Abstract
The gastrointestinal mucosa is an important route of entry for microbial pathogens. The immune cells of Peyer’s patch (PP) compartments contribute to the active immune response against infection. Although local anesthetics are widely used in clinical practice, it remains unclear whether local anesthetics such as lidocaine affect PP T cell functions. The aim of this study was to examine if lidocaine has any effects on mouse PP T cell functions. To test this, freshly isolated mouse Peyer’s patch T cells were incubated with lidocaine. The effects of lidocaine on concanavalin A-stimulated PP T cell proliferation and cytokine production were assessed. The effect of lidocaine on PP T cell mitogen-activated protein kinase (MAPK) activation was also assessed. The results indicate that lidocaine suppresses cell proliferation, cytokine production, and MAPK activation in PP T cells. Furthermore, we found that the chronic in vivo exposure to lidocaine increases bacterial accumulation in PP. The enhanced immunosuppressive effects of lidocaine on PP T cell functions could contribute to the host’s enhanced susceptibility to infection.
INTRODUCTION
Local anesthetics (LAs) are widely used for analgesia in patients with trauma and surgical injury. Lidocaine has been used since the decade of the 1960s for several indications including regional blocks, antiarrhythmia, analgesic for neuropathic and central pain, and as adjuvant for postoperative pain, including postoperative pain refractory to opioids. Although lidocaine is an old drug, it is still used in many areas in the clinical setting. Indeed, the AHA 2005 Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care recommends it for the therapy of ventricular fibrillation, ventricular tachycardia, and PVC1. In addition, lidocaine is the most frequently used local anesthetic in all types of local and regional anesthesia even now2. Pain is a major problem for critically ill patients. Pain from surgical incisions, trauma, and invasive procedures may hinder the effectiveness of ventilation and lead to complication. Epidural analgesia can provide excellent postoperative as well as intraoperative analgesia. Adequate pain relief reduces perioperative complications3. LAs are well known for their effect on immune functions. Although LAs have antibacterial actions and beneficial effects on inflammatory response1-3, they also have harmful effects on immune function3-5. Previous studies have shown that LAs induce neutrophil4,6, natural killer cell7, and monocyte5 dysfunction. Consequently, LAs have been considered as a double-edged sword for the immune response.
The gastrointestinal mucosa plays a crucial role for host antibacterial defense. The gastrointestinal mucosa is an important route of entry for microbial pathogens, and cells of gastrointestinal mucosa rapidly initiate innate and acquired immune response after bacterial invasion. The function of Peyer’s patches (PP), one of the gut-associated lymphoid tissues (GALT), is impaired following thermal injury8, ischemia-reperfusion injury9, or in the absence of enteral feeding10. PP dysfunction might disrupt intestinal immune defense and therefore contribute to bacterial translocation from the gut lumen11 to mesenteric lymph nodes (MLN) and other extra-intestinal sites including spleen, liver, lung, and blood. Furthermore, intestinal immune dysfunction might be associated with septic complication. After bacterial invasion, the intestinal mucosa produces mediators that orchestrate the onset of an early inflammatory response such as increased production and release of chemokines, cytokines and nitric oxide within the first few hours. These molecules can act as early signals to activate an acute mucosal inflammatory response and enhance the ability of epithelial cells to produce cytokines that regulate mucosal immune responses for preventing bacterial translocation12,13.
Immune cells of PP compartments contribute to the active immune response against infection. Although local anesthetics are widely used in clinical practice, even in patients with impaired organ functions, it remains unclear whether local anesthetics such as lidocaine affect PP T cell functions. The aim of this study was to examine if lidocaine has any effects on mouse PP T cell functions.
MATERIALS and METHODS
Mice
Male C3H/HeN mice (Charles River Laboratories, Wilmington, MA) 6–8 weeks old and weighing 20–25 g, were used in the experiments. Mice were allowed to acclimatize to the animal facility for one week before experiments. Animal experiments were conducted in accordance with guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Isolation and culturing of T cells from PP
Mice were anesthetized, and intestine was exposed via midline incision. PP were carefully excised from the intestine wall, then dissociated using the neutral protease enzyme collagenase type V (Sigma, St Louis, Mo; 40 U/mL) in Hank’s balanced salt solution (HBSS; Fisher Scientific, Atlanta, GA) containing 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin, and 100 μg/ml streptomycin for 60 min at 37° in a water shaker (1 50 rpm). After collagenase digestion, the cell suspension was incubated with nylon wool-packed columns. These columns were pre-equilibrated with HBSS containing 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% fetal calf serum (FCS). Columns containing cells were incubated at 37°C for 50–60 m in. T cells were obtained by eluting columns with 15 ml of HBSS at a flow rate of 1 drop/sec8,14. Lymphocytes were resuspended in RPMI-1640 (Fisher Scientific) supplemented with 2 mM L-glutamine, 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS, then counted. Our flow cytometric analysis demonstrated that cells contained >97% CD3-positive T cells. Cell suspensions were stained with antibody against surface marker CD3 (clone: 17A2, eBioscience, San Diego, CA). Cells were analyzed using a BD LSRII (BD Biosciences, San Diego, CA).
Lidocaine
We purchased lidocaine hydrochloride from Sigma (St. Louis, MO). Because it is water soluble, we dissolved and diluted lidocaine hydrochloride in culture medium (RPMI-1640 supplemented with with 2 mM L-glutamine, 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS) for in vitro studies. For in vivo study, lidocaine hydrochloride was dissolved and diluted in saline, and same volume (1 ml) of saline was given to control mice.
Cell viability assay
The cell survival assay of PP T cells (1×106 cells/ml, each) was performed up to 3 days in the presence or absence of lidocaine. Cells were stained with trypan blue, and percentages of viable cells were calculated by dividing the number of live cells by the total cell number. Change in the percentages of viable cells was calculated on the basis of the number of cells added to the culture on day 0.
Cell proliferation assay
ELISA (Amersham Biosciences, Piscataway, NJ) kit was used to determine the proliferation of PP T cells. Briefly, PP T cells (1×106 cells/ml) were plated into a 96-well plate with or without concanavalin A (ConA; 5 μg/ml) stimulation at various concentrations of lidocaine, and cultured at 37°C, 5% CO 2 and 95% humidity for 48 hr. 5-bromo-2’-deoxyuridine (BrdU) was added to the cell suspension and incubated for an additional 8 hr for PP T cells. Plates were then centrifuged at 2000 rpm, 4°C for 10 min, supernatan ts were carefully removed, and cells dried using a hairdryer for 15 min. The cells were incubated with blocking buffer for 30 min at room temperature after which blocking buffer was removed by centrifugation. Peroxidase-labeled anti-BrdU reagent was then added, and plates were incubated for an additional 90 min at room temperature. Plates were washed 3 times with phosphate-buffered saline (PBS), and after the addition of 3.3’ 5.5’-tetramethylbenzidine and 1 M sulfuric acid, the intensity of the color was measured with a Bio Tek (PowerWave, Winooski, VT) plate reader.
Determination of cytokine production
The levels of interleukin (IL)-2, IL-4, IL-5 and interferon (IFN)-γ in culture supernatants were determined by cytometric bead array (BD Bioscience, San Diego, CA) according to the manufacturer’s instruction.
Detection of phosphorylated (activated) mitogen-activated protein kinase (MAPK) signaling molecules by fluorescein-activated cell sorter analysis (FACS)
We measured intracellular signaling molecules by FACS, as described previously15. After preparation of PP T cells, cells were incubated with lidocaine for 1 hr. Cells were then incubated with 5 μg/ml of ConA for 15 min. After incubation, cells were rapidly fixed with 2% paraformaldehyde for 10 min at 37°C, permea bilized with ice-cold methanol (100%) for an additional 10 min, and washed with PBS supplemented with 1% bovine serum albumin and 0.1% sodium azide. Prior to antibody staining, samples were incubated with Fc block antibody (eBiosciences, San Diego, CA) to prevent nonspecific binding, then stained with unlabeled, primary mouse monoclonal antibodies specific for phosphorylated (active) forms of p38, extracellular signal-regulated kinase (ERK)1/2, or stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK; Cell Signaling, Beverly, MA). An isotype-matched mouse IgG was used as a nonspecific staining control. Cells were then washed and stained with a goat anti-mouse FITC-labeled secondary antibody (Jackson ImmunoResearch, West Grove, PA). Cells were subsequently washed twice and resuspended in 0.5% paraformaldehyde. Flow cytometry was performed using the LSR II instrument (BD Biosciences), and results were analyzed using the accompanying FACSDiva software (BD Biosciences).
The effect of in vivo exposure of lidocaine on bacterial translocation
After in vivo exposure of mice to lidocaine by i.p. inoculations (0.25 mg/10 g body weight) 4 times a day for 7 days as described by Dickenstein et al.16, animals were anesthetized and killed. The abdominal cavity was exposed under aseptic conditions. MLN, spleen, and blood were collected. Organs were weighed and homogenized in sterile glass tissue grinders (Fisher Scientific). Equal volume of blood (10 μl) or tissue homogenate (10 μl) from various experimental groups was cultured separately on Tryptic soy agar plates (Difco, BD Diagnostic Systems, Franklin Lakes, NJ). The agar plates were incubated for 48 hr at 37° for the growth of bacteria. Bacte rial colony-forming units (CFU) were counted. If the plates did not show any bacterial growth up to 48 hr, the organ was considered negative for the presence of bacteria.
Statistical analysis
Data were presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) and Tukey’s test were used for comparison between groups, and differences were considered significant at p<0.05.
RESULTS
Peyer’s patch T cell viability
Lidocaine less than 100 μM had no effect on PP T cell viability, as determined by the exclusion of the vital stain trypan blue. However, lidocaine at 1000 μM decreased cell viability of PP T cells after incubation for 2-3 days (Fig. 1). In view of this, subsequent experiments were performed with less than 100 μM lidocaine.
Fig. 1. Changes in viability of Peyer’s patch T cells after incubation with lidocaine.
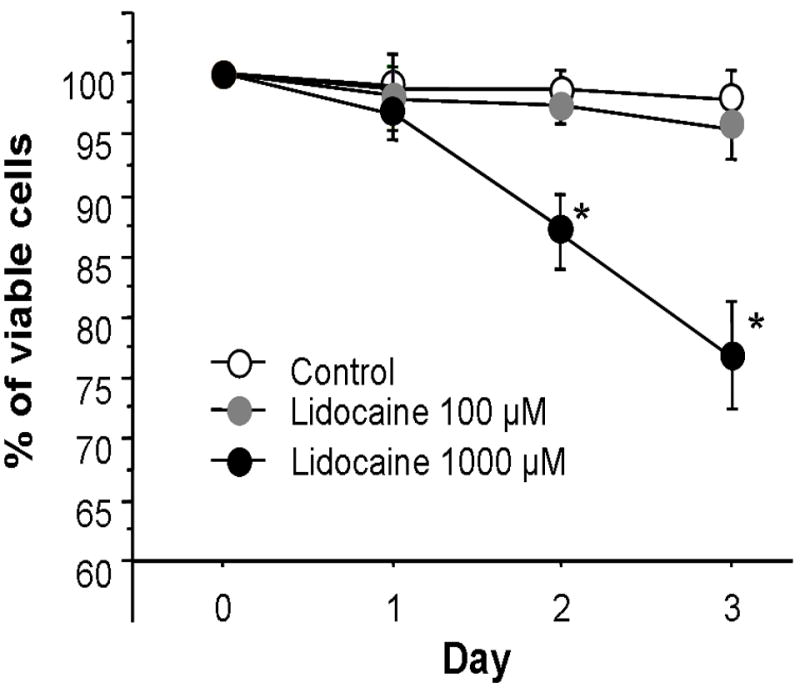
Data are mean ± SE from 6 experiments in each group. ANOVA, *p<0.05 compared to equivalent control (0 μM lidocaine).
Peyer’s patch T cell proliferation
The effect of lidocaine on the ability of PP T cells to proliferate in response to stimulation with ConA is shown in Fig. 2. Proliferation of PP T cells in response to stimulation with ConA was increased compared to non-stimulated controls. Lidocaine induced a reduction in PP T cell proliferation in a dose-dependent manner.
Fig. 2. The effect of lidocaine on proliferation of Peyer’s patch T cells with or without ConA stimulation.
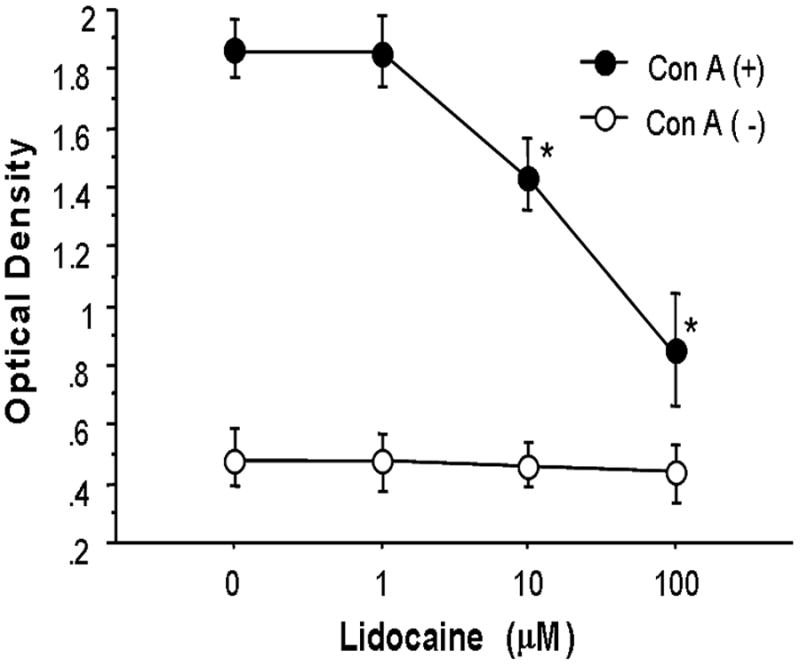
Data are mean ± SE from 6 experiments in each group. ANOVA, *p<0.05 compared to equivalent control (0 μM lidocaine).
Peyer’s patch T cell cytokine production
Fig. 3 shows the effect of lidocaine on PP T cell cytokine production. Production of Th1 (IL-2 and IFN-γ) cytokines by PP T cells was significantly increased in response to stimulation with ConA compared to non-stimulated controls. Lidocaine induced a reduction in PP T cell Th1 cytokine production in a dose-dependent manner. In addition to Th1 cytokine production, the PP T cell Th2 (IL-4 and IL-5) cytokine production was significantly higher in response to stimulation with ConA compared to non-stimulated controls. Lidocaine induced a decrease in PP T cell Th2 cytokine production in a dose-dependent manner.
Fig. 3. The effect of lidocaine on Peyer’s patch T cell cytokine production with ConA stimulation.
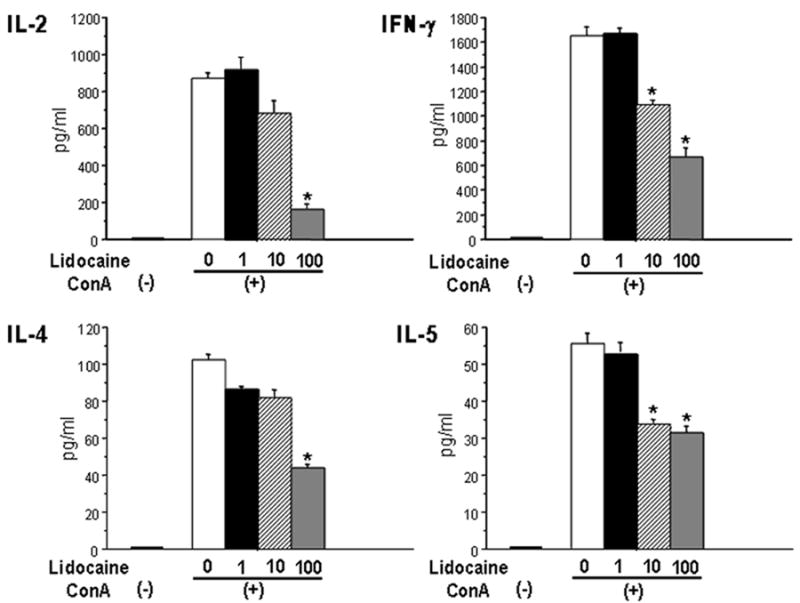
Data are mean ± SE from 6 mice in each group. ANOVA, *p<0.05 compared to equivalent sham; #p<0.05 compared to no lidocaine.
Effect of lidocaine on activation of p38, ERK1/2, and SAPK/JNK
Since ConA was used to stimulate cytokine production by PP T cells, we examined whether impaired cytokine production in the presence of lidocaine is associated with impaired phosphorylation of MAPK in response to ConA. To evaluate activation of p38, ERK1/2, and SAPK/JNK, we used a flow cytometric approach as previously described (15). Fig. 4 shows the effect of lidocaine on activation of p38, ERK1/2, and SAPK/JNK. Phosphorylation of p38, ERK1/2, and SAPK/JNK was detected in isolated PP T cells in the absence of ConA stimulation. Unstimulated cells did not exhibit any significant differences in levels of phosphorylated p38, ERK1/2, and SAPK/JNK between lidocaine-treated PP T cells and non-treated PP T cells. Activation of p38 MAPK and ERK1/2 was significantly increased in response to ConA stimulation. Lidocaine (100 μM) induced suppression of p38 MAPK and ERK1/2 activation in PP T cells. However, the activation of SAPK/JNK was not changed in response to ConA stimulation and lidocaine had no effect on SAPK/JNK activation of PP T cells.
Fig. 4. The effect of lidocaine on MAPK activation in Peyer’s patch T cells.
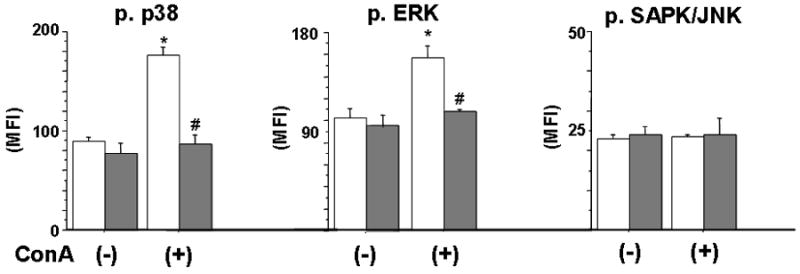
Open bar: lidocaine (-); dark bar: lidocaine (+). Data are mean ± SE from 6 mice in each troup. ANOVA, *p<0.05 compared to ConA (-); #p<0.05 compared to no lidocaine.
Effect of in vivo exposure of lidocaine on bacterial numbers
Bacterial numbers were determined by culturing blood and homogenates prepared from MLN and spleen. As shown in Table 1, the number of bacteria in MLN of sham animals was 1.5 ± 0.7 CFU. The number of bacteria in MLN from lidocaine-treated mice (10 ± 2.1) was significantly higher compared with MLN of sham animals. No bacterial growth was noted in the blood or in homogenates prepared from spleens of animals from any experimental group. There were no significant differences in body weight between the groups at the beginning of the experiment and the end of the experiment.
Table 1.
The effect of in vivo exposure to lidocaine on bacterial numbers.
| Tissue/Organ | Lidocaine (-) | Lidocaine (+) |
|---|---|---|
| MLN | 1.5 ± 0.7 | 10 ± 2.1* |
| Spleen | ND | ND |
| Blood | ND | ND |
Bacterial colony-forming units (CFU) were determined by culturing blood and homogenates prepared from mesenteric lymph nodes (MLN) and spleen. Data are mean ± SE from 6 mice in each group.
ANOVA, p<0.05 compared to no lidocaine; ND, not detected.
DISCUSSION
In this study, we observed a significant decrease in PP T cell proliferation after incubation with lidocaine. The suppressive effect of lidocaine on PP T cell proliferation was evident at lidocaine concentration of more than 10 μM. This was accompanied with suppression of PP T cell cytokine production. Results also suggest that lidocaine likely mediates suppression of PP T cell proliferation and cytokine production by inhibiting p38 and ERK1/2 phosphorylation. Furthermore, we found that chronic in vivo exposure of lidocaine increases bacterial accumulation in PP. These findings collectively suggest that lidocaine diminishes PP T cell functions, resulting in bacterial multiplication and their accumulation in PP.
The concentrations of lidocaine used in this study ranged from 1-100 μM (0.25-25 μg/ml). Lidocaine concentration in human plasma reaches 2.2 μg/ml after epidural administration20. After 2 mg/kg intravenous administration, the peak plasma concentration of lidocaine reaches 1.5-1.9 μg/ml (6-8 μM) within 15 min21. Plasma concentration of lidocaine above 10 μg/ml tends to produce systemic toxicity such as cardiac arrhythmia, hypotension, and cardiovascular collapse22,23. A dose of 8 mg/kg lidocaine has been used to measure the function of the nonembolized lobes of the liver22. Lidocaine has also been shown to enhance apoptosis and suppress mitochondrial functions in human neutrophils in vitro24. In this study, the suppressive effects of lidocaine appeared at 10 μM. Furthermore, although we did not measure plasma lidocaine concentration in chronic lidocaine-exposed mice, systemic toxicities of lidocaine were not observed in any mice. Previous study by Bassan et al.25 showed that the plasma concentration of lidocaine reaches 2-6.5 μg/ml after 1 mg/kg initial dose and subsequent 2-3 mg/min continuous intravenous administration in human adult. In this setting, the total dose of lidocaine is 2,950-4,390 mg/day for a 70-kg human. In our study, the dose of lidocaine administered to the mice may be the equivalent of 1,750 mg/day for a 70-kg human. We could not measure tissue lidocaine concentration; however, the plasma lidocaine concentration seemed to be less than 2-6.5 μg/ml in chronic lidocaine-exposed mice. Therefore, we assumed that lidocaine suppresses PP T cell function at the concentration observed in the clinical setting. Local anesthetics are frequently applied over a period of days or weeks and it is known that an increasing susceptibility of leukocytes to local anesthetics results with increasing exposure time. Therefore, even lower local anesthetic concentrations may result in compromised leukocyte function in cases involving prolonged exposure times. In accordance with our results, MacGregor et al.26 also demonstrated that 5 of 6 animals infused with lidocaine died within 48 hr from Staphylococcus aureus inoculation, whereas only 1 of 6 non-infused animals died. Taken together, our study indicates that lidocaine, even at clinically relevant concentrations, may induce bacterial contamination of the peritoneal cavity, if prolonged infusion of lidocaine is carried out.
Many previous studies demonstrated that T cell-mediated immunity is critical to defense against bacterial infection, including bacteria derived from intestine. Owens and Berg17 have shown an increase in spontaneous bacterial translocation from intestine to extra-intestinal organs in athymic homozygous (nu/nu) nude mice compared with heterozygous strains (nu/+). Furthermore, Choudhry et al.18 have shown that depletion of T cells in healthy rats resulted in increased bacterial accumulation in MLN. A few bacteria are known to translocate to MLN even in healthy conditions, however, these bacteria do not survive because of intact immune defense. Thus, MLN from healthy animals remains relatively sterile. Because T cell mediated-immunity is critical to defense against enteric bacteria, impaired PP T cell functions as observed after lidocaine exposure would be expected to contribute to decreased bacterial clearance and increased bacteria multiplication, resulting in their accumulation.
Recent studies have demonstrated intracellular signaling to regulate many aspects of cellular functions. MAPK pathways, including p38, ERK1/2, and SAPK/JNK, play significant roles in mediating signals triggered by cytokines and growth factors, and are involved in cell proliferation, cell differentiation, and cell death19-21. Therefore, we hypothesized that lidocaine suppresses MAPK activation in PP T cells, leading to reductions in PP T cell functions. Although the role of SAPK/JNK along with p38 and ERK1/2 has been suggested in T cell proliferation and IL-2 production, the present study demonstrated that SAPK/JNK may not be critical to T cell suppression by lidocaine, because there was no change in SAPK/JNK activity after lidocaine treatment. Thus, the suppression in p38 and ERK1/2 likely plays a major role in lidocaine-mediated suppression of T cell proliferation and IL-2 production. Also, p38 activation has been observed in T cell activation by IL-12, which is known to allow for differentiation of T cells to Th1 subtypes and promote Th1 cell production of cytokines including IL-2 and IFN-γ. We found significant suppression in PP T cell IFN-γ production following lidocaine treatment. Previous studies showed that IFN-γ produced by intestinal T cells helps in resolution of Yersinia enterocolitia22 and S. typhimurium23 infection. Furthermore, IFN-γ-deficient mice showed impaired ability to kill bacteria24. These studies and our results together suggest that alterations in IFN-γ response via suppression of p38 will result in dysfunction of the host defense against bacteria.
Conversely, our findings demonstrated that lidocaine suppresses not only PP T cell Th1 cytokine (IL-2 and IFN-γ) production, but also PP T cell Th2 cytokine (IL-4 and IL-5) production. Previously, Wu et al.25 demonstrated that intestinal IgA levels are correlated with intestinal Th2 cytokine concentration. They found intravenous total parenteral nutrition decreases production of Th2 cytokines and induces severely impaired mucosal immunity. Recent study also showed that intravenous total parenteral nutrition blunts GALT lymphocyte ERK1/2 phosphorylation and this may be an important mechanism underlying impaired immunologic and physiologic barrier of the gut in parenterally fed animals10. Moreover, phosphorylation of ERK1/2 is essential for proper expression of the tight junction proteins to normal gut barrier function26. The Ras-ERK MAPK cascade regulates GATA3 protein which has a crucial role for the differentiation of Th2 cells27. Thus, depressed Th2 cytokine production by lidocaine in PP T cells shown in this study may be mediated via ERK1/2 activation suppression. Blunted ERK1/2 activation might also be associated with impairment of intestinal immune function.
Our results suggest that lidocaine-induced immune dysfunction of PP T cells may be due to the suppression of MAPK activation. It has been demonstrated that lidocaine inhibits IP3 receptor-mediated intracellular Ca2+ release28 and protein kinase C (PKC) activity29,30. Sayeed et al.31 indicated downregulation of Ca2+ signaling in T cells as an important factor in T cell suppression. The proximal signaling mechanisms and downstream Ca2+ signaling are implicated in regulation of IL-2 gene, respectively, via PKC- and calcineurin-mediated pathways in T cells32. Moreover, recent studies have indicated that the MAPK pathway may also be modulated by activation of Ca2+ signaling33. Thus, it seems reasonable to surmise that lidocaine-induced signaling derangements in T cells may be linked to crosstalk between MAPK and Ca2+ signaling mechanisms. Therefore, we assume that inhibition of PKC and Ca2+ signaling is another possible mechanism by which lidocaine suppresses PP T cell function. In this regard, a previous study has shown that neither ropivacaine nor bupivacaine inhibit PKC activity44; it is possible that both ropivacaine and bupivacaine do not depress PP T cell immune functions. However, it would appear appropriate to investigate the effect of these local anesthetics on immune responses in PP T cells, and we hope to conduct those studies in the future.
It could be argued that there is much work emerging regarding the role of the central nervous system in intestinal immune function, therefore, vagus nerve stimulation can modulate intestinal immune injury. The vagus nerve has a counter-inflammatory role in a number of model systems. While the majority of these anti-inflammatory effects have been ascribed to the activation of nicotinic receptors on macrophages45, little is known about the role of the vagus in modulating the activity of other cells involved in inflammatory responses. Recently, Karimi et al.46 demonstrated that vagal input downregulates T cell function through action at nicotinic receptors. The local anesthetic lidocaine blocks voltage-gated Na+ channels and suppresses action potential generation and propagation of neurons. Previous study47 showed that lidocaine blocks vagus nerve conduction; however, it is unclear whether lidocaine affects the nicotinic receptor on T cells. Further studies are required to confirm whether lidocaine suppresses PP T cell function via vagus nerve system.
It would be possible that i.p. lidocaine could alter oral nutritional intake and this is associated with altered gut immunity and increased translocation of bacteria to PP. Previous studies13,35 demonstrated that nutrition provides an important role in maintaining gut immunity. In our study, there were no significant differences in body weight between the groups at the beginning of the experiment and the end of the experiment. Therefore, we believe that alteration of intake does not cause increased translocation of bacteria to PP in lidocaine treated mice.
In summary, we investigated the effect of lidocaine on PP T cell functions. Our in vitro results show an association of impaired PP T cell proliferation capacity and cytokine production ability and impaired MAPK activation in the presence of lidocaine. Furthermore, in vivo study demonstrated that chronic exposure to lidocaine promoted bacterial translocation from intestine to extra-intestinal organs. Impaired PP T cell functions as observed after lidocaine exposure could contribute to the host’s enhanced susceptibility to infection. Careful attention must therefore be paid to the effects of lidocaine on host defense mechanisms.
Acknowledgments
The authors wish to thank Bobbi Smith for assistance in preparing this manuscript.
This study was supported by NIH grant RO1 GM37127 (IHC) and Grants-in-Aid for Scientific Research (C2-16591575 to TK) from the Ministry of Education, Science, Sports, and Culture, Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(IV):1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 2.Jin P, Min JC. Local anesthetics. In: Dunn PF, editor. Clinical anesthesia procedures of the Massachusetts General Hospital. 7. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 238–246. [Google Scholar]
- 3.Carli F, Mayo N, Klubien K, Schricker T, Trudel J, Belliveau P. Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: results of a randomized trial. Anesthesiology. 2002;97:540–549. doi: 10.1097/00000542-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg PH, Renkonen OV. Antimicrobial activity of bupivacaine and morphine. Anesthesiology. 1985;62:178–179. doi: 10.1097/00000542-198502000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Grimmond TR, Brownridge P. Antimicrobial activity of bupivacaine and pethidine. Anaesth Intensive Care. 1986;14:418–420. doi: 10.1177/0310057X8601400415. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–875. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 7.Hyvonen PM, Kowolik MJ. Dose-dependent suppression of the neutrophil respiratory burst by lidocaine. Acta Anaesthesiol Scand. 1998;42:565–569. doi: 10.1111/j.1399-6576.1998.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki T, Kawasaki C, Ogata M, Shigematsu A. The effect of local anesthetics on monocyte mCD14 and human leukocyte antigen-DR expression. Anesth Analg. 2004;98:1024–1029. doi: 10.1213/01.ANE.0000104480.04856.93. [DOI] [PubMed] [Google Scholar]
- 9.Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, Niwa Y. Inhibitory effect of local anaesthetics on reactive oxygen species production by human neutrophils. Acta Anaesthesiol Scand. 1997;41:524–528. doi: 10.1111/j.1399-6576.1997.tb04735.x. [DOI] [PubMed] [Google Scholar]
- 10.Takagi S, Kitagawa S, Oshimi K, Takaku F, Miura Y. Effect of local anaesthetics on human natural killer cell activity. Clin Exp Immunol. 1983;53:477–481. [PMC free article] [PubMed] [Google Scholar]
- 11.Ravindranath T, Al-Ghoul W, Namak S, Fazal N, Durazo-Arvizu R, Choudhry M, Sayeed MM. Effects of burn with and without Escherichia coli infection in rats on intestinal vs. splenic T cell responses. Crit Care Med. 2001;29:2245–2250. doi: 10.1097/00003246-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Fukatsu K, Sakamoto S, Hara E, Ueno C, Maeshima Y, Matsumoto I, et al. Gut ischemia-reperfusion affects gut mucosal immunity: a possible mechanism for infectious complications after severe surgical insults. Crit Care Med. 2006;34:182–187. doi: 10.1097/01.ccm.0000196207.86570.16. [DOI] [PubMed] [Google Scholar]
- 13.Maeshima Y, Fukatsu K, Kang W, Ueno C, Moriya T, Saitoh D, Mochizuki H. Lack of enteral nutrition blunts extracellular-regulated kinase phosphorylation in gut-associated lymphoid tissue. Shock. 2007;27:320–325. doi: 10.1097/01.shk.0000239760.13206.18. [DOI] [PubMed] [Google Scholar]
- 14.Nieuwenhuijzen GA, Deitch EA, Goris RJ. Infection, the gut and the development of the multiple organ dysfunction syndrome. Eur J Surg. 1996;62:259–273. [PubMed] [Google Scholar]
- 15.Kramer DR, Sutherland RM, Bao S, Husband AJ. Cytokine mediated effects in mucosal immunity. Immunol Cell Biol. 1995;73:389–396. doi: 10.1038/icb.1995.61. [DOI] [PubMed] [Google Scholar]
- 16.Ramsay AJ. Genetic approaches to the study of cytokine regulation of mucosal immunity. Immunol Cell Biol. 1995;73:484–488. doi: 10.1038/icb.1995.78. [DOI] [PubMed] [Google Scholar]
- 17.Choudhry MA, Ahmad S, Thompson KD, Sayeed MM. T-lymphocyte Ca2+ signalling and proliferative responses during sepsis. Shock. 1994;1:267–272. doi: 10.1097/00024382-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 19.Dickstein R, Kiremidjian-Schumacher L, Stotzky G. Effect of lidocaine on the function of immunocompetent cells. II. Chronic in vivo exposure and its effects on mouse lymphocyte activation and expression of immunity. Immunopharmacology. 1985;9:127–19. doi: 10.1016/0162-3109(85)90008-6. [DOI] [PubMed] [Google Scholar]
- 20.Burm AG, van Kleef JW, Gladines MP, Olthof G, Spierdijk J. Epidural anesthesia with lidocaine and bupivacaine: effects of epinephrine on the plasma concentration profiles. Anesth Analg. 1986;65:1281–1284. [PubMed] [Google Scholar]
- 21.Tsai PS, Buerkle H, Huang LT, Lee TC, Yang LC, Lee JH. Lidocaine concentrations in plasma and cerebrospinal fluid after systemic bolus administration in humans. Anesth Analg. 1998;87:601–604. doi: 10.1097/00000539-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Fink BR. Acute and chronic toxicity of local anaesthetics. Can Anaesth Soc J. 1973;20:5–16. doi: 10.1007/BF03025560. [DOI] [PubMed] [Google Scholar]
- 23.Tominaga M, Ku Y, Iwasaki T, Fukumoto T, Muramatsu S, Kusunoki N, et al. Effect of portal vein embolization on function of the nonembolized lobes of the liver: Evaluation by first-pass hepatic lidocaine extraction in dogs. Surgery. 2002;132:424–430. doi: 10.1067/msy.2002.126015. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki C, Kawasaki T, Ogata M, Sata T, Chaudry IH. Lidocaine enhances apoptosis and suppresses mitochondrial functions of human neutrophil in vitro. J Trauma. 2010;68:401–408. doi: 10.1097/TA.0b013e3181af6e56. [DOI] [PubMed] [Google Scholar]
- 25.Bassan MM, Weinstein SR, Mandel WJ. Use of lidocaine by continuous infusion. Am Heart J. 1974;87:302–303. doi: 10.1016/0002-8703(74)90069-6. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor RR, Thorner RE, Wright DM. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood. 1980;56:203–209. [PubMed] [Google Scholar]
- 27.Owens WE, Berg RD. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980;27:461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 29.Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med. 2000;28:N67–N77. doi: 10.1097/00003246-200004001-00008. [DOI] [PubMed] [Google Scholar]
- 30.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:20–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 32.Kempf VA, Bohn E, Noll A, Bielfeldt C, Autenrieth IB. In vivo tracking and protective properties of Yersinia-specific intestinal T cells. Clin Exp Immunol. 1998;113:429–437. doi: 10.1046/j.1365-2249.1998.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao S, Beagley KW, France MP, Shen J, Husband AJ. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology. 2000;99:464–472. doi: 10.1046/j.1365-2567.2000.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229:662–667. doi: 10.1097/00000658-199905000-00008. discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005;31:709–717. doi: 10.1007/s00134-005-2601-9. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, et al. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem. 2005;280:29409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 38.Haines KA, Reibman J, Callegari PE, Abramson SB, Philips MR, Weissmann G. Cocaine and its derivatives blunt neutrophil functions without influencing phosphorylation of a 47-kilodalton component of the reduced nicotinamide-adenine dinucleotide phosphate oxidase. J Immunol. 1990;144:4757–4764. [PubMed] [Google Scholar]
- 39.Tomoda MK, Tsuchiya M, Ueda W, Hirakawa M, Utsumi K. Lidocaine inhibits stimulation-coupled responses of neutrophils and protein kinase C activity. Physiol Chem Phys Med NMR. 1990;22:199–210. [PubMed] [Google Scholar]
- 40.Mikawa K, Akamarsu H, Nishina K, Shiga M, Obara H, Niwa Y. Effects of ropivacaine on human neutrophil function: comparison with bupivacaine and lidocaine. Eur J Anaesthesiol. 2003;20:104–110. doi: 10.1017/s026502150300019x. [DOI] [PubMed] [Google Scholar]
- 41.Fazal N, Choudhry MA, Sayeed MM. Inhibition of T cell MAPKs (Erk 1/2, p38) with thermal injury is related to down-regulation of Ca2+ signaling. Biochim Biophys Acta. 2005;1741:113–119. doi: 10.1016/j.bbadis.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 43.Manninen A, Renkema GH, Saksela K. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J Biol Chem. 2000;275:16513–16517. doi: 10.1074/jbc.M910032199. [DOI] [PubMed] [Google Scholar]
- 44.Mikawa K, Akamatsu H, Nishina K, Shiga M, Obara H, Niwa Y. Effects of ropivacaine on human neutrophil function: comparison with bupivacaine and lidocaine. Eur J Anaesthesiol. 2003;20:104–110. doi: 10.1017/s026502150300019x. [DOI] [PubMed] [Google Scholar]
- 45.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 46.Karimi K, Bienenstock J, Wang L, Forsythe P. The vagus nerve modulates CD4+ T cell activity. Brain Behav Immun. 2010;24:316–323. doi: 10.1016/j.bbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Fink BR, Cairns AM. Differential peripheral axon block with lidocaine: unit studies in the cervical vagus nerve. Anesthesiology. 1983;59:182–186. [PubMed] [Google Scholar]


