Abstract
Rationale: Mice with genetic deletion of the cholesterol efflux transporter, ATP-binding cassette (ABC) G1, have pulmonary lipidosis and chronic pulmonary inflammation. Whether ABCG1 regulates host defense is unknown.
Objectives: To determine whether ABCG1 regulates pulmonary innate immunity and host defense, and to investigate the underlying molecular/cellular mechanisms.
Methods: Abcg1+/+ and Abcg1−/− mice were challenged with intrapulmonary lipopolysaccharide (LPS) or Klebsiella pneumoniae, intravenous K. pneumoniae, or intraperitoneal LPS. Phenotypic responses were profiled. Bone marrow chimeras and in vitro assays were used to differentiate and characterize the role of hematopoietic versus nonhematopoietic ABCG1 in host defense.
Measurements and Main Results: Unexposed Abcg1−/− mice had normal numbers of circulating neutrophils, but increased neutrophil recruitment to the airspace and lung parenchyma, and increased airspace cytokines and chemokines in the steady state. After intrapulmonary LPS or K. pneumoniae, Abcg1−/− mice displayed exaggerated further neutrophil recruitment to and degranulation in the airspace, and elevated airspace cytokine/chemokine induction. Alveolar macrophage ABCG1 was critical, as ABCG1 deficiency in hematopoietic cells was sufficient to enhance responses in vivo, and Abcg1−/− alveolar macrophages adopted a “foam cell” phenotype, and were hyperresponsive ex vivo. Pulmonary compartmentalization and clearance of K. pneumoniae were increased in Abcg1−/− mice, indicating enhanced host defense. By contrast, Abcg1+/+ and Abcg1−/− mice had equivalent responses to intravenous K. pneumoniae and intraperitoneal LPS, suggesting that ABCG1 regulates innate immunity in a tissue-selective manner.
Conclusions: Abcg1−/− mice have an enhanced pulmonary host defense response driven predominantly by hematopoietic cells.
Keywords: ATP binding cassette G1, cholesterol, innate immunity, lung, pneumonia
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The cholesterol transporter ATP-binding cassette (ABC) G1 regulates cholesterol efflux and Toll-like receptor responses in macrophages, and cholesterol homeostasis in the lung. Whether ABCG1 regulates host defense is undetermined.
What This Study Adds to the Field
We report that ABCG1 negatively regulates innate immunity and antibacterial host defense in the lung, but not the bloodstream. ABCG1 deficiency in hematopoietic cells is sufficient to enhance cytokine induction in and neutrophil recruitment to the infected lung, enhancing bacterial clearance. These results identify ABCG1 as a link between cholesterol homeostasis and host defense in the lung.
Cellular cholesterol potentiates several inflammatory functions of the macrophage. Overloading of cultured macrophages with exogenous cholesterol induces cytokines through endoplasmic reticulum stress (1, 2), and metabolic overloading through deficiency of the cholesterol efflux transporter, ATP-binding cassette (ABC) G1 expands lipid raft microdomains of the plasma membrane, priming the macrophage for much more robust responses to Toll-like receptor (TLR) ligands (3). On the other hand, depletion of raft cholesterol attenuates TLR4 activation (3). Whereas in vivo investigations have greatly advanced our understanding of interactions between cholesterol and innate immunity in macrophages and other cells of the arterial wall, little is known about whether cholesterol impacts immune responses in the macrophage-rich lung. Given the high prevalence of dyslipidemia and lower respiratory tract infection in the United States, and reports that cholesterol-active therapies impact pulmonary innate immunity (4, 5), interactions in the lung between cholesterol and host defense may indeed bear great therapeutic and public health significance.
ABCG1, a plasmalemmal transporter that regulates cellular cholesterol levels by effluxing cholesterol to high-density lipoprotein particles, is highly expressed in both alveolar macrophages and alveolar epithelial type II cells (6–8). Of interest, Abcg1−/− mice have marked pulmonary lipidosis and increased surfactant phospholipids (8), and patients with pulmonary alveolar proteinosis have deficient alveolar macrophage ABCG1 (9), indicating the pivotal importance of steady-state cholesterol trafficking by this transporter to lung physiology. Moreover, reminiscent of the inflammatory effects of cholesterol loading of isolated macrophages, it has recently been reported that Abcg1−/− mice have basal induction of cytokines and chemokines in the lung driven by alveolar macrophage cholesterol overload (2, 10). The role of ABCG1 in regulation of pulmonary innate immunity and host defense is, however, undescribed; indeed, we are unaware of any prior investigation of ABCG1 in infection. We hypothesized that, given the marked pulmonary lipidosis phenotype, ABCG1 deficiency would selectively “prime” the lung for more robust responses to both lipopolysaccharide (LPS) and gram-negative bacteria, enhancing host defense in the lung, but not in the bloodstream.
Herein, we confirm a suppressive role for ABCG1 in the pulmonary host defense response. Unexposed Abcg1−/− mice have normal numbers of circulating neutrophils (PMNs), but increased steady-state PMN recruitment to the airspace and lung parenchyma, and basal induction in the airspace of cytokines. After inhalation of LPS or Klebsiella pneumoniae, Abcg1−/− mice develop a more robust immune response in the airways than their wild-type (WT) counterparts, characterized by enhanced production of multiple cytokines, increased PMN recruitment, and enhanced degranulation of PMNs into the airspace. Alveolar macrophage ABCG1 plays a critical role in the phenotype, as hematopoietic deficiency of ABCG1 is sufficient to enhance responses to bacterial exposure in vivo, and ABCG1-deficient alveolar macrophages display enhanced responses ex vivo. Abcg1−/− mice are more effective at clearing bacteria from the lungs and at limiting extrapulmonary bacterial dissemination, indicating enhanced pulmonary host defense. On the other hand, they have normal responses to systemic LPS and K. pneumoniae, suggesting selectivity of the phenotype for route of infection. Together, these data indicate that ABCG1 plays a critical regulatory role in the response to gram-negative bacteria in the lung. Some of the results of these studies have been previously reported in the form of an abstract (11).
METHODS
Reagents
Escherichia coli 0111:B4 LPS, penicillin, and streptomycin were from Sigma (St. Louis, MO). K. pneumoniae 43,816 (serotype 2), Dulbecco's modified Eagles medium, and fetal bovine serum were from American Type Culture Collection (Rockville, MD). Macrophage inflammatory protein (MIP)–2 and keratinocyte-derived chemokine (KC) were from Peprotech (Rocky Hill, NJ). Tumor necrosis factor (TNF)–α and IL-6 ELISA kits were from Ebioscience (San Diego, CA). LPS-induced CXC chemokine (LIX) ELISA was from R&D systems (Minneapolis, MN). Bioplex assays for KC, MIP-2, monocyte chemoattractant protein (MCP)–1, IL-17, TNF-α, IL-6, and the Bio-Rad protein assay were from Bio-Rad (Hercules, CA). The Hema 3 staining system was from Fisher Scientific (Pittsburgh, PA).
Animals
Age- (8–12 wk) and sex-matched mice were used for all experiments. Abcg1−/− mice backcrossed more than six generations onto C57BL/6 (98.2–99.1% C57BL/6 across 110 microsatellite markers [data not shown]) were generated as described previously (6). C57BL/6 control animals were purchased from Jackson Laboratory (Bar Harbor, ME). All experiments were performed in accordance with the Animal Welfare Act and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals after approval by the Animal Care and Use Committee of the National Institute of Environmental Health Sciences. Anesthesia was induced by flow-regulated isofluorane, and animals were killed by intraperitoneal Fatal-Plus solution (Vortech Pharmaceuticals, Ltd.; Dearborn, MI).
In Vivo Exposures
Mice were exposed to aerosolized E. coli 0111:B4 LPS (300 μg/ml, 30 min), as previously described (5). In other experiments, mice received intraperitoneal LPS (2 mg/kg in phosphate-buffered saline [PBS]). K. pneumoniae was delivered by oropharyngeal aspiration under anesthesia (500 or 2,000 cfu), or intravenously (7 × 104 cfu in 200 μl PBS) into the retro-orbital venous plexus during anesthesia.
Bronchoalveolar Lavage Fluid Collection and Analysis
Bronchoalveolar lavage fluid (BALF) was collected as previously described (5). Quantitation and differential staining of leukocytes (white blood cells [WBCs]) was as previously described (5). BALF protein was quantified by the method of Bradford (12), and BALF myeloperoxidase (MPO) by EnzChek MPO assay kit from Invitrogen (Carlsbad, CA). BALF cytokines/chemokines were measured by ELISA or Bio-Plex assay.
Bacterial Quantification
Bacteria were quantified as previously described (5). Briefly, lung and spleen were homogenized in sterile PBS (2.0 ml) immediately after death, and serial dilutions of these or blood were plated in duplicate on tryptic soy agar. After overnight incubation (37°C), cfu were counted.
Generation of Bone Marrow Chimeras
For bone marrow transplantation, Abcg1+/+ mice that were congenic for CD45 (stock no. 002014; Jackson Laboratory) were used. Recipients were lethally irradiated (900 rad) by a Model 431 irradiator using a 137Cs source (JL Shepherd and Associates, San Fernando, CA). Within 4 hours after irradiation, donor-derived bone marrow from femurs and tibias (2 × 106 cells) was injected intravenously into recipients. The efficiency of donor stem cell engraftment was determined by flow cytometry for CD45.1 (Abcg1+/+) 9 weeks after transfer on circulating PMNs (Gr-1+) and B lymphocytes (B220+), and at 10 weeks (death) on alveolar macrophages and dendritic cells (CD11c+). Engraftment efficiency within all experimental animals was greater than 94% (see Figure E1 in the online supplement).
Statistical Analysis
A two-tailed Student's t test was applied for comparisons of two groups (GraphPad Prism software; GraphPad Inc., San Diego, CA). Survival was tested by a parametric analysis method that assumed a Weibull distribution. Data are represented as means (±SEM). For all tests, a P value of less than 0.05 was considered significant.
Additional methodological details are provided in the online supplement.
RESULTS
Increased Steady-State Leukocyte Recruitment to and Cytokine Induction in Abcg1−/− Lungs
Recent reports have revealed that Abcg1−/− mice develop chronic pulmonary inflammation that is accompanied by progressive pulmonary lipidosis (2, 8, 10). Consistent with these reports, we found that BALF from unexposed Abcg1−/− mice contained a 2.1-fold increase in total WBCs compared with BALF from Abcg1+/+ mice (Figure E2A). As expected, BALF WBCs from Abcg1+/+ mice were mostly (99.72 ± 0.20%) alveolar macrophages, with a small number of lymphocytes, but no detectable PMNs. In contrast, in addition to increases in the absolute number of alveolar macrophages and lymphocytes, PMNs were readily detectable in BALF from naive Abcg1−/− mice (3.89 ± 1.49% of total WBCs). MPO, a granule protein that serves as a biomarker of PMNs (4), was also higher in lung homogenates from Abcg1−/− mice compared with Abcg1+/+ mice (Figure E2B), suggesting elevated total lung parenchymal PMN burden. Alveolar macrophages from Abcg1−/− mice were large and foamy in appearance (Figure E2C), consistent with accumulation of intracellular lipids, as previously reported (2, 8, 10). Multiple cytokines and chemokines known to recruit PMNs to the lung, including TNF-α, IL-6, MIP-2, KC, and LIX, were elevated in BALF from naive Abcg1−/− mice (Figure E2D). Finally, BALF total protein, a surrogate indicator of compromised alveolocapillary barrier integrity (4), was significantly higher in Abcg1−/− compared with WT mice (Figure E2E). Taken together, these findings indicate that ABCG1 deficiency induces marked steady-state pulmonary inflammation.
Abcg1−/− Mice Have Enhanced Responses to Inhaled, but Not Systemic, LPS
In light of these steady-state findings, we hypothesized that Abcg1−/− mice would be primed for a more robust pulmonary innate immune response upon challenge. However, it also appeared plausible that the ABCG1-deficient lung might be hyporesponsive. For example, lungs preinflamed by exposures, including hyperoxia, ozone, and LPS, are reported to have attenuated subsequent responses to LPS (13–15). Moreover, the lungs in Abcg1−/− mice are reported to have increased apoptosis and apoptotic cell clearance (efferocytosis) (10), and efferocytosis reduces responses in the lung to LPS and bacteria (16, 17).
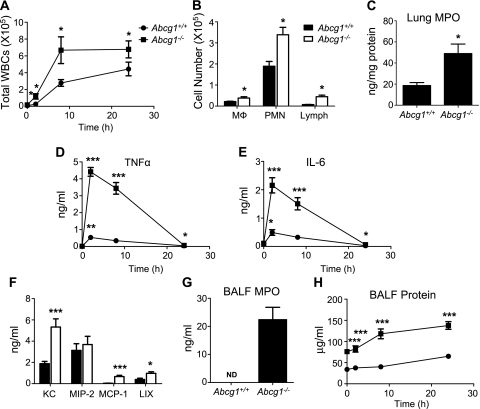
To resolve this issue, we exposed mice to aerosolized LPS by an established method (5). An enhanced response to LPS was indeed observed in Abcg1−/− mice (Figure 1). Enhanced WBC recruitment into the airways was observed as early as 2 hours after exposure, and continued throughout a 24-hour time course (Figure 1A). The increase in BALF WBCs predominantly reflected PMN recruitment; however, significantly higher numbers of macrophages and lymphocytes were also present in the airway (Figure 1B). MPO activity was also significantly higher in Abcg1−/− than in Abcg1+/+ lung parenchymal homogenates 8 hours after LPS inhalation (Figure 1C). Higher levels of TNF-α and IL-6 were present in BALF from Abcg1−/− mice at all time points compared with Abcg1+/+ mice (Figures 1D–1E). Increased levels of KC, a PMN chemokine, and MCP-1, a macrophage chemokine, were also observed (Figure 1F). In addition, an increase in LIX, a CXC chemokine of epithelial origin that promotes PMN migration to the lung (18), was found in BALF from Abcg1−/− mice, whereas BALF MIP-2 was equivalent between Abcg1−/− and Abcg1+/+. Given that cytokines promote PMN degranulation (19), which can, in turn, promote further tissue injury, we measured cell-free levels of MPO in BALF. Whereas soluble MPO was below the limit of detection in BALF 8 hours after LPS exposure in Abcg1+/+ mice (a time point at which there were significant numbers of BAL PMNs present [Figure 1A]), it was readily detectable in BALF from Abcg1−/− mice (Figure 1G), suggesting higher PMN activation. BALF total protein content was higher in Abcg1−/− than Abcg1+/+ mice at all time points after LPS exposure (Figure 1H).
Figure 1.
The pulmonary response to inhaled lipopolysaccharide (LPS) is enhanced in ATP-binding cassette (Abc) g1−/− mice. Abcg1+/+ and Abcg1−/− mice were challenged with aerosolized LPS. (A) BALF total leukocytes (white blood cells [WBCs]) were counted at 2, 8, and 24 hours after LPS exposure (*P < 0.05). (B) Bronchoalveolar lavage fluid (BALF) macrophages (Mϕ), polymorphonuclear neutrophils (PMNs), and lymphocytes (Lymph) were counted 8 hours after LPS exposure (*P < 0.05). (C) Myeloperoxidase (MPO) activity was quantified in whole-lung homogenates 8 hours after LPS (*P < 0.05). (D and E) BALF tumor necrosis factor (TNF)–α and IL-6 protein was quantified by ELISA at 2, 8, and 24 hours after LPS exposure (*P < 0.05; **P < 0.01; ***P < 0.001). (F) BALF keratinocyte-derived chemokine, macrophage inflammatory protein (MIP)–2, and monocyte chemoattractant protein (MCP)–1 protein levels were quantified by Bioplex assay (*P < 0.05; ***P < 0.001), and BALF LPS-induced CXC chemokine by ELISA (*P < 0.05). (G) BALF MPO was quantified 8 hours after LPS (*P < 0.05). (H) BALF total protein content was quantified at 2, 8, and 24 hours after LPS by Bradford assay (***P < 0.001). Data shown are representative of two independent experiments involving 10 mice per genotype.
To rule out the possibility that enhanced PMN recruitment after LPS exposure simply reflected relative circulating neutrophilia in Abcg1−/− mice, we enumerated peripheral leukocyte populations in the blood of Abcg1+/+ and Abcg1−/− mice. There was no difference between genotypes in the numbers of PMNs, monocytes, lymphocytes, or eosinophils in blood (Table 1), suggesting that the pulmonary phenotype observed in Abcg1−/− mice reflects regulatory defects specific either to the lung or to leukocyte trafficking to the lung.
TABLE 1.
PERIPHERAL BLOOD LEUKOCYTE AND DIFFERENTIAL COUNT IN Abcg1+/+ AND Abcg1−/− MICE
| Cell Count x 103/μl |
||
|---|---|---|
| Cell Type | Abcg1+/+ (n = 9) | Abcg1−/− (n = 9) |
| WBCs | 3.7 ± 0.9 | 3.5 ± 0.5 |
| Neutrophils | 0.42 ± 0.11 | 0.49 ± 0.23 |
| Monocytes | 0.02 ± 0.04 | 0.05 ± 0.06 |
| Lymphocytes | 2.68 ± 0.6 | 2.88 ± 0.3 |
| Eosinophils | 0.10 ± 0.08 | 0.11 ± 0.07 |
Definition of abbreviations: Abcg1 = ATP-binding cassette G1; WBC, white blood cells.
Values are means ± SD.
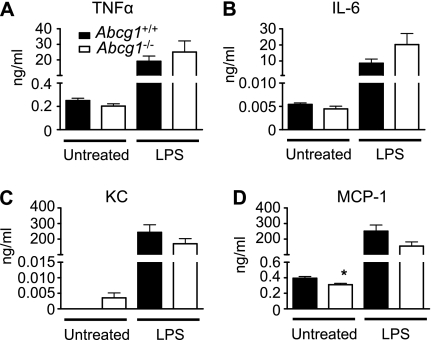
To determine whether extrapulmonary responses to bacterial stimulation were also enhanced in Abcg1−/− mice, we administered LPS systemically via intraperitoneal injection and measured proinflammatory cytokine/chemokine levels in blood 2 hours later. Intraperitoneal LPS resulted in a dramatic increase in serum concentrations of TNF-α, IL-6, KC, and MCP-1 over baseline levels. However, no difference was observed between Abcg1+/+ and Abcg1−/− mice (Figure 2). This observation suggests that, unlike the lung, ABCG1 does not regulate the response to LPS in the systemic compartment. Taken together, our results suggest that ABCG1 regulates responses to bacterial stimulation in a tissue-selective manner.
Figure 2.
ATP-binding cassette (Abc) g1−/− mice have a normal serum response to systemic lipopolysaccharide (LPS). Abcg1+/+ and Abcg1−/− mice (n = 12/group) received intraperitoneal LPS (2 mg/kg) or were left untreated. Serum levels of cytokines (A and B) and chemokines (C and D) were quantified by Bioplex assay 2 hours after injection (*P < 0.05).
ABCG1 Does Not Directly Regulate PMN Migration
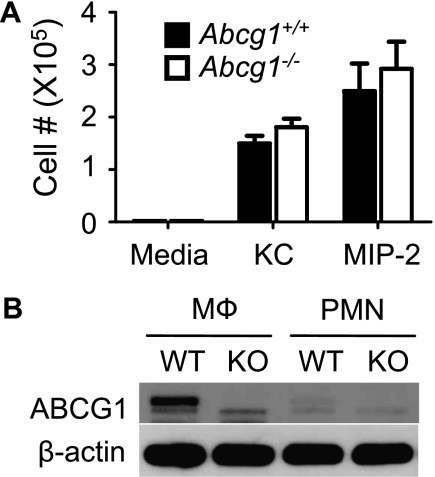
To determine whether ABCG1 also directly regulates intrinsic PMN migratory function in response to chemokines, we analyzed chemotaxis of bone marrow–derived PMNs from Abcg1−/− and Abcg1+/+ mice in vitro. As shown in Figure 3A, there was no difference, compared with WT, in the ability of Abcg1−/− PMNs to undergo directional migration toward KC or MIP-2. Indeed, immunoblotting revealed that, compared with WT peritoneal macrophages, WT bone marrow–derived PMNs express very little ABCG1 protein (Figure 3B), suggesting that genetic deletion of ABCG1 may not significantly impact native PMN functions. These results indicate that the exaggerated recruitment of PMNs into the airways of Abcg1−/− mice is likely due to the observed enhancement of airspace chemokines.
Figure 3.
ATP-binding cassette (ABC) G1 does not directly regulate neutrophil migration. (A) Chemotaxis of bone marrow–derived neutrophils isolated from Abcg1+/+ and Abcg1−/− mice toward keratinocyte-derived chemokine (25 ng/ml) and macrophage inflammatory protein (MIP)–2 (5 ng/ml) was measured using a Transwell system. (B) Protein immunoblot analysis of ABCG1 and β-actin control in whole-cell lysates of peritoneal exudate macrophages (Mϕ) and bone marrow–derived neutrophils (PMNs) from Abcg1+/+ (wild type [WT]) and Abcg1−/− (knockout [KO]) mice. Data are representative of three separate experiments.
ABCG1 Negatively Regulates Pulmonary Responses to K. pneumoniae
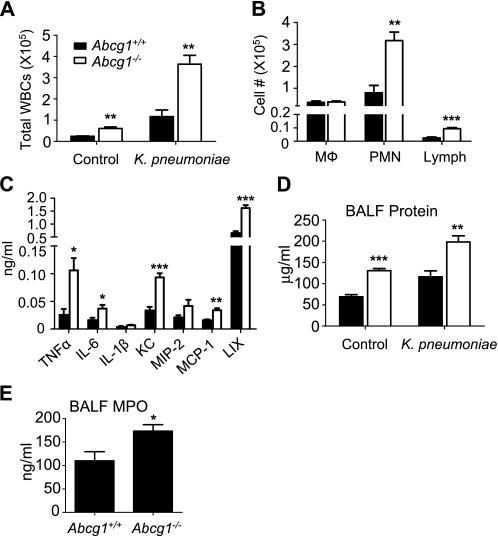
In light of the exaggerated response to inhaled LPS, we predicted that Abcg1−/− mice would have enhanced responses to intrapulmonary gram-negative bacteria. To test this, we exposed mice intratracheally to intact K. pneumoniae. An exaggerated response was indeed observed in the airways of Abcg1−/− mice after exposure, with a 3.2-fold higher BAL total WBC count 24 hours after inoculation (Figure 4A). Like the response to LPS, the increased inflammatory infiltrate was due, in large part, to PMN recruitment, although a significant increase in the numbers of lymphocytes in the airways was also observed (Figure 4B). The levels of several cytokines and chemokines were significantly increased in BALF from Abcg1−/− mice 24 hours after inoculation as compared with Abcg1+/+ mice (Figure 4C). Similar to the response to LPS exposure, higher BALF protein was also found in Abcg1−/− mice after bacterial inoculation (Figure 4D). Finally, we analyzed extracellular MPO in the airways after bacterial inoculation by measuring MPO in cell-free BALF. Extracellular MPO was significantly higher in the airways of Abcg1−/− than Abcg1+/+ mice at 24 hours after inoculation (Figure 4E). As extracellular MPO has significant bactericidal activity (20), this finding suggests the potential for enhanced host defense function in Abcg1−/− mice.
Figure 4.
The pulmonary response to inhaled Klebsiella pneumoniae is enhanced in ATP-binding cassette (Abc) g1−/− mice. Abcg1+/+ and Abcg1−/− mice were inoculated intratracheally with 2,000 cfu of K. pneumoniae and then analyzed 24 hours after exposure. (A) Total BAL leukocytes (white blood cells [WBCs]) and (B) leukocyte subsets (macrophages [Mϕ], polymorphonuclear neutrophils [PMNs], lymphcytes [Lymph]) were counted (**P < 0.01; ***P < 0.001). (C) Bronchoalveolar lavage fluid (BALF) cytokine and chemokine protein levels were quantified by Bioplex assay (LPS-induced CXC chemokine [LIX] quantified by ELISA) (*P < 0.05; **P < 0.01; ***P < 0.001). (D) BALF total protein was quantified by Bradford assay (**P < 0.01; ***P < 0.001). (E) Myeloperoxidase (MPO) activity was measured in cell-free BALF (*P < 0.05). Data shown are representative of two independent experiments involving 10 mice per genotype.
Hematopoietic Deficiency of ABCG1 Is Sufficient for Enhanced Pulmonary Responses to K. pneumoniae
ABCG1 is highly expressed in both alveolar macrophages (of hematopoietic origin) and type 2 pneumocytes (of structural origin) (8). Both cell types are known to contribute to pulmonary leukocyte recruitment after bacterial exposure through production of cytokines and chemokines (5). Therefore, we investigated the relative contributions of hematopoietic (radiosensitive) and structural (radioresistant) ABCG1 in the pulmonary response to K. pneumoniae. Bone marrow chimeric mice were generated by bone marrow transfer after lethal irradiation, and were exposed to K. pneumoniae. As shown in Figure 5, hematopoietic deficiency of ABCG1 (ie, Abcg1−/− marrow) was sufficient to cause enhanced responses in the lung 24 hours after K. pneumoniae. Compared with Abcg1+/+ recipient (R) mice reconstituted with Abcg1+/+ donor (D) bone marrow (WT [D]→WT [R]), a significant increase in lung recruitment of WBCs was only observed in the groups reconstituted with Abcg1−/− bone marrow (knockout [KO]→WT and KO→KO) (Figure 5A). A similar result was observed when BALF protein content was analyzed (Figure 5B). In contrast, compared with WT→WT mice, no difference in either of these parameters was observed in Abcg1−/− mice reconstituted with Abcg1+/+ bone marrow (WT→KO), chimeras that lack ABCG1 expression in radioresistant (structural) cells only. This suggests that deficiency of ABCG1 in structural cells is not sufficient to impact K. pneumoniae–induced WBC recruitment or microvascular injury. Compared with Abcg1−/− mice reconstituted with Abcg1−/− bone marrow (KO→KO), less WBC recruitment and BALF protein was observed in the KO→WT group, suggesting that, in the setting of hematopoietic ABCG1 deficiency, structural cell ABCG1 deficiency further enhances lung responses.
Figure 5.
Hematopoietic and nonhematopoietic ATP-binding cassette (ABC) G1 play distinct roles in pulmonary response to Klebsiella pneumoniae. ABCG1 bone marrow chimeras, generated by bone marrow reconstitution of lethally irradiated recipients (donor → recipient), were inoculated intratracheally with 2,000 cfu of K. pneumoniae. Mice were killed at 24 hours after exposure for analysis (10–12 mice/genotype). (A) Total bronchoalveolar lavage fluid (BALF) leukocytes (white blood cells [WBCs]) were enumerated (**P < 0.01; ***P < 0.001). (B) Total protein content was measured in BALF by Bradford assay (**P < 0.01; ***P < 0.001). (C) BALF WBC differentials were counted (macrophages [Mϕ], polymorphonuclear neutrophils [PMNs], and lymphocytes [Lymph]) (*P < 0.05; **P < 0.01; ***P < 0.001). (D) BALF cytokines and chemokines were quantified by Bioplex assay (LPS-induced CXC chemokine [LIX] quantified by ELISA) (*P < 0.05; **P < 0.01; ***P < 0.001). (E and F) Alveolar macrophages (E) and peritoneal exudate macrophages (F) from Abcg1+/+ and Abcg1−/− mice (n = 6/genotype) were harvested and either left untreated or treated with lipopolysaccharide (LPS) in vitro. Tumor necrosis factor (TNF)–α protein levels in culture supernatant was quantified by ELISA (*P < 0.05; ***P < 0.001).
To more precisely characterize the role of hematopoietic and structural cell ABCG1 in WBC recruitment to the exposed lung, we measured WBC subsets in BALF from chimeric mice 24 hours after infection (Figure 5C). Compared with WT→WT mice, significant increases in BALF PMNs and lymphocytes were only observed in the KO→WT and KO→KO groups, suggesting that hematopoietic deficiency of ABCG1 is sufficient to enhance recruitment of these cell types to the airspace. Significantly increased BALF PMNs were observed in KO→KO mice compared with KO→WT mice, whereas there was no difference in lymphocytes, suggesting that ABCG1 expression in lung structural cells also regulates PMN, but not lymphocyte recruitment. By contrast, compared with the WT→WT group, only the WT→KO and KO→KO groups had significantly more macrophages, with equivalent increases in these two chimeras, suggesting that structural and not hematopoietic ABCG1 regulates macrophage numbers in the airspace after infection.
Finally, we measured the levels of several cytokines/chemokines in BALF harvested from chimeric mice after infection with K. pneumoniae (Figure 5D). Similar to WBC recruitment, hematopoietic ABCG1 deficiency was sufficient to enhance the expression of almost all cytokines and chemokines surveyed. Notably, this was also true of LIX, a chemokine of epithelial origin, suggesting Abcg1−/− macrophage-to-epithelial intercellular signals. As found for total WBCs and BALF protein, significantly higher levels of several cytokines (TNF-α, KC, MIP-2, MCP-1, LIX, and IL-17) were observed in KO→KO BALF as compared with KO→WT BALF. Taken together, these results suggest that ABCG1 expression in both hematopoietic and structural cells contributes to cytokine/chemokine production, leukocyte trafficking, and tissue injury induced by K. pneumoniae in the lungs of Abcg1−/− mice.
ABCG1 Regulates LPS-induced Cytokine Production from Macrophages
Given that alveolar macrophages are the predominant hematopoietic cell present in the airspace at rest, and had an abnormal foam cell phenotype in Abcg1−/− mice (Figure E2), we predicted, from the bone marrow chimera experiments, that alveolar macrophages might be responsible, at least in part, for the excessive production of inflammatory mediators in the airways of Abcg1−/− mice after infection. To investigate this prediction, we harvested macrophages from mice and stimulated them with LPS ex vivo. Indeed, compared with Abcg1+/+, significantly enhanced levels of TNF-α were produced by Abcg1−/− alveolar (Figure 5E) and peritoneal (Figure 5F) macrophages, suggesting that alveolar macrophages are critical in the infection phenotype of Abcg1−/− mice.
Macrophages are highly efficient at internalizing bacteria through phagocytosis. Furthermore, phagocytosis of apoptotic cells is reportedly enhanced in ABCG1-deficient alveolar macrophages (10). To determine whether ABCG1 regulates phagocytosis of bacteria by macrophages, we analyzed the ability of peritoneal exudate macrophages and alveolar macrophages to internalize fluorescein-labeled E. coli bioparticles. In contrast to the LPS response, we observed no difference in phagocytosis between Abcg1+/+ and Abcg1−/− macrophages (Figure E3). Taken together, these results suggest that ABCG1 regulates the cytokine/chemokine, but not phagocytosis, functions of macrophage host defense.
ABCG1 Negatively Regulates Pulmonary Host Defense against K. pneumoniae
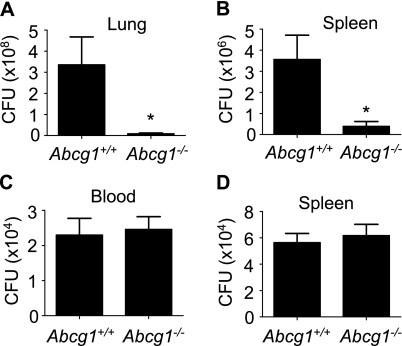
As PMNs, a critical cellular effector of host defense against K. pneumoniae (21), were increased in the Abcg1−/− lung, as were BALF levels of the bactericidal PMN granule protein MPO, we predicted that pulmonary host defense against K. pneumoniae would be enhanced in Abcg1−/− mice. To address this, mice were inoculated intratracheally with K. pneumoniae and bacterial burden in the lung and spleen was measured 24 hours later. Bacterial burden in the lungs of Abcg1−/− mice was indeed 40-fold less than that in Abcg1+/+ mice (Figure 6A), confirming enhanced pulmonary bacterial clearance. There was also a ninefold reduction in bacterial burden in the spleens of Abcg1−/− mice (Figure 6B), consistent with reduced extrapulmonary dissemination. To test for possible enhanced clearance of bacteremia in Abcg1−/− mice, we next injected K. pneumoniae intravenously and quantified bacterial burden in the blood and spleen after injection. There was no difference between Abcg1+/+ and Abcg1−/− mice in bacterial burden 4 hours (Figures 6C–6D) or 24 hours (data not shown) after injection in either tissue. Taken together with our finding of equivalent serum cytokines in Abcg1+/+ and Abcg1−/− mice after intraperitoneal LPS (Figure 2), these results suggest that pulmonary, but not systemic, innate immunity and host defense are enhanced in Abcg1−/− mice.
Figure 6.
ATP-binding cassette (Abc) g1−/− mice have reduced bacterial burden in lung and spleen after infection with inhaled Klebsiella pneumoniae. (A and B) Abcg1+/+ and Abcg1−/− mice (n = 20/group) were inoculated intratracheally with 2,000 cfu of K. pneumoniae. Bacterial cfu in lung (A) and spleen (B) homogenates were quantified 48 hours after exposure (*P < 0.05). (C and D) Abcg1+/+ and Abcg1−/− mice (n = 12/group) were inoculated intravenously with 70,000 cfu of K. pneumoniae. Bacterial cfu in the blood (C) and in splenic homogenates (D) were quantified 4 hours after inoculation.
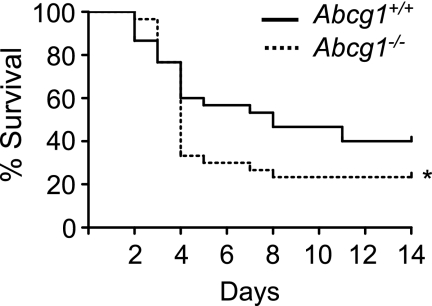
As K. pneumoniae was more effectively cleared in Abcg1−/− mice, we predicted that a reduced mortality rate would be observed among these mice after infection. To address this, we infected mice intratracheally with K. pneumoniae and monitored survival. Surprisingly, ABCG1 deficiency did not improve survival. Rather, a significantly higher mortality rate was observed among Abcg1−/− mice after inoculation (Figure 7). Taken together with our findings of enhanced lung injury in infected Abcg1−/− lungs (Figure 4), the higher mortality rate among Abcg1−/− mice in the face of reduced pulmonary and extrapulmonary bacterial burden may reflect respiratory failure caused by overexuberant lung inflammation during infection. In support of this, histopathologic scoring confirmed significantly enhanced disease changes in Abcg1−/− lungs 3 days after K. pneumoniae inoculation (Figure E4A). Abcg1−/− lungs displayed significantly increased PMN infiltration, interstitial thickening, and intra-alveolar edema (Figure E4B), as well as increased pleuritis, perivascular edema, and focal bronchiolar mural changes (data not shown).
Figure 7.
ATP-binding cassette (Abc) g1−/− mice have reduced survival after pulmonary infection with Klebsiella pneumoniae. Abcg1+/+ and Abcg1−/− mice (n = 30/group) were inoculated intratracheally with 500 cfu of K. pneumoniae, and survival was monitored (*P < 0.05).
DISCUSSION
The lung is not widely conceived of as a cholesterol-sensitive organ. However, cholesterol does play a pivotal role in physiological processes that are unique to the lung, including surfactant production by alveolar epithelial cells (22, 23) and regulation of the biophysical properties of surfactant (24). Moreover, the importance of steady-state cholesterol flux to maintenance of normal lung homeostasis was recently revealed by reports of profound cholesterol overload of the lungs in Abcg1−/− mice (2, 8, 10). As cholesterol overload potentiates innate immune and inflammatory responses of the macrophage (1–3, 25), and pharmacologic treatments reducing cellular cholesterol attenuate pulmonary innate immunity (4, 5), we predicted that the Abcg1−/− lung would be primed for more robust host defense responses.
Herein, we report that Abcg1−/− mice indeed display enhanced pulmonary responses to both inhaled LPS and K. pneumoniae, characterized by increased airspace cytokine and chemokine levels and increased PMN recruitment. ABCG1, previously shown to be expressed in both alveolar macrophages and alveolar epithelial cells (8), plays distinct roles in hematopoietic and nonhematopoietic (i.e., structural) pulmonary cells. Hematopoietic ABCG1 deficiency suffices to enhance pulmonary responses to K. pneumoniae; nonhematopoietic ABCG1 deficiency is not sufficient, but further enhances responses in the setting of hematopoietic ABCG1 deficiency (Figure 5). An underlying role for the alveolar macrophage in the Abcg1−/− phenotype was confirmed, as Abcg1−/− alveolar macrophages produced increased TNF-α when stimulated with LPS ex vivo.
Of interest, indirect regulatory roles for ABCG1 in intercellular communications in the lung were also identified. Nonhematopoietic, but not hematopoietic, ABCG1 deficiency was sufficient to increase the number of alveolar macrophages during K. pneumoniae infection. Hematopoietic, but not nonhematopoietic (e.g., epithelial), ABCG1 deficiency enhanced K. pneumoniae–induced BALF expression of LIX (CXCL5), a PMN-active chemokine of exclusive epithelial origin in the lung (18), perhaps through parallel increases in IL-17 (Figure 5D), a known inducer of LIX and PMN recruitment in the lung (26). The normal chemotaxis that we observed in PMNs from Abcg1−/− mice suggests that increased PMN recruitment is likely secondary to up-regulated chemokines in the ABCG1-deficient lung. Moreover, the relative deficiency of ABCG1 in WT PMNs, as compared with WT macrophages (Figure 3), suggests the interesting opportunity for future studies aiming to contrast the relative role of this protein in PMN versus macrophage biology.
Increased PMN recruitment to and degranulation within the infected Abcg1−/− lung led us to investigate whether clearance of K. pneumoniae was also enhanced. PMNs are the critical cellular effector of host defense against extracellular bacteria, and extracellular MPO has bactericidal activity through catalyzing the formation of hypochlorous acid (20). Abcg1−/− mice indeed displayed enhanced compartmentalization and clearance of bacteria in the lung. Interestingly, regulation of innate immunity by ABCG1 in vivo appears to be tissue selective, as Abcg1+/+ and Abcg1−/− mice had equivalent responses to intraperitoneal LPS and intravenous K. pneumoniae. Moreover, whereas Abcg1−/− mice had enhanced basal recruitment of PMNs to the airspace and lung parenchyma, they displayed normal circulating PMN counts.
Alveolar macrophages from Abcg1−/− mice displayed a cell-intrinsic enhanced innate immune response (Figure 5E), consistent with prior investigations of ABCG1-deficient macrophages (2, 3, 10). However, it seems likely that the “nonnaive” basal state of the ABCG1-deficient lung, characterized by increased numbers of macrophages and neutrophils, and up-regulation of cytokines known to prime leukocyte host defense functions (e.g., degranulation), likely also contributes to poising the lung for enhanced host defense. As seen in other reported models, we speculate that the increased mortality in infected Abcg1−/− mice (Figure 7) may stem from overexuberant lung inflammation (Figure E4). The increased BALF protein in Abcg1−/− mice (Figure 5B) also suggests that compromise of pulmonary microvascular integrity may contribute. In support of this premise, ABCG1 has recently been shown to promote endothelial function in systemic blood vessels (27). Finally, future studies will need to discern whether surfactant in Abcg1−/− mice, previously reported to have abnormal composition (8), also has defective function.
Although others have demonstrated in vitro that the TLR hyperresponses of ABCG1-deficient macrophages stem from their increased cholesterol (3), we did not confirm this intermediate mechanistic link in vivo in the present study. As macrophages deficient in ABCA1, another ABC family cholesterol transporter, accumulate levels of cholesterol that are similar to those of Abcg1−/− macrophages, but produce significantly lower levels of cytokines (25), factors other than simple cholesterol accumulation are likely to be involved. Subcellular distribution of cholesterol is likely important. For example, endoplasmic reticulum stress induced by free cholesterol overload is reported to result in nuclear factor–κB activation in macrophages (1). On the other hand, TLR4 is up-regulated on the surface of cholesterol-laden Abcg1−/− macrophages, but not Abca1−/− macrophages, suggesting the importance of plasma membrane cholesterol. Of interest, as LPS itself down-regulates ABCG1 in human macrophages (28), promoting cellular cholesterol loading, it is possible that disinhibition of the macrophage via ABCG1 down-regulation may even play a role in the LPS response of WT macrophages. Future studies will be necessary to better clarify how the spatial organization of intracellular cholesterol impacts the inflammatory state and maturation of macrophages, and how ABCG1 contributes to these events.
The observation that pulmonary innate immunity is modulated by ABCG1 may bear important clinical implications. Interestingly, the human lung disease pulmonary alveolar proteinosis has been linked to ABCG1 deficiency, likely stemming from neutralizing autoantibodies against granulocyte-macrophage colony–stimulating factor, a macrophage growth factor required for ABCG1 induction (9, 29). Pulmonary alveolar lipoproteinosis associated with macrophage infiltration has also been noted in Niemann-Pick disease, a lipid storage disorder characterized by macrophage cholesterol overload (30, 31). Taken together, these rare diseases suggest that more prevalent functional polymorphisms of ABCG1 may possibly also influence pulmonary inflammation and host defense in human subjects. At least two single-nucleotide polymorphisms (SNPs) have recently been investigated in preliminary studies in humans: G2457A, a SNP of uncertain functional significance, and −257T > G, a promoter SNP reported to reduce ABCG1 transcription and to associate with severity of coronary artery disease among Japanese men (32, 33).
As ABCG1 is a target gene of the nuclear receptor, liver X receptor (LXR), and endogenous LXR ligands synthesized in the lung are reportedly reduced in chronic lung disease in human subjects (34), it is also possible that relative deficiency of ABCG1 may promote or sustain common chronic inflammatory lung diseases. Conversely, a recent report from our group that synthetic LXR agonists reduce pulmonary innate immune responses (5) suggests that such agents may operate, at least in part, through ABCG1 up-regulation, and that ABCG1 may be a manipulable molecular target in lung disease. Of potential concern, statins, which are now undergoing study as potential therapeutics in human acute lung injury and chronic obstructive pulmonary disease, reportedly reduce ABCG1 expression in macrophages through depletion of endogenous LXR ligands (35, 36), an effect that could possibly mitigate their anti-inflammatory potential in the human lung.
In summary, we identify ABCG1 as a potential link between cholesterol homeostasis and host defense responses in the lung. We propose that ABCG1-mediated cholesterol homeostasis serves as an important negative regulator of leukocyte trafficking to the lung and pulmonary inflammatory responses to the environment. However, future investigations will be necessary to discern whether ABCG1 influences pulmonary inflammatory phenotypes through cholesterol-independent mechanisms, to better clarify the mechanisms by which cellular cholesterol regulates inflammatory functions in alveolar cells, and to determine whether pharmacological manipulation of ABCG1 and cholesterol in the lung may be used to improve respiratory health in human subjects.
Supplementary Material
Acknowledgments
The authors thank Kathleen Smoak for assistance with ex vivo cell studies, Grace E. Kissling for assistance with statistical analysis, Laura Miller DeGraff for assistance with animal dosing, and William P. Fitzgerald for assistance with irradiation.
Supported in part by Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences grant Z01 ES102005.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200910-1580OC on April 15, 2010
Author Disclosure: D.W.D. is an employee of the National Institutes of Health (NIH). J.H.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.H.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.T.R. received more than $100,001 from KineMed Inc. for a peptide drug development project–NIH-approved cooperative research agreement (CRADA), $5,001–$10,000 from AlphaCore Pharma for a recombinant Lecithin-cholesterol acyltransferase development project–NIH-approved CRADA, and $5,001–$10,000 from VirxSys Inc. for a trans-splicing gene delivery project–NIH-approved CRADA. M.B.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and MAP kinase–dependent inflammation in advanced atherosclerosis. J Biol Chem 2005;280:21763–21772. [DOI] [PubMed] [Google Scholar]
- 2.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol 2008;180:3560–3568. [DOI] [PubMed] [Google Scholar]
- 3.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter–deficient macrophages: free cholesterol accumulation, increased signaling via Toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008;118:1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, Worthen GS. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med 2005;171:606–615. [DOI] [PubMed] [Google Scholar]
- 5.Smoak K, Madenspacher J, Jeyaseelan S, Williams B, Dixon D, Poch KR, Nick JA, Worthen GS, Fessler MB. Effects of liver x receptor agonist treatment on pulmonary inflammation and host defense. J Immunol 2008;180:3305–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 2005;1:121–131. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem 2005;280:30150–30157. [DOI] [PubMed] [Google Scholar]
- 8.Baldan A, Tarr P, Vales CS, Frank J, Shimotake TK, Hawgood S, Edwards PA. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem 2006;281:29401–29410. [DOI] [PubMed] [Google Scholar]
- 9.Thomassen MJ, Barna BP, Malur AG, Bonfield TL, Farver CF, Malur A, Dalrymple H, Kavuru MS, Febbraio M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res 2007;48:2762–2768. [DOI] [PubMed] [Google Scholar]
- 10.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol 2008;180:4273–4282. [DOI] [PubMed] [Google Scholar]
- 11.Draper DW, Madenspacher JH, DeGraff LM, Fessler MB. ATP binding cassette transporter G1 is a negative regulator of pulmonary innate immunity [abstract]. Am J Respir Crit Care Med 2009;179:A1022. [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 13.Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R III. Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol 2003;171:955–963. [DOI] [PubMed] [Google Scholar]
- 14.Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, Fong A, Foster WM, Schwartz DA. Ambient ozone primes pulmonary innate immunity in mice. J Immunol 2007;179:4367–4375. [DOI] [PubMed] [Google Scholar]
- 15.Natarajan S, Kim J, Remick DG. Chronic pulmonary LPS tolerance induces selective immunosuppression while maintaining the neutrophilic response. Shock (Augusta, Ga 2009. [DOI] [PMC free article] [PubMed]
- 16.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 2002;109:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med 2009;206:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol 2005;32:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klebanoff SJ, Vadas MA, Harlan JM, Sparks LH, Gamble JR, Agosti JM, Waltersdorph AM. Stimulation of neutrophils by tumor necrosis factor. J Immunol 1986;136:4220–4225. [PubMed] [Google Scholar]
- 20.Britigan BE, Ratcliffe HR, Buettner GR, Rosen GM. Binding of myeloperoxidase to bacteria: effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim Biophys Acta 1996;1290:231–240. [DOI] [PubMed] [Google Scholar]
- 21.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 2001;25:335–340. [DOI] [PubMed] [Google Scholar]
- 22.Nistor A, Simionescu M. Uptake of low density lipoproteins by the hamster lung: interactions with capillary endothelium. Am Rev Respir Dis 1986;134:1266–1272. [DOI] [PubMed] [Google Scholar]
- 23.Pian MS, Dobbs LG. Lipoprotein-stimulated surfactant secretion in alveolar type ii cells: mediation by heterotrimeric G proteins. Am J Physiol 1997;273:L634–L639. [DOI] [PubMed] [Google Scholar]
- 24.Leonenko Z, Gill S, Baoukina S, Monticelli L, Doehner J, Gunasekara L, Felderer F, Rodenstein M, Eng LM, Amrein M. An elevated level of cholesterol impairs self-assembly of pulmonary surfactant into a functional film. Biophys J 2007;93:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 2007;117:3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, et al. An IL-17f/a heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol 2007;179:7791–7799. [DOI] [PubMed] [Google Scholar]
- 27.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest 2008;118:3701–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009;119:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 1999;190:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikegami M, Dhami R, Schuchman EH. Alveolar lipoproteinosis in an acid sphingomyelinase–deficient mouse model of Niemann-Pick disease. Am J Physiol Lung Cell Mol Physiol 2003;284:L518–L525. [DOI] [PubMed] [Google Scholar]
- 31.Bjurulf B, Spetalen S, Erichsen A, Vanier MT, Strom EH, Stromme P. Niemann-Pick disease type C2 presenting as fatal pulmonary alveolar lipoproteinosis: morphological findings in lung and nervous tissue. Med Sci Monit 2008;14:CS71–CS75. [PubMed] [Google Scholar]
- 32.Rujescu D, Giegling I, Dahmen N, Szegedi A, Anghelescu I, Gietl A, Schafer M, Muller-Siecheneder F, Bondy B, Moller HJ. Association study of suicidal behavior and affective disorders with a genetic polymorphism in ABCG1, a positional candidate on chromosome 21q22.3. Neuropsychobiology 2000;42:22–25. [DOI] [PubMed] [Google Scholar]
- 33.Furuyama S, Uehara Y, Zhang B, Baba Y, Abe S, Iwamoto T, Miura S, Saku K. Genotypic effect of Abcg1 gene promoter −257T>G polymorphism on coronary artery disease severity in Japanese men. J Atheroscler Thromb 2009;16:194–200. [DOI] [PubMed] [Google Scholar]
- 34.Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lutjohann D, Diczfalusy U, Bjorkhem I. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res 1999;40:1417–1425. [PubMed] [Google Scholar]
- 35.Wong J, Quinn CM, Brown AJ. Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. Arterioscler Thromb Vasc Biol 2004;24:2365–2371. [DOI] [PubMed] [Google Scholar]
- 36.Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008;196:180–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.