Abstract
The levels of proteins required for methyl-directed mismatch repair appear to decline in stationary-phase and nutritionally-deprived cells of Escherichia coli. It has been hypothesized that error-correction by the system also declines, and this decline is responsible for adaptive or stationary-phase mutations. However, evidence in support of this hypothesis is lacking. The mismatch repair system is no less effective in correcting errors during prolonged selection than it is during growth. Furthermore, mismatch repair proteins supplied in excess reduce both growth-dependent and adaptive mutation.
Keywords: Mutation, Adaptive mutation, Mismatch repair, Spontaneous mutation, Stationary-phase mutation, DNA polymerase error, Stationary phase
1. Introduction
The best-studied example of stationary-phase or adaptive mutation is a strain of Escherichia coli, FC40, that cannot metabolize lactose (Lac−) because of a frameshift mutation affecting the lacZ gene carried on its episome. Two days after plating FC40 cells on minimal lactose medium, Lac+ colonies appear that are due to mutations that occurred during nonselected growth prior to plating. For several days thereafter, Lac+ colonies continue to appear at a fairly constant rate although the Lac− population is not increasing. These late-arising revertants are called ‘adaptive’ [1].
The mutations that give rise to adaptive Lac+ revertants of FC40 are made by DNA polymerase III [2] and consist mainly of –1 base pair frameshifts in runs of iterated bases [3,4]. Because such polymerase errors are typically corrected by methyl-directed mismatch repair (MMR), it was hypothesized that MMR might be limiting in stationary-phase and nutritionally-deprived cells, giving rise to the adaptive mutations [3,4]. This hypothesis was supported by the finding that levels of the MMR proteins, MutS and MutH, but not MutL, appear to decline in stationary phase and nutritionally deprived cells [5]. However, the levels of MMR activity that remain might be entirely sufficient for the amount of DNA synthesis that normally takes place.
Prior to the publication of Ref. [5], we reported that overproduction of the MMR proteins, MutL and MutS, decrease adaptive mutation to Lac+ in FC40 [2,6]. We had found that overproduction of either protein was slightly inhibitory, but we published only the larger decrease observed when MutL and MutS were overproduced together. But, because excess MMR proteins also decreased the frequency of early-arising, growth-dependent Lac+ mutants, we concluded that these experiments did not demonstrate that MMR activity was specifically limiting in stationary phase cells [2]. Subsequently, Harris et al. [7] claimed that MMR activity was, indeed, specifically limiting in FC40 during lactose selection and that MutL was the limiting protein. Although in their experiments MutS had no effect, they found, as had we, that overproduction of MutL decreased the frequency of both early and late-arising Lac+ mutants. But, they dismissed the decrease in early-arising mutants as an artifact.
The data presented here support our previous findings, refute the conclusions of Harris et al. [7], and suggest that loss of MMR activity is not responsible for the majority of adaptive mutations in FC40 or in other strains of E. coli.
2. Loss of MMR has as large an effect on adaptive mutations as it does on growth-dependent mutations
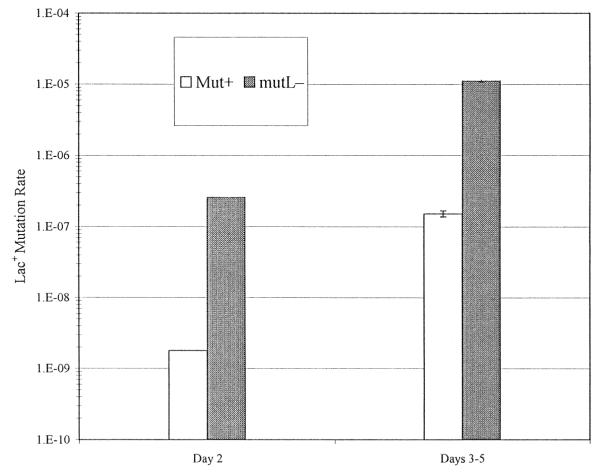
In E. coli, adenines in dGATC sequences are methylated by the Dam methylase. Because methylation lags behind replication, newly synthesized DNA is hemimethylated. The MMR enzymes promote the correction of mismatches in hemimethylated DNA in favor of the parental methylated strand, increasing in the fidelity of DNA replication [8]. Fig. 1 shows that loss of MutL results in about a 100-fold increase in the growth-dependent mutation rate to Lac+ (Day 2) in FC722, a close derivative of FC40 [9]. As half of the growth-dependent Lac+ mutations are –1 base pair frameshifts that are, in principal, correctable by MMR [3], this increase represents the true capacity of MMR to correct mismatches during growth. Loss of MutL results in a similar 100-fold increase in the rate of adaptive mutation to Lac+ (Days 3–5). Thus, at normal levels MMR is no less effective in correcting errors during lactose selection than during growth. Similar results have been obtained with other E. coli strains [10–12], and when the Lac− frameshift allele is on the chromosome instead of the episome [13]. Defects in each of the three major MMR proteins, MutS, MutL, and MutH, as well as overproduction of the Dam methylase, have the same mutagenic phenotype in FC40 [6,14,15], indicating that during lactose selection DNA is methylated as normal.
Fig. 1.
The effect of loss of MutL on growth-dependent and adaptive reversion of an episomal Lac− allele. The results are from fluctuation tests (25 cultures for wild type and 48 cultures for the mutL− derivative). Growth-dependent mutation rates (Day 2, white) are Lac+ mutations per cell per generation, calculated from the proportion of cultures giving no Lac+ colonies 2 days after plating on lactose minimal medium. Adaptive mutation rates (Days 3–5, grey) are the mean Lac+ mutations per cell per day (±S.E.), calculated from the numbers of Lac+ colonies appearing on days 3 to 5 after plating on lactose minimal medium.
Additional evidence also indicates that MMR activity is not globally limiting in nutritionally deprived or stationary-phase cells. MutL and MutS participate in another mismatch repair pathway called very-short patch repair (VSR). [16]. Overproduction of the Vsr endonuclease is mutagenic, probably because it depletes MMR activity [17]. Overproduction of Vsr increases adaptive mutation about 100-fold in wild type FC40 but not in a mutL− derivative [18]. If MMR were not active during lactose selection excess Vsr would have no effect.
3. Overproduction of MMR proteins affects both growth-dependent and adaptive mutations
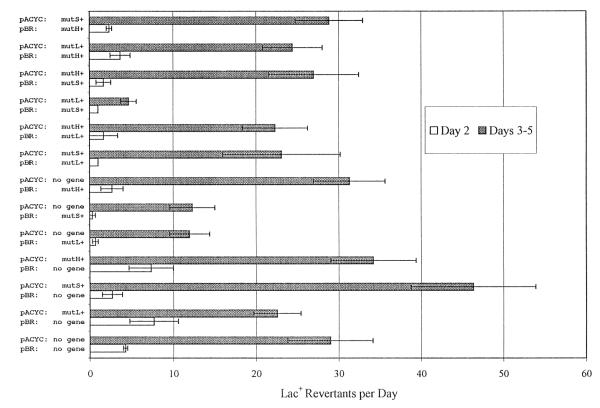
Fig. 2 shows that overexpression of MutL or MutS from pBR322 plasmids reduces adaptive mutation in FC722 about 2-fold. However, overproduction of MutL and MutS together has a stronger antimutagenic effect, reducing adaptive mutation about 6-fold when MutS is in excess (i.e., when MutS and MuL are on pBR322- and pACY184-based plasmids, respectively). Thus, neither MutL nor MutS can be said to be limiting as the effect of each protein appears to depend on the relative level of the other. We conclude that the different vectors and constructions used by Harris et al. [7] precluded their observing an effect of MutS overproduction.
Fig. 2.
The effect of overproduction of MMR proteins on growth-dependent and adaptive reversion of the episomal Lac− allele. The results are the mean number of Lac+ colonies per day appearing on Day 2 (white). and Days 3–5 (gray) after plating on lactose minimal medium, determined from 3 to 4 cultures of each strain. Each strain carried both a pACYC- and a pBR-derived plasmid; the genes on the plasmids are listed to the left of the graph. Error bars are ±S.E., and in some cases are too small to be seen. Plasmids carrying MMR proteins were obtained from M.G. Marinus [2,6].
Fig. 2 also shows that overproduction of MutL and MutS, together or separately, reduces the number of Lac+ mutants appearing on Day 2, which are due to growth-dependent mutations. Thus, MMR activity can be improved in growing cells as well as in nutritionally deprived cells (see below).
4. Overproduction of MMR proteins also decreases growth-dependent TetR mutations
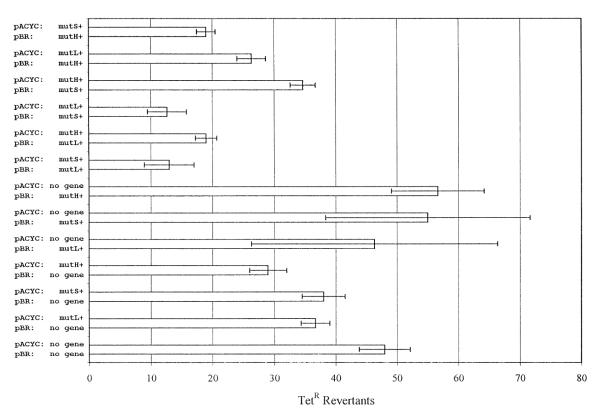
Because this last finding was disputed by Harris et al. [7], we repeated the experiment with a different mutational target. Strain FC722 carries on its episome a TetS allele with the same type of frameshift mutation as the Lac− allele [9]. Because tetracycline is bacteriostatic, the TetR mutants that appear after plating a culture on tetracycline-containing medium must have arisen during nonselective growth prior to plating. Although the vast majority of TetR colonies appear after two days of incubation, plates were scored for an additional 2 days to insure that any slow-growing mutants produced colonies. Fig. 3 shows that, unlike reversion to Lac+, reversion to TetR is not sensitive to any one MMR protein, at least within the limitations of a small experiment. However, overproduction of various combinations of MMR proteins reduced the frequency of TetR mutants about 4-fold. This result again demonstrates that excess MMR proteins affect mutation in growing cells, and that limitation of MMR activity is not confined to stationary-phase or nutritionally deprived cells.
Fig. 3.
The effect of overproduction of MMR proteins on growth-dependent reversion of an episomal TetS allele. The results are the mean number of TetR colonies appearing 2 to 5 days after plating on glycerol minimal medium with tetracycline, determined from 3 to 4 cultures of each strain. Each strain carried both a pACYC- and a pBR-derived plasmid; the genes on the plasmids are listed to the left of the graph. Error bars are ±S.E. Plasmids were as in Fig. 2 except their TetR genes were deleted if required. The control pBR322 TetS plasmid was obtained from S.T. Lovett.
5. The reduction in growth-dependent mutation to Lac+ is not due to slow growth
Harris et al. [7] claimed that the reduction in the number of Lac+ revertants appearing on day 2 was not because excess MMR reduces the growth-dependent mutation rate. Rather, they claimed that Lac+ cells carrying the MMR genes on a plasmid took longer to form colonies than Lac+ cells carrying only the vector. Based on this, they included in their calculation of growth-depended mutation rates an unreported number of late-arising Lac+ mutants. However, as shown in Table 1, the 12% increase in t50, the ‘time at which half of the colony-forming units have produced visible colonies’, that they reported for cells overproducing MutL and MutS is not statistically significant. Indeed, statistical analysis of their results with 8 strains in 7 experiments shows that none of the differences in t50 reported by Harris et al. are statistically significant (F=1.44, df=7, P=0.25). Thus, the differential corrections that they made to the mutation rates are not justified.
Table 1.
Statistical analysis of the effect of MMR proteins on growth of Lac+ colonies
| Experiment | Time to 50% colony formation of Lac+ clones (h) |
Paired two sample t-test for means |
||
|---|---|---|---|---|
| Control | pMutSL | |||
| 1 | 52 | 71 | Degrees of freedom |
2 |
| 2 | 56 | 58 | t Statistic | 1.16 |
| 3 | 67 | 67 |
t Critical, one-tail |
2.92 |
| Mean | 58 | 65 |
t Critical, two-tail |
4.30 |
| Variance | 60 | 44 |
P(T≤t), one-tail |
0.18 |
| Mean difference |
+7 |
P(T≤t), two-tail |
0.37 | |
Data are from Ref. [7], Table 2.
The t50 results reported by Harris et al. [7] do indicate that the growth rate of Lac+ cells was significantly reduced by their vector. In our experience, the vast majority of Lac+ revertants of FC40 make visible colonies in 48 h. We did observe that Lac+ cells with excess MMR grew somewhat poorly in competition with Lac− wild type cells, and compensated for this by using ‘scavenger’ Lac− cells that carried the same plasmids [2]. In the experiments reported here, no scavengers at all were used.
6. An alternative explanation for the antimutagenic effect of overproduction of MMR proteins
Overproduction of MMR proteins could reduce mutations by mechanisms unrelated to error correction. In vivo, MMR prevents recombination between homeologous DNA, presumably by binding to mismatches in the DNA and aborting recombination [8]. Thus, excess MMR proteins bound to mismatches could prevent the recombination required for adaptive mutation in FC40. This would not be detected as a general decline in homologous recombination, as few recombining DNA molecules would contain mismatches, but would produce a decline in recombination-dependent mutations because the mismatched DNA would give rise to the mutations. In vitro, MutS blocks RecA-catalyzed branch migration of recombination intermediates, and this blockage is enhanced by the presence of MutL [19]. If MMR proteins also block branch migration by other enzymes [20], this could explain why excess MMR proteins have a 200-fold greater effect on adaptive mutations in a recG− mutant, which is missing one of the pathways for branch migration, than in wild type cells [6].
7. Conclusions
Many spontaneous mutations that commonly occur are, in principal, correctable by MMR (e.g., see Ref. [21]). Thus, MMR repair capacity must never be in great abundance. MMR is rather easily saturated in growing cells, although there is some disagreement about which protein becomes limiting [5,17,22,23]. As MutS and MutL interact with each other even in the absence of mismatches [24], and also interact with other proteins, some of these differences may be attributable to different levels of gene expression, the different alleles and constructions used, and/or the physiological state of the cells. Although the levels of MutS and MutH appear to decline in stationary-phase cells [5], the MMR activity that remains in most cells appears to be sufficient for the amount of DNA synthesis that occurs. It may be difficult to prove or disprove the hypothesis that MMR is marginally more deficient in stationary-phase cells, or a subset of them, than in growing cells. But, to date, the hypothesis that MMR activity is purposely limited or down-regulated in stationary-phase or nutritionally deprived cells, and that this gives rise to adaptive mutations, has no published experimental support.
Acknowledgements
I thank M.G. Marinus and S.T. Lovett for plasmids, and W.A. Rosche for helpful discussions. I also appreciate the thoughtful comments of an anonymous reviewer. Supported by NSF Grant MCB 97838315.
Footnotes
Manuscript invited by Edward L. Loecher. A commentary in response to this one has been invited by the editors for a future issue of the journal.
References
- [1].Cairns J, Foster PL. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991;128:695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Foster PL, Gudmundsson G, Trimarchi JM, Cai H, Goodman MF. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7951–7955. doi: 10.1073/pnas.92.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Foster PL, Trimarchi JM. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science. 1994;265:407–409. doi: 10.1126/science.8023164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rosenberg SM, Longerich S, Gee P, Harris RS. Adaptive mutation by deletions in small mononucleotide repeats. Science. 1994;265:405–407. doi: 10.1126/science.8023163. [DOI] [PubMed] [Google Scholar]
- [5].Feng G, Tsui H-CT, Winkler ME. Depletion of the cellular amounts of the MutS and MutH methyl-directed mismatch repair proteins in stationary-phase Escherichia coli K-12 cells. J. Bacteriol. 1996;178:2388–2396. doi: 10.1128/jb.178.8.2388-2396.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Foster PL, Trimarchi JM, Maurer RA. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics. 1996;142:25–37. doi: 10.1093/genetics/142.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harris RS, Feng G, Ross KJ, Sidhu R, Thulin C, Longerich S, Szigety SK, Winkler ME, Rosenberg SM. Mismatch repair protein MutL becomes limiting during stationary-phase mutation. Genes Dev. 1997;11:2426–2437. doi: 10.1101/gad.11.18.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu. Rev. Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- [9].Foster PL. Nonadaptive mutations occur on the F’ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 1997;179:1550–1554. doi: 10.1128/jb.179.5.1550-1554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boe L. Mechanism for induction of adaptive mutations in Escherichia coli. Mol. Microbiol. 1990;4:597–601. doi: 10.1111/j.1365-2958.1990.tb00628.x. [DOI] [PubMed] [Google Scholar]
- [11].Jayaraman R. Cairnsian mutagenesis in Escherichia coli: genetic evidence for two pathways regulated by mutS and mutL genes. J. Genet. 1992;71:23–41. [Google Scholar]
- [12].Reddy M, Gowrishankar J. A genetic strategy to demonstrate the occurrence of spontaneous mutations in non-dividing cells within colonies of Escherichia coli. Genetics. 1997;147:991–1001. doi: 10.1093/genetics/147.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rosche WA, Foster PL. unpublished results.
- [14].Foster PL, Cairns J. Mechanisms of directed mutation. Genetics. 1992;131:783–789. doi: 10.1093/genetics/131.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Foster PL. unpublished results.
- [16].Lieb M, Bhagwat AS. Very short patch repair: reducing the cost of cytosine methylation. Mol. Microbiol. 1996;20:467–473. doi: 10.1046/j.1365-2958.1996.5291066.x. [DOI] [PubMed] [Google Scholar]
- [17].Macintyre G, Doiron KM, Cupples CG. The Vsr endonuclease of Escherichia coli: an efficient DNA repair enzyme and a potent mutagen. J. Bacteriol. 1997;179:6048–6052. doi: 10.1128/jb.179.19.6048-6052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Foster PL, Rosche WA. Levels of the Vsr endonuclease do not regulate stationary-phase reversion of a Lac− frameshift allele in Escherichia coli. J. Bacteriol. 1998;180:1944–1946. doi: 10.1128/jb.180.7.1944-1946.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Worth L, Clark S, Radman M, Modrich P. Mismatch repair proteins MutS and MutL inhibit RecA-catalyzed strand transfer between diverged DNAs. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3238–3241. doi: 10.1073/pnas.91.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zahrt TC, Maloy SR. Barriers to recombination between closely related bacteria: MutS and RecBCD inhibit recombination between Salmonella typhimurium and Salmonella typhi. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9786–9791. doi: 10.1073/pnas.94.18.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cupples CG, Cabrera M, Cruz C, Miller JH. A set of lacZ mutations in Escherichia coli that allow rapid detection of specific frameshift mutations. Genetics. 1990;125:275–280. doi: 10.1093/genetics/125.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schaaper RM, Radman M. The extreme mutator effect of Escherichia coli mutD5 results from saturation of mismatch repair by excessive DNA replication errors. EMBO J. 1989;8:3511–3516. doi: 10.1002/j.1460-2075.1989.tb08516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maas WK, Wang C, Lima T, Hach A, Lim D. Multicopy single-stranded DNA of Escherichia coli enhances mutation and recombination frequencies by titrating MutS protein. Mol. Microbiol. 1996;19:505–509. doi: 10.1046/j.1365-2958.1996.392921.x. [DOI] [PubMed] [Google Scholar]
- [24].Wu T-H, Marinus MG. Personal communication.