Summary
NOV-002 is a glutathione disulfide (GSSG) mimetic that is in Phase III clinical trials for the treatment of advanced non-small cell lung cancer and other oncology indications. GSSG is reduced by glutathione reductase (GR) to form glutathione (GSH), thereby maintaining redox homeostasis. The purpose of the study was to report the pharmacokinetic properties of NOV-002 and evaluate the effect that NOV-002 elicits in redox homeostasis. The pharmacokinetic analysis and tissue distribution of NOV-002 and GSH was evaluated in mice following a dose of 250 mg/kg, i.p. The redox potential and total protein thiol status was calculated. Here we show that NOV-002 is a substrate for GR and that GSH is a primary metabolite. Nonlinear pharmacokinetic modeling predicted that the estimated absorption and elimination rate constants correspond to a half-life of ~13 mins with an AUC of 1.18 μg.h/ml, a Cmax of 2.16 μg/ml and a volume of distribution of 42.61 L/kg. In addition, measurement of the redox potential and total protein thiol status indicated the generation of a transient oxidative signal in the plasma compartment after administration of NOV-002. These results indicate that NOV-002 exerts kinetic and dynamic effects in mice consistent with the GSSG component as the active pharmacological constituent of the drug. A longer-lasting decrease in total plasma free thiol content was also seen, suggesting that the oxidative effect of the GSSG from NOV-002 was impacting redox homeostasis.
Keywords: glutathione, glutathionylation, pharmacokinetics
INTRODUCTION
Oxidized glutathione (GSSG) is a homodimer of glutathione (GSH), a tripeptide composed of γ-glutamyl cysteine, glutamic acid and glycine. The GSSG/GSH redox couple is considered to be the most important determinant for regulation of cellular redox control [1]. In addition to its well-studied role in cell detoxification [2], this redox couple is now understood to be an important physiological regulator of a wide variety of cell functions. Key to this regulatory role is the ability of GSH, under certain conditions, to form reversible, mixed disulfides with low pK cysteine residues in a variety of proteins, a reaction termed protein S-glutathionylation. This post-translational modification can have an impact on a number of critical cellular processes including signaling events [3] [4].
Although all tissues have the capacity to synthesize GSH, the liver is the primary biosynthetic and storage organ for supply via sinusoidal efflux into the bloodstream. In rodents, hepatic GSH undergoes a turnover with a half-life of < 2hr [5] while in humans this value approximates 3hr [6]. Essentially no GSSG is released into the plasma, instead being directed into the cannalicular effluent and then into bile. However, GSSG is a substrate for both glutathione reductase (GR) and γ-glutamyl transpeptidase (GGT) and thus is a source of recycled GSH. In most cases the intracellular ratio of GSH: GSSG is maintained at around 100:1, but in some intracellular organelles such as the endoplasmic reticulum, there is a significant shift towards the oxidized state. In blood and plasma, the GSH: GSSG ratio is maintained at approximately 100:1, though the concentration of GSH can in some instances be 1000-fold higher in erythrocytes compared to plasma, and a significant amount of protein-bound glutathione (GSSP, ie S-glutathionylated proteins) can be present [7]. We and others have shown that the protein thiol pool is important for redox signaling events [8];[3, 9]. In fact, Hansen et al., propose that protein thiols contribute to the redox active pool as substantially as GSH and GSSG.
NOV-002, a GSSG mimetic presents a novel pharmacological approach to manipulate both the GSSG/GSH redox couple and protein S-glutathionylation for therapeutic applications. In particular, it is the subject of Phase III clinical trials for the treatment of advanced non-small cell lung cancer (NSCLC) and other oncology indications [10, 11]. Our previous studies with NOV-002, which contains GSSG and cisplatin in a 1000:1 molar ratio, have shown that in tumor cells, NOV-002 stimulates the S-glutathionylation of such target proteins as actin and also influences a number of kinase-mediated processes [9]. As previously described, the cisplatin content of NOV-002 is not sufficient to exert cytotoxicity and may act to enhance GSSG exposure and/or activity in vivo. In in vivo tumor models, NOV-002 has exhibited immunomodulatory actions including increased infiltration of memory T cells into tumors, increased cellular immune response to tumor antigen and suppression of myeloid derived suppressor cell function (manuscripts in preparation). Here we report the plasma pharmacokinetics of GSSG and GSH after NOV-002 administration to mice, as well as effects on the total pool of plasma protein thiols.
MATERIALS AND METHODS
Glutathione reductase assay
10 μg/mL NOV-002 or GSSG was incubated at 37°C in the presence of 200 μM NADPH with 0 or 0.4 ug/mL GR in McIlvaine buffer for 15 min. Samples were acidified with 100μl perchloric acid (10% v/v), spiked with the internal standard (IS), Glu-Val-Phe (1μg/ml) and transferred to borosilicate HPLC vials (MicroSolv, Eatontown, NJ) with maximum recovery inserts (Waters, Milford, MA) and 7.5μl were injected for UPLC-MS/MS analysis.
Animals
The pharmacokinetic profile of NOV-002 was studied in 6–10 week old C57BL/6 mice (N=7 animals / time point). Animals were treated with a single 250 mg/kg NOV-002 (i.p.) and sampled through retro-orbital puncture at 0, 5, 10, 15, 30, and 240 min. Blood was collected in heparin-coated tubes and immediately centrifuged at 7000×g for 5 min. Arterial plasma was removed and utilized for analysis.
Tissue distribution
Control and NOV-002 treated animals were sacrificed 30 min following administration. Liver, lung, kidney, and spleen were removed and placed in liquid nitrogen for protein thiol concentration or homogenized in 20mM formic acid for GSH/GSSG measurement. After homogenization, samples were deproteinated with 100μl perchloric acid (10% v/v) and centrifuged at 16 000 × g for 10 min. The cleared lysates (95μl), with 5μl of the IS (1μg/ml), were transferred to borosilicate HPLC vials (MicroSolv, Eatontown, NJ) with maximum recovery inserts (Waters, Milford, MA) and 7.5μl were injected for UPLC-MS/MS analysis.
UPLC-MS/MS analysis
All solvents and water were HPLC grade and were purchased from Fisher Scientific (USA). The internal standard (IS), Glu-Val-Phe, GSH and GSSG was purchased from Sigma (St-Louis, MO) and NOV-002 was a gift from Novelos Pharmaceutical. The IS, GSSG and GSH were dissolved in HPLC grade water at 10mg/ml and standard stock solutions were prepared by dilution of the standard stock solutions with HPLC grade water. A GSSG and GSH standard curve (including the IS) ranging from 0 – 40 μg/ml and 0 – 80 μg/ml were generated with R2 = 0.99.
An Acquity UPLC coupled to a Quattro Premier XE mass spectrometer (Waters, Milford, MA) was used to measure NOV-002 concentrations. Chromatographic separation was performed on an Acquity UPLC HSS C18 2.1 × 100mm (1.8μm) column preceded by a Acquity UPLC HSS C18 (1.8μm) pre-column. The mobile phases were 20mM formic acid (pH 2.2) (A) and 100% acetonitrile (B). The 7.50 min gradient was programmed as follow: 0 min, 98% A; 2 min, 98% A; 2.50 min, 50% A; 4.75 min 50% A; 4.90 min 98% A; and 7.50 min, 98% A. The flow rate was 0.2 ml/min. The mass spectrometer was operated in positive ion mode with capillary voltage 4.25 kV, source temperature 120°C, desolvation temperature 400°C and nitrogen gas flow at 1000 L/Hr. Data acquisition was performed using MassLynx 4.1 and quantification using QuanLynx 4.1 (Waters, Milford, MA). The multiple reaction monitoring (MRM) transitions were as follow: IS m/z 394 → 165.8, GSSG m/z 612.9 → 354.95 and GSH m/z 307.9 → 178.7. The cone voltages were 23V, 35V and 25V, and the collision energy 13V, 22V and 12V respectively.
PK modeling
Non-linear, least square regression analysis of plasma GSSG and GSH concentrations was performed using the pharmacokinetic modeling program WinNonLin v.4.1 (Pharsight, Cary, NC). The purpose of the modeling was to estimate standard pharmacokinetic parameters for parent drug and metabolite. Various approaches to fitting polyexponential equations to the data were tried, including the addition of a lag time and subtraction of the mean GSSG concentration at baseline (Time 0) from subsequent concentration measures.
Detection of protein thiols in plasma and tissues
100ug of protein from plasma or cell lysate was passed through BioSpin6 (BioRad, Hercules, CA) micro-column that retains both GSH and GSSG. Then 20 μl of eluent was added to 2 ml of 40 mM PB (pH=7.4) in a quartz cuvette of a PTI QM-8 spectrofluorometer (PTI Inc., Birmingham, NJ) under constant stirring at 37°C. The emission (513 nm, excitation at 379 nm) of each sample was recorded for 1 min (background) before and 2 min after an addition of 5 μM (final concentration) of ThioGlo-1 (Calbiochem). Each sample fluorescence saturation value corresponds to a concentration of free thiols. At the end of each experiment 1 μM reduced glutathione was added to the sample to ensure that saturation was not associated with the concentration of ThioGlo-1. The data represent mean ± SE (SigmaStat 10, SyStat, MA) of at least three independent measurements per condition.
Data analysis
The mean and SD were computed for each treatment group. Statistically significant differences between control and treatment groups were detected by the One Way ANOVA Test and with Tukey’s Multiple Comparison Test for all pair-wise multiple comparisons using SigmaStat 10 (SyStat, MA), [12] or SPSS 14.0 (SPSS, IL).
RESULTS
Drug pharmacokinetic studies
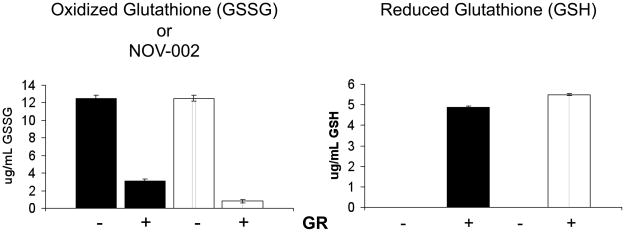
GSSG is the active pharmacological component of NOV-002 [10, 11], and GSH is proposed as a primary metabolite. Glutathione reductase (GR) is a flavoprotein that catalyzes the NAPDH-dependent reduction of GSSG to GSH and as such, the activity of GR is important in maintaining the ratio of GSSG / GSH. Here we validate that NOV-002 is a substrate of GR and is converted to GSH in vitro, Figure 1. In the absence of GR, neither GSSG nor NOV-002 yielded detectable levels of GSH. However, following 15 min incubation with GR and NADPH, GSSG was diminished from 12.5± 0.35 ug/mL to 0.8 ± 0.2 ug/mL; while the GSSG component of NOV-002 equally diminished 12.5± 0.4 ug/mL to 3.1 ± 0.2 ug/mL. Conversion of GSSG or NOV-002 by GR led to the formation of 5.5 ± 0.05 ug GSH/mL and 4.9 ± 0.08 ug GSH/mL, respectively. These results suggest that NOV-002 can be metabolized in vivo to GSH via GR. Activity of GR maintains the molar ratio of GSSG:GSH blood and bodily fluids [13].
Figure 1. NOV-002 is a substrate for glutathione reductase.
10 μg/ml NOV-002 was treated with 0 or 0.4 μg/ml glutathione reductase in the presence of 200 μM NADPH. Mean concentration profiles for (A) GSSG and (B) GSH were obtained by HPLC-MS analysis. Data are represented as mean ± SEM of n = 3 per group.
Using HPLC-MS detection, we measured the pharmacokinetics of the active component (GSSG) of NOV-002 and its metabolite (GSH) following a single administration (250 mg/kg, i.p.). Data available for pharmacokinetic analysis included 33 plasma concentration measurements of GSSG and 31 values for GSH derived from blood sampled from individual animals at time 0 (before drug administration) 5, 10, 15, and 30 minutes following drug administration. Seven additional concentration values determined from samples collected 4 hours following drug administration confirmed that GSSG and GSH values had returned to baseline by this point and these values were not subjected to further analysis.
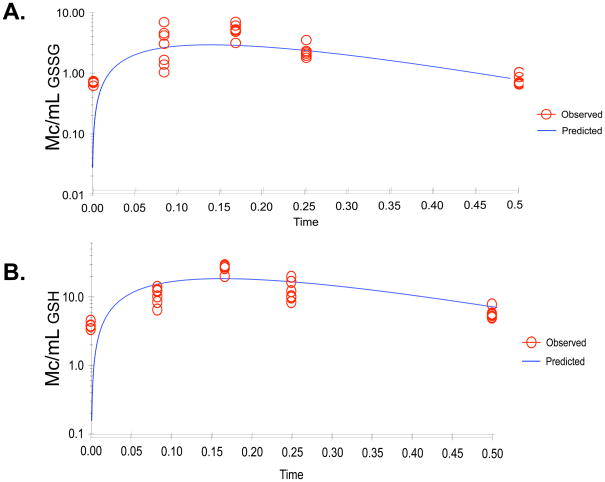
Both the parent compound (GSSG) and primary metabolite (GSH) were measurable at all time points and the concentration of GSH exceeded that of GSSG. Concentrations increased following drug administration to a peak and then declined in an apparent log-linear manner. Based on this observation, an initial structural model consisted of a one-compartment open model with first-order absorption and elimination, Figure 3. Estimates of standard pharmacokinetic parameters are given in Table 1 and the concentration versus time course is shown in Figure 3 for the observed versus the predicted data. For the metabolite, the area under the concentration versus time curve was calculated to the last measured time point using the trapezoidal rule and compared to that of the parent drug. Regression analysis of metabolite data allowed estimation of a terminal elimination rate constant. As the dose of metabolite depends upon the fraction of drug biotransformed to individual metabolites, we were unable to estimate clearance of GSH.
Figure 3. Non-linear pharmacokinetic estimation of NOV-002.
The observed (O) and expected (---) plasma concentrations are plotted as a function of time for A) GSSG, the active component of NOV-002 and B) GSH, the primary metabolite. Satisfactory algorithm convergence and acceptance of the modeling results was based on standard methods, including the likelihood ratio test, Akaike’s Information Criterion, goodness of fit plots, and visual inspection of the observed versus predicted concentration-time values. Data were best fit by the initial one-compartment open model with first order absorption and elimination.
Table 1.
Estimated pharmacokinetic parameters for NOV-002 (GSSG) and its primary metabolite (GSH)
| PK Parameter | GSH | GSSG |
|---|---|---|
| Co (ug/mL) | 4.10 | 0.65 |
| Cmax (ug/mL) | 18.30 | 2.16 |
| Tmax (hr) | 0.16 | 0.15 |
| Volume of distribution, V/F (L/kg) | 42.61 | |
| K01 (absorption rate constant), hr−1 | 6.42 | |
| K10 (elimination rate constant), hr−1 | 5.81 | 6.40 |
| AUC 0–30min (ug.h/mL) | 6.37 | 1.18 |
| Clearance, CL/F (L/hr/kg) | 273.15 |
As expected, the range of concentrations showed greater variability in the initial samples following administration when variability in the absorption is likely to be the highest. The estimated absorption and elimination rate constants correspond to an absorption and elimination half-life of about 6.5 minutes. This similarity suggests that the elimination of GSSG may be rate-limited by its absorption. The relatively rapid elimination suggests multiple doses or infusions may be needed to sustain drug concentrations in an effective range. The slightly slower elimination of the metabolite (elimination half-life of 5.8 minutes) compared to GSSG is consistent with the expectation that metabolites are cleared more slowly than they are formed and in keeping with the substantially higher plasma concentrations reflected by an AUC ratio of metabolite to parent drug of 5.39. The values for clearance and for volume of distribution of GSSG may be inflated by an unknown contribution of incomplete bioavailability (F) from non-systemic drug administration. Overall, the data are consistent with a rapidly absorbed and metabolized drug with considerable variability in clearance. These estimates provide initial and fundamental values of disposition parameters in a rodent species to aid future experimental design.
GSSG and GSH were measured in the liver, lung, kidney and spleen 30 min following treatment, Table 2. NOV-002 treatment led to significant increases in the GSSG content in all tissues, while the GSH concentrations were significantly elevated in the kidney. This is consistent with our previous findings that NOV-002 is nephroprotective against cisplatin-induced kidney damage [14].
Table 2.
Effects on GSSG and GSH levels in tissues 30 min following NOV-002 treatment. Data are represented as the mean ± SEM
| Tissue | GSH ng/ug | GSSG (ng/ug) | |
|---|---|---|---|
| Liver | Control | 29.9 ± 7.9 | 1.22 ± 0.19 |
| NOV-002 | 25.4 ±4.9 | 2.36 ± 0.35 * p= 0.01 | |
| Kidney | Control | 6.8 ±0.6 | 0.29 ± 0.03 |
| NOV-002 | 11.5 ±1.5 * p=0.01 | 1.30 ± 0.32 * p= 0.01 | |
| Lung | Control | 3.2 ±0.9 | 0.13 ± 0.01 |
| NOV-002 | 2.9 ±0.4 | 3.55 ± 1.00 * p= 0.005 | |
| Spleen | Control | 14.6 ± 5.1 | 1.12 ± 0.19 |
| NOV-002 | 12.6 ± 1.9 | 5.41 ± 1.19 * p= 0.003 | |
Redox Modulation
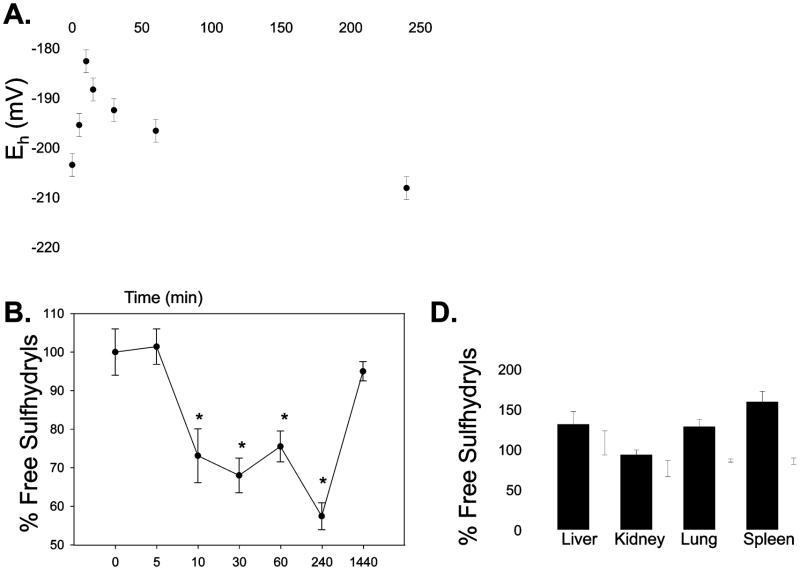
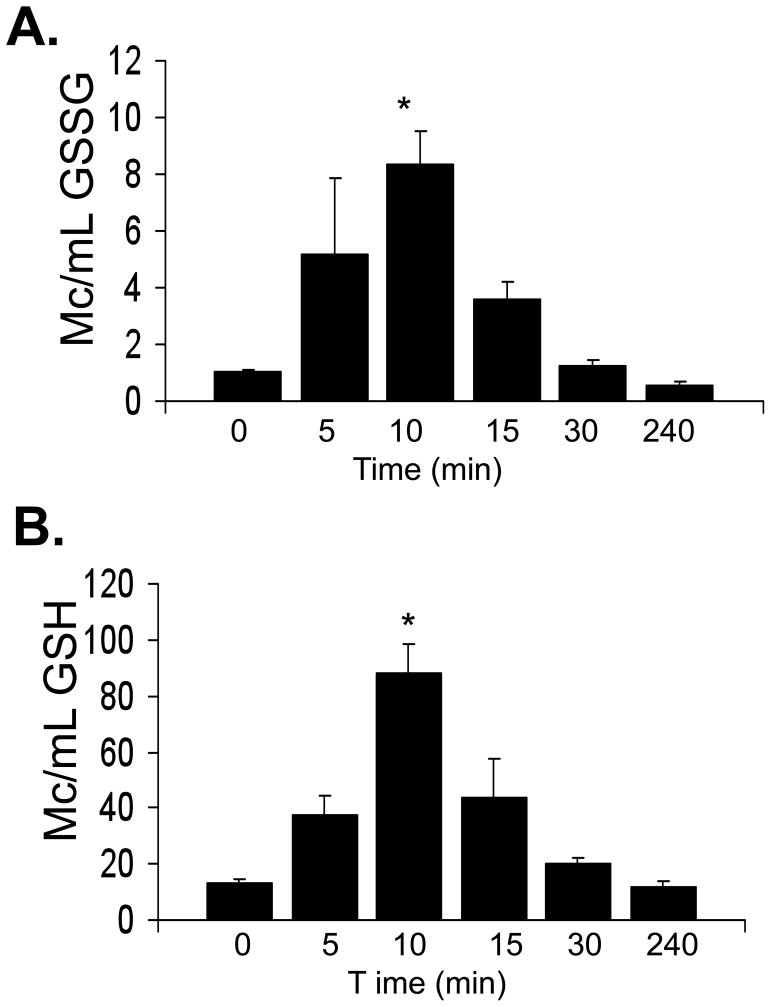
The ratio of GSSG/GSH is an important determinant of redox homeostasis. We measured the redox potential in the arterial plasma prior to and following NOV-002 treatment, as previously described [15]. Initial concentrations (Co) were 4.1 ± 0.2 ug/mL (GSH) and 0.65 ± 0.92 (GSSG) which correspond to a redox potential (Eh) of −203 ± 1.6 mV. Peak plasma concentrations of GSH and GSSG were observed at 10 minutes and were ~6–8 fold higher than Co, respectively. The corresponding redox potential (Eh = − 182 ± 4.6 mV) was significantly different, suggesting a shift towards oxidizing conditions (Figure 4). Plasma concentrations of both GSH and GSSG were restored to baseline levels within 30 min. In a separate group of animals, we assessed the bone marrow, liver and kidney toxicity following NOV-002 treatment. The complete blood counts and blood chemistry analysis of renal and liver function were indistinguishable in the control and NOV-002 treated groups (data not shown). These data are consistent with clinical results showing the drug is not toxic toward normal cells [10, 11].
Figure 4. Effect of NOV-002 on plasma redox potential and free sulfhydryls.
Mice were treated with 250 mg/kg NOV-002 (i.p.). Orbital blood was collected at 0, 5, 10, 15, 30 and 240 min. A) The Eh GSSG/GSH was calculated from the GSSG and GSH concentrations using the Nernst equation. B) Schematic illustrating how the fluorescent probe, ThioGlo™, binds to free thiols. S-Glutathionylated cysteine residues cannot bind ThioGlo and thereby have lower fluorescence intensities. C) Free protein thiol content of plasma proteins was measured at 0, 5, 10, 15 and 30 min following 250 mg/kg (i.p.). D) Free protein thiol content was measured in control (black) and NOV-002 treated (white) tissues 30 minutes following treatment. The ThioGlo-1 emission (at 513nm) for each treatment group was averaged and plotted as mean ± SD (n = 6); (*=p≤0.001).
In prior studies, we showed that NOV-002 led to a transient oxidative state in human myeloid cells that was concurrent with an increase in proliferation rate and S-glutathionylation of proteins [9]. We propose that oxidation of essential cysteine residues in plasma proteins may reflect in vivo effects of NOV-002 that outlast increased plasma levels of GSH or GSSG. In fact, Hansen et al., (2009) demonstrated that the contribution of protein thiols in living cells could be of equivalent importance to that of GSH/GSSG [8]. To address this issue, we measured the global status of protein thiols in arterial plasma and tissues. We measured total free thiol (SH) groups in plasma using a fluorescent molecular probe, ThioGlo™-1 which shows enhanced fluorescence upon binding to free thiols as depicted in the cartoon in panel B of Figure 4 [16, 17]. We observed a significant oxidation of protein SH groups in plasma from NOV-002 treated animals. Within 10 min, only 73 ± 6% of pre-dose SH groups were detectable. Interestingly, the oxidation of protein SH groups persisted for > 4h after NOV-002 administration, even though plasma GSH/GSSG levels were restored to pre-dose levels within 30 min. Plasma protein free thiols returned to pre-dose levels within 24h. In contrast, the protein thiols in the liver, lung and brain following NOV-002 had fewer oxidized proteins. Concurrently, the GSSG pools in the liver, lung, spleen and kidney were significantly elevated relative to untreated animals.
DISCUSSION and CONCLUSIONS
The studies presented here add to our understanding of the preclinical pharmacology of NOV-002, the presumed mechanism of action of which is based on a growing understanding of the role of the glutathione system in the regulation of cell function in both normal and tumor cells [5,6,7]. Our results confirm that, even at a relatively high single-dose level compared to that used in clinical studies (250 mg/kg and ~1 mg/kg, respectively), NOV-002 had no apparent toxicity in mice.
In common with GSSG itself, NOV-002 results in oxidative signaling, protein S-glutathionylation and changes in signaling pathway protein function in both cell-free and cellular systems [18, 19]. Indeed, although generally impermeable to cells, exogenous GSSG can cause a rapid change in intracellular GSH levels by shifting the equilibrium towards the formation of mixed disulfides with protein thiols [20]. Such responses could be mediated by specific trans-membrane proteins, rich in cysteine residues, which transduce the oxidizing power of GSSG across the plasma membrane through a thiol/disulfide exchange. Indeed, we have shown that S-glutathionylation of cell surface proteins and intracellular levels of GSSG are increased after cells are exposed in vitro to NOV-002 [9]. Significantly, the signaling pathway regulatory effects of redox changes (including those inducible by GSSG itself) are dependent on the type of cell and its physiological state with inhibitory, stimulatory or no effects on pathway elements being possible. Some examples of effects on gene expression include regulation of gene transcription factors such as NFkB, Nrf2 and AP-1, which have roles in the regulation of many genes involved in immune and inflammatory responses, including cytokines and growth factors [21, 22]. The activities of other immune/inflammation regulatory proteins are also sensitive to GSH/GSSG (e.g. mitogen-activated MAP kinases; [20]). In addition, changes in GSH/GSSG can modulate protein phosphorylation in signaling pathways, further amplifying the impact of redox changes on cell function [19]. In this report, we have presented data demonstrating that NOV-002 also resulted in generation of an oxidative signal in the plasma compartment after i.p. administration in vivo.
Assessment of plasma levels of GSSG and GSH in mice after i.p. administration of NOV-002 revealed a rapid and transient increase in both analytes associated with a significant shift of redox potential in the plasma compartment towards a more oxidized state. In contrast to this transient redox potential change which peaked at 10 minutes post-dose, NOV-002 administration also resulted in a significant reduction in plasma protein free thiol content lasting at least 4 hours post-dose and returning to normal by 24 hours. This decrease in free thiol content reflects S-glutathionylation of plasma proteins after NOV-002 and, particularly if it also occurs in other tissues, could be responsible for pharmacological effects that substantially outlast increases in plasma levels of GSSG or GSH.
Figure 2. Effect of NOV-002 on plasma GSSG and GSH.
Mice were treated with 250 mg/kg NOV-002 (i.p.). Orbital blood was collected at 0, 5, 10, 15, 30 and 240 min. Mean plasma concentration-time profiles for (A) GSSG and (B) GSH were obtained by HPLC-MS analysis. Data are represented as mean ± SD of n=7 animals per time-point.
Acknowledgments
This study was supported by the National Cancer Institute grants CA08660 and CA117259. We thank the Drug Metabolism and Pharmacokinetics and Proteomics Core Facilities of the Hollings Cancer Center.
Abbreviations
- GGT
γ-glutamyl transpeptidase
- GSH
glutathione reduced
- GSSG
oxidized
- GR
glutathione reductase
- Cmax
peak plasma concentration
- C0
baseline plasma concentration
- Tmax
time to reach peak plasma concentration
- AUC 0–30min
total area under the plasma concentration-time curve from time zero to 30 minutes
- CL
average time for total body clearance
- K01
absorption rate constant
- K10
elimination rate constant
- i.p
intraperitoneal injection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 2.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54(16):4313–20. [PubMed] [Google Scholar]
- 3.Townsend DM. S-Glutathionylation: What are the Consequences to Cell Injury and the Unfolded Protein Response? Molecular Interventions. 2007;7:313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalle-Donne I, et al. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43(6):883–98. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–44. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 6.Burgunder JM, Lauterburg BH. Decreased production of glutathione in patients with cirrhosis. Eur J Clin Invest. 1987;17(5):408–14. doi: 10.1111/j.1365-2362.1987.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman WA, et al. Protein glutathiolation in human blood. Biochem Pharmacol. 2003;65(5):741–6. doi: 10.1016/s0006-2952(02)01560-5. [DOI] [PubMed] [Google Scholar]
- 8.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A. 2009;106(2):422–7. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend DM, He L, Hutchens S, VandenBerg TE, Pazoles CJ, Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Research. 2008;68:2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend DM, Pazoles CJ, Tew KD. NOV-002, a mimetic of glutathione disulfide. Expert Opin Investig Drugs. 2008;17(7):1075–83. doi: 10.1517/13543784.17.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend DM, Tew KD. Pharmacology of a mimetic of glutathione disulfide, NOV-002. Biomed Pharmacother. 2009;63(2):75–8. 12. doi: 10.1016/j.biopha.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tukey JW. Comparing individual means in the analysis of variance. Biometrics. 1949;5(2):99–114. [PubMed] [Google Scholar]
- 13.Manso C, Wroblewski F. Glutathione reductase activity in blood and body fluids. J Clin Invest. 1958;37(2):214–8. doi: 10.1172/JCI103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenderny S, et al. Protective effects of a glutathione disulfide mimetic (NOV-002) against cisplatin induced kidney toxicity. Biomed Pharmacother. 2009 doi: 10.1016/j.biopha.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones DP, et al. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28(4):625–35. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 16.Townsend DM, et al. Novel role for glutathione S-transferase {pi}: regulator of protein S-glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284(1):436–45. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabisiak JP, Sedlov A, Kagan VE. Quantification of oxidative/nitrosative modification of CYS(34) in human serum albumin using a fluorescence-based SDS-PAGE assay. Antioxid Redox Signal. 2002;4(5):855–65. doi: 10.1089/152308602760599016. [DOI] [PubMed] [Google Scholar]
- 18.Droge W, et al. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994;8(14):1131–8. [PubMed] [Google Scholar]
- 19.Filomeni G, et al. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells. Free Radic Biol Med. 2005;39(3):345–54. doi: 10.1016/j.freeradbiomed.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Filomeni G, Rotilio G, Ciriolo MR. Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. FASEB J. 2003;17(1):64–6. doi: 10.1096/fj.02-0105fje. [DOI] [PubMed] [Google Scholar]
- 21.Pineda-Molina E, et al. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40(47):14134–42. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 22.Rahman I. Regulation of nuclear factor-kappa B, activator protein-1, and glutathione levels by tumor necrosis factor-alpha and dexamethasone in alveolar epithelial cells. Biochem Pharmacol. 2000;60(8):1041–9. doi: 10.1016/s0006-2952(00)00392-0. [DOI] [PubMed] [Google Scholar]