Abstract
Background
Epidemiological and controlled intervention trials suggest that omega-3 (n-3) fatty acid deficiency represents a reversible risk factor for recurrent affective disorders. However, there is limited comparative information available regarding the n-3 fatty acid status and associated mood symptoms in medication-free patients with major depressive disorder (MDD) and bipolar disorder (BD).
Methods
The fatty acid composition of erythrocyte membranes from adult male and female healthy controls (n=20) and medication-free patients with MDD (n=20) and BD (n=20) was determined by gas chromatography. Associations with depression and mania symptom severity scores were determined.
Results
After correction for multiple comparisons, both MDD (-20%) and BD (-32%) patients exhibited significantly lower erythrocyte docosahexaenoic acid (DHA, 22:6n-3) composition relative to healthy controls, and there was a trend for lower DHA in BD patients relative to MDD patients (-15%, p=0.09). There were no gender differences for DHA in any group. Other n-3 fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3) and docosapentanoic acid (22:5n-3), and n-6 fatty acids, including arachidonic acid (AA, 20:4n-6), were not different. Erythrocyte DHA composition was inversely correlated with indices of delta-9 desaturase activity (18:1/18:0), and associated elevations in oleic acid (18:1n-9) composition, and delta-6 desaturase activity (20:3/18:2). DHA composition was not significantly correlated with depression or mania symptom severity scores.
Limitations
Data regarding diet and life style factors (cigarette smoking) were not available to evaluate their contribution to the present findings.
Conclusions
Male and female patients with MDD and BD exhibit selective erythrocyte DHA deficits relative to healthy controls, and this deficit was numerically greater in BD patients. Selective DHA deficits are consistent with impaired peroxisome function, which has implications for n-3 fatty acid interventions aimed at preventing or reversing this deficit.
Keywords: Bipolar disorder, Major depressive disorder, Erythrocyte, Docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA), Arachidonic acid (AA), Fatty acid
1. Introduction
Evidence from cross-national and cross-sectional epidemiological surveys suggest that greater habitual dietary omega-3 (n-3) fatty acid intake from fish/seafood, principally eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), is associated with reduced prevalence rates for major depressive disorder (MDD), particularly among women (Hibbeln, 1998, 2002; Peet, 2004; Raeder et al., 2007; Tanskanen et al., 2001; Timonen et al., 2004), and bipolar disorders (BD) (Noaghiul & Hibbeln, 2003). Prospective longitudinal studies have found that baseline n-3 fatty acid intake is associated with reduced adjusted risk for emergent depressive symptoms (Golding et al., 2009; Kamphuis et al., 2006; Rees et al., 2009). In one of the largest prospective studies conducted to date, greater EPA and DHA intake from fish at baseline was associated with a lower adjusted risk for depression at 10 years among 3,317 young adults (mean age: 32 years) residing in the U.S, and this inverse association was stronger in women (Colangelo et al., 2009). Additionally, independent meta-analyses have also found a significant advantage of EPA+DHA supplementation over placebo for reducing depression symptom severity (Freeman et al., 2006; Lin & Su, 2007). These data suggest that n-3 fatty acid deficiency may represent a preventable and reversible risk factor for recurrent mood disorders.
Because erythrocyte EPA+DHA composition is positively correlated with habitual dietary EPA+DHA intake (Cao et al., 2006; Sands et al., 2005; Itomura et al., 2008), it represents a peripheral biomarker of n-3 fatty acid status. Prior case-control studies have observed significant erythrocyte or plasma EPA and/or DHA deficits in patients with MDD (Maes et al., 1996, 1999; Peet et al., 1998; Edwards et al., 1998; Tiemeier et al., 2003) and BD (Chiu et al., 2003; Ranjekar et al., 2003) relative to healthy subjects residing in western European and Asian countries. Only one case-control study has been conducted in the U.S., and found no significant difference in plasma free or esterified DHA or EPA in a small sample (n=10) of medication-free manic patients (Sublette et al., 2009). Some studies have observed an inverse correlation between DHA and/or EPA, and positive correlations between plasma n-6:n-3 ratios, principally AA:EPA, and measures of depression and/or manic symptom severity (Adams et al., 1996; Edwards et al., 1998; Conklin et al., 2007; Maes et al., 1996; Sublette et al., 2009). However, there have been no studies directly comparing MDD and BD patients to identify potential distinguishing features in erythrocyte fatty acid composition and associated mood symptoms to evaluate the diagnostic utility of this biomarker.
In the present study, we determined erythrocyte fatty acid composition in healthy adult male and female controls without a history of psychiatric illness (n=20) and medication-free patients with MDD (n=20) or BD (n=20). We used medication-free patients because preclinical evidence suggests that antidepressant and mood-stabilizer medications alter membrane fatty acid turnover and/or biosynthesis (Qu et al., 2006; Raeder et al., 2006), and may confound associations between fatty acid status and symptom severity scores. We additionally evaluated the effects of gender because gonadal hormones have a significant influence on n-3 fatty acid biosynthesis and erythrocyte composition (Bakewell et al., 2006; Giltay et al., 2004; McNamara et al., 2009; Pawlosky et al., 2003), and we have observed gender differences in postmortem cortical DHA deficits in patients with MDD (female > male)(McNamara et al., 2007). Additionally, we evaluated the relationship between erythrocyte fatty acid compositions and depression and manic symptom severity scores on the Hamilton Depression Rating Scale (HDRS, Hamilton, 1967) and the Clinician-Administered Rating Scale for Mania (CARS-M, Altman et al., 1994), respectively. Based on the evidence reviewed above, our primary hypothesis was that erythrocyte DHA composition would be significantly lower in MDD and BD patients relative to age-matched healthy controls, and inversely correlated with depression symptom severity scores.
2. Method
2.1. Subject demographics
These studies were conducted in hospitalized patients with BD (n=20) and MDD (n=20) admitted to the Psychiatric Clinical Research Center, as part of the General Clinical Research Center (GCRC), University of Illinois at Chicago. This study was approved by the Institutional Review Board of the University of Illinois at Chicago. Healthy adult male and female controls with no history of psychiatric illness (n=20) were recruited from the greater Chicago area. Patients were kept drug-free up to 2-weeks prior to blood collection to permit sufficient washout for the majority of antidepressant and mood-stabilizer medications. Although some patients were receiving fluoxetine, and norfluoxetine has a long elimination half-life (7-15 days, Altamura et al., 1994), we have previously found that chronic treatment with fluoxetine resulting in clinically-relevant plasma fluoxetine and norfluoxetine concentrations did not alter rat erythrocyte fatty acid composition (Able et al., 2009). A comparison of group demographic variables is presented in Table 1. Date regarding smoking status and diet were not obtained. There were no significant group differences in age, F(2,54)=1.3, p=0.28. Mean depression (HDRS) and manic (CARS-M) symptom severity scores are presented.
Table 1. Subject demographics.
| Variable | HC | MDD | BD |
|---|---|---|---|
| Age (years), mean ± SD | 36.2 ± 8.9 | 34.6 ± 11.0 | 30.7 ± 11.7 |
| Gender | 10M, 10F | 6M, 11F | 12M, 6F |
| Race | 3AS, 7AA, 1H, 9C | 8AA, 9C | 6AA, 12C |
| HDRS | - | 26.4 ± 7.9 | 17.6 ± 13.3 |
| CARS-M | - | 5.0 ± 4.7 | 15.0 ± 12.3 |
2.2. Erythrocyte fatty acid composition
Whole venous blood (40 ml) was collected into tubes containing 4 ml of sodium citrate (3.8%), and centrifuged at 210 ×g for 15 min at 4°C. Plasma and the platelet rich interface were removed, and erythrocytes washed twice with 0.9% saline and stored at -80°C. Erythrocyte membrane total fatty acid composition was determined with a Shimadzu GC-2014 (Shimadzu Scientific Instruments Inc., Columbia MD) using the saponification and methylation procedure described previously (Mecalfe et al., 1966). Analysis of fatty acid methyl esters was based on area under the curve calculated with Shimadzu Class VP 4.3 software. Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). To avoid potential variability associated with the manual addition of an internal standard, we did not calculate total mass (nmol/g). However, we have previously found that weight percent data and total mass (nmol/g) data are highly correlated using the direct saponification method (McNamara et al., 2008). All samples were processed by a technician blinded to group identity.
2.3. Statistical analysis
Analyses of variance (ANOVA) were performed using GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD). Pending a significant main effect of group (HC, MDD, BD) after Bonferroni correction for multiple comparisons (α=0.05/17 fatty acids and ratios = 0.003), Bonferroni corrected post-hoc tests (2-tailed) were performed to evaluate individual group differences. Gender × group interactions were evaluated with a two-factor ANOVA using Illness (HC, MDD, BD) and Gender (male, female) as the main factors. Parametric linear regression analyses were performed to determine the interrelationship between erythrocyte fatty acid composition and relevant fatty acids ratios and depression (HDRS) and mania (CARS-M) symptom severity scores, and Bonferroni adjusted for multiple comparisons (α=0.05/16 comparisons = 0.003).
3. Results
3.1. Fatty acid composition
Erythrocyte samples from three MDD patients and two BD patients were excluded from the final analyses because of evidence for gross abnormalities in membrane fatty acid composition, including the absence of DHA. Therefore, the analyses were performed on n=20 healthy controls, n=17 MDD patients, and n=18 BD patients. After correction for multiple comparisons, significant main effects of group were observed for DHA (22:6n-3), F(2,54)=11.6, p≤0.0001, oleic acid (18:1n-9), F(2,54)=7.6, p=0.001, and vaccenic acid (18:1n-7), F(2,54)=8.5, p=0.0006 (Table 2). Pairwise comparisons found that both MDD (-20%, p=0.006) and BD (-32%, p≤0.0001) patients exhibited significantly lower DHA composition compared with controls. Both MDD (+8%, p=0.0018) and BD (+14%, p=0.0013) patients also exhibited significantly greater vaccenic acid composition compared with controls, and BD patients exhibited significantly greater oleic acid composition compared with controls (+10%, p=0.0004). A significant main effect of group was also observed for EPA+DHA, F(2,54)=11.1, p≤0.0001, and both MDD (-21%, p=0.006) and BD (-31%, p≤0.0001) patients exhibited significantly lower EPA+DHA composition compared with controls. Significant main effects of group were also observed for AA:DHA, F(2,54)=11.6, p≤0.0001, AA:EPA+DHA, F(2,54)=10.3, p=0.0002, and total n-3 (20:5+22:5+22:6)/total n-6 (18:2+20:3+20:4+22:4), F(2,54)=9.6, p=0.0003, ratios. Only BD patients exhibited significantly greater AA:DHA (+35%, p≤0.0001), AA:EPA+DHA (+34%, p≤0.0001), and n-6/n-3 (+23%, p=0.0003) ratios relative to healthy controls. Although there was a trend for greater DHA deficits (-9%, p=0.09) and elevations in the AA:DHA (+19%, p=0.04) and AA:EPA+DHA (+18%, p=0.05) ratios in BD patients relative to MDD patients, these were not statistically significant after correction for multiple comparisons.
Table 2. Erythrocyte fatty acid composition.
| Fatty acid1 | HC | MDD | BD | F2 | HC vs. MDD | HC vs. BD | MDD vs. BD |
|---|---|---|---|---|---|---|---|
| Saturated fatty acids | |||||||
| Palmitic acid (16:0) | 16.9 ± 0.2 | 17.7 ± 0.3 | 17.6 ± 0.4 | 0.158 | 0.03 | 0.12 | 0.89 |
| Stearic acid (18:0) | 16.4 ± 0.3 | 17.0 ± 0.6 | 16.2 ± 0.3 | 0.331 | 0.32 | 0.61 | 0.21 |
| Monounsatutated fatty acids | |||||||
| Oleic acid (18:1n-9) | 11.9 ± 0.2 | 13.0 ± 0.2 | 13.2 ± 0.3 | 0.001 | 0.0018 | 0.0013 | 0.59 |
| Vaccenic acid (18:1n-7) | 1.2 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.0 | 0.0006 | 0.012 | 0.0004 | 0.12 |
| Polyunsaturated fatty acids | |||||||
| Linoleic acid (18:2n-6) | 10.9 ± 0.3 | 9.5 ± 0.3 | 10.0 ± 0.4 | 0.018 | 0.0014 | 0.10 | 0.32 |
| Homo-gamma-linoleic (20:3n-6) | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 0.1 | 0.065 | 0.71 | 0.02 | 0.11 |
| Arachidonic acid (AA, 20:4n-6) | 16.9 ± 0.3 | 16.2 ± 0.6 | 17.0 ± 0.4 | 0.417 | 0.31 | 0.87 | 0.29 |
| Docosatetraenoic acid (22:4n -6) | 4.0 ± 0.1 | 3.8 ± 0.2 | 4.1 ± 0.1 | 0.597 | 0.46 | 0.85 | 0.38 |
| Docosapentaenoic acid (22:5n-6) | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.503 | 0.45 | 0.23 | 0.78 |
| Eicoapentaenoic acid (EPA, 20:5n-3) | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.095 | 0.09 | 0.07 | 0.83 |
| Docosapentaenoic acid (22:5n-3) | 2.3 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 0.251 | 0.11 | 0.23 | 0.66 |
| Docosahexaenoic acid (DHA, 22:6n-3) | 4.4 ± 0.2 | 3.5 ± 0.2 | 3.0 ± 0.2 | 0.0001 | 0.007 | 0.0001 | 0.09 |
| EPA+DHA (Omega-3 index) | 4.8 ± 0.3 | 3.8 ± 0.2 | 3.3 ± 0.2 | 0.0001 | 0.006 | 0.0001 | 0.12 |
| n-6/n-3 ratios | |||||||
| AA:DHA | 4.0 ± 0.2 | 5.0 ± 0.4 | 6.2 ± 0.4 | 0.0001 | 0.02 | 0.0001 | 0.04 |
| AA:EPA | 46.5 ± 5.2 | 55.1 ± 5.0 | 59.2 ± 5.0 | 0.196 | 0.24 | 0.09 | 0.58 |
| AA:EPA+DHA | 3.7 ± 0.2 | 4.6 ± 0.3 | 5.6 ± 0.3 | 0.0002 | 0.03 | 0.0001 | 0.05 |
| n-6/n-3 | 7.1 ± 0.3 | 8.5 ± 0.8 | 9.2 ± 0.4 | 0.0003 | 0.08 | 0.0003 | 0.46 |
Weight percent total fatty acids (g/100 g) expressed as mean ± S.E.M.
P-values form the one-way ANOVA.
3.2. Product:precursor ratios
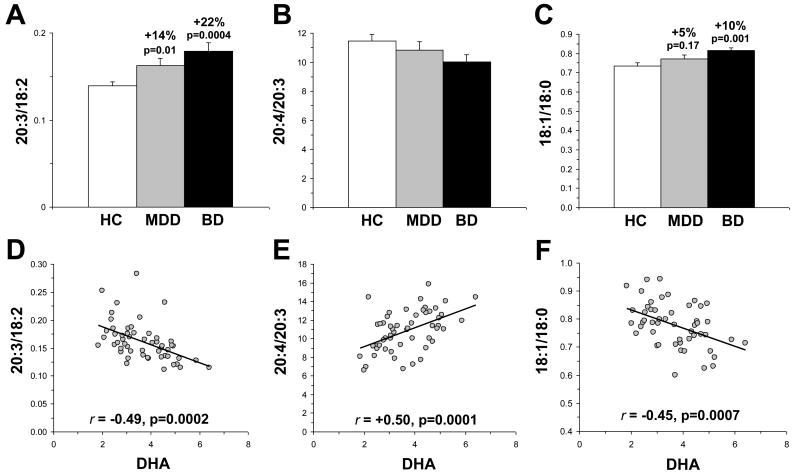
For the 20:3/18:2 ratio (an index of delta-6 desaturase activity), there was a significant main effect of group, F(2,54)=7.5, p=0.0014. Both MDD (+15%, p=0.01) and BD (+22%, p=0.0004) patients exhibited a greater 20:3/18:2 ratio relative to controls, and this ratio did not differ significantly between MDD and BD patients (p=0.21)(Fig. 1A). The main effect of group was not significant for either the 20:4/20:3 ratio (an index of delta-5 desaturase activity), F(2,54)=2.2, p=0.11 (Fig. 1B) or the 22:4/20:4 ratio (an index of elongase activity), F(2,54)=0.03, p=0.97. For the 18:1/18:0 ratio (an index of delta-9 desaturase activity), there was a significant main effect of group, F(2,54)=5.4, p=0.007 (Fig. 1C). BD patients (+10%, p=0.001), but not MDD patients (p=0.17), exhibited a greater 18:1/18:0 ratio relative to controls, and there was a trend for a greater ratio in BD patients relative to MDD patients (+5%, p=0.09). For the 22:6/22:5 ratio, there was a significant main effect of group, F(2,54)=7.3, p=0.0016. Both MDD (-15%, p=0.04) and BD (-28%, p=0.0006) patients exhibited a smaller 22:6/22:5 ratio relative to controls, and this ratio did not differ significantly between MDD and BD patients (p=0.10). Among all subjects (n=55), DHA was inversely correlated with the 20:3/18:2 ratio, r = -0.49, p=0.0002 (Fig. 1D), and positively correlated with the 20:4/20:3 ratio, r = +0.50, p=0.0001 (Fig. 1E). DHA was not correlated with the 22:4/20:4 ratio, r = +0.01, p=0.93. Erythrocyte DHA was inversely correlated with the 18:1/18:0 ratio, r = -0.45, p=0.0007 (Fig. 1F).
Figure 1.
Comparison of the 20:3/18:2 ratio (A), an index of delta-6 desaturase activity, and the 20:4/20:3 ratio (B), an index of delta-5 desaturase activity, and the 18:1/18:0 ratio, an index of delta-9 desaturase activity (C), in healthy controls (HC) and patients with MDD or BD. Correlations between erythrocyte DHA composition and the 20:3/18:2 ratio (D), the 20:4/20:3 ratio (E), and the 18:1/18:0 ratio (F) among all subjects (n=55). Percent difference from controls and associated p-values (two-tailed) are presented in A-C, and Pearson correlation coefficients and p-values (two-tailed) are presented in D-F. Values are group mean ± S.E.M.
3.3. Gender and age
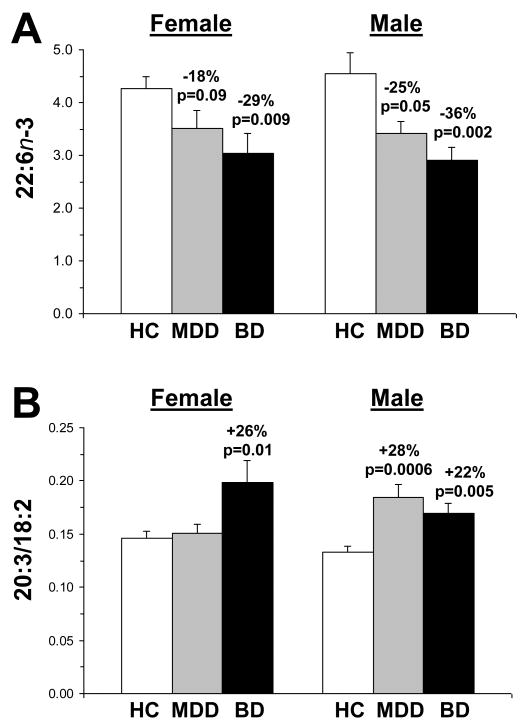
For n-3 fatty acids, there were no significant Gender × group interactions for DHA (22:6n-3)(Fig. 2A), F(2,54)=0.23, p=0.79, EPA (20:5n-3), F(2,54)=1.0, p=0.36, EPA+DHA, F(2,54)=0.31, p=0.73, DPA (22:5n-3), F(2,54)=0.95, p=0.39, or total n-3, F(2,54)=0.30, p=0.74. For n-6 fatty acids, there were no significant Gender × group interactions for linoleic acid (18:2n-6), F(2,54)=0.29, p=0.75, whereas trends were found for 20:3n-6, F(2,54)=2.1, p=0.056, and 20:4n-6, F(2,54)=3.9, p=0.02. The Gender × group interaction was significant for the 20:3/18:2 ratio, F(2,54)=5.1, p=0.009 (Fig. 2B). Specifically, female BD patients exhibited a greater 20:3/18:2 ratio relative to both female controls (+26%, p=0.01) and female MDD patients (+24%, p=0.02), but not male BD patients (p=0.16). Male MDD patients exhibited a greater 20:3/18:2 ratio relative to female MDD patients (+18%, p=0.03). Among all subjects (n=55), age was not significantly correlated with individual n-3 fatty acid compositions, including DHA, r = +0.15, p=0.26, individual n-6 fatty acids, individual monounsaturated fatty acids, or individual saturated fatty acids.
Figure 2.
(A) Comparison of erythrocyte DHA (22:6n-3) composition in male and female healthy controls (HC) and patients with MDD or BD. Note that there are no gender differences in erythrocyte DHA composition in any group. (B) Comparison of the 20:3/18:2 ratio in male and female healthy controls (HC) and patients with MDD or BD. Note that unlike female patients with BD, female patients with MDD do not exhibit a greater 20:3/18:2 ratio relative to female controls. Percent difference from controls and associated p-values (two-tailed) are presented. Values are group mean ± S.E.M.
3.4. Symptom severity
The relationship between fatty acid compositions and ratios and depression (HDRS) and manic (CARS-M) symptom severity scores were determined by linear regression analysis (Table 3). After correcting for multiple comparisons, there were no significant correlations between relevant fatty acid compositions, including DHA, and depression or mania symptom severity scores.
Table 3. Correlations with HDRS and CARSM scores.
| MDD | BD | |||
|---|---|---|---|---|
| r | p | r | p | |
| HDRS | ||||
| 22:6n-3 (DHA) | 0.04 | 0.89 | -0.31 | 0.22 |
| 20:5n-3 (EPA) | -0.11 | 0.69 | 0.09 | 0.71 |
| EPA+DHA | 0.02 | 0.94 | -0.26 | 0.31 |
| 18:2n-6 | 0.26 | 0.38 | -0.22 | 0.40 |
| 20:3/18:2 | -0.21 | 0.47 | 0.43 | 0.09 |
| 20:4n-6 (AA) | -0.38 | 0.19 | 0.37 | 0.15 |
| AA:EPA | -0.13 | 0.67 | 0.03 | 0.93 |
| AA:EPA+DHA | -0.12 | 0.69 | 0.46 | 0.06 |
| CARSM | ||||
| 22:6n-3 (DHA) | 0.58 | 0.03 | 0.14 | 0.62 |
| 20:5n-3 (EPA) | -0.06 | 0.83 | -0.23 | 0.41 |
| EPA+DHA | 0.54 | 0.04 | 0.09 | 0.74 |
| 18:2n-6 | 0.09 | 0.74 | 0.41 | 0.13 |
| 20:3/18:2 | 0.07 | 0.80 | -0.64 | 0.009 |
| 20:4n-6 (AA) | -0.03 | 0.91 | -0.12 | 0.66 |
| AA:EPA | 0.29 | 0.33 | 0.14 | 0.62 |
| AA:EPA+DHA | -0.56 | 0.04 | -0.13 | 0.65 |
4. Discussion
The main finding of the present study is that medication-free MDD and BD patients exhibit selective erythrocyte DHA deficits compared with demographically similar healthy controls residing in the U.S. The deficit was specific to DHA, as there were no significant deficits observed for other principal n-3 fatty acids including EPA and DPA or n-6 fatty acids including AA. Although we did not observe significant differences between MDD and BD patients for any fatty acid, there were clear trends for greater DHA deficits, and a larger AA:DHA ratio, in BD patients compared with MDD patients. There were no significant gender differences in erythrocyte DHA composition in controls or patients with MDD or BD, and erythrocyte DHA composition was not correlated with age. Erythrocyte DHA composition was not significantly correlated with depression or mania symptom severity. The magnitude of erythrocyte DHA deficits observed in MDD (-20%) and BD (-32%) patients are comparable to those previously observed in patients residing in Asia and western Europe (Chiu et al., 2003; Edwards et al., 1998; Peet et al., 1998; Ranjekar et al., 2003), thus providing cross-national evidence for erythrocyte DHA deficits in MDD and BD. Moreover, the present finding is also consistent with our prior finding of DHA deficits in the postmortem PFC (BA10) gray matter of patients with MDD and BD (McNamara et al., 2007, 2008).
Prior studies have observed inverse correlations between DHA and/or EPA, and positive correlations between plasma n-6:n-3 ratios, principally AA:EPA, and measures of depression and/or manic symptom severity (Adams et al., 1996; Edwards et al., 1998; Conklin et al., 2007; Maes et al., 1996; Sublette et al., 2009). In the present study, neither DHA nor the AA:EPA ratio were significantly correlated with either depression or mania symptom severity scores. However, healthy subjects commonly exhibit low scores on both depression and mania scales, indicating that erythrocyte DHA levels are ultimately inversely correlated with depression and mania symptom severity. Furthermore, although these patients were drug-free up to 2-weeks prior to blood collection, carry over effects of medications on both mood and erythrocyte fatty acid composition cannot be ruled out. Nevertheless, because erythrocyte membrane-esterified DHA composition has a long half life, free plasma fatty acid levels may be more sensitive to acute and more subtle variations in mood symptom severity (Sublette et al., 2009).
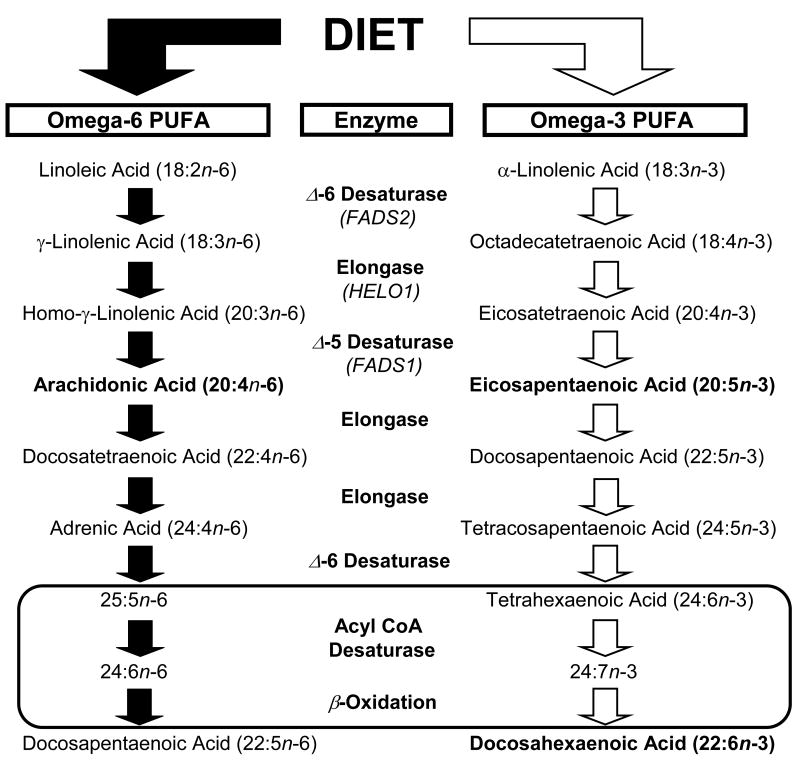
Selective reductions in dietary n-3 fatty acid intake are associated with elevations in delta-6 (FADS2) and delta-5 (FADS1) desaturase gene expression and activity in rat liver, and elevations in n-6 fatty acid biosynthesis and DPA (22:6n-6) tissue composition (Igarashi et al., 2008). In the present study, the erythrocyte 20:3/18:2 ratio, an index of delta-6 desaturase activity (Harmon et al., 2003; Obukowicz et al., 1998), was significantly elevated in patients with MDD and BD, and inversely correlated with erythrocyte DHA. Moreover, the erythrocyte 20:4/20:3 ratio, an index of delta-5 desaturase activity, and the 202:4/20:4 ratio, an index of elongase activity, did not differ between groups. Because delta-6 desaturase is the rate-limiting enzyme in the biosynthesis of n-3 and n-6 fatty acids from alpha-linolenic acid (ALA, 18:3n-3) and linoleic acid (18:2n-6), respectively (Fig. 3), pharmacological inhibition of delta-6 desaturase results in significant reductions of both DHA and AA (Harmon et al., 2003; Obukowicz et al., 1998). In the present study, however, erythrocyte AA and the down-stream n-3 fatty acid metabolites of ALA, EPA (20:5n-3) and DPA (22:5n-3), were not significantly reduced in MDD and BD patients. These data suggest that the DHA deficits observed in MDD and BD patients cannot be wholly attributed to reductions in desaturase or elongase activity. Indeed, the selective DHA deficit, reduction in the DHA/DPA (22:5n-3) ratio, and lack of a reciprocal increase in 22:5n-6 in MDD and BD patients is consistent with a defect in peroxisome function (Sprecher & Chen, 1999). This is supported by the finding that peroxisomal biogenesis disorders are also associated with erythrocyte DHA deficits and no corresponding elevation in 22:5n-6, and normal levels of n-3 fatty acid precursors of DHA (Martinez, 1994; Moser et al., 1999).
Figure 3.
Diagram illustrating the biosynthetic pathways of omega-6 and omega-3 PUFAs from dietary precursors linolenic acid (LA, 18:2n-6) and alpha-linolenic acid (ALA, 18:3n-3), respectively. The biosynthesis of long-chain fatty acid products of LA and ALA requires a series of common microsomal elongation and delta (Δ) 5- and 6-desaturase-mediated reactions, and the final synthesis of DHA and DPA (22:5n-6) requires additional enzymatic modifications within peroxisomes. It is proposed that a defect in peroxisomal function could account for the selective DHA deficits, normal levels of n-3 fatty acid precursors of DHA including EPA (20:5n-3) and DPA (22:5n-3), and lack of reciprocal elevations in 22:5n-6 in patients with MDD and BD.
Delta-9 desaturase (stearoyl-CoA desaturase, SCD) mediates the biosynthesis of oleic acid (18:1) from stearic acid (18:0) (Ntambi & Miyazaki, 2004), and oleic acid is a major component of triglycerides (Attie et al., 2002). SCD gene expression is repressed by n-3 fatty acids at the level of transcription and mRNA stability (Ntambi et al., 1999), and is up-regulated in the rat liver in response to moderate and selective reductions in n-3 fatty acids (Igarashi et al., 2008). In the present study, we observed an inverse correlation between erythrocyte DHA and EPA+DHA and the 18:1/18:0 ratio, and this ratio and oleic acid composition were significantly elevated in BD patients relative to controls. Prior studies have found that the 18:1/18:0 ratio is positively correlated with triglyceride levels (Attie et al., 2002), and that regular consumption of fish (Harris, 1989) or preformed EPA+DHA (McKenney & Sica, 2007) are associated with a 20-40% reduction in plasma triglyceride levels. Although we did not determine triglyceride levels in the present study, the present findings may therefore have mechanistic implications for the significantly elevated triglyceride levels observed in BD patients (Fiedorowicz et al., 2008; Sicras et al., 2008).
There is now substantial evidence that lower erythrocyte EPA+DHA composition (the ‘omega-3 index’) is a preventable risk factor for coronary heart disease (Harris, 2008), and emerging findings suggest that erythrocyte EPA+DHA deficiency may contribute to the elevated rate of coronary heart disease comorbidity in MDD (McNamara, 2009). Based on data from prospective longitudinal studies investigating sudden cardiac death as an endpoint, erythrocyte EPA+DHA composition of ≤4% was determined to be associated with a 10-fold greater risk for sudden cardiac death compared with >8% (Harris & von Schacky, 2004). In the present study, erythrocyte EPA+DHA composition in healthy controls (4.8%±1.1 %) was similar to that previously observed in a larger cohort of healthy subjects residing in the U.S. (4.9%±2.1%, Sands et al., 2005), and approximately one-half that observed in healthy Japanese adults (8.5±1.8%, Itomura et al., 2008). Furthermore, 65% of MDD patients and 78% of BD patients in the present study exhibited an erythrocyte EPA+DHA composition of ≤4.0%, compared with 25% of controls. Moreover, the mean erythrocyte EPA+DHA composition observed in MDD (3.8%±1.6%) and BD (3.3%±1.6%) patients resemble that observed in patients with acute coronary syndrome (3.4%±1.6%; Block et al., 2008). These data therefore suggest that the majority of MDD and BD patients in the present study are at high risk for sudden cardiac death.
Prior preclinical evidence suggests that mood-stabilizer medications reduce AA turnover in rat brain (Rapoport et al., 2009), and we reported that AA deficits found in the postmortem PFC of medication-free BD patients were partially normalized in patients treated with mood-stabilizer medications (McNamara et al., 2008). Furthermore, chronic lithium treatment, resulting therapeutically-relevant plasma concentrations, was found to significantly increased rodent erythrocyte membrane AA composition (McNamara et al., 2006). In the present study, we did not observe significant alterations in erythrocyte AA composition in medication-free BD patients, which is consistent with prior case-control studies (Ranjekar et al., 2003; Sublette et al., 2007). However, another case-control study found that medicated BD patients exhibit significant deficits in erythrocyte AA (Chiu et al., 2003). In view of these conflicting findings, and the confounding influence of mood-stabilizer medications on erythrocyte AA composition, future studies investigating erythrocyte AA composition in medication-naïve first-episode manic patients are warranted.
4.1. Implications
These findings corroborate prior case-control studies finding deficits in erythrocyte DHA composition in MDD and BD patients, and therefore provide additional evidence implicating n-3 fatty acid deficiency in the pathophysiology of recurrent affective disorders. Erythrocyte DHA composition is therefore a robust and reliable biomarker of n-3 fatty acid deficiency in patients with mood disorders. In view of the recent finding that erythrocyte DHA composition is positively correlated with functional activity in the PFC (McNamara et al., 2010), the present findings may additionally have implications for cerebral hypoperfusion observed in patients with mood disorders (Ito et al., 1996). The present data also suggest that a defect in peroxisome function may contribute to erythrocyte DHA deficits in MDD and BD. If true, this would have important implications for n-3 fatty acid interventions aimed at reversing this deficit. Specifically, preformed DHA, rather than pre-peroxisome precursors of DHA including EPA, would be required. This is supported by the findings that preformed DHA corrects erythrocyte and cortical DHA deficits in patients with peroxisomal biogenesis disorders (Martinez et al., 2000; Moser et al., 1999). Moreover, this may explain in part why supplementation with DHA in addition to EPA (Stoll et al., 1999), but not EPA alone (Keck et al., 2006), is efficacious in the treatment of mood symptoms in BD patients. Additionally, increasing erythrocyte DHA to levels observed in the Japanese population (6.8%, Itomura et al., 2008), where the lifetime prevalence rates if MDD and BD are among the lowest in the world, may represent an appropriate target for future primary and secondary prevention trials (McNamara, 2009).
4.2. Limitations
This study has two primary limitations. First, data regarding habitual dietary n-3 fatty acid intake was not obtained to evaluate its contribution to the observed erythrocyte DHA deficits. However, as discussed above, the precursors of DHA, EPA and DPA, were not significantly altered in MDD or BD patients, suggesting comparable dietary intake. Second, data regarding cigarette smoking status was not obtained, and prior studies have observed an effect of cigarette smoking on erythrocyte DHA composition (Hibbeln et al., 2003). However, other studies in healthy adults (Sands et al., 2005) and BD patients (Chiu et al., 2003) have not observed an association between cigarette smoking and erythrocyte DHA composition.
4.3. Conclusions
This study demonstrates that young adult patients with MDD or BD exhibit significant and selective deficits in erythrocyte DHA composition relative to healthy controls residing in the U.S. These data are consistent with the findings of fatty acid studies conducted in other countries, and add to a growing body of evidence implicating n-3 fatty acid deficiency in the pathophysiology and potentially pathogenesis of recurrent affective disorders. The present data also suggest that erythrocyte DHA deficits, and associated elevations in delta-6 and delta-9 desaturase activity, are greater in BD patients than MDD patients. The present data additionally suggest that MDD and BD may be associated with a defect in peroxisomal function, which has implications for n-3 fatty acid interventions aimed at preventing or reversing this deficit.
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIMH Grant MH083924 and MH074858 to R.K.M, and MH56528 to G.N.P, and DK59630 to P.T.; the NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest: No conflict declared.
Contributors. R. McNamara designed the study and wrote the manuscript. R. Jandacek, T. Rider, and P. Tso performed the gas chromatography. Y. Dwivedi and G. Pandey obtained and supplied the biological samples. All authors contributed to and have approved the final manuscript.
Conflict of Interest Statement. None of the authors have any actual or potential conflict of interest, including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence, or be perceived to influence, their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Able J, Jandacek R, Rider T, Tso P, McNamara RK. Chronic treatment with risperidone, but not fluoxetine, preferentially increases omega-3 fatty acid composition in rat erythrocytes and prefrontal cortex: Evidence for augmented biosynthesis. Biological Psychiatry. 2009;65:27S. doi: 10.1016/j.schres.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(Suppl):S157–61. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Moro AR, Percudani M. Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- Bakewell L, Burdge GC, Calder PC. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr. 2006;96:93–99. doi: 10.1079/bjn20061801. [DOI] [PubMed] [Google Scholar]
- Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–2272. doi: 10.1373/clinchem.2006.072322. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition. 2009 doi: 10.1016/j.nut.2008.12.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, Miller del D, Haynes WG. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–137. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20:598–603. doi: 10.1097/EDE.0b013e31819d6a57. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Harmon SD, Kaduce TL, Manuel TD, Spector AA. Effect of the delta6-desaturase inhibitor SC-26196 on PUFA metabolism in human cells. Lipids. 2003;38:469–476. doi: 10.1007/s11745-003-1086-9. [DOI] [PubMed] [Google Scholar]
- Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Harris WS. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res. 1989;30:785–807. [PubMed] [Google Scholar]
- Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Makino KK, Martin CE, Dickerson F, Boronow J, Fenton WS. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry. 2003;53:431–441. doi: 10.1016/s0006-3223(02)01549-4. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Ito H, Kawashima R, Awata S, Ono S, Sato K, Goto R, Koyama M, Sato M, Fukuda H. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J Nucl Med. 1996;37:410–414. [PubMed] [Google Scholar]
- Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]
- Kamphuis MH, Geerlings MI, Tijhuis MA, Kalmijn S, Grobbee DE, Kromhout D. Depression and cardiovascular mortality: a role for n-3 fatty acids? Am J Clin Nutr. 2006;84:1513–1517. doi: 10.1093/ajcn/84.6.1513. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, Mintz J, McElroy SL, Freeman MP, Suppes T, Frye MA, Altshuler LL, Kupka R, Nolen WA, Leverich GS, Denicoff KD, Grunze H, Duan N, Post RM. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60:1020–1022. doi: 10.1016/j.biopsych.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20:4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Martinez M, Mougan I, Roig M, Ballabriga A. Blood polyunsaturated fatty acids in patients with peroxisomal disorders. A multicenter study Lipids. 1994;29:273–280. doi: 10.1007/BF02536332. [DOI] [PubMed] [Google Scholar]
- Martínez M, Vázquez E, García-Silva MT, Manzanares J, Bertran JM, Castelló F, Mougan I. Therapeutic effects of docosahexaenoic acid ethyl ester in patients with generalized peroxisomal disorders. Am J Clin Nutr. 2000;71:376S–385S. doi: 10.1093/ajcn/71.1.376s. [DOI] [PubMed] [Google Scholar]
- McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy. 2007;27:715–728. doi: 10.1592/phco.27.5.715. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford K, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatric Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult mice: prevention by chronic lithium treatment. J Psychiatr Res. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK. Membrane omega-3 fatty acid deficiency as a preventable risk factor for comorbid coronary heart disease in major depressive disorder. Cardiovascular Psychiatry and Neurology. 2009;9:1–13. doi: 10.1155/2009/362795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK. Evaluation of docosahexaenoic acid deficiency as a preventable risk factor for recurrent affective disorders: current status, future directions, and dietary recommendations. Prostaglandins Leukot Essent Fatty Acids. 2009;81:223–231. doi: 10.1016/j.plefa.2009.05.017. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen J, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010 doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- Moser AB, Jones DS, Raymond GV, Moser HW. Plasma and red blood cell fatty acids in peroxisomal disorders. Neurochem Res. 1999;24:187–197. doi: 10.1023/a:1022549618333. [DOI] [PubMed] [Google Scholar]
- Nara TY, He WS, Tang C, Clarke SD, Nakamura MT. The E-box like sterol regulatory element mediates the suppression of human Delta-6 desaturase gene by highly unsaturated fatty acids. Biochem Biophys Res Commun. 2002;296:111–117. doi: 10.1016/s0006-291x(02)00851-3. [DOI] [PubMed] [Google Scholar]
- Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- Obukowicz MG, Welsch DJ, Salsgiver WJ, Martin-Berger CL, Chinn KS, Duffin KL, Raz A, Needleman P. Novel, selective delta6 or delta5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J Pharmacol Exp Ther. 1998;287:157–166. [PubMed] [Google Scholar]
- Pawlosky R, Hibbeln J, Lin Y, Salem N. n-3 fatty acid metabolism in women. Br J Nutr. 2003;90:993–994. doi: 10.1079/bjn2003985. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404–408. doi: 10.1192/bjp.184.5.404. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chang L, Klaff J, Seemann R, Greenstein D, Rapoport SI. Chronic fluoxetine upregulates arachidonic acid incorporation into the brain of unanesthetized rats. Eur Neuropsychopharmacol. 2006;16:561–571. doi: 10.1016/j.euroneuro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Raeder MB, Fernø J, Glambek M, Stansberg C, Steen VM. Antidepressant drugs activate SREBP and up-regulate cholesterol and fatty acid biosynthesis in human glial cells. Neurosci Lett. 2006;395:185–190. doi: 10.1016/j.neulet.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245–249. doi: 10.1016/j.jad.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees AM, Austin MP, Owen C, Parker G. Omega-3 deficiency associated with perinatal depression: case control study. Psychiatry Res. 2009;166:254–259. doi: 10.1016/j.psychres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- Sicras A, Rejas J, Navarro R, Serrat J, Blanca M. Metabolic syndrome in bipolar disorder: a cross-sectional assessment of a Health Management Organization database. Bipolar Disord. 2008;10:607–616. doi: 10.1111/j.1399-5618.2008.00599.x. [DOI] [PubMed] [Google Scholar]
- Sprecher H, Chen Q. Polyunsaturated fatty acid biosynthesis: a microsomal-peroxisomal process. Prostaglandins Leukot Essent Fatty Acids. 1999;60:317–321. doi: 10.1016/s0952-3278(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- Sublette EM, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, Parsey RV, John Mann J. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. Prostaglandins Leukot Essent Fatty Acids. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin-Jones S, Russ MJ, Rapoport SI. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamäki H, Lehtonen J, Vartiainen E. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MM. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]