from 5 to 11 ng/g. A limitation of this investigation was the lack of inclusion of other cocaine metabolites in the placenta method besides BE. Addition of other biomarkers (ecgonine methyl ester, m-OH-benzoylecgonine) may have improved identification of cocaine exposure in placenta specimens, although no data are yet available on cocaine and metabolites concentrations in this alternative matrix. With regard to opiates, morphine concentrations were much higher in meconium (1163 and 1185 ng/g) than in placenta (9 ng/g).
Few published placenta drug data following in utero exposure are available 32–35, with only 3 studies also reporting meconium results 32–34. Alprazolam and its metabolite α-hydroxyalprazolam were detected in meconium and placenta in nearly the same concentrations (7–9 ng/g) in a mother who reported consumption of 1.5-mg/day alprazolam during pregnancy 32. In another case, a mother drinking 1L/day of homemade mate containing 930 mg caffeine and 620 mg theobromine had higher placenta (caffeine 56.9 ng/g, theobromine 45.3 ng/g) than meconium concentrations (caffeine 27.0 ng/g, theobromine 40.1 ng/g) 33. In the third report, 6 women chewed betel nut (arecoline) during pregnancy but no data on amount, timing, and duration of drug exposure were recorded. In these cases, arecoline was detected in all placenta specimens (9–15 ng/g) but only in 4 of 6 meconium specimens albeit at similar concentrations (6–22 ng/g) 34. These 3 case reports showed good concordance between placenta and meconium, whereas our results indicate that meconium identifies drug exposure better than placenta.
In the present study, placenta concentrations were much lower than meconium concentrations for BUP and metabolites (from 15 to 70-fold), for morphine (130-fold), and although cocaine was detected in meconium, it was not identified in placenta. The nature of the specimen is completely different; placenta tissue is dynamic, constantly infused with maternal and fetal blood, and metabolically active whereas meconium is more static, acting as a depot from as early as the 12th week of gestation. Differences in drug accumulation in placenta and meconium are dependent on physicochemical properties, pharmacokinetic factors, placenta diffusion and metabolism, and fetal metabolism. If an analyte is lipophilic and subject to enterohepatic circulation, there will be more accumulation in meconium than placenta. The timing and frequency of drug exposure also are key factors.
Our recent research indicates that meconium primarily reflects in utero drug exposure in the 3rd trimester 23, 36, but there are few data describing the placental window of detection. Lozano et al. 37 suggested that placenta reflects drug exposure for only several hours, but our data document a window of opiate detection of at least 1 week; morphine was quantified in just one placenta from a mother, participant A, whose last opiate positive urine specimen was 7 days prior to delivery (Table 2). Comparing maternal urine drug tests throughout gestation and drugs in placenta demonstrated that high drug consumption in the 3rd trimester is an important factor influencing incorporation of drugs in placenta. For example, participant A had 74% opiate-positive urine specimens and an opiate positive placenta specimen (Table 2). According to urine test results, all mothers, except participant E, relapsed to cocaine during pregnancy, with the last cocaine positive urine 8–82 days prior to delivery. Cocaine and metabolites were not detected in any placenta (n=5). Mother A had 40.7% cocaine-positive urine specimens in the 3rd trimester (Table 2), with the last cocaine positive urine 8 days prior to delivery, suggesting that the window of drug detection in placenta may be less than one week for cocaine, or that a cocaine biomarker other than cocaine and BE could be important for placental identification of cocaine exposure. Additional research with a larger cohort is needed to establish cutoff concentrations and windows of detection for drugs in placenta. Although meconium offers several advantages over placenta including higher drug concentrations and a longer window of drug detection, placenta can be useful in cases where meconium is not available, or for the identification of recent drug consumption. Meconium appears to be a static repository for drugs comsumed primarily in the 3rd trimester, while placenta has a more dynamic role in drug metabolism and protection of the fetus.
To the best of our knowledge only two articles described correlations between BUP and metabolite concentrations in meconium and neonatal outcomes 24, 25, and the present work is the first describing correlations with placenta levels. Marquet et al. 25 reported that the meconium of infants who experienced withdrawal (n=9) tended to have higher BUP concentrations than those who did not (n=6), but no relationship was noted for NBUP. Kacinko et al. 24 observed statistically significant negative correlations between free and percentage of free BUP concentrations in meconium and head circumference in 10 neonates of BUP-maintained women (5 of whom were included in the present study). Also, there was a negative correlation between free BUP/free NBUP ratio and time to NAS onset, and free NBUP% and time to peak NAS score. Total BUP, free BUP/free NBUP ratio, and total BUP/total NBUP ratio were all significantly correlated with the percentage of NAS scores >4 24.
Although the number of placenta specimens (n=5) in this research was limited, statistically significant correlations between BUP metabolite concentrations and neonatal outcomes were observed with high correlation coefficients (r>0.83). NBUP-Gluc concentrations in placenta correlated positively with time to NAS onset, and negatively with duration of NAS, and the NBUP/NBUP-Gluc ratio correlated positively with maximum NAS scores and negatively with the length of the newborn. In this group of pregnant women receiving BUP treatment in similar doses, higher NBUP-Gluc concentrations in placenta predicted better neonatal outcomes, indicating that less BUP reaches the fetus. The placenta may protect the fetus from BUP exposure metabolizing BUP to NBUP, with further transformation to NBUP-Gluc that accumulated in placenta. The maternal-placenta-fetal unit is a complex system, with enzymes, transporters, and a drug’s physicochemical properties modifying biomarker concentrations. Furthermore, heroin and morphine were shown to induce CYP19 activity, 38, while tobacco smoking had no affect 39.
These data require replication due to the small number of specimens. Also, the affect of concurrent cocaine and opiate use on outcome results should be investigated further. However, analysis of placenta, an alternative biological matrix, available at time of birth in adequate amounts, appears to provide valuable data on neonatal outcomes. Treatment of this vulnerable population of in utero exposed infants could be improved if the onset and severity of NAS could be predicted.
Acknowledgments
We would like to thank the participants and their families and the clinical staff at CAP and JHBMC. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), and NIDA grant RO1 DA12220.
References
- 1.Drug Addiction Treatment Act 2000, p. 111, STAT. 1101
- 2.Lejeune C, Simmat-Durand L, Gourarier L, et al. Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Depend. 2006;82:250–7. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G. Treatment of opioid dependence in pregnant women. Addiction. 2000;95:1141–1144. doi: 10.1046/j.1360-0443.2000.95811411.x. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop A, Panjari M, O’Sullivan H, et al. Clinical guidelines for the use of buprenorphine in pregnancy. Fitzroy, Vic: Turning Point Alcohol and Drug Centre; 2003. [Google Scholar]
- 5.Jones HE, Martin PR, Heil SH, et al. Treatment of opioid-dependent pregnant women: clinical and research issues. J Subst Abuse Treat. 2008;35:245–59. doi: 10.1016/j.jsat.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iribarne C, Picart D, Dreano Y, et al. Involvement of cytochrome P450 3A4 in n-dealkylation of buprenorphine in human liver microsomes. Life Sci. 1997;60(22):1953–1964. [Google Scholar]
- 7.King CD, Green MD, Rios GR, et al. The glucuronidation of exogenous and endogenous compounds by stably expressed rat and human UDP-glucuronosyltransferase 1.1. Arch Biochem Biophys. 1996;332:92–100. doi: 10.1006/abbi.1996.0320. [DOI] [PubMed] [Google Scholar]
- 8.Rios G, Tephly T. Inhibition and active sites of UDP-glucuronosyltransferases 2B7 and 1A1. Drug Metab Dispos. 2002;30:1364–7. doi: 10.1124/dmd.30.12.1364. [DOI] [PubMed] [Google Scholar]
- 9.Rouguieg K, Picard N, Sauvage F-L, et al. Contribution of the Different UDP-Glucuronosyltransferase (UGT) Isoforms to Buprenorphine and Norbuprenorphine Metabolism and Relationship with the Main UGT Polymorphisms in a Bank of Human Liver Microsomes. Drug Metab Dispos. 2010;38:40–45. doi: 10.1124/dmd.109.029546. [DOI] [PubMed] [Google Scholar]
- 10.Pasanen M. The expression and regulation of drug metabolism in human placenta. Adv Drug Deliv Rev. 1999;38:81–97. doi: 10.1016/s0169-409x(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh SV, Nanovskaya TN, Ahmed MS. Aromatase is the major enzyme metabolizing buprenorphine in human placenta. J Pharmacol Exp Ther. 2003;306:1099–105. doi: 10.1124/jpet.103.053199. [DOI] [PubMed] [Google Scholar]
- 12.Pasanen M, Pelkonen O. The expression and environmental regulation of P450 enzymes in human placenta. Crit Rev Toxicol. 1994;24:211–229. doi: 10.3109/10408449409021606. [DOI] [PubMed] [Google Scholar]
- 13.Kitawaki J, Inoue S, Tamura T, et al. Increasing aromatase cytochrome P-450 level in human placenta during pregnancy: studied by immunohistochemistry and enzyme-linked immunosorbent assay. Endocrinology. 1992;130:2751–2757. doi: 10.1210/endo.130.5.1572292. [DOI] [PubMed] [Google Scholar]
- 14.Collier AC, Ganley NA, Tingle MD, et al. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol. 2002;63:409–19. doi: 10.1016/s0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- 15.Pelkonen O. Biotransformation of xenobiotics in the fetus. Pharmacol Ther. 1980;10:261–81. doi: 10.1016/0163-7258(80)90083-2. [DOI] [PubMed] [Google Scholar]
- 16.Hakkola J, Pelkonen O, Pasanen M, et al. Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity. Crit Rev Toxicol. 1998;28:35–72. doi: 10.1080/10408449891344173. [DOI] [PubMed] [Google Scholar]
- 17.Lacroix D, Sonnier M, Moncion A, et al. Expression of CYP3A in the human liver--evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247:625–34. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 18.Garland M, Abildskov KM, Taylor S, et al. Fetal morphine metabolism and clearance are constant during late gestation. Drug Metab Dispos. 2006;34:636–46. doi: 10.1124/dmd.105.007567. [DOI] [PubMed] [Google Scholar]
- 19.Schinkel A, Jonker J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 20.Nekhayeva IA, Nanovskaya TN, Hankins GD, et al. Role of human placental efflux transporter P-glycoprotein in the transfer of buprenorphine, levo-alpha-acetylmethadol, and paclitaxel. Am J Perinatol. 2006;23:423–30. doi: 10.1055/s-2006-951301. [DOI] [PubMed] [Google Scholar]
- 21.Nanovskaya T, Deshmukh S, Brooks M, et al. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, et al., editors. Primary Pediatric Care. St. Louis: Mosby Year Book; 1992. pp. 1367–1378. [Google Scholar]
- 23.Kacinko SL, Jones HE, Johnson RE, et al. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin Pharmacol Ther. 2008;84:604–12. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kacinko S, Jones H, Johnson R, et al. Correlations of Maternal Buprenorphine Dose, Buprenorphine, and Metabolite Concentrations in Meconium with Neonatal Outcomes. Clin Pharmacol Ther. 2008 doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquet P, Lavignasse P, Gaulier J, et al. Case study of neonates born to mothers undergoing buprenorphine maintenance treatment. In: Kintz P, Marquet P, editors. Buprenorphine Therapy of Opiate Addiction. Totowa, New Jersey: Humana Press; 2002. pp. 125–135. [Google Scholar]
- 26.Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Signorini DF, Leung O, Simes RJ, et al. Dynamic balanced randomization for clinical trials. Stat Med. 1993;12:2343–2350. doi: 10.1002/sim.4780122410. [DOI] [PubMed] [Google Scholar]
- 28.Mechoulam R, Peters M, Murillo-Rodriguez E, et al. Cannabidiol--recent advances. Chem Biodivers. 2007;4:1678–92. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- 29.de Castro A, Concheiro M, Shakleya D, et al. Simultaneous Quantification of Methadone, Cocaine, Opiates, and Metabolites in Human Placenta by Liquid Chromatography-Mass Spectrometry. J Anal Toxicol. 2009;33:243–252. doi: 10.1093/jat/33.5.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Concheiro M, Jones H, Johnson RE, et al. Confirmatory Analysis of Buprenorphine, Norbuprenorphine, and Glucuronide Metabolites in Plasma by LCMSMS. Application to Umbilical Cord Plasma from Buprenorphine-maintained Pregnant Women. J Chromatogr B. 2009 doi: 10.1016/j.jchromb.2009.11.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12:577–581. [PubMed] [Google Scholar]
- 32.Garcia-Algar O, Lopez-Vilchez MA, Martin I, et al. Confirmation of gestational exposure to alprazolam by analysis of biological matrices in a newborn with neonatal sepsis. Clin Toxicol (Phila) 2007;45:295–8. doi: 10.1080/15563650601072191. [DOI] [PubMed] [Google Scholar]
- 33.Martin I, Lopez-Vilchez MA, Mur A, et al. Neonatal withdrawal syndrome after chronic maternal drinking of mate. Ther Drug Monit. 2007;29:127–9. doi: 10.1097/FTD.0b013e31803257ed. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Algar O, Vall O, Alameda F, et al. Prenatal exposure to arecoline (areca nut alkaloid) and birth outcomes. Arch Dis Child Fetal Neonatal Ed. 2005;90:F276–f277. doi: 10.1136/adc.2004.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luck W, Nau H, Hansen R, et al. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- 36.Casanova OQ, Lombardero N, Behnke M, et al. Detection of cocaine exposure in the neonate. Analyses of urine, meconium, and amniotic fluid from mothers and infants exposed to cocaine. Arch Pathol Lab Med. 1994;118:988–993. [PubMed] [Google Scholar]
- 37.Lozano J, Garcia-Algar O, Vall O, et al. Biological matrices for the evaluation of in utero exposure to drugs of abuse. Ther Drug Monit. 2007;29:711–34. doi: 10.1097/FTD.0b013e31815c14ce. [DOI] [PubMed] [Google Scholar]
- 38.Zharikova O, Deshmukh S, Kumar M, et al. The effect of opiates on the activity of human placental aromatase/CYP19. Biochem Pharmacol. 2007;73:279–286. doi: 10.1016/j.bcp.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DR, Koymans L, Kamataki T, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]