Abstract
Rationale: Extensively drug-resistant (XDR) tuberculosis (TB) may arise in individuals on treatment for multidrug-resistant (MDR) TB. Preventing this amplification of resistance will likely improve clinical outcomes and delay the secondary spread of XDR-TB.
Objectives: To measure the proportion of individuals that develops XDR-TB during the course of MDR-TB treatment, and to identify those factors associated with the development of XDR.
Methods: We performed a retrospective analysis of 608 consecutive patients with documented MDR-TB who were started on MDR-TB treatment between September 10, 2000 and November 1, 2004 in the Tomsk Oblast TB Treatment Services in Western Siberia, Russian Federation.
Measurements and Main Results: A total of 6% of patients were observed to develop XDR-TB while on MDR-TB treatment. These patients were significantly less likely to be cured or to complete treatment. Using Cox proportional hazard models, we found that the presence of bilateral and cavitary lesions was associated with a greater than threefold increase in hazard (adjusted hazard ratio [HR], 3.47; 95% confidence interval [CI], 1.32–9.14). Prior exposure to a second-line injectable antibiotic was associated with a greater than threefold increase in hazard (adjusted HR, 3.65; 95% CI, 1.81–7.37), and each additional month in which a patient failed to take at least 80% of their prescribed drugs was associated with nearly an additional 20% hazard of developing XDR-TB (adjusted HR, 1.17; 95% CI, 1.01–1.35).
Conclusions: Early and rapid diagnosis, timely initiation of appropriate therapy, and programmatic efforts to optimize treatment adherence during MDR-TB therapy are crucial to avoiding the generation of excess XDR-TB in MDR-TB treatment programs.
Keywords: antibiotic resistance, acquired resistance, epidemiology, adherence, tuberculosis
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The emergence of extensively drug-resistant (XDR) tuberculosis (TB) is one of the threats to global TB control, and there are ample data documenting greater mortality and treatment failure rates compared with other multidrug-resistant (MDR) TB cases. Identifying risk factors associated with XDR-TB is necessary to design interventions to prevent the emergence of XDR-TB within MDR-TB treatment programs. Yet, to date, there are no published data describing factors associated with the emergence of XDR-TB during treatment for MDR-TB.
What This Study Adds to the Field
This work adds to the field by: (1) identifying the factors associated with developing XDR-TB within a cohort of MDR-TB cases receiving treatment in Tomsk, Russia; and (2) discussing how these findings may be used to inform policy and programmatic efforts to curb the emergence of XDR-TB within MDR-TB treatment programs.
The sporadic appearance and subsequent selection of drug-resistant Mycobacterium tuberculosis mutants is an unintended and unavoidable consequence of using anti-tuberculosis (TB) antibiotics (1). The emergence of multidrug-resistant (MDR) TB—defined as strains resistant to at least isoniazid and rifampin—has introduced challenging, but surmountable, complexities to TB programs that have responded by treating MDR-TB with second-line drugs. Whether through formal MDR-TB treatment programs or unregulated means, such as over-the-counter and “black-market” sources, patients with MDR-TB have gained increased access to second-line anti-TB agents. Regardless of the mechanism of access, a subset of individuals inevitably will fail to respond to MDR-TB therapy, and, in the process, may acquire additional resistance to second-line drugs (2–4). Extensively drug-resistant (XDR) TB—defined as MDR-TB with additional resistance to the fluoroquinolones and a second-line injectable—is the result of this sequential mutation–selection process, and compromises the effectiveness of even the most tailored individualized regimens (5–7).
A critical conundrum for programs and practitioners is how to balance MDR-TB treatment efforts with the dangers of selecting for increasingly drug-resistant strains, in particular among individuals who have previously failed conventional, first-line TB treatment. Identifying baseline patient characteristics and MDR-TB treatment–related factors associated with developing XDR-TB is crucial to the design and implementation of strategies that minimize the development of XDR-TB among individuals receiving MDR-TB therapy.
The Tomsk Oblast Tuberculosis Treatment Services in Western Siberia was one of the first wide-scale MDR-TB treatment programs to be implemented in a resource-poor setting. Since its inception in 2000, cure or treatment completion has been achieved in 66% of all MDR-TB cases, and in 48% of individuals who had baseline XDR-TB (5). Although this program offers a successful model for other MDR-TB treatment programs in Russia and elsewhere, several questions remain. In this retrospective analysis, we sought to explore the phenomenon of XDR-TB that arises and is diagnosed during MDR-TB therapy. In doing so, we hope to address the following questions: what proportion of individuals develops XDR-TB during the course of MDR-TB treatment within the context of a strong MDR-TB treatment program, and which factors are associated with the development of XDR?
METHODS
Study Location
The Tomsk Oblast Tuberculosis Services (TOTBS) provides treatment to the Tomsk Oblast in western Siberia, Russia, with a population of approximately 1.1 million inhabitants. Since 2000, TOTBS has collaborated with the Tomsk TB Prison Hospital, Partners in Health, Massachusetts State Laboratory Institute, and the Open Society Institute to provide MDR-TB treatment. In 2003, TB incidence in Tomsk was 93.4 per 100,000; of new cases surveyed from 2003 to 2005, 7.2% were MDR-TB (8). Details of this program, including patient selection criteria and treatment principles, have been described elsewhere (5, 9). In general, patients submit sputum at baseline and monthly during treatment for smear and culture. Individuals with a positive culture after 4 or more months of treatment are routinely assessed for possible treatment failure, including repeat drug susceptibility testing (DST) and consideration of regimen reinforcement, when possible.
Study Participants and Case Definitions
Of 636 patients who were consecutively treated for MDR-TB between September 10, 2000 and November 1, 2004, we performed a retrospective case series of the 608 patients with documented MDR-TB. For this analysis, we excluded individuals who had baseline XDR-TB and those who did not have sufficient pretreatment drug susceptibility data to rule out XDR-TB before initiating MDR-TB treatment (e.g., individuals without testing to second-line drugs). Drugs included in the individualized treatment regimens were determined based on available DST results and previous treatment histories. Per TOTBS protocol, any positive culture found after an individual received 4 or more months of MDR-TB treatment was sent for additional DST. However, a few individuals had positive cultures after at least 4 months of treatment, and did not receive a repeat DST (n = 8), possibly for clinical or programmatic reasons. Because we could not confirm whether these individuals did or did not develop XDR-TB, we excluded them from the current analysis.
XDR-TB was defined as resistance to isoniazid, rifampicin, any fluoroquinolone, and either kanamycin or capreomycin. DST to amikacin was not available. An individual was considered to have baseline XDR-TB if XDR-TB was documented at any time before starting MDR-TB therapy. Acknowledging that the observation of newly documented XDR-TB during treatment could be due to either resistance amplification or reinfection, we use the term “developed XDR-TB” to refer to any case that was confirmed not to have XDR-TB at baseline, and was subsequently diagnosed with XDR-TB during MDR-TB treatment. Individuals who had any DST performed during treatment that did not document XDR-TB, and those who were culture negative after 4 months of therapy, were presumed not to have developed XDR-TB. Time to developed XDR-TB was defined as the number of days from the start of MDR-TB therapy to the date of sample collection in which XDR-TB was documented.
Data Collection and Analysis
We abstracted data from clinical reporting forms that were prospectively completed by TB providers. In addition, we performed retrospective chart reviews of all patients, and obtained additional data from the TOTBS registry and laboratory databases. Data were entered into the “DOTS-Plus” Electronic Medical Record (Boston, MA), which used a Microsoft SQL 2000 server (Microsoft Corp., Seattle, WA) and exported data into an Access 2000 database (Microsoft Corp.). Analysis was conducted with SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Variables considered included baseline characteristics, such as: sociodemographic variables; site of treatment initiation (e.g., prison versus civilian and inpatient hospital versus other sites); comorbid conditions, including human immunodeficiency virus (HIV) and physician-diagnosed alcoholism; indicators of clinical severity (e.g., low body mass index, both bilateral and cavitary lesions on chest radiograph, and respiratory insufficiency as documented by physicians); and prior treatment exposure to second-line agents. We also assessed on-treatment factors, including alcohol use ever documented during MDR-TB therapy, and several time-varying covariates calculated for each month in MDR-TB treatment, as follows: cumulative hospital days (i.e., number of days ever hospitalized in the current and all prior months of treatment); cumulative prison days (i.e., number of days ever incarcerated in the current and all prior months of treatment); adjunctive surgery (having had surgery in the current or any prior month of treatment); and cumulative nonadherent months (number of months in which less than 80% of prescribed doses were actually taken).
Final treatment outcomes (i.e., cured, completed treatment, failed, defaulted, died), as defined by the Stop TB MDR-TB Working Group, were used for this study (10).
We used Cox proportional hazards regression models to identify factors associated with time to developed XDR-TB. We considered variables that were suspected to contribute to the development of XDR-TB due to clinical or programmatic factors, including known risk factors for poor treatment outcome (e.g., HIV, alcoholism, severe radiographic lesions), prior exposure to second-line drugs, and duration of institutional exposure while on treatment. For non–time-varying variables, we evaluated the proportional hazards assumption using the Kolmogorov-type supremum test (11), as implemented in SAS (PROC PHREG). Variables that were associated with time to developed XDR-TB at a P value of less than 0.2 in univariable analysis, as well as age and sex, were considered as candidates for the multivariable model. We used a forward stepwise approach to determine the final model; variables were retained in the final multivariable model if they predicted time to XDR-TB at a P value of 0.1 or less.
The Harvard School of Public Health and the Siberian State Medical University granted institutional review board approval for this study.
RESULTS
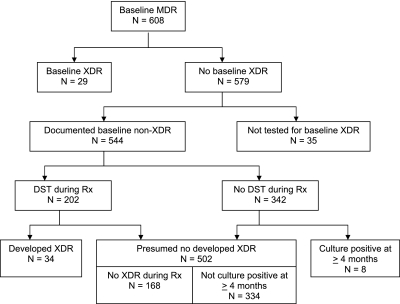
As shown in Figure 1, of 608 patients with MDR-TB treated during the study period, 29 individuals had baseline XDR-TB. Of the remaining 579 individuals, 544 had sufficient drug susceptibility data to document that they did not have XDR-TB at baseline. Of these, 202 patients had at least one DST performed during MDR-TB treatment. We found that 34 of these patients developed XDR-TB, whereas 168 did not. Of the 342 patients without a DST during treatment, 334 (97.7%) did not have a positive TB culture after 4 months of MDR-TB therapy, whereas 8 individuals had a positive culture after 4 months of therapy, and were excluded from analysis. Therefore, subsequent analysis was based on a cohort of 536 individuals, of whom 34 (6.3%) developed XDR-TB during treatment, and 502 (93.7%) were considered not to have developed XDR-TB. Among these 34 individuals, the median time to diagnosis of developed XDR-TB was 182 days (quartile [Q] 1, Q3: 122, 287). The median number of DSTs performed during MDR-TB treatment was 6.5 (Q1, Q3: 3, 11) among those who developed XDR, compared with 2 DSTs among those that did not develop XDR-TB (Q1, Q3: 1, 4).
Figure 1.
Study flow.
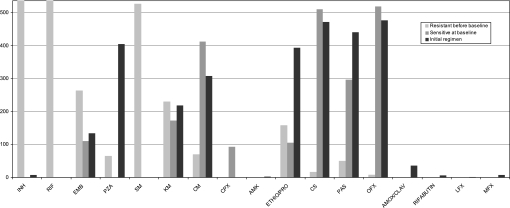
Table 1 presents baseline characteristics of the study participants. The cohort was comprised of young individuals who were predominantly male. Approximately 27% initiated treatment in prison, whereas the remainder started treatment within civilian services. Almost all individuals had received previous treatment for TB; prior regimens included a second-line injectable in 30% of the cases, and a fluoroquinolone in 14% of the cases. Alcoholism was common (42%), and HIV was rare (1%). The majority of individuals had advanced pulmonary disease, manifested by respiratory insufficiency (55%), as well as bilateral and cavitary lesions on chest radiograph (61%). As shown in Figure 2, baseline drug resistance to first- and second-line drugs was common.
TABLE 1.
BASELINE AND TREATMENT CHARACTERISTICS OF COHORT
| Characteristic | No. (%), Unless Specified |
|---|---|
| Female sex | 95 (17.7) |
| Age, yr* | 34.2 (26.6, 45.0) |
| Treatment initiation site | |
| Civilian | 392 (73.1) |
| TB hospital | 275 (51.3) |
| Day hospital/polyclinic in Tomsk | 94 (17.5) |
| Sites outside of Tomsk city | 23 (4.3) |
| Prison | 144 (26.9) |
| Year of treatment initiation | |
| 2000 | 33 (6.2) |
| 2001 | 89 (16.6) |
| 2002 | 117 (21.8) |
| 2003 | 150 (28.0) |
| 2004 | 147 (27.4) |
| Receiving disability pension | 226 (42.2) |
| Homeless | 22 (4.1) |
| Previous or present incarceration (n = 534) | 282 (52.8) |
| Low body mass index (n = 535) | 218 (40.8) |
| HIV (n = 533) | 5 (0.9) |
| Alcoholism, per physician assessment (n = 463) | 192 (41.5) |
| Illicit drug use | 95 (17.7) |
| Any prior TB treatment | 533 (99.4) |
| Median no. previous TB treatments† | 2 (1, 3) |
| Prior TB treatment with second-line injectable (n = 532) | 160 (30.1) |
| Prior TB treatment with fluoroquinolone (n = 531) | 72 (13.6) |
| Previous TB surgery (n = 534) | 54 (10.1) |
| Respiratory insufficiency (n = 528) | 289 (54.7) |
| Bilateral and cavitary lesions, (n = 529) | 322 (60.9) |
| No. of drugs to which resistant* | |
| First-line drugs | 4 (3, 4) |
| Second-line drugs | 1 (0, 2) |
| Alcohol use during treatment | 205 (38.2) |
| No. of hospital days during treatment*† | 205 (111, 316) |
| No. of prison days during treatment*† | 554 (474, 594) |
| Adjunctive surgery during MDR-TB treatment | 51 (9.5) |
| Percentage of treatment months with >80% adherence* | 89 (80, 96) |
Definition of abbreviations: HIV = human immunodeficiency virus; MDR = multidrug resistant; TB = tuberculosis.
If not otherwise specified, n = 536.
Continuous variable, median (quartile 1, quartile 3) presented.
Among those with any time spent in these locations during follow-up.
Figure 2.
Baseline drug resistance and drugs used in multidrug-resistant (MDR) treatment regimens (n = 536).
The median time to culture conversion was 1.97 months (Q1, Q3: 0.98, 2.95) among those who did not develop XDR-TB on treatment, compared with 3.93 months (Q1, Q3: 1.97, 4.92) for those who developed XDR-TB (P = 0.001). Final treatment outcomes were also poor among individuals who developed XDR-TB. In contrast to a 68.5% response rate among individuals who did not develop XDR-TB, only 14.7% of those who developed XDR-TB were cured or completed treatment (P < 0.0001). Among those who did not develop XDR-TB, 21.1% defaulted, 4.8% died, and only 5.6% failed treatment. Of the 34 individuals who developed XDR-TB, 20.6% defaulted, 11.8% died, and 52.9% failed treatment.
We performed an analysis of factors associated with time to developed XDR-TB (Table 2). Based on results from the Kolmogorov-type supremum tests, we concluded that the proportional hazard assumption was satisfied for baseline variables. In the univariable analysis, initiating treatment at the TB hospital was associated with an increased risk of developing XDR-TB, with an unadjusted hazard ratio (HR) of 2.28 (95% confidence interval [CI], 1.11–4.68). Baseline respiratory insufficiency (HR, 2.85; 95% CI, 1.29–6.30), baseline bilateral and cavitary lesions (HR, 3.01; 95% CI, 1.51–10.09), prior exposure to a second-line injectable (HR, 3.98; 95% CI, 1.99–7.95), and prior exposure to a quinolone (HR, 3.31; 95% CI, 1.61–6.79) were also associated with developing XDR-TB in the univariable analysis. Among time-varying covariates, we found that the number of cumulative nonadherent months was a statistically significant predictor (HR, 1.16; 95% CI, 1.002–1.34), whereas, the cumulative number of hospital days, cumulative number of prison days, and use of adjunctive MDR-TB surgery were not significantly associated with developing XDR-TB.
TABLE 2.
FACTORS ASSOCIATED WITH TIME TO DIAGNOSIS OF EXTENSIVELY DRUG-RESISTANT TUBERCULOSIS
| Characteristic | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Male sex* | 0.59 (0.27–1.25) | 0.37 (0.17–0.81) |
| Age, yr† | 1.01 (0.98–1.04) | 1.01 (0.98–1.04) |
| Year of treatment initiation | 0.80 (0.61–1.05) | — |
| Started in prison | 0.52 (0.22–1.27) | — |
| Started in TB hospital | 2.28 (1.11–4.68) | — |
| Baseline low body mass index (n = 535) | 1.17 (0.59–2.30) | — |
| Baseline HIV (n = 533) | 3.11 (0.43–22.71) | — |
| Baseline alcoholism (n = 463) | 1.63 (0.83–3.20) | — |
| Alcohol use during treatment | 1.58 (0.80–3.11) | — |
| Baseline respiratory insufficiency (n = 528)* | 2.85 (1.29–6.30) | — |
| Baseline bilateral and cavitary lesions (n = 529)* | 3.01 (1.51–10.09) | 3.47 (1.32–9.14) |
| Prior TB treatment with a second-line injectable (n = 532)* | 3.98 (1.99–7.95) | 3.65 (1.81–7.37) |
| Prior TB treatment with a quinolone (n = 531)* | 3.31 (1.61–6.79) | — |
| Cumulative no. of prison days | 1.00 (0.99–1.01) | — |
| Cumulative no. of hospital days | 1.00 (1.00–1.01) | — |
| Cumulative no. of months with <80% adherence* | 1.16 (1.00–1.34) | 1.17 (1.01–1.35) |
Definition of abbreviations: CI = confidence interval; HIV = human immunodeficiency virus; HR = hazard ratio; MDR = multidrug resistant; TB = tuberculosis.
If not otherwise specified, n = 536.
Entered into multivariable model.
Forced into multivariable model.
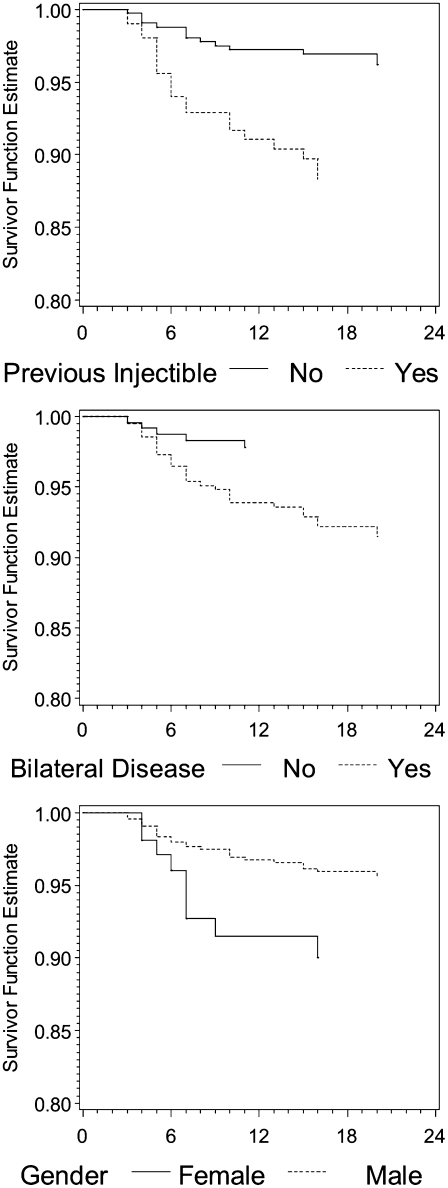
Multivariable analysis retained the following variables as significant predictors of time to developing XDR-TB, adjusting for other variables in the model: male sex (adjusted HR, 0.37; 95% CI, 0.17–0.80); bilateral and cavitary lesions (adjusted HR, 3.47; 95% CI, 1.32–9.14); prior exposure to a second-line injectable (adjusted HR, 3.65; 95% CI, 1.81–7.37); and cumulative nonadherent months (adjusted HR, 1.17; 95% CI, 1.01–1.35). Figure 3 displays the multivariable-adjusted time to developing XDR-TB, stratified by selected non–time-varying variables.
Figure 3.
Comparison of the time to diagnosis of extensively drug resistant (XDR) for baseline factors found to be significantly associated with hazard in the final multivariable model. Results are adjusted for non–time-varying factors included in the final model (including age at treatment initiation).
DISCUSSION
We studied the development of XDR-TB among 536 patients with MDR-TB initiating therapy within a well established MDR-TB treatment program, which provides care that is fully compliant with the World Health Organization drug-resistant TB treatment guidelines. Despite overall excellent clinical outcomes, we found that 6% of patients with MDR-TB developed XDR-TB during treatment.
Not surprisingly, outcomes were worse among patients with MDR-TB who developed XDR-TB. Less than 15% of patients who developed XDR-TB during treatment were cured or completed treatment, compared with almost 70% for patients who did not develop XDR-TB, and 48.3% for individuals with XDR-TB before the initiation of treatment (5).
We identified several baseline and time-varying risk factors for developing XDR-TB on MDR-TB therapy. In our final multivariable model, the presence of bilateral and cavitary lesions on baseline chest radiograph was associated with more than a threefold increased hazard of developing XDR-TB. The increased risk associated with the presence of extensive and cavitary disease could be explained by the increased bacillary burden within cavitary lesions, in which the likelihood of spontaneous mutations associated with drug resistance is greater (11), and/or the existence of subpopulations of bacilli that survive either due to metabolic dormancy or exposure to subinhibitory drug concentrations (12, 13).
Almost all of the patients in this cohort had previously been treated for TB; those who had previously received second-line injectable agents had more than a threefold increased hazard of developing XDR-TB. Whereas prior exposure to fluoroquinolones was significantly associated in our univariable analysis, this factor was no longer associated with the hazard of developing XDR-TB after controlling for other variables included in the multivariate model. Baseline resistance to second-line parenteral agents was relatively common among those who had prior exposure to one of these drugs (79/160, or 49%), whereas baseline fluoroquinolone resistance was relatively rare among individuals with prior exposure to a fluoroquinolone (24/72, or 33%). This difference in the association between prior treatment history and baseline drug resistance may help to explain our finding that prior exposure to second-line parenteral agents, but not fluoroquinolones, was associated with developing XDR-TB. The interpretation of baseline resistance with the hazard of developing XDR-TB is difficult, because baseline resistance (at least in part) is determined by which drugs were included in individualized treatment regimens (Figure 2).
The final model also indicated that male sex was independently associated with a reduced hazard of developing XDR-TB. Women have been associated with worse MDR-TB treatment outcomes in other cohorts (6), an observation that may be explained by sex differences in unmeasured socioeconomic, clinical, or lifestyle risk factors.
Finally, nonadherence to MDR-TB therapy was a strong risk factor for developing XDR-TB. The overall adherence in this cohort was high (median, 89.7%; Q1, Q3: 80.1%, 95.7%), likely due to the implementation of strict directly-observed treatment (DOT) and adherence enablers. However, for individuals who did not adhere to treatment, each additional month in which they failed to take greater than 80% of prescribed doses increased the hazard of developing XDR-TB by approximately 17%. This finding highlights the fact that programmatic efforts to optimize adherence to MDR-TB therapy should not only provide adherence support to all individuals—such as DOT, enablers, and incentives—but also implement additional aggressive strategies to bolster adherence among individuals who, despite standard measures, cannot adhere to treatment (14).
Based on these findings, we conclude that the development of XDR-TB during MDR-TB treatment is related to two potentially modifiable factors: baseline chronic disease and nonadherence to MDR-TB therapy. Extensive cavitary disease and baseline resistance to second-line agents are often the result of delayed diagnosis of drug resistance, and treatment with prior regimens that include a few second-line chemotherapeutic agents, but not enough to bring about cure. Programmatic efforts to diagnose and treat drug-resistant disease aggressively with appropriate second-line regimens could avert the creation of individuals with advanced disease who, when finally treated with appropriate regimens, may develop XDR-TB and experience poor treatment outcomes (15). In addition, MDR-TB treatment programs must address potential barriers to treatment adherence (e.g., patient side effects, socioeconomic factors), including targeted interventions for high-risk patients who may need intensive support to overcome the structural barriers to adherence that are associated with poverty and social marginalization (16–20).
A major strength of our analysis is that we were able to rule out XDR-TB at baseline among this cohort of patients with MDR-TB with external quality-assured DSTs. In addition, the strong treatment program in Tomsk, with well defined protocols for obtaining culture and DST during treatment, allowed us to assess the time to development of XDR-TB. In our cohort, only a very small fraction of patients (∼1.5%) did not have a repeat DST on a positive culture after 4 months of MDR-TB treatment (Figure 1).
Our study has several limitations. It is possible that some patients did develop XDR-TB, but culture converted and responded to MDR-TB therapy; however, such patients are of lesser clinical importance. The occurrence of XDR-TB in individuals can result from either acquired or transmitted resistance. The only available approach for identifying cases of transmitted resistance in our cohort of previously treated patients would be through molecular typing (21). Because genetic typing of isolates was not performed, we cannot rule out the possibility that some of these patients were reinfected by XDR-TB strains circulating in the community or in congregate settings. We note that a previous analysis in this same community identified hospitalization at the beginning or during TB treatment as a significant risk factor for an increased hazard of developing MDR-TB (16). However, neither the initiation of treatment in hospitals or in prisons nor the cumulative number of days in hospital or in prisons while on MDR-TB treatment was significantly associated with the hazard of developing XDR-TB in this analysis. If XDR-TB becomes increasingly prevalent within nosocomial settings in Tomsk, time spent in these facilities could, in the future, become associated with risk of infection or reinfection with XDR-TB, as has been observed in other epidemic locations (22). In addition to reinfection during MDR treatment, it is also possible that heteroresistance (clonal heterogeneity with subpopulations of MDR and XDR variants) (23) or mixed infection with different MDR and XDR strains were present at baseline (24–27). If the XDR strains were relatively rare within an individual, standard drug resistance testing may not have detected XDR that was present at the time of treatment initiation for MDR disease.
The treatment of drug-resistant TB with second-line drugs is an important component of TB control, and is an integral part of the World Health Organization Stop TB Strategy (28). Our findings point to the importance of early and rapid diagnosis of drug resistance, prompt initiation of therapy with appropriate second-line drugs, and strategies to ensure treatment adherence as fundamental programmatic elements.
Acknowledgments
The authors acknowledge the contributions of the following individuals to this research: Natasha Arlyapova, Donna Barry, Valentina Ivanovna Berezina, Vera Golubchikova, Alexander Golubkov, Olga Yurievna Khristenko, Gwyneth Jones, Oleg Petrovich Karpeichik, Tom Nicholson, Michael Nikiforov, Vera Egorovna Pavlova, Gennady Giorgevich Peremitin, Oksana Ponomarenko, Ekaterina Pushkareva, Dmitry Yurievich Shegercov, Olga Sirotkina, Ekaterina Petrovna Stepanova, Galina Yanova, Askar Yedilbayev, and Natalia Aleksandrovna Zemlyanaya.
Supported by the Bill and Melinda Gates Foundation and from the Eli Lilly Foundation. I.G., S.K., S.S.S., and A.P. received partial salary support and/or travel support from the Bill and Melinda Gates Foundation and from the Eli Lilly Foundation. S.K. and S.S.S. received salary support from the Frank Hatch Fellowships in Global Health Equity at Brigham and Women's Hospital. S.S.S. received additional salary support from the Infectious Disease Society of America, The Heiser Foundation, and the National Institute of Allergy and Infectious Diseases; S.K. also received partial salary support, research, and travel funding from the John D. and Catherine T. MacArthur Foundation, and Partners in Health; M.F.F. received support from National Institute of Allergy and Infectious Diseases Pre-Doctoral Training Program in the Epidemiology of Infectious Diseases and Biodefense (T32 AI007535); T.C. received support from National Institutes Office of the Director award DP2OD006663.
Originally Published in Press as DOI: 10.1164/rccm.200911-1768OC on April 22, 2010
Author Disclosure: S.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.K. received more than $100,001 from the Eli Lilly Foundation in industry-sponsored grants. I.Y.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.F.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.K.S. is deceased and is unable to provide a financial disclosure statement. Y.G.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.D.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.P.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gillespie SH. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrob Agents Chemother 2002;46:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox HS, Kalon S, Allamuratova S, Sizaire V, Tigay ZN, Rusch-Gerdes S, Karimovich HA, Kebede Y, Mills C. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: treatment complexity and XDR-TB among treatment failures. PLoS ONE 2007;2:e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pillay M, Sturm AW. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in Kwazulu-Natal, South Africa. Clin Infect Dis 2007;45:1409–1414. [DOI] [PubMed] [Google Scholar]
- 4.Raviglione M. XDR-TB: entering the post-antibiotic era? Int J Tuberc Lung Dis 2006;10:1185–1187. [PubMed] [Google Scholar]
- 5.Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Atwood S, Mukherjee JS, Rich ML, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet 2008;372:1403–1409. [DOI] [PubMed] [Google Scholar]
- 6.Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, Fitzmaurice GM, Alcantara Viru FA, Appleton SC, Bayona JN, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med 2008;359:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sotgiu G, Ferrara G, Matteelli A, Richardson MD, Centis R, Ruesch-Gerdes S, Toungoussova O, Zellweger JP, Spanevello A, Cirillo D, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J 2009;33:871–881. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Anti-tuberculosis drug resistance in the world: fourth global report. Geneva: World Health Organization; 2008.
- 9.Shin SS, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Mishustin S, Barnashov A, Karpeichik Y, Andreev YG, Golubchikova VT, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis 2006;10:402–408. [PubMed] [Google Scholar]
- 10.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, Zarovska E, Rich ML, Fraser HS, Alarcon E, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005;9:640–645. [PubMed] [Google Scholar]
- 11.Yew WW, Chau CH. Drug-resistant tuberculosis in the 1990s. Eur Respir J 1995;8:1184–1192. [DOI] [PubMed] [Google Scholar]
- 12.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84:29–44. [DOI] [PubMed] [Google Scholar]
- 13.Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Med 2007;4:e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelmanova IY, Taran D, Golubkov A, Mazitov R, Mishustin S, Golubchikova VT, Nicholson T, Keshavjee S. Introducing “Sputnik”: a model of patient-centered TB treatment in Tomsk, Russia. IUATLD World Conference on Lung Health. Capetown, South Africa: International Journal of Tuberculosis and Lung Disease; 2007. p. S1.
- 15.World Health Organization. Guidelines for the programmatic management of drug-resistant TB. Geneva: World Health Organization; 2008.
- 16.Gelmanova IY, Keshavjee S, Golubchikova VT, Berezina VI, Strelis AK, Yanova GV, Atwood S, Murray M. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ 2007;85:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubowiak W, Bogorodskaya E, Borisov S, Danilova I, Kourbatova E. Treatment interruptions and duration associated with default among new patients with tuberculosis in six regions of Russia. Int J Infect Dis 2009;13:362–368. [DOI] [PubMed] [Google Scholar]
- 18.Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Lomakina OB, Kourbatova EV. Impact of socio-psychological factors on treatment adherence of TB patients in Russia. Tuberculosis (Edinb) 2008;88:495–502. [DOI] [PubMed] [Google Scholar]
- 19.Keshavjee S, Gelmanova IY, Pasechnikov AD, Mishustin SP, Andreev YG, Yedilbayev A, Furin JJ, Mukherjee JS, Rich ML, Nardell EA, et al. Treating multidrug-resistant tuberculosis in Tomsk, Russia: developing programs that address the linkage between poverty and disease. Ann N Y Acad Sci 2008;1136:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee JS, Rich ML, Socci AR, Joseph JK, Viru FA, Shin SS, Furin JJ, Becerra MC, Barry DJ, Kim JY, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet 2004;363:474–481. [DOI] [PubMed] [Google Scholar]
- 21.Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev 2006;19:658–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews JR, Gandhi NR, Moodley P, Shah NS, Bohlken L, Moll AP, Pillay M, Friedland G, Sturm AW. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis 2008;198:1582–1589. [DOI] [PubMed] [Google Scholar]
- 23.Cullen MM, Sam NE, Kanduma EG, McHugh TD, Gillespie SH. Direct detection of heteroresistance in Mycobacterium tuberculosis using molecular techniques. J Med Microbiol 2006;55:1157–1158. [DOI] [PubMed] [Google Scholar]
- 24.Garcia de Viedma D, Alonso Rodriguez N, Andres S, Ruiz Serrano MJ, Bouza E. Characterization of clonal complexity in tuberculosis by mycobacterial interspersed repetitive unit-variable-number tandem repeat typing. J Clin Microbiol 2005;43:5660–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamputa IC, Jugheli L, Sadradze N, Willery E, Portaels F, Supply P, Rigouts L. Mixed infection and clonal representativeness of a single sputum sample in tuberculosis patients from a penitentiary hospital in georgia. Respir Res 2006;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamputa IC, Rigouts L, Eyongeta LA, El Aila NA, van Deun A, Salim AH, Willery E, Locht C, Supply P, Portaels F. Genotypic and phenotypic heterogeneity among Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients. J Clin Microbiol 2004;42:5528–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rie A, Victor TC, Richardson M, Johnson R, van der Spuy GD, Murray EJ, Beyers N, Gey van Pittius NC, van Helden PD, Warren RM. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med 2005;172:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. The stop TB strategy: building on and enhancing DOTS to meet the TB-related millenium development goals. Geneva: World Health Organization; 2006.