Abstract
The earliest cortical location at which attention influences visual processing is controversial. To address this issue, the C1 and P1 components of cue-elicited ERPs were examined in a spatially-cued task under high and low levels of attentional load (active vs. passive viewing). Cues were presented either to the left or right visual field in separate trials (unilateral presentation), or to both visual fields simultaneously (bilateral presentation). For the unilateral presentation, C1 (peak latency approximately 80 ms) was not modulated by attentional load, whereas P1 (peak latency approximately 120-140 ms) was larger for high- relative to low-load condition. Bilateral presentation of the stimuli enhanced the amplitude of the C1 component relative to unilateral presentation; however, the increase of signal/noise ratio of C1 revealed no attentional load effect on C1. Results show that attentional load modulates visual processing in the P1, but not in the C1 time range, regardless of the increased signal/noise ratio by bilateral presentation. While it remains unclear about the conditions under which a C1 attentional effect is reliably elicited, the present results suggest that the direct manipulation of attentional load under a voluntary attention task seems not crucial for eliciting C1 attentional effect.
Keywords: Voluntary attention, attentional load, Event-related potentials (ERPs), Signal/noise ratio, Passive viewing, Active viewing
Introduction
The role of striate cortex in early visual processing is controversial—is it a passive receptor that registers the physical properties of the stimuli, or an active processor that directly modulates stimulus processing? Both single-unit recording (Ito and Gilbert, 1999; McAdams and Reid, 2005; Motter, 1993; Roelfsema et al., 1998) and brain imaging techniques (Gandhi et al., 1999; Hopf et al., 2004; Somers et al., 1999) have shown that attention affects neural activity in the striate cortex. However, the majority of event-related potential (ERP) studies have found that the C1 component—the first visual cortical response that originates in striate cortex (Clark et al., 1995; Mangun, 1995) -- is insensitive to attention. The earliest attentional modulation of ERPs is observed on the later visual P1 component generated in the extrastriate cortex (e.g., Clark and Hillyard, 1996; Clark et al., 1995; Heinze et al., 1994). A delayed feedback hypothesis has been advanced to reconcile the ERP evidence of no attentional effect on C1 and the brain imaging evidence of an attentional effect on striate cortex (Lamme and Spekrejse, 2000; Mehta et al., 2000a; Mehta et al., 2000b; Martinez et al., 1999, 2001; Noesselt et al., 2002; Super et al., 2001). According to this hypothesis, striate cortex is unaffected by attention at the initial processing stage (approximately 80 ms after stimulus onset); however, striate cortex is affected by attention at a later processing stage (approximately 150 ms after stimulus onset) via a re-entrant or a feedback mechanism.
In contrast, some recent studies have found that an early and direct attentional modulation of the C1 component occurs under certain experimental conditions (e.g., Fu et al., 2008; Fu et al., 2009; Kelly et al., 2008; Khoe et al., 2005; Rauss et al., 2009; Wu et al., 2005; see also Poghosyan et al., 2005 for MEG evidence). Specifically, manipulations of both attentional and perceptual load have been effective in eliciting a C1 attentional effect (Fu et al., 2009; Fu et al., 2010; Rauss et al., 2009). Rauss et al. (2009) presented both central task-relevant stimuli and peripheral task-irrelevant stimuli. They found that the C1 evoked by peripheral task-irrelevant stimuli was enhanced when the central task-relevant stimuli required low but not high attentional load. Recently, we observed C1 to task-relevant stimuli was modulated by attention when the perceptual load of the stimuli was high (Fu et al., 2009; Fu et al., 2010). Note that the load manipulation in these two studies was quite different. While Rauss et al. (2009) manipulated the attentional load of the central stimuli and measured the effect on task-irrelevant peripheral stimuli, Fu et al. (2009) manipulated the perceptual load of the task-relevant stimuli. In addition, the types of attention involved in these two studies are different. In Fu et al. (2009), involuntary attention paradigm was used and participants' attention was automatically or reflexively attracted by the onset of the peripheral cues (Yantis and Jonides, 1990). In contrast, in Rauss et al. (2009), a voluntary attention paradigm was used and the focus of attention “spilled over” to the peripheral task-irrelevant stimuli when the attentional load of the central task was low.
Considered together, these two studies indicate that interactions between load and type of attention may contribute to the early attentional effect on C1. Even under voluntary attention condition, some previous studies have found no C1 attentional modulation (e.g., Clark and Hillyard, 1996; Di Russo et al., 2003; Martinez et al., 1999; Martinez et al., 2001; Noesselt et al., 2002), whereas other recent studies have shown C1 modulation (e.g., Kelly et al., 2008; Rauss et al., 2009; see Fu et al., 2008, for a negative C1 attentional effect). Therefore, some of the mixed results on attention modulation of C1 may be affected by several contributory factors, such as load (perceptual or attentional) and the type of attention involved.
We investigated this further by assessing whether C1 is attention sensitive in a voluntary attention task with direct manipulation of attentional load. The term “attentional load” usually refers to the amount of cognitive processing to the same stimulus (Rauss et al., 2009) and thus differs from “perceptual load”, which involves processing of different stimuli (e.g., Lavie, 1995). In other words, perceptual load is intrinsic to difference in stimulus property, whereas attentional load is determined by difference in cognitive processing to the same stimulus. Importantly, the present manipulation of attentional load differed from Rauss et al. (2009). In Rauss et al. (2009), the attentional load on the central task-relevant stimuli was manipulated as high or low, and the effect of that load on peripheral task-irrelevant stimuli (stimuli-of-interest) was evaluated. The hypothesis was that, for capacity-limited attentional resources, higher attentional load in the central task would demand more attentional resources, thereby leaving less attentional resources available to process the peripheral stimuli-of-interest, and vice versa. In other words, in Rauss et al. (2009) the effect of attentional load on the stimuli-of-interest was controlled indirectly by manipulating attentional load of the central stimuli. In contrast, in the present study we sought to directly manipulate the attentional load of the stimuli-of-interest via task assignments of active and passive viewing of the same stimuli. Our hypothesis was that active viewing would require more attentional resources and thus would result in a higher attentional load to the viewed stimuli, and vice versa. Therefore, the present manipulation of attentional load is consistent with Rauss et al. (2009) in terms of allocation of attentional resource but differs in the way the attentional resources are allocated.
In addition to the direct manipulation of attentional load, the present study also improved the ability to resolve and measure C1 and P1. Specifically, bilateral stimuli were included to improve the C1 and/or P1 signal/noise (S/N) ratio of signals from posterior midline electrodes 1. This is proposed because (1) C1 has a small amplitude (approximately 0.5-1 μV, see e.g., Di Russo et al., 2003; Fu et al., 2001; Fu et al., 2005; Martinez et al., 2001; Noesselt et al., 2002); (2) the polarity of C1 is negative when stimuli are presented in the left or right visual field above the horizontal meridian (e.g., Kelly et al, 2008; Fu et al., 2008); and (3) the C1 is generally large and clear at the posterior midline electrodes for stimuli presented in the left or right visual field (e.g., Clark and Hillyard, 1996; Martinez et al., 1999). Therefore, one would expect an enhancement of the C1amplitude at posterior midline electrodes for a stimulus presented simultaneously in both left and right visual fields relative to a stimulus presented only in one visual field when the C1s for LVF (left visual field) and RVF (right visual field) stimuli have the same polarity. In order to elicit a negative C1 that can be separated from the subsequent P1 component, the stimuli were presented at appropriate locations in accordance with the cruciform model of striate cortex (Jeffreys and Axford, 1972; Mangun 1995) and empirical studies (e.g., Clark and Hillyard, 1996; Di Russo et al., 2003; Martinez et al., 2001). In addition, to avoid cue-target ERP overlap, only the ERPs to the cue stimuli in a Posner-type peripheral cued-attention task were analyzed. The ERPs to the target stimuli were dropped from analyses, because (1) the C1 and P1 of the target stimuli could be contaminated by the ERPs to the cues; and (2) target stimuli were not presented bilaterally and thus the effect of signal/noise ratio could not be addressed.
We predicted that attentional load (manipulated by active vs. passive viewing) would affect stimulus processing at an early stage. Specifically, the P1 would be larger for active relative to passive viewing, as suggested by the “sensory gain control” hypothesis of visual attention (Hillyard et al., 1998). We also predicted that bilateral presentation would improve the S/N ratio of C1, so that its amplitude would be larger for bilateral relative to unilateral stimuli. Finally, as to the controversial C1 attentional effect, an enlarged C1 would be predicted for stimuli under the high attentional load (i.e., active viewing) condition, if voluntary attention enhances C1 in a way that more allocation of attentional resources to a stimulus elicits a larger C1 (e.g., Kelly et al., 2008; Rauss et al., 2009). In contrast, if voluntary attention does not modulate C1 (e.g., Clark and Hillyard, 1996; Di Russo et al., 2003), then greater allocation of attentional resources (attentional load) should have no effect on the amplitude of C1, even if the S/N ratio of the C1 is improved by bilateral stimuli.
Method
Participants
18 healthy participants (1 male) from the student population at George Mason University took part in this study as paid volunteers. Participants were between 18 and 28 years of age (mean age 21.6 years) and had normal or corrected to normal vision. 17 participants were right-handed, and 1 female participant was left-handed. Informed consent was obtained from all participants.
Stimuli and Procedure
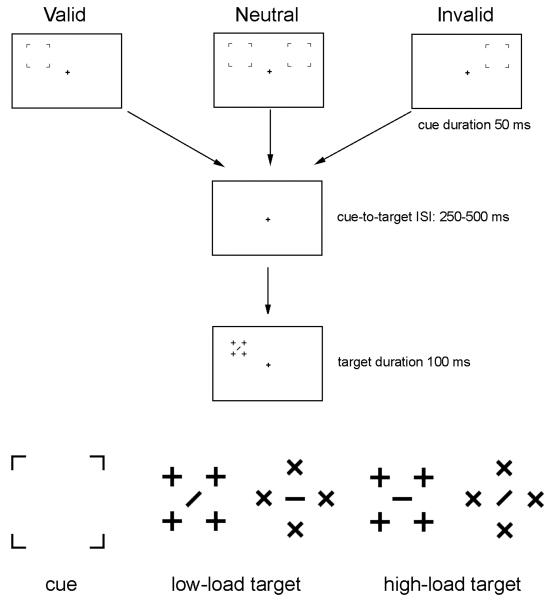
The stimuli used were similar to that described in Fu et al. (2010). A black fixation cross (0.31° × 0.31°) was presented on a white background at the center of the monitor throughout the entire recording session. Each trial consisted of a cue (4 brackets) and a target array (Figure 1). The location of the cues was valid, invalid, or neutral (bilateral) in predicting the location of the subsequent stimulus array. Each cue type appeared with equal probability. In the bilateral cuing condition, the cue (3.53° × 3.53°) appeared simultaneously in both the left and right visual fields. The target array (2.87° × 2.87° for diamond-shaped and 2.29° × 2.29° for square-shaped targets) appeared randomly on the left or right of the screen, centered 2.05° above and 7.07° to the side of the fixation cross. The durations of the cue and the target array were 50 ms and 100 ms, respectively. Cue-to-target interstimulus intervals (ISIs) were between 250 and 500 ms. These relatively long ISIs were designed to avoid ERP overlap between cue and target. The ITI (inter-trial interval) between the offset of the target array and the next cue ranged between 1100 and 1600 ms.
Figure 1.
Illustrations of the valid, neutral, and invalid trials (upper panel), and the cue and target stimuli (lower panel) used in the present study. For the unilateral (valid and invalid) cues, the cue stimulus appeared only in the left or right visual field. For the bilateral (neutral) cues, the cue stimulus appeared simultaneously in both the left and right visual fields. The perceptual load of the target stimuli was low or high with equal probability. The cue-elicited ERPs for the left, right, and bilateral cues were analyzed under active and passive viewing conditions, respectively. The ERPs elicited by the target stimuli were not analyzed in the present study.
Participants were tested under both active and passive viewing task conditions in separate experimental blocks. Under the passive viewing condition, participants were required to maintain fixation on the central cross, with no task assignment. The active viewing task was similar to Fu et al. (2010), but with longer cue-to-target ISIs. Stimuli are illustrated in Figure 1. Participants were required to discriminate the orientation of the bar in the center of the target array. The target stimuli were presented under two levels of perceptual load, high or low. For the high-load targets, the central bar was surrounded by distractors having the same elements, i.e., a central horizontal or vertical bar surrounded by crosses, or a central forward or backward slash surrounded by Xs. For the low-load targets, the central target bar and the distracters did not share any feature elements in common – i.e., a central horizontal or vertical target bar was surrounded by Xs, and a centered forward or backward slash was surrounded by crosses. Therefore, from the feature detection point of view (Treisman, 1980), the target element should “pop out” from the background distracters for the low-load stimuli, whereas the high-load stimuli should require focused attention to detect the target. The location of the four distractors was designed to avoid collinearity between the central bar and the distractors. The two load conditions were presented with equal probability. Note that in the present study, we were interested in the effect of attentional load (i.e., the difference between active and passive viewing) of the cue-elicited ERPs rather than the effect of perceptual load of the target-elicited ERPs. In other words, the manipulation of perceptual load of the target stimuli served only as a control for active viewing.
Participants were required to press “z” or “n” for a forward (“\”) and a backward bar (“/”), respectively. Response keys were counterbalanced across participants. Participants were instructed to respond as quickly as possible while maintaining high accuracy. They were also informed that the location of the cue did not predict the location of the array. To minimize eye movement, participants were asked to fixate on the central plus sign throughout the trial. During practice sessions, the experiment operator watched participants' eyes to ensure that they were able to perform the task while maintaining central fixation. There were 80 practice trials and 1280 experimental trials for active and passive viewing conditions, respectively. The sequence of trials in each block and the sequence of active and passive viewing blocks were randomized for each participant.
EEG recording and data analysis
Sixty-four channels of EEGs were recorded. The EEG recording procedure and the configuration of the electrodes were the same as in a previous study (Fu et al., 2008). The physical reference electrode was approximately 2 cm posterior to CZ, and the EEG data were re-referenced to the average of left and right mastoid (LM and RM). HEOG was monitored by placing electrodes lateral to the left and right orbits, and VEOG by placing two electrodes approximately 1.5 cm below and above the left eye. EEGs were digitized at 500 Hz and were filtered with a band pass of 0.1-40 Hz. A 200 ms pre-stimulus epoch of EEG was used as the baseline. Only the ERPs for the cue stimuli were analyzed in the present study, and since components C1 and P1 to the cue stimuli occurred before target onset (250-500 ms ISIs between the cue and target stimuli), there was no overlap between ERPs to cues and target. The ERPs for the target stimuli were excluded from further analyses because they were overlapped by the ERPs for the cue stimuli, and they were presented unilaterally (i.e., there were no bilateral targets to improve the C1 signal). Peak amplitudes of the C1 (60-100 ms) and P1 (100-160 ms) components were measured. Data from electrodes PZ and POZ were used for C1 analysis, and data from electrodes P3/P4 and PO5/PO6 were used for P1 analysis. For behavioral data analyses, only the RTs for the correct trials were analyzed by means of repeated measures ANOVA on the factors of perceptual load (high vs. low) and visual field (left vs. right). Electrophysiological data were analyzed by means of repeated measures ANOVA on the factors of electrode, presentation conditions (left vs. right vs. bilateral), hemisphere (left vs. right) and attention (passive vs. active viewing). For the midline electrodes, no factor of hemisphere was included.
There were several reasons for comparing the effect of stimulus presentation (unilateral vs. bilateral) on the attentional effect on ERPs. First, if the C1 attentional effect were evident with unilateral stimulation, despite the relatively low S/N ratio, that would suggest a relatively robust attentional effect. Second, it would be informative should it be shown that improving the S/N ratio by means of bilateral presentation helps to detect the C1 attentional effect. Finally, ability to observe an interaction between presentation and attention would be informative about how S/N ratio of the C1 component influences the attentional effect. Greenhouse-Geisser correction for degrees of freedom was applied wherever necessary.
Results
Behavioral measures
The overall mean accuracy for target discrimination was 88.63%. Participants responded faster to the low-load stimuli relative to the high-load stimuli [557 vs. 596 ms, F (1, 17) = 96.790, p < .0005]. Reaction times (RTs) for the valid, neutral and invalid trials were 571, 578, and 581 ms respectively. Participants responded fastest to valid trials relative to both the invalid [F (1, 17) = 10.465, p < .005] and the neutral trials [F (1, 17) = 6.770, p < .019]. The RT difference between neutral and invalid trials was not significant.
Electrophysiological measures
Figure 2 shows the ERPs for the left, right and bilateral stimulus under active and passive viewing. There is a clear negative C1 (peak at approximately 80 ms) and a posterior P1 component. It is also clear that attention affected the amplitudes of the P1 component.
Figure 2.
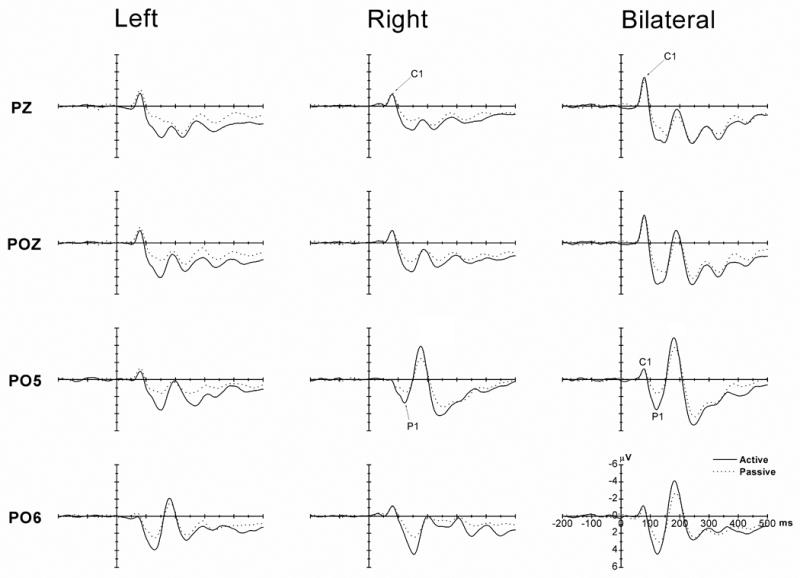
The grand-averaged ERPs for the left (left column), right (middle column) and bilateral (right column) stimuli under the active (solid lines) and passive (dotted line) viewing conditions. The ERPs on two midline electrodes (PZ and POZ, top 2 rows) and two lateral electrodes (PO5 and PO6, bottom 2 rows) are shown. For the unilateral cue stimuli (left and middle columns), no C1 difference was found between the active and passive viewing conditions at PZ and POZ (top 2 rows). The increase of the C1 amplitude by bilateral presentation (right column, top 2 rows) did not reveal any attentional effect on C1. In contrast, the P1 attentional effect (active vs. passive viewing) was consistently observed on both midline and lateral electrodes, regardless of whether the stimuli were presented unilaterally or bilaterally. Therefore, a dissociation between C1 and P1 components as a function of attentional load was observed in the present study.
1. C1 component
The C1 component (peak latency approximately 80 ms) was observed in the occipital, parietal, and central areas. The largest C1 component was observed at the midline electrode site PZ (Figure 2). The data from PZ and another midline electrode, POZ, are listed in Table 1.
Table 1.
The peak latencies (ms) and amplitudes (μV) of the C1 component (with standard errors) for the bilateral, left, and right stimuli at the PZ and POZ sites.
| Bilateral | Left | Right | ||||
|---|---|---|---|---|---|---|
| Active | Passive | Active | Passive | Active | Passive | |
| Latencies: | ||||||
| PZ | 79 (0.7) | 81 (0.9) | 79 (1.2) | 80 (1.9) | 79 (0.7) | 79 (1.7) |
| POZ | 79 (0.7) | 81 (0.9) | 78 (1.3) | 80 (1.6) | 78 (1.3) | 81 (2.0) |
| Amplitudes: | ||||||
| PZ | -3.5 (0.4) | -3.2 (0.4) | -1.6 (0.3) | -2.1 (0.3) | -1.5 (0.2) | -1.7 (0.2) |
| POZ | -3.3 (0.5) | -3.2 (0.5) | -1.6 (0.4) | -2.0 (0.4) | -1.6 (0.3) | -1.4 (0.2) |
1.1 Latency
The attention main effect on C1 latency was not significant, and no other attention-related interaction was significant. This indicates that the attention manipulation in the present study did not affect the latencies of the C1 component. No other main effects or interactions were significant.
1.2 Amplitude
The attention main effect on C1 amplitude was not significant, and no other attention-related interaction was significant. Thus the amplitude of the C1 component was insensitive to the attention manipulation, i.e., whether participants were required to actively or passively view the stimuli (Figure 2). The non-significant attention main effect and the non-significant interaction between attention and presentation also suggest that the increase of C1 signal by bilateral presentation reveals no attentional effect. This is further confirmed by a non-significant attention main effect [F (1, 17) = 0.495, p < .491] and a non-significant attention by electrode interaction [F (1, 17) = 1.692, p < .211] for the separate analysis of the bilateral presentation condition only. However, the presentation main effect was significant [F (2, 34) = 19.703, p < 0.0005, ε= 0.719], and further separate analysis suggests that the bilateral stimuli elicited larger C1 relative to either left or right unilateral stimuli [F(1, 17) = 63.445, p < .0005, and F(1, 17) = 26.861, p < .0005, respectively] (see Table 1 and Figures 2 and 3). The amplitudes of C1 between the left and right stimuli showed no significant difference at these two midline electrodes.
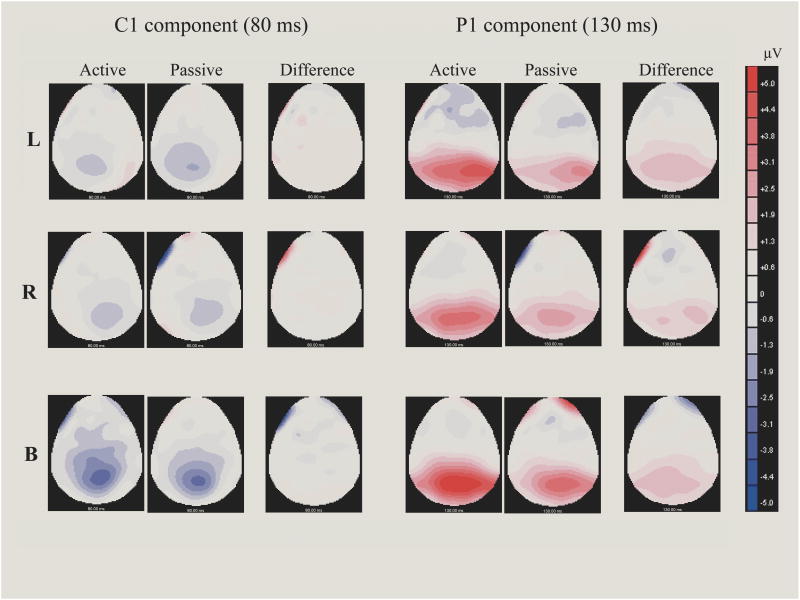
Figure 3.
The scalp voltage maps of the C1 (80 ms) and P1 (130 ms) components for the left, right, and bilateral stimuli under the active and passive viewing conditions. The difference maps showed the scalp distribution of the difference waveform, which were obtained by subtracting ERPs under the passive viewing condition from ERPs under the active viewing condition. It can be seen that C1 shows no attentional effect (left panels), whereas P1 is larger under the active viewing condition (right panels). It is also clear that bilateral stimuli enhance the C1 component.
2. P1 component
The P1 component can be seen on the posterior lateral electrodes, and is largest at the PO5/PO6 electrodes (Figure 3). The data from PO5/PO6 and P3/P4 are listed in Table 2.
Table 2.
The peak latencies (ms) and amplitudes (μV) of the P1 component (with standard errors) for the bilateral and unilateral (contralateral and ipsilateral) stimuli at the P3/P4 and PO5/PO6 sites.
| Bilateral | Unilateral | |||||
|---|---|---|---|---|---|---|
| Active | Passive | Active | Passive | |||
| Contra. | Ipsi. | Contra. | Ipsi. | |||
| Latencies: | ||||||
| P3/P4 | 124 (3.1) | 131 (3.8) | 126 (2.8) | 142 (3.7) | 126 (3.0) | 138 (3.1) |
| PO5/PO6 | 122 (2.8) | 127 (3.1) | 126 (2.6) | 144 (3.1) | 127 (2.8) | 142 (3.3) |
| Amplitudes: | ||||||
| P3/P4 | 4.7 (0.5) | 3.0 (0.5) | 3.9 (0.5) | 4.2 (0.5) | 2.4 (0.3) | 2.4 (0.3) |
| PO5/PO6 | 5.1 (0.7) | 3.2 (0.5) | 4.4 (0.7) | 4.7 (0.5) | 2.8 (0.4) | 2.6 (0.3) |
2.1 Latency
The presentation main effect on P1 latency was significant [F (2, 34) = 6.221, p < 0.008, ε= 0.830], suggesting an earlier P1 for bilateral relative to unilateral stimuli (Table 2). The attention main effect was not significant, but the presentation by attention interaction was significant [F (2, 34) = 5.072, p < 0.012, ε= 0.971]. We thus separately analyzed the bilateral and unilateral stimulus conditions.
For the bilateral stimulus, the attention main effect was significant [F (1, 17) = 8.394, p < 0.010], suggesting that the P1 was earlier for active relative to passive viewing condition (Table 2). No other interactions related to attention were significant.
For the unilateral stimuli, neither the attention nor the presentation main effects were significant, suggesting that the peak latency of P1 was unaffected by either attention or by presentation location (left vs. right). However, presentation location did affect the peak latency of the contralateral and ipsilateral P1. This is supported by a significant presentation by hemisphere interaction [F (1, 17) = 32.179, p < 0.0005], with the P1 peak latency earlier for contralateral compared to ipsilateral P1 components (Table 2).
2.2 Amplitude
P1 was larger under active relative to passive viewing conditions, as suggested by a significant main effect of attention [F (1, 17) = 43.076, p < 0.0005] (Figure 2). No other main effects or interactions were significant. Specifically, no significant P1 difference was observed between the bilateral and the unilateral stimuli, suggesting that bilateral stimuli did not enhance the amplitude of the P1 component. This is because we measured the peak amplitude of the P1 from both hemispheres, and the ipsilateral P1 of the unilateral stimuli had a later peak but comparable amplitude as compared to the bilateral stimuli (Table 2). The scalp voltage distribution of the P1 under active and passive viewing conditions and the distribution of the P1 difference between active and passive viewing are shown in Figure 3.
Discussion
The present study investigated how attentional load affects visual stimulus processing at early processing stages as indexed by the visual C1 and P1 components. Our manipulation of attentional load by active vs. passive viewing of the stimuli was more direct than the manipulation used in a recent study (Rauss et al., 2009), which indirectly imposed attentional load on peripheral stimuli by varying attentional involvement at central stimuli. The bilateral presentation of the stimuli also resulted in improved signal/noise ratios of the early ERP components. First, the bilaterally presented stimuli produced nearly a doubling of C1 amplitudes relative to the unilaterally presented stimuli. Second, the analyses of the cue-elicited ERPs were immune to ERP overlap problems because the cue-evoked C1 and P1 occurred well before the target stimulus was presented. Results pointed to a dissociation of the C1 and P1 components under voluntary attention for both the unilateral and bilateral stimuli, regardless of the signal/noise ratio. Overall we found that P1 was enhanced by increasing attentional load, whereas C1 was not.
Dissociation of C1 and P1 as a function of attentional load
The behavioral data show that the typical cue validity effect was observed, with participants responding faster to the valid trials relative to the neutral and invalid trials. Consistent with previous studies (Mueller and Rabbitt, 1989; Yantis and Jonides, 1990), this suggests that the onsets of the cue stimuli attracted participants' attention automatically to the cued location. However, the behavioral data provide no information about the participants' attentional allocation under the passive viewing condition, because overt responses were not made. ERPs provide a way to compare the effect of attention between the active and passive viewing conditions. Under active viewing, participants needed to respond to the targets, in addition to maintaining gaze on the central fixation. Thus it can be assumed that more attentional resources were allocated to the stimuli under the active relative to the passive viewing condition. Consistent with the well-established literature showing attention enhances the amplitude of P1 (e.g., Clark and Hillyard, 1996; Hillyard et al., 1998), the P1 was larger for the active relative to the passive viewing condition. This indicates that the manipulation of the active and passive viewing was successful in controlling participants' attentional allocation.
In contrast to the P1 attentional effect, no C1 attentional effect was observed for the unilateral stimuli in the present study. That C1 amplitude was similar for the active and passive viewing conditions suggests that under active conditions, participants were processing the stimuli (most likely at the sensation/perception stage) much as they did under passive viewing conditions -- otherwise no C1, or at least a smaller C1 would be expected. The absence of an attentional effect on C1 is consistent with previous studies (e.g., Clark and Hillyard, 1996; Martinez et al., 1999; Noesselt et al., 2002; Di Russo et al., 2003). Moreover, even the larger C1 following bilateral stimuli (almost doubled in amplitude) did not reveal any attention enhancement on the C1 component. Therefore, regardless of the signal/noise ratio, a dissociation between the C1 and P1 component as a function of attention was observed, with P1, but not C1, showing sensitivity to attentional load.
Bilateral presentation and the enhancement of the early ERP components
The present study used bilateral stimuli to investigate whether heightening the C1 signal helps to reveal attentional effects on the early ERP components. It was predicted that the bilateral stimuli would evoke a larger C1 relative to the unilateral stimuli, because the C1 of the LVF and RVF stimuli are both negative-going (e.g., Fu et al., 2005; Fu et al., 2008; Martinez et al., 2001; Noesselt et al., 2002) and are summed together for the bilateral stimuli. Consistent with that prediction, the C1 was larger for the bilateral relative to the unilateral stimuli, under both active and passive viewing conditions. However, the increase of the S/N ratio had no effect on the attentional effect on C1. That is, the C1 for the bilateral stimuli was larger for bilateral stimuli, but the increase of C1 was comparable between the active and passive viewing conditions and thus no C1 effect of attentional load was observed. An extension of this observation would be to use a stimulus with two or more elements in the same visual field, such as the cluttered stimuli used in previous studies (e.g., Fu et al., 2005; Fu et al., 2008; Fu et al., 2009; Rauss et al., 2009). Presumably a larger C1 would be elicited by these cluttered stimuli relative to the stimuli having only one element. Therefore, although the enhancement of C1 did not help to reveal the attentional effects on the C1 component in the present study, this provides a practical solution to improve the C1 signal in future studies.
Attention type and the locus of attentional selection
C1 modulation by attention has been observed in studies involving both involuntary attention (Rauss et al., 2009; Fu et al., 2009) and voluntary attention (Kelly et al., 2008; Poghosan et al., 2005). Because involuntary attention is automatic (Mueller and Rabbitt, 1989) and develops faster relative to voluntary attention (Eimer, 2000), we have proposed that involuntary attention is a contributing factor for eliciting early attentional modulation on the C1 component (Fu et al., 2005). This is supported by our previous finding that the C1 is larger for valid relative to invalid trials, when the perceptual load of the stimuli is high (Fu et al., 2009; Fu et al., 2010). Consistent with this hypothesis, it is possible that central directional cues also evoke involuntary attention (e.g., Ristic et al., 2002; Driver et al., 1999) and may play a role in studies reporting C1 modulation by central cues (e.g., Kelly et al., 2008). Nevertheless, the lack of the C1 attentional effect in the present study does not necessarily contradict the C1 attention effect under involuntary attention tasks (e.g., Fu et al., 2009; Fu et al. 2010), because the manipulation of active and passive viewing involves mainly different levels of voluntary attention. In two recent studies showing C1 attentional effect under voluntary attention tasks (Kelly et al., 2008; Rauss et al., 2009), the way attentional load is manipulated (direct vs. indirect) seems not essential. This is because C1 attentional effect has been observed under both direct manipulation of attention load by central cueing (Kelly et al., 2008) and indirect manipulation of attention load (Rauss et al., 2009). The present results contribute to this discussion by suggesting that not only the way attentional load is manipulated (direct vs. indirect), but also the manipulation of attentional load itself, might be irrelevant to the C1 attentional effect. However, the present results do not exclude the possibility of other contributing factors, such as stimulus property and configuration, arousal, involuntary part of the cues, and etc., may play a role in these two studies (Kelly et al., 2008; Rauss et al., 2009; see Fu et al., 2009 for discussion on possible contributory factors of C1 attentional effect). Nevertheless, the lack of the C1 attentional effect in the present study, consistent with the majority of previous ERP studies (e.g., Clark and Hillyard, 1996; Di Russo et al., 2003; Martinez et al., 1999; Martinez et al., 2001; Noesselt et al., 2002), does question whether C1 attentional effect can be obtained under voluntary attention task.
Dissociable mechanisms for voluntary and involuntary attention
The P1 amplitude and latency differences between active and passive viewing conditions in the present study also shed light on the relationship between voluntary and involuntary attention. Because the validity of the cue stimuli was only 50%, the behavioral validity effect should be elicited by involuntary attention, i.e., the automatic attraction of attention by stimulus onset. Moreover, the involuntary attention elicited by the onset of the cue stimuli was presumably comparable between the active and passive viewing because same stimuli were used. Thus, the attentional difference as indicated by the P1 amplitude difference was presumably from voluntary attention involved in active viewing. That is, although the cue stimuli “attracted” attention automatically to the cued location, regardless whether the stimuli were actively or passively viewed, this attraction of attention was not powerful enough to consume all attentional resources. Voluntary attention can exert its effects on involuntary attention, at an early processing stage, as indicated by the P1 component (approximately 120-150 ms). Presumably, this process originates in the extra-striate cortex (see, e.g., Clark and Hillyard, 1996; and Di Russo et al., 2003, for source locations of the posterior P1 component). This suggests that voluntary and involuntary attention involve at least partially dissociable mechanisms.
Summary
The present study showed a dissociation of C1 and P1 components of the visual ERP under different levels of attentional load, manipulated directly in active and passive viewing tasks. The earliest attentional load effect was observed on the P1 component for both the unilateral and the bilateral conditions, with enhanced P1 seen under active viewing. No C1 effect of attentional load was observed, even with the greater C1 amplitudes seen with bilateral stimulation. Therefore, the C1 and P1 components showed a dissociation as a function of attentional load, regardless of the S/N ratio. The bilateral presentation may provide a useful way to improve C1 signals in future study. Overall, results are consistent with the view that initial processing in the striate cortex (presumably the generator of the C1 component), is not modulated by voluntary attention, at least under the present experimental settings. Attentional load modulates stimulus processing at a later processing stage in the extrastriate cortex level (presumably the generator of the P1 component). Therefore, attentional load seems not a crucial factor for eliciting C1 attentional effect under a voluntary attention task.
Acknowledgments
This research was supported by NIH grant AG19653 to RP. We thank Autumn Brown for help in data collection.
Footnotes
One anonymous reviewer correctly pointed out that the bilateral cues differ from the unilateral cues not only in the signal strength of early component(s), but also in the complexity of the stimuli—i.e., the bilateral cues were more complex relative to the unilateral cues. This confounding of signal strength and stimulus complexity, similar to that in our previous study using stimuli with different perceptual load (Fu et al., 2009), is because the enhancement of C1 by stimulation is accompanied by different physical property of the stimuli. Therefore, should there be any attentional effect on C1, further studies are required to identify whether this is due to enhanced signal/noise ratio per se or due to the difference between physical property of the stimuli, such as complexity or perceptual load. The present study used the signal/noise ratio difference throughout the manuscript for simplicity purpose, but it should be noted that this was accompanied by other perceptual differences, such as complexity, between the bilateral and unilateral cues.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Human Brain Mapping. 1995;2:170–187. [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual cognition. 1999;6:509–540. [Google Scholar]
- Eimer M. The time course of spatial orienting elicited by central and peripheral cues: evidence from event-related brain potentials. Biological Psychology. 2000;53:253–258. doi: 10.1016/s0301-0511(00)00049-1. [DOI] [PubMed] [Google Scholar]
- Fu S, Greenwood PM, Parasuraman R. Brain mechanisms of involuntary visuospatial attention: an event-related potential study. Human Brain Mapping. 2005;25:378–390. doi: 10.1002/hbm.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Zinni M, Squire P, Kumar R, Caggiano DM, Parasuraman R. When and where perceptual load interacts with voluntary visuospatial attention: an event-related potential and dipole modeling study. NeuroImage. 2008;39:1345–1355. doi: 10.1016/j.neuroimage.2007.09.068. [DOI] [PubMed] [Google Scholar]
- Fu S, Huang Y, Luo Y, Wang Y, Fedota J, Greenwood PM, Parasuraman R. Perceptual load interacts with involuntary attention at early processing stages: event-related potential studies. NeuroImage. 2009;48:191–199. doi: 10.1016/j.neuroimage.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Fedota J, Greenwood PM, Parasuraman R. Early interactions between perceptual load and involuntary attention: An event-related potential study. Neuroscience Letters. 2010;468:68–71. doi: 10.1016/j.neulet.2009.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Noesselt T, Tempelmann C, Braun J, Schoenfeld A, Heinze HJ. Popout modulates focal attention in the primary visual cortex. NeuroImage. 2004;22:574–582. doi: 10.1016/j.neuroimage.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron. 1999;22:593–604. doi: 10.1016/s0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys D, Axford J. Source locations of pattern-specific component of human visual evoked potentials. I: Component of striate cortical origin. Experimental Brain Research. 1972;16:1–21. doi: 10.1007/BF00233371. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial attention modulates initial afferent activity in human primary visual cortex. Cerebral cortex. 2008;2008:2629–2636. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoe W, Mitchell J, Reynolds J, Hillyard SA. Exogenous attentional selection of transparent superimposed surfaces modulates early event-related potentials. Vision Research. 2005;45:3004–3014. doi: 10.1016/j.visres.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Spekreijse H. Contextual modulation in primary visual cortex and scene perception. In: Gazzaniga MS, editor. The new cognitive neuroscience. Cambridge, MA: MIT press; 2000. pp. 279–290. [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Martinez A, DiRusso F, Anllo-Vento L, Sereno MI, Buxton RB, Hillyard SA. Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Research. 2001;41:1437–1457. doi: 10.1016/s0042-6989(00)00267-4. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. The Journal of Neuroscience. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I: distribution and timing of effects across visual areas. Cerebral Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. II: physiological mechanisms of modulation. Cerebral Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. Journal of Neurophysiology. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- Poghosyan V, Shibata T, Loannides AA. Effects of attention and arousal on early responses in striate cortex. European Journal of Neuroscience. 2005;22:225–234. doi: 10.1111/j.1460-9568.2005.04181.x. [DOI] [PubMed] [Google Scholar]
- Rauss KS, Pourtois G, Vuilleumier P, Schwartz S. Attentional load modifies early activity in human primary visual cortex. Human Brain Mapping. 2009;30:1723–1733. doi: 10.1002/hbm.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin & Review. 2002;9:507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nature Neuroscience. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen J, Han S. Neural mechanisms of attentional modulation of perceptual grouping by collinearity. Neuroreport. 2005;16:567–570. doi: 10.1097/00001756-200504250-00010. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:121–124. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]