Abstract
Amides as neutral and hydrophobic internucleoside linkages in RNA are highly interesting modifications for RNA interference. However, testing amides in siRNAs is hampered by the shortage of efficient methods to synthesize the monomeric building blocks, the nucleoside amino acid equivalents. This paper reports an efficient synthesis of protected ribonucleoside 5'-amino 3'-carboxylic acids from d-xylose in 14 steps 7% overall yield. The key features that ensure efficiency and ease of operations are chemoselective reduction of the ester and minimization of protecting group manipulation.
Keywords: RNA, Modified nucleoside, Amide internucleoside linkage, Carbohydrate synthesis, Selective reduction of ester
1. Introduction
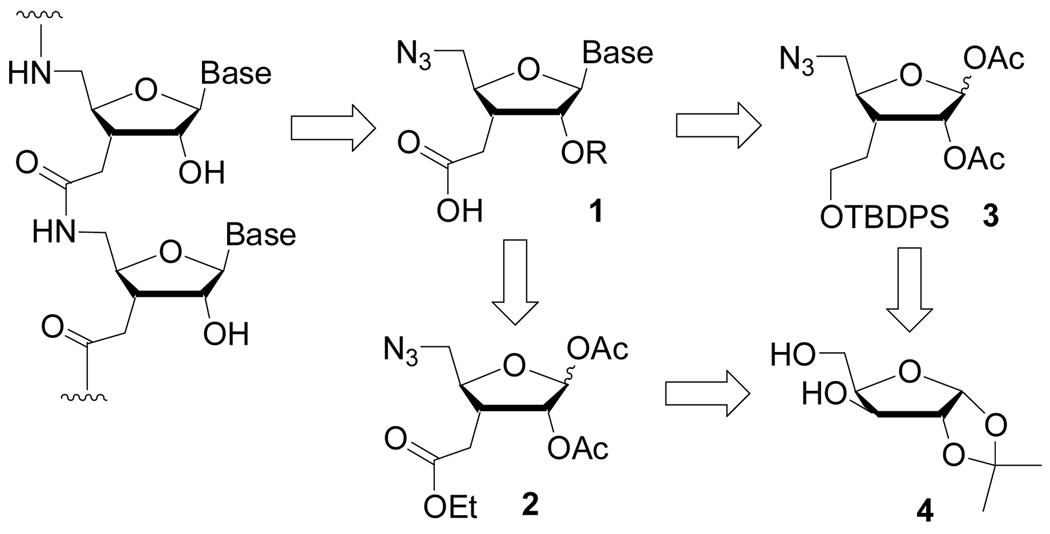
The potential of RNA interference (RNAi) to become a new therapeutic strategy has revitalized the interest in chemical modifications of RNA. To be useful as therapeutic agents short interfering RNAs (siRNAs) need to be chemically modified to optimize their potency, enzymatic stability, cellular uptake, biodistribution, and pharmacokinetics.1 We have been interested in hydrophobic non-ionic mimics of phosphate backbone, such as formacetals2 and amides3 (Figure 1). Non-ionic phosphate mimics may offer several advantages for siRNAs: (1) the absence of natural phosphate will confer high nuclease resistance, (2) the reduction of charge may facilitate crossing of cellular membranes and (3) the increased hydrophobicity of siRNAs may improve their biodistribution and pharmacokinetics.
Figure 1.
Structure and retrosynthetic analysis of amide linked RNA.
In previous studies we found that replacement of selected phosphates with amide linkages (Figure 1) did not change the overall conformation and UV melting temperature (thermal stability) of RNA double helix.3 The amide linkages appeared to be excellent mimics of phosphate backbone in RNA and were expected to be compatible with RNAi machinery. In accord with such expectation, Iwase et al.4 recently reported that an siRNA having two amide linkages at the overhanging uridines retained high activity in RNAi assays. These results suggest a hypothesis that siRNAs may tolerate even more extensive amide modification, which may realize the potential advantages of the hydrophobic RNA modifications. The bottleneck of testing the above hypothesis is the shortage of efficient and large scale syntheses of enantiomerically pure C3’-homologated carboxylic acids 1 (Figure 1), the monomers for introduction of consecutive amide linkages in siRNAs.
In their study of amide modified siRNAs, Iwase et al.4 used uridine carboxylic acids, which were prepared from uridine following a procedure by Robins and co-workers.5 The problem with such an approach is that four independent synthetic routes must be pursued to prepare all four nucleoside carboxylic acids, which is time and resources consuming. Robins and co-workers have also developed synthesis of 1 starting from d-glycose or d-xylose. 6 The sugar route diverges at the common intermediate 2 (Figure 1) on which the desired heterocylces can be installed in a straightforward manner. However, we found that Robin’s route from sugars was complicated at the finals steps by the need to cleave the ester (see 2) and, consequently, laborious reintroduction of 2’-OH and heterocycle protecting groups, which being base labile, were lost during the ester cleavage.
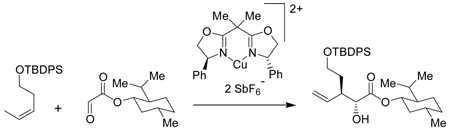
Recently, we reported an asymmetric de novo synthesis of 1 starting from small molecules and proceeding through the common intermediate 3.7 The final oxidation of alcohol led to straightforward and efficient preparation of carboxylic acids 1. However, we encountered difficulties in scaling up the enantioselective reactions during the starting steps of de novo route. The de novo route started with a double asymmetric ene reaction that could be performed on a 4 g scale (13 mmol) of 1-(tert-butyldiphenylsilanoxy)-3-pentene, but required an excess of 28 g (130 mmol) of (1'S, 2'R, 5'S)-menthyl glyoxalate.
Preparation of pure menthyl glyoxylate8 involved column chromatography and vacuum distillation and was not practical on scales larger than 28 g. The double asymmetric reaction involving large excess of menthyl glyoxylate was necessary to insure good yields and high enantiomeric purity (>98:2) of the product and it appeared unlikely that this reaction could be scaled up to >50 mmol required to support our planed studies.
Herein we report optimization of our synthetic route7 by adopting the initial steps from the Robin’s synthesis to prepare the key intermediate 3 starting from d-xylose.6 The key features that ensure efficiency of the new route are chemoselective reduction of the ester and minimization of protecting group manipulation.
2. Results and discussion
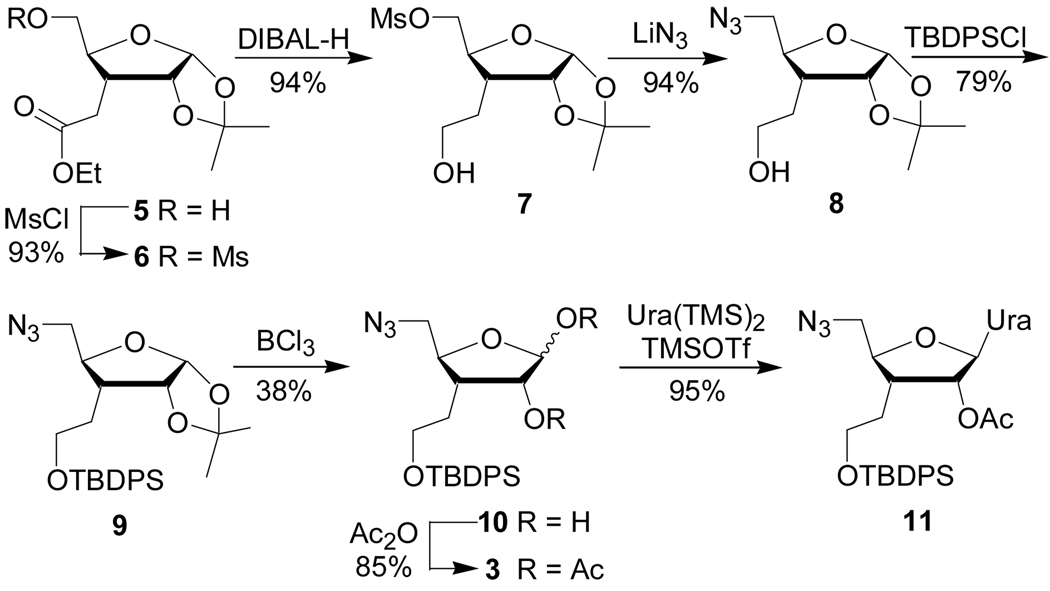
Our synthesis (Scheme 1) started with the C3’-homologated ribose derivatives 5 and 6, which were prepared as described by Robins and co-workers.6 The plan was to reduce the ester before installation of the azide and then oxidize the alcohol after the nucleoside synthesis as was successfully done in our de novo route.7 In this way we would avoid the cleavage of ester under basic conditions after the nucleoside synthesis. Direct reduction of 5 would generate two primary alcohols, which would be difficult (if at all possible) to selectively functionalize. To avoid additional protecting groups we decided to use the mesyl group (Ms, Scheme 1) as both differentiating functionality for the 5-OH in 5 and leaving group for later nucleophilic substitution with azide.8 Treatment of alcohol 5 with methanesulfonyl chloride, as described by Robins and co-workers, gave mesylate 6.6
Scheme 1.
Synthesis of C3’-homologated uridine.
The challenge with such an approach was the selective reduction of the ester in the presence of potentially reactive mesyl group. Of several reducing agents tried (Table 1), the best results were obtained with DIBAL-H at 0 °C.10 Borane dimethyl sulfide complex11 also performed well but required elevated temperature (60 °C) and longer reaction time. While LiAlH4 and Ca(BH4)2 (formed in situ from NaBH4 and CaCl2 12) gave good yields, LiBH4 with catalytic amounts of 9BBN13 gave complex reaction mixture containing no target product.
Table 1.
Results of selective reduction of ester 6.
| Reagent | Solvent | Temp. (°C) |
Time (h) |
Yield (%) |
|
|---|---|---|---|---|---|
| 1 | DIBAL-H | CH2Cl2, hexane | 0 | 1 | 94 |
| 2 | BH3·Me2S | THF | 60 | 2 | 93 |
| 3 | LiAlH4 | Dimethoxyethane | 0 | 2 | 78 |
| 4 | NaBH4, CaCl2 | THF | 25 | 24 | 74 |
| 5 | LiBH4-9BBN | THF, toluene | 100 | 24 | 0 |
Importantly, the mesylation and DIBAL-H reduction steps gave products in acceptable purity without the need for column chromatography. Treatment of mesylate 7 with lithium azide gave alcohol 8, which was purified by silica gel column chromatography. Treatment of 8 with TBDPCl gave 9, which was used in next step again without chromatographic purification.
The most challenging step in our synthesis was the removal of the 1,2-isopropylydene protecting group. Cleavage of 1,2-acetals in carbohydrates generally requires relatively harsh conditions. In our case, the TBDPS ether, which was relatively close on the same side of the tetrahydrofurane ring (see 9 in Scheme 1), further complicated the reaction. Typical protic acid hydrolysis procedures (Table 2) gave either no reaction (entry 1) or led to concurrent cleavage of the TBDPS group (entries 2–7).
Table 2.
Results of cleavage of isopropylydene in 9.
| Reagent | Temp (°C) |
Time (h) | Yield (%) |
|
|---|---|---|---|---|
| 1 | Amberlyst-15/MeOH | 25 | overnight | NR a |
| 2 | THF/TFA/H2O (5:4:1) | 25 | overnight | 0 b |
| 3 | 80% Acetic acid | 80 | overnight | 0 b |
| 4 | 5% methanesulfonic acid/MeOH | 25 | overnight | 0 b |
| 5 | 0.3% H2SO4/MeOH | 25 | overnight | 0 b |
| 6 | 0.5M HCl/THF | 50 | overnight | 0 b |
| 7 | H2SO4/AcOAc/AcOH (1:2:60) | 25 | overnight | 29% c |
| 8 | BBr3 in hexanes/CH2Cl2 (3:40) | −78 to 0 | 0.5 | 0 c |
| 9 | BCl3 in hexanes/CH2Cl2 (3:40) | 0 | 0.5 | 38% d |
No reaction,
Cleavage of TBDPS,
Decomposition to polar by-products,
Based on recovered starting material.
Acceptable yield of 10 was achieved using BCl3 14 after recycling of the recovered starting material. Acylation with pyridine/acetic anhydride gave the glycosyl donor 3, which serves as a common intermediate in synthesis of the modified nucleosides.
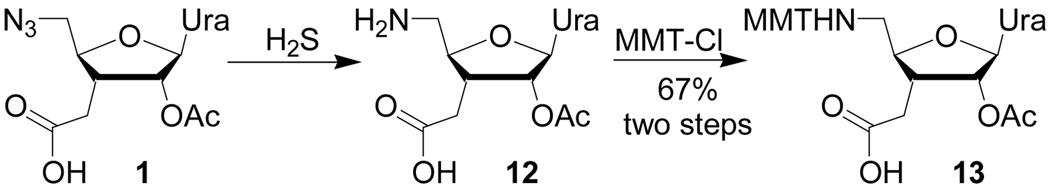
Final steps in preparation of all four ribonucleoside 5'-amino 3'-carboxylic acids from the common intermediate 3 have been previously reported by us.7 Following these procedures we used 3, prepared from d-xylose, to synthesize uridine derivative 11. The product obtained was identical to 11 previously synthesized via the de novo route.7 Following the reported procedures 11 was further converted to the uridine azido acid 1 (Scheme 2). Reduction of azide with hydrogen sulfide was immediately followed by protection of the amino group with methoxytrityl chloride to give the amino acid 13, suitable for solid phase synthesis of oligoamides.
Scheme 2.
Synthesis of uridine 5'-amino 3'-carboxylic acid.
3. Conclusion
In summary, we have developed a new synthesis of uridine 5'-azido 3'-carboxylic acid 1 from d-xylose in 14 steps 7% overall yield. One step reduction followed by protection of amino group gave the target 5’-amino 3’-carboxyl uridine 13 in 67% yield. Although the yield of the new route is somewhat lower than that of our previous de novo synthesis (9 steps and 19% to 1),7 the ability to produce enantiomerically pure material on larger scales is an important advantage for our current studies on solid phase synthesis of oligoamides. While the start of our de novo route was limited to ca 13 mmol scale in the first step, the new route could be easily started with 200 mmols (30 g) of d-xylose. We envision that the new route should allow future preparation of the target nucleoside 5'-azido 3'-carboxylic acids on a gram scale.
The route presents formal synthesis of all four ribonucleoside 5'-azido 3'-carboxylic acids (1) because it makes the common intermediate 3 described in our previous work.7 The key features that ensure efficiency and ease of operations are chemoselective reduction of the ester, minimization of protecting group manipulation and minimization of silica gel column purification steps. The efficiency may be further increased by optimization of the removal of the 1,2-isopropylydene protecting group. The new route should facilitate synthesis and testing of amide-modified siRNAs, which have shown promising results in initial studies.4
4. Experimental section
4.1. 3-Deoxy-3-(2-hydroxyethyl)-1,2-O-isopropylidene-5-O-(methanesulfonyl)-α-D-ribofuranose (7)
DIBAL-H (46.5 mL, 45.6 mmol, 25% in hexanes) was added slowly to a solution of 6 (7.88 g, 23.2 mmol) in anhydrous CH2Cl2 (120 mL) at 0 °C. The solution was stirred at 0 °C for 1 h. The reaction was quenched by extracting with diluted HCl (0.2%, 150 mL), the organic layer was separated, filtered to remove solid particles and dried with anhydrous Na2SO4. Evaporation gave the title compound 7 (6.43 g, 94 %) as a colorless oil, which could be used in next step without further purification. 1H NMR (CDCl3, 360 MHz) δ 5.78 (d, J = 3.6 Hz, 1H), 4.69 (m, 1H), 4.44 and 4.26 (ABX, J = 2.2, 4.3, 11.5 Hz, 2H), 4.03 (m, 1H), 3.74 (m, 2H), 3.04 (s, 3H), 2.13 (m, 1H), 1.91 (sb, 1H), 1.86 (m, 1H), 1.63 (m, 1H), 1.47 (s, 3H), 1.30 (s, 3H). 13C NMR (CDCl3, 90 MHz) δ 111.8, 104.9, 81.0, 79.2, 68.6, 60.6, 41.6, 37.6, 29.6, 27.5, 26.4. MS (ESI) Calcd for 2×C11H20O7S+Na 615.2; found 614.7.
4.2. 5-Azido-3,5-dideoxy-3-(2-hydroxyethyl)-1,2-O-isopropylidene-α-D-ribofuranose (8)
LiN3 (4.48 g, 89.5 mmol) was added to a solution of 7 (5.30 g, 17.9 mmol) in anhydrous DMF (50 mL). The solution was heated to 60 °C and stirred at this temperature for 14 h. The solution was concentrated in vacuum, the residue was dissolved in ethyl acetate (40 mL) and washed with brine (30 mL) and water (3 × 30 mL), the organic layer was dried with Na2SO4, filtered and evaporated in vacuum. The crude product was purified on silica gel column using hexane/ethyl acetate (4:1 v/v) to give the title compound (4.11 g, 94 %) as a colorless oil. 1H NMR (CDCl3, 360 MHz) δ 5.81 (d, J = 3.1 Hz, 1H), 4.67 (m, 1H), 3.98 (m, 1H), 3.75 (m, 2H), 3.62 (dd, J = 3.0, 13.7 Hz, 1H), 3.23 (dd, J = 4.7, 13.7 Hz, 1H), 2.12 (m, 1H), 1.84 (m, 1H), 1.58 (m, 1H), 1.49 (s, 3H), 1.32 (s, 3H). 13C NMR (CDCl3, 90 MHz) δ 111.7, 104.8, 81.2, 80.4, 60.9, 51.5, 42.5, 27.6, 26.6, 26.3.
4.3. 5-Azido-3,5-dideoxy-3-[2-(tert-butyldiphenylsilyoxy)-ethyl]-1,2-O-isopropylidene-α-D-ribofuranose (9)
To a solution of 8 (2.41 g, 9.9 mmol) in imidazole (1.47 g, 21.6 mmol) in anhydrous DMF (20 mL), was added tert-butyldiphenylsilyl chloride (3.1 mL, 11.8 mmol). The solution was stirred at room temperature over night. The reaction was then quenched with water, evaporated in vacuum and the residue was dissolved in EtOAc (20 mL) and washed once with brine (15 mL) then with water (3 × 15 mL). The organic layer was dried with Na2SO4, filtered and evaporated in vacuum to give the title compound 9 (3.75 g, 79%) as a crude colorless oil whose purity was acceptable for the next step. 1H NMR (CDCl3, 360 MHz) δ 7.71 (m, 4H), 7.44 (m, 6H), 5.77 (d, J = 3.4 Hz, 1H), 4.42 (m, 1H), 3.98 (m, 1H), 3.82 (m, 2H), 3.61 (dd, J = 3.0, 13.7 Hz, 1H), 3.20 (dd, J = 4.8, 13.2 Hz, 1H), 2.17 (m, 1H), 1.84 (m, 1H), 1.55 (m, 1H), 1.49 (s, 3H), 1.29 (s, 3H), 1.11 (s, 9H). 13C NMR (CDCl3, 90 MHz) δ 135.6, 129.7, 127.6, 111.6, 104.9, 80.9, 61.9, 51.6, 42.3, 27.6, 26.8, 19.1. MS (ESI) Calcd for C26H35N3NaO4Si 504.2; found 504.2.
4.4. 5-Azido-3,5-dideoxy-3-[2-(tert-butyldiphenylsilyoxy)-ethyl]-α-D-ribofuranose (10)
A solution of 9 (1.505 g, 3.12 mmol) in anhydrous CH2Cl2 (40 mL) was cooled to 0 °C and then treated with BCl3 (3.12 mL, 3.12 mmol, 1M solution in hexanes) for 30 minutes. The reaction was quenched with saturated NaHCO3 (50 mL) and brought to room temperature, the aqueous layer was extracted with CH2Cl2 (3 × 50 mL). The organic layers were pooled together, dried with Na2SO4, filtered and evaporated in vacuum. The residue was purified on silica gel column using hexane/ethyl acetate (4:1 v/v) to give the title compound 10 (0.309g, 0.718 mmol, 23%) as a colorless oil. After chromatography 0.624g, 40% of 9 was recovered bringing the yield of 10 to 38% based on the recovered starting material. The spectroscopic data are for mixture of α and β diastereomers. 1H NMR (CDCl3, 360 MHz) δ 7.70-7.64 (m, 4H), 7.47-7.39 (m, 6H), 5.48 and 5.38 (d, J = 3.4 Hz, 1H), 4.31-4.25 (m, 1H), 4.10-4.04 (m, 1H), 3.83-3.65 (m, 2H), 3.57-3.48 (m, 1H), 3.31 (dd, J = 5.5, 13.2 Hz), 3.12 and 3.03 (dd, J = 4.3, 13.2 Hz), 2.40-2.30 and 2.23-2.14 (m, 1H), 1.97-1.85 (m, 1H), 1.68-1.50 (m, 1H), 1.07 (s, 9H). 13C NMR (CDCl3, 90 MHz) δ 135.5, 132.5, 130.0, 127.8, 102.8, 98.2, 82.9, 80.0, 71.8, 63.3, 54.2, 52.4, 42.8, 28.0, 26.7, 18.9.
4.5. 5-Azido-3,5-dideoxy-3-[2-(tert-butyldiphenylsilyoxy)-ethyl]-1,2-O-diacetyl-α-D-ribofuranose (3)
Compound 10 (0.8 g, 1.81 mmol) was dissolved in pyridine/acetic anhydride (6 mL, 1:1 v/v) and stirred at room temperature for 12 hours. The solution was concentrated in vacuum, the residue was dissolved in EtOAc (50 mL) and washed with saturated aqueous NaHCO3 (3 × 50 mL). The organic layer was dried with anhydrous Na2SO4, filtered and evaporated under vacuum to give the title compound 3 (0.809g, 1.54 mmol, 85%) as colorless oil of purity acceptable for the next step. The spectroscopic data are for mixture of α and β diastereomers. 1H NMR (CDCl3, 360 MHz) δ 7.69-7.61 (m, 4H), 7.46-7.36 (m, 6H), 6.38 and 6.08 (d, J = 3.1 Hz, 1H), 5.24-5.16 (m, 1H), 4.24-4.18 and 4.13-4.08 (m, 1H), 3.74-3.58 (m, 3H), 3.27-3.23 and 3.18-3.14 (dd, J = 4.3, 13.7 Hz, 1H), 2.77-2.68 and 2.55-2.46 (m, 1H), 2.09 (s, 3H), 2.03 (s, 3H), 1.81-1.50 (m, 2H), 1.06 (s, 9H). 13C NMR (CDCl3, 90 MHz) δ 135.5, 129.7, 127.7, 98.7, 83.8, 82.7, 61.7, 38.0, 29.7, 27.4, 26.8, 20.6.
4.6. 2’-O-Acetyl-5’-azido-3’-[2-(tert-butyldiphenylsiloxy) ethyl]-3’,5’-dideoxyuridine (11)
The title compound 11 was prepared from 3 (0.222 g, 0.412 mmol) as previously described by us7 in 95% yield (0.226 g). The 1H and 13C NMR matched the reported data.7 Elemental analysis (C29H35N5O6Si): calculated C, 60.29; H, 6.11; N, 12.12; found C, 60.44; H, 6.17; N, 11.78.
4.7. 2’-O-Acetyl-3’,5’-dideoxy-3’-carboxymethyl-5’-(monomethoxytrityl)amino-uridine triethylammonium salt (13)
Hydrogen sulfide was passed through a solution of 1 (275 mg, 0.78 mmol) in pyridine/water (25 mL, 4:1 v/v) for 1 h at room temperature. The solution was stirred over night and evaporated in vacuum. The residue was dissolved in water (50 mL) and washed with CH2Cl2 (4 × 50 mL). The organic layers were discarded and the water phase was evaporated to give amine 12 (~230 mg, ~0.70 mmol, ~90%), which was used in the next step without further purification. Amine 12 was dissolved in anhydrous pyridine (10 mL) and 4-methoxytrityl chloride (1.08g, 3.5 mmol) was added. The solution was stirred at room temperature over night. The reaction was quenched with saturated aqueous triethylammonium bicarbonate (1 mL) and evaporated in vacuum. The residue was dissolved in CH2Cl2 (50 mL) and extracted with saturated aqueous NaHCO3 (3 × 50 ml). The organic phase was dried with Na2SO4, filtered and concentrated in vacuum. The residue was purified on silica gel column eluting with a stepwise gradient of MeOH (0–10%) in CH2Cl2 containing 100 ppm of triethylamine to give the title compound 13 (362 mg, 0.52 mmol, 67 % for two steps). 1H NMR (CDCl3, 300 MHz) δ 7.65 (d, J = 8.6 Hz, 1H), 7.29 (m, 12H), 6.80 (d, J = 9.0 Hz, 2H), 5.81 (s, 1H), 5.60 (d, J = 8.1 Hz, 1H), 5.46 (d, J = 5.7 Hz, 1H), 4.04 (m, 1H), 3.76 (s, 3H), 2.95 (q, J = 7.1 Hz, 6H), 2.72 (m, 2H), 2.26 (m, 3H), 2.06 (s, 3H), 1.17 (t, J = 7.14 Hz, 9H). 13C NMR (CDCl3, 72 MHz) δ 175.7, 169.6, 163.7, 158.2, 150.7, 150.1, 146.2, 146.1, 140.4, 137.9, 130.1, 128.7, 128.1, 126.6, 113.5, 102.5, 90.3, 84.3, 78.4, 70.5, 55.4, 45.4, 45.2, 39.6, 31.2, 29.9, 20.8, 8.6. HRMS (ESI) Calcd for C33H33N3NaO8, 622.2165; Found, 622.2157.
Supplementary Material
Acknowledgments
We thank Binghamton University and NIH (R01 GM071461) for support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at …
References and notes
- 1.Watts JK, Deleavey GF, Damha MJ. Drug Discovery Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]; Corey DR. J. Clin. Invest. 2007;117:3615–3622. doi: 10.1172/JCI33483. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rozners E. Curr. Org. Chem. 2006;10:675–692. [Google Scholar]; Manoharan M. Curr. Opin. Chem. Biol. 2004;8:570–579. doi: 10.1016/j.cbpa.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kolarovic A, Schweizer E, Greene E, Gironda M, Pallan PS, Egli M, Rozners E. J. Am. Chem. Soc. 2009;131:14932–14937. doi: 10.1021/ja904926e. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rozners E, Katkevica D, Strömberg R. ChemBioChem. 2007;8:537–545. doi: 10.1002/cbic.200600515. [DOI] [PubMed] [Google Scholar]; Rozners E, Strömberg R. J. Org. Chem. 1997;62:1846–1850. [Google Scholar]
- 3.Rozners E, Katkevica D, Bizdena E, Strömberg R. J. Am. Chem. Soc. 2003;125:12125–12136. doi: 10.1021/ja0360900. [DOI] [PubMed] [Google Scholar]
- 4.Iwase R, Toyama T, Nishimori K. Nucleosides, Nucleotides & Nucleic Acids. 2007;26:1451–1454. doi: 10.1080/15257770701542389. [DOI] [PubMed] [Google Scholar]; Iwase R, Miyao H, Toyama T, Nishimori K. Nucleic Acids Symposium Series. 2006:175–176. doi: 10.1093/nass/nrl087. [DOI] [PubMed] [Google Scholar]
- 5.Peterson MA, Nilsson BL, Sarker S, Doboszewski B, Zhang W, Robins MJ. J. Org. Chem. 1999;64:8183–8192. doi: 10.1021/jo9908647. [DOI] [PubMed] [Google Scholar]
- 6.Robins MJ, Doboszewski B, Timoshchuk VA, Peterson MA. J. Org. Chem. 2000;65:2939–2945. doi: 10.1021/jo991399g. [DOI] [PubMed] [Google Scholar]
- 7.Rozners E, Liu Y. J. Org. Chem. 2005;70:9841–9848. doi: 10.1021/jo0515879. [DOI] [PubMed] [Google Scholar]; Rozners E, Liu Y. Org. Lett. 2003;5:181–184. doi: 10.1021/ol027229z. [DOI] [PubMed] [Google Scholar]
- 8.Whitesell JK, Lawrence RM, Chen H-H. J. Org. Chem. 1986;51:4779–4784. [Google Scholar]
- 9.Matin MM. J. Appl. Sci. Res. 2008;4:1478–1482. [Google Scholar]
- 10.Yoon NM, Gyoung YS. J. Org. Chem. 1985;50:2443–2450. [Google Scholar]
- 11.Brown HC, Choi YM, Narasimhan S. J. Org. Chem. 1982;47:3153–3163. [Google Scholar]
- 12.Hou D, Reibenspies JH, Burgess K. J. Org. Chem. 2001;66:206–215. doi: 10.1021/jo001333h. [DOI] [PubMed] [Google Scholar]
- 13.Piers E, Chong JM. J. Org. Chem. 1982;47:1604–1606. [Google Scholar]
- 14.Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4672–4685. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.