Abstract
NKT cells are key regulators of autoimmunity, tumor immune surveillance, and the immune response to pathogens. The role of NKT cells in regulating adaptive immunity to cutaneous Ags is largely unknown. This study explores the role of CD1d-restricted NKT cells in cross-priming of CD8 effector T cells to OVA expressed in epithelial keratinocytes (K5mOVA transgenic mouse). In a skin grafting model, we show that NKT cells enhance the rejection of K5mOVA skin grafts by promoting generation of OVA-specific CD8 effector T cells in the skin-draining lymph nodes. This is associated with a decrease in the proportion of both Th17 cells and IL-17–producing NKT cells within the lymph node, thereby inducing a Th1-biased response by increasing the ratio of IFN-γ to IL-17 production. Administration of a strong agonist ligand (α-galactosylceramide) for NKT cells induced higher levels of local IFN-γ production, enhancing the rate of K5mOVA graft rejection. Thus, NKT cells can promote adaptive immunity to cell-associated Ag expressed in skin by local regulation of IFN-γ production in secondary lymphoid tissue during cross-priming of effector CD8 T cells.
Skin integrity is controlled by a complex immunosurveillance network, vital for host survival. Maintaining a balance of active defense mechanisms to clear pathogens and prevent tumor development, and tolerogenic pathways to prevent chronic inflammation and autoimmunity, is critical to achieve immune homeostasis. Th17 cytokines have recently been associated with inflammatory processes in skin (1–3), once attributed solely to Th1 responses. Th1 and Th17 T cells are often colocalized in inflammatory skin environments; however, uncertainty exists in the relative contributions of IFN-γ and IL-17 and whether these cytokines work in synergism or are antagonistic (4–10).
NKT cells regulate adaptive immunity to infections, cancer, and autoimmune reactions through production of Th1 (particularly IFN-γ) or Th2 (IL-4, IL-10, IL-13) cytokines, and have been implicated in skin diseases such as atopic dermatitis, psoriasis, and UV-induced skin cancer (reviewed in Ref. 11). Recent reports have provided convincing evidence that NKT cells are also capable of producing Th17 cytokines such as IL-21 (12), IL-22 (13), and IL-17 (14–19). IL-17 production by NKT cells was first characterized in a model of airway neutrophilia, revealing an NK1.1−IL-17+ NKT subset in lungs (19). Further reports have confirmed that IL-17–producing NKT are largely CD4−NK1.1−, RORγt+, and are most commonly found in skin-draining lymph nodes (DLNs) where they respond to inflammation in skin (14, 15).
The functional consequences of NKT cell activation and associated IFN-γ/IL-17 cytokine production to skin-derived Ags is largely unknown. IL-17 and IFN-γ production by NKT cells is mutually exclusive (14), suggesting that different inflammatory stimuli may provoke differential cytokine production, resulting in diverse immunological responses in skin diseases. In an inflammatory skin grafting model, this study explored the role of NKT cells within skin-DLN in cross-priming of CD8 T cells to epithelial cell-derived Ag. We show that NKT cells enhance rejection of skin grafts expressing OVA by promoting generation of Ag-specific CD8 effector T cells. We also reveal that the effector T cell response in skin graft rejection is associated with IFN-γ, but not IL-17 production by NKT cells.
Materials and Methods
Mice
Inbred C57BL/6 mice were obtained from the Animal Resources Center (Perth, Australia). K5mOVA mice, transgenic for K5 promoter-driven, membrane-bound OVA, were provided by H. Azukizawa (Osaka, Japan) (20). IFN-γ knock-out (KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and IL-17KO mice were kindly provided by Y. Iwakura (Tokyo, Japan). NKT cell-deficient CD1dKO and Jα18KO mice were obtained from M.Smyth (Melbourne, Australia) and maintained locally at the Princess Alexandra Hospital Biological Research Facility (Brisbane, Australia). OT-1 mice carrying a MHC class I-restricted transgenic TCR for the OVA257–264 (SIINFEKL) peptide were originally obtained from F. Carbone (Melbourne, Australia) and crossed with CD45.1 congenic C57BL/6.SJL-Ptprc mice (Animal Resources Center) to generate mice bearing CD45.1+ OT-1 cells. All mice were housed under specific pathogen-free conditions at the Princess Alexandra Hospital Biological Research Facility, were sex-matched for all experiments and were used at 6–12 wk of age. All animal procedures were approved by the University of Queensland Animal Ethics Committee.
Reagents and flow cytometry
OVA257–264 peptide, a H-2Kb–restricted epitope with amino acid sequence SIINFEKL, was purchased from Auspep (Melbourne, Australia) with >80% purity, dissolved in 100% DMSO and stored at −20°C. Anti-mouse mAbs to CD3 (145-2C11),CD4 (RM4-4),CD8 (53-6.7),CD69 (H1.2F3),CD44 (IM7), CD45.1 (A20), TCR-Vα2 (B20.1), NK1.1 (PK136), IFN-γ (XMG1.2), IL-17 (TC11), and associated isotype control Igs were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), and Serotec (Raleigh, NC). The synthetic NKT cell ligand, α-galactosylceramide (αGalCer) was purchased from Alexis Biochemicals (San Diego, CA), dissolved in pyridine, and stored at −20°C until use. Preparation of αGalCer-loaded CD1d tetramer (Tet) is described elsewhere (21) and was kindly provided by D. Godfrey (Melbourne, Australia). Cells were stained at predetermined optimal concentrations of Ab for 30 min at 4°C. For intracellular staining of cytokines, permeabilization, and fixation of cells was conducted using the BD Cytofix/Cytoperm kit according to the manufacturers’ instructions (BD Biosciences). Monensin (BioLegend, San Diego, CA) was added to the cells to inhibit cytokine release from the Golgi/endoplasmic reticulum complex. Stained cells were analyzed on a FACScalibur or FACScanto flow cytometer (BD Biosciences) and analyzed with Summit software (Dako, Glostrup, Denmark).
Skin grafting
Donor ear skin was grafted onto recipient flanks as previously described (22, 23). Briefly, skin from transgenic mice, taken from dorsal and ventral surfaces of the ear (~1 cm2), was placed onto the thoracic flank region of an anesthetized nontransgenic but otherwise syngeneic recipient. Grafts were held in place with antibiotic-permeated gauze (Bactigras; Smith and Nephew, London, U.K.) and bandaged with micropore tape and Flex-wrap (Lyppard, Queensland, Australia). Bandages were removed after 7 d and grafts were observed at least three times weekly. Grafts were considered rejected if there was a loss of distinct border and visible signs of ulceration and/or necrosis to >80% of the graft area.
In vivo assays and immunizations
To assess OVA-specific CD8+ T cell proliferation in vivo, CD45+ OT-1 cells were labeled with 2.5 µM CFSE and injected i.v. (1 × 107) into the tail vein of wild-type (wt) or NKT cell-deficient mice. Seven days later, recipient spleens were harvested and CFSE dilution in CD45.1+/Vα2+/CD8+ cells was assessed by FACS. Indices of proliferation were generated using ModFit LT software (Verity Software House, Topsham, ME). For transfer of activated OT-1 cells, one round of in vitro stimulation was performed as described (24), and cells were administered at 1 × 106 cells/mouse. To determine responsiveness to soluble Ag challenge, mice were immunized s.c. at the tail base with 50 µg OVA protein (grade 5; Sigma-Aldrich, St. Louis, MO) and 20 µg QuilA adjuvant (Soperfos Biosector DK-Vedback, Denmark). For NKT cell stimulation in vivo, mice were treated once with 2 µg αGalCer i.p. 2 d postskin grafting. Vehicle (pyridine and PBS) was used for control treatments.
Adoptive transfers of pure NKT cell populations
For NKT cell transfers, mononuclear cells pooled from liver, thymus, inguinal lymph nodes (LNs), and spleen of wt, IFN-γKO, or IL-17KO C57BL/6 mice were sorted by flow cytometry (MoFlo, BD Biosciences) based on dual CD3+ and CD1d-Tet+ staining. CD3+CD1d-Tet+ T cell purity after sorting was consistently >90%. For NKT cell reconstitution of Jα18KO recipient mice, 2 × 105 pure NKT cells were injected i.v. into the tail vein 3 d prior to skin grafting.
IFN-γ ELISPOT and cytometric bead array
For ELISPOT assays, cell suspensions isolated from skin-DLN of immunized or grafted recipients were cultured overnight in complete RPMI 1640 medium in the presence of 5 ng/ml recombinant mouse IL-2 (BD Biosciences) and with or without addition of 0.01 µM SIINFEKL peptide. Skin-DLNs were identified by their proximity to the skin graft and increased size relative to the uninvolved, contralateral LN. The IFN-γ ELISPOT procedure has been previously described (25). Secreted levels of IFN-γ and IL-17 were detected by Th1/Th2/Th17 cytometric bead array according to the manufacturers’ protocol (BD Biosciences). Where indicated, cells were stimulated in vitro for 4 h with 25 ng/ml PMA and 1 µg/ml ionomycin prior to collecting supernatant for cytokine detection. Samples were analyzed on a FACSarray (BD Biosciences).
Statistics
Kaplan-Meier plots were used to analyze skin graft survival and a log-rank test was performed to assess the statistical significance of differences between survival curves. For all other data in which statistics were performed, a two-tailed t test or nonparametric Mann-Whitney U test, as indicated, was used for assessment of differences between groups. Differences were considered to be significant when the p value was <0.05. Prism (Graphpad Software, La Jolla, CA) software was used to prepare graphs and for statistical analysis.
Results
Invariant NKT cells promote rejection of OVA-expressing skin grafts
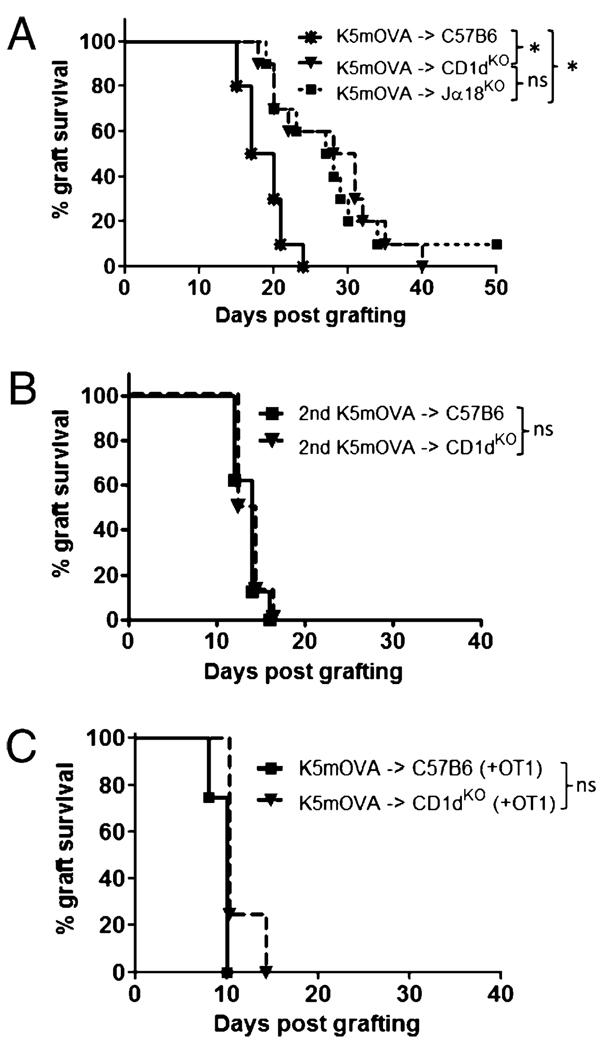
In immunocompetent mice, rapid CD8 T cell-mediated rejection of skin grafts expressing OVA in epithelial keratinocytes (K5mOVA transgenic skin) occurs after cross-presentation of Ag in the skin-DLN (20). To determine the role for NKT cells in induction of the immune response to Ag expressed in skin, we grafted K5mOVA skin onto NKT cell-deficient CD1dKO and Jα18KO recipients. Skin graft rejection was significantly delayed in both CD1dKO and Jα18KO mice when compared with wt C57B6 mice (Fig. 1A). Similar median rejection times on Jα18KO and CD1dKO mice confirmed that the invariant Vα14+/Jα18+ NKT cell population was responsible for the enhanced response in wt mice. Although initial graft rejection was delayed in NKT deficient mice, all experimental groups had similar numbers of rejected grafts by day 40 suggesting a similar antigraft immune response at this point. Second K5mOVA grafts applied at day 50 after the first graft were then rejected with similar kinetics in the presence or absence of NKT cells (Fig. 1B) suggesting that the effector/memory CD8 T cell response to graft tissue is not affected by NKT cells. To further delineate the role of NKT cells in augmenting CD8 T cell priming versus effector function, we transferred in vitro activated OT-1 cells into C57B6 and CD1dKO mice in conjunction with grafting of K5mOVA skin. In bypassing the requirement for cross-priming of an endogeneous OVA-specific CD8 T cell repertoire, delayed K5mOVA graft rejection in NKT cell-deficient mice was overcome (Fig. 1C). Therefore, NKT cells assist in priming responses to skin-derived Ag, but do not measurably enhance or inhibit effector functions necessary for skin graft rejection from an Ag primed host.
FIGURE 1.
Delayed K5mOVA rejection in NKT cell-deficient recipients. A, Kaplan-Meier survival curves of OVA-expressing skin grafts (K5mOVA) placed onto wt C57B6 and NKT cell-deficient CD1dKO and Jα18KO recipients. Median K5mOVA graft survival: C57B6 recipient = 19 d; CD1dKO recipient = 30 d; Jα18KO recipient = 28 d (n = 10 mice per group). B, Survival of second K5mOVA grafts placed 50 d after the initial K5mOVA graft on C57B6 and CD1dKO recipients. Median K5mOVA graft survival: C57B6 recipient = 14 d; CD1dKO recipient = 13 d (n = 8 mice per group). C, Survival of K5mOVA grafts after adoptive transfer of 1 × 106 in vitro activated OT-1 T cells into C57B6 and CD1dKO recipients, 1 d prior to grafting. Median K5mOVA graft survival for both C57B6 and CD1dKO recipients = 10 d (n = 4 mice per group). *p = 0.002, log-rank test.
Generation of OVA-specific CD8 T cells to skin-derived Ag is enhanced by NKT cells
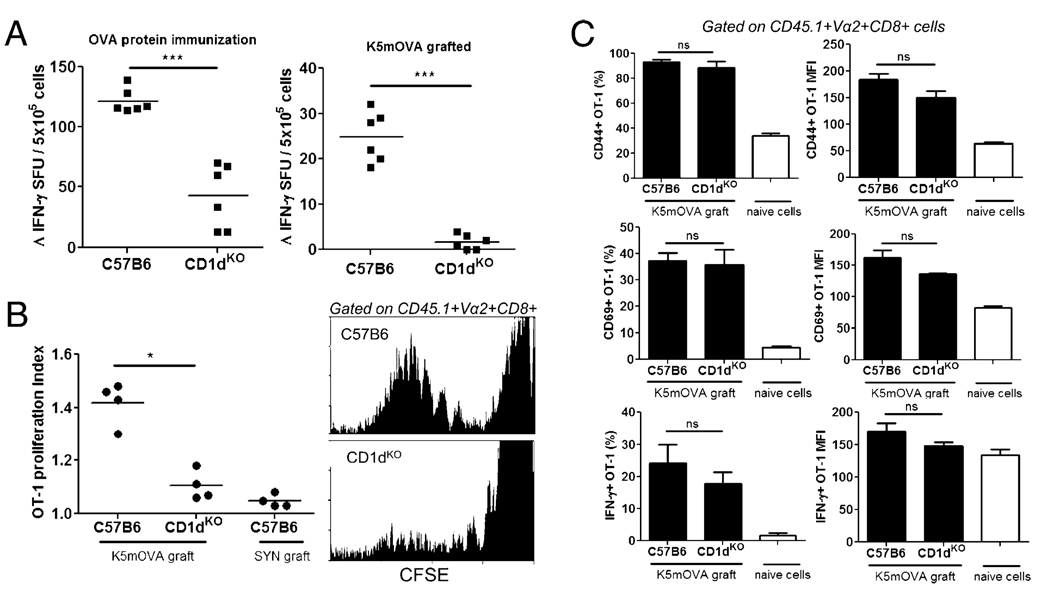
To further investigate the capacity for NKT cells to promote cross-priming of CD8 T effector cells in skin-DLN, we measured the endogenous OVA-specific CD8 T cell response in C57B6 and CD1dKO mice after K5mOVA skin grafting. OVA-specific, IFN-γ secreting CD8 T cell numbers generated in skin-DLN after grafting of K5mOVA skin, or OVA immunization, were lower in NKT cell-deficient animals (Fig. 2A), confirming the ability of NKT cells to promote cross-priming of CD8 T cells. NKT cells also weakly enhanced the proliferation of transferred OT-1 cells in LN specifically draining K5mOVA skin grafts (Fig. 2B). The absence of NKT cells in CD1dKO recipients did not, however, adversely affect the extent of in vivo OT-1 cell activation or cytokine production in response to nonspecific mitogen stimulation (Fig. 2C). Thus, NKT cells assist in the generation of endogenous, Ag-specific CD8 T cell responses in skin-DLNs; however, NKT cell activity is not critical for the activation of transferred CD8 T cells containing a high OVA-specific precursor frequency.
FIGURE 2.
NKT cells enhance the generation of endogenous OVA-specific CD8 T cells. A, Ag-specific IFN-γ production by CD8 T cells isolated from skin-DLN of C57B6 and CD1dKO mice 7 d after primary stimulation with either s.c. OVA protein immunization (left panel) or grafting with K5mOVA skin (right panel). IFN-γ–producing cells were measured by ELISPOT after overnight restimulation with SIINFEKL peptide and IL-2. Δ IFN-γ is the corrected spot forming unit (SFU) value after subtracting counts obtained from control wells containing no peptide stimulation. Background counts from control wells were consistently <10 SFU. Each dot represents the mean value from triplicate wells from one mouse. ***p = 0.002, Mann-Whitney U test. B, In vivo proliferation of CFSE-labeled, CD45.1 congenic OT-1 cells 7 d after transfer into C57B6 or CD1dKO recipients that were grafted with K5mOVA skin 1 d after cell transfer. Transplanted, syngeneic C57B6 skin expressing SYN peptide was used as irrelevant Ag graft controls. *p = 0.03, Mann-Whitney U test. OT-1 cells in the skin-DLNs of grafted mice were examined at day 7 after transfer. Flow cytometry histograms (right panels) show representative CFSE dilution within the gated CD45.1+/Vα2+/CD8+ transferred OT-1 T cell population in K5mOVA grafted C57B6 and CD1dKO recipients. C, Activation phenotype, based on CD44 (top) and CD69 (middle) expression, and IFN-γ cytokine production (bottom) of CD45.1+/Vα2+/CD8+ gated OT-1 T cells, isolated from skin-DLNs of C57B6 and CD1dKO recipients 7 d after cell transfer and grafting with K5mOVA skin. IFN-γ production was measured by intracellular staining after 4 h in vitro stimulation with PMA/ionomycin. Data are presented both as percentage of cell expression (left column) and levels of expression by mean fluorescence intensity (right column). White bars in each graph illustrate baseline expression of activation markers/cytokines from nontransferred, naive OT-1 T cells (means + SEM; n = 4 mice per group). All graphs are representative of two independent experiments.
The ratio of IFN-γ– to IL-17–producing T cells and NKT cells is increased after skin grafting
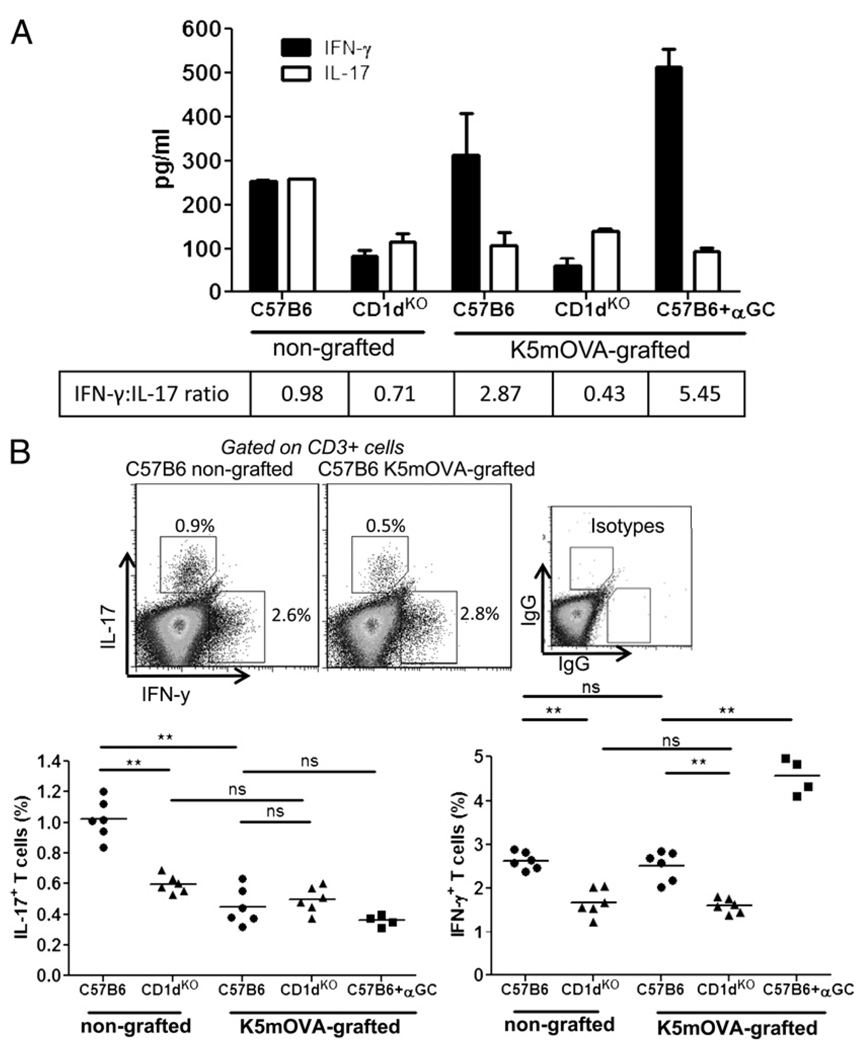
Both IFN-γ and IL-17 have been implicated in T cell immune responses to skin diseases. To determine whether NKT cells influence T cell differentiation into IFN-γ– or IL-17–producing effector cells after skin grafting, we examined the ability of T cells from skin-DLN of NKT-replete and NKT-deficient mice to secrete IFN-γ and IL-17 in response to PMA/ionomycin stimulation. A nonspecific T cell stimulus was used to determine whether a general bias in T cell cytokine production resulted from the presence or absence of NKT cells and grafting. The data indicate that NKT-replete recipients, independent of grafting, secrete higher levels of IFN-γ in response to a nonspecific stimulus than NKT-deficient recipients (Fig. 3A). The addition of the activating NKT ligand, α-GalCer, to the K5mOVA-grafted mice increased the production of IFN-γ within NKT-replete mice. The other key observation related to a grafting effect. K5mOVA skin grafts onto C57B6 mice resulted in LN cells that secreted less IL-17 than LN cells from mice that were not grafted (Fig. 3A). In contrast, if NKT cells were absent in the recipient mice, than IL-17 secretion was unchanged before and after K5mOVA grafting. Consequently, NKT cells appear to enhance the IFN-γ response of LN cells and provide an environment that suppresses IL-17 production during skin grafting with K5mOVA skin.
FIGURE 3.
IFN-γ dominates IL-17 production in wt, but not CD1dKO recipients after grafting of K5mOVA skin grafts. A, Secretion of IFN-γ (black bars) and IL-17 (white bars) by cells isolated from the skin-DLNs of recipient C57B6 and CD1dKO mice without skin grafts (nongrafted) or grafted with K5mOVA skin (K5mOVA-grafted). Cytokine was measured in the supernatant from in vitro cell cultures stimulated with PMA/ionomycin for 4 h. Bars show mean + SEM values from three mice per group, whereas values in box (bottom) represent the ratio of IFN-γ to IL-17 production for each recipient. B, Upper panels are representative dot plots of intracellular cytokine staining for IL-17 and IFN-γ in CD3+ cells from nongrafted and K5mOVA-grafted C57B6 mice. Cells were cultured in vitro with PMA/ionomycin for 4 h before staining for IL-17 and IFN-γ. The lower panels represent the percentage of CD3+ T cells isolated from skin-DLN of K5mOVA grafted or nongrafted C57B6 and CD1dKO mice, producing IL-17 (left graph) or IFN-γ (right graph) after PMA/ionomycin stimulation in vitro. Each dot represents intracellular staining from one mouse. Production of IL-17 and IFN-γ was also measured in cells derived from K5mOVA-grafted C57B6 mice after stimulation in vitro with α-GalCer (+αGC). Data were pooled from two independent experiments. **p < 0.01; Mann-Whitney U test.
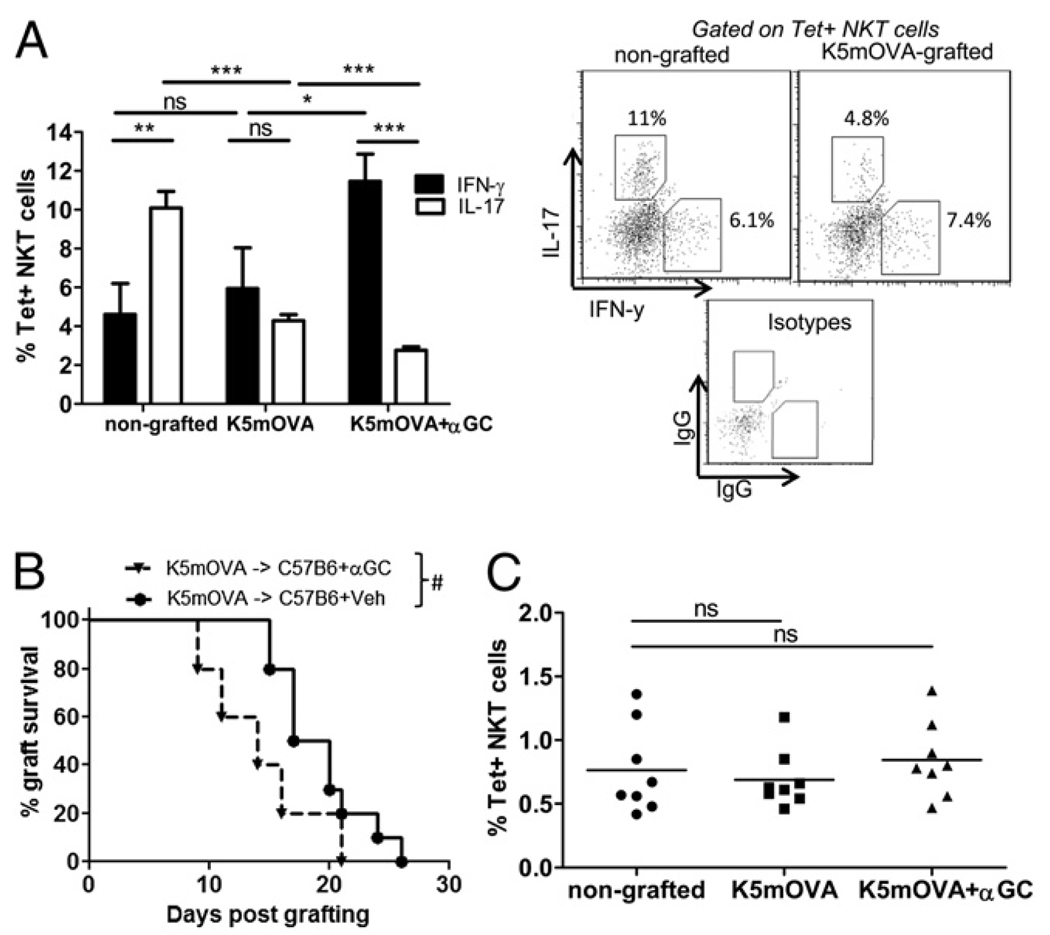
To determine whether the relative changes in IL-17 and IFN-γ production reflected a change in cytokine production by the NKT cells or by T cells, each population was individually examined for cytokine production using intracellular staining. Consistent with the cytokine secretion data in Fig. 3A, the presence of NKT cells resulted in increased numbers of CD3+ LN cells that produced IFN-γ, irrespective of grafting (Fig. 3B). Also consistent with Fig. 3A was the reduced IL-17–producing capacity of CD3+ cells and CD1d Tet+ cells after grafting with K5mOVA skin grafts onto C57B6 recipients (Figs. 3B, 4A). Treatment of C57B6 mice bearing K5mOVA grafts with α-GalCer resulted in an elevated fraction of IFN-γ–producing cells without altering the proportion of IL-17–producing cells (Fig. 4A). K5mOVA skin graft rejection was also accelerated by treatment of the mice with α-GalCer (Fig. 4B), despite the proportion of NKT cells in the DLNs remaining unchanged with grafting or α-GalCer treatment. The data suggested that a dominance of IFN-γ over IL-17 production in the T cells and NKT cells of the LNs after K5mOVA grafting might lead to accelerated graft rejection.
FIGURE 4.
A switch from IL-17– to IFN-γ–producing NKT cells in skin-DLNs correlates with rapid K5mOVA rejection. A, Intracellular cytokine staining for IL-17 (white bars) and IFN-γ (black bars) was performed on LN cells isolated from nongrafted C57B6 mice or C57B6 mice grafted for 7 d with K5mOVA skin with or without a single injection of α-GalCer (αGC) at day 2 postgrafting. The percentage of CD3+/CD1d-Tet+ NKT cells producing either cytokine is shown after 4 h PMA/ionomycin treatment in vitro. Bars depict mean + SEM from n = 8 mice per group, pooled from two independent experiments. *p < 0.05; **p < 0.005; ***p < 0.0005; two-tailed t test. Representative dot plots of IL-17– and IFN-γ–producing NKT cells from nongrafted and grafted mice are shown in the panels, along with isotype control staining for both IL-17 and IFN-γ. B, Survival of K5mOVA skin grafts on C57B6 recipients after i.p. treatment with α-GalCer (αGC) or a vehicle control (Veh). Median K5mOVA graft survival: C57B6 and Veh recipient = 19 d; C57B6 and αGC recipient = 14 d (n = 10 mice per group; #p = 0.04). C, Percentage of CD3+/CD1d-Tet+ NKT cells out of the gated lymphocyte-sized population in skin-DLNs of nongrafted C57B6 mice and C57B6 mice grafted with K5mOVA skin with or without α-GalCer treatment (two-tailed t test).
IFN-γ but not IL-17 production by NKT cells contributes to enhanced rejection of K5mOVA grafts
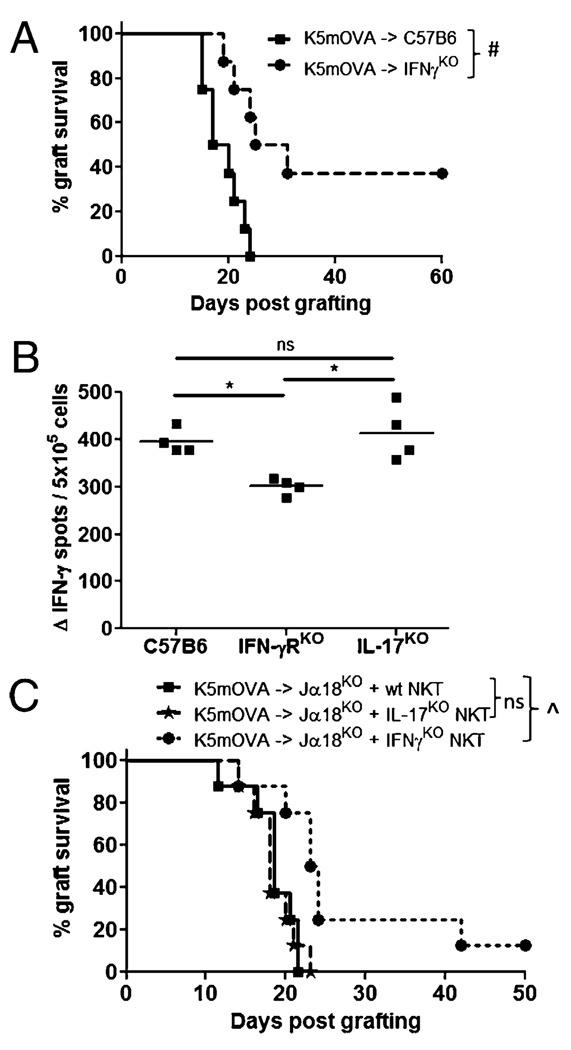
Having observed an IFN-γ bias over IL-17 by LN cells from K5mOVA grafted mice when stimulated nonspecifically in vitro, we next examined the in vivo role of IFN-γ production from NKT cells in graft rejection. As a first step, the ability to produce IFN-γ was removed from all cells of the recipient mice. Recipient mice deficient in IFN-γ rejected K5mOVA grafts with delayed kinetics relative to grafted, wt recipients consistent with IFN-γ promoting skin graft rejection (Fig. 5A). Mice deficient in IFN-γR signaling also produced fewer IFN-γ–secreting, OVA-specific CD8 T cells relative to wt mice, whereas mice deficient in IL-17 had no change in the CD8 T cell response (Fig. 5B). Again, this suggested that IFN-γ signaling was important in generating an OVA-specific CD8 T cell response that is an important component of graft rejection.
FIGURE 5.
IFN-γ production from NKT cells enhances rejection of K5mOVA grafts in reconstituted Jα18KO mice. A, Graft survival curves for K5mOVA skin grafts on wt C57B6 (rectangles) or IFN-γKO recipient mice (circles). Percent K5mOVA graft survival: C57B6 recipient = 0% (median survival = 18 d); IFN-γKO recipient = 38% (median survival = 28 d) (n = 8 mice per group; #p = 0.003). B, OVA-specific, IFN-γ production by CD8 T cells isolated from skin-DLNs of C57B6, IFN-γRKO, and IL-17KO mice, 7 d after s.c. immunization with OVA protein in adjuvant. IFN-γ–producing cells were measured by ELISPOT after overnight in vitro restimulation with SIINFEKL peptide and IL-2. Δ IFN-γ is the corrected SFU value after subtracting counts obtained from control wells containing no peptide stimulation. Each dot represents the mean value from triplicate wells from one mouse. *p = 0.02; Mann-Whitney U test. C, Graft survival curves for K5mOVA grafts placed onto Jα18KO recipient mice that were reconstituted 3 d prior to grafting with purified NKT cells isolated from either wt, IFN-γKO or IL-17KO mice. Median K5mOVA graft survival: Jα18KO and wtNKT and Jα18KO and IL-17KO NKT recipients both = 18 d; Jα18KO and IFN-γKO NKT recipient = 24 d (n = 8 mice per group; ^p = 0.005).
To examine the specific contribution of IFN-γ produced by NKT cells to promote the rejection of K5mOVA skin grafts, we assessed rates of graft rejection in Jα18KO mice after reconstitution with purified populations of NKT cells that were isolated from wt C57B6, IFN-γKO, or IL-17KO mice. Rejection of grafts in recipients that were reconstituted with IFN-γ–replete NKT cells was significantly faster than for animals reconstituted with IFN-γKO NKT cells (Fig. 5C). In contrast, lack of IL-17 production by NKT cells did not alter the kinetics of graft rejection. Therefore, IFN-γ but not IL-17 produced from NKT cells contributes to enhanced graft rejection in NKT-replete animals.
The dominant cytokine-producing NKT cell in the skin-DLN of grafted mice is an NK1.1 negative subset
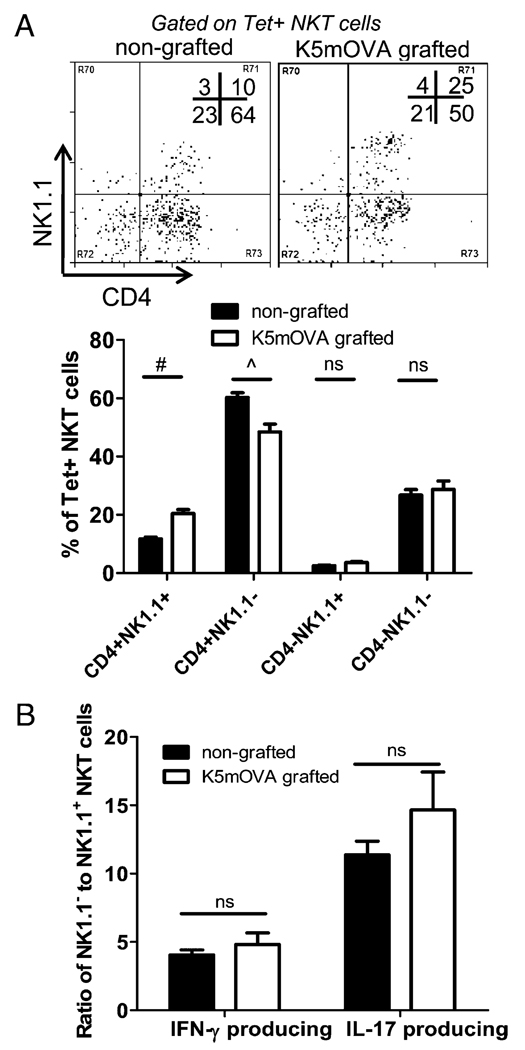
Having established a role for IFN-γ–producing NKT cells in K5mOVA graft rejection, we next wished to identify the NKT subset involved in this rejection. CD1d-restricted NKT cells can be divided into functionally distinct subsets (26, 27). We observed that the CD4+NK1.1− NKT cell subset was dominant in the skin-DLNs of normal skin, and remained dominant after grafting with K5mOVA skin, despite being reduced slightly in number (Fig. 6A). To assess whether the proportions of IFN-γ– and IL-17–producing NKT cells pre- and postgrafting was determined by changes in these distinct NKT subsets, we characterized CD1d-Tet reactive, cytokine-producing cells based on NK1.1 expression. Both IFN-γ and IL-17 was produced mostly from NK1.1− NKT cells as indicated by the high ratios of NK1.1− to NK1.1+ cells producing either cytokine (Fig. 6B). NK1.1− cells were the primary source of IL-17 in resting LNs, as they outnumbered NK1.1+ IL-17+ cells by 10- to 15-fold, and this was not significantly altered after grafting (Fig. 6B). Therefore, the decrease in IL-17 production observed within the NKT cell population after grafting (Fig. 4A) may be explained by the specific decrease of NK1.1− NKT cells (Fig. 6A), because reduction in this subset would have large impact on IL-17 secretion.
FIGURE 6.
NK1.1 negative NKT cells are the dominant IFN-γ– and IL-17–producing subset in skin-DLNs. A, The upper panels are representative dot plots of CD4 and NK1.1 Ab staining among CD1d-tetramer+ (Tet+) NKT cells isolated from nongrafted C57B6 mice and C57B6 mice grafted with K5mOVA skin for 7 d. The lower panel summarizes the percentages of each subset of NKT cells displayed in the upper panels (mean + SEM; n = 6 mice). B, The ratio of NK1.1− to NK1.1+ NKT subsets producing IFN-γ or IL-17 for nongrafted (black bars) and K5mOVA grafted mice (white bars) at day 7 postgrafting are shown (mean + SEM; n = 4 mice). Skin-DLN cells were stimulated for 4 h in vitro with PMA/ionomycin prior to staining with CD1d-Tet, Ab staining for NK1.1 and intracellular staining for cytokines. The percentage of NK1.1−IFN-γ+ cells of total CD3+CD1d-Tet+ cells was divided by the percentage of NK1.1+IFN-γ+ cells of total CD3+CD1d-Tet+ cells to obtain the ratio displayed on the y-axis for IFN-γ–producing cells. A similar calculation was performed for IL-17–producing cells. #p = 0.004; ^p = 0.002; Mann-Whitney U test.
Discussion
In this study, we demonstrate that CD1d-restricted NKT cells in skin-DLN promote clearance of epithelial cell-associated Ag by enhancing the levels of IFN-γ and reducing the IL-17 production during grafting. Although it is well established that NKT cells can enhance cell-mediated immunity to pathogens and tumors through production of IFN-γ and other cytokines (reviewed in Refs. 28 and 29), little is known on the role of NKT cells in regulating cutaneous immunity.
To investigate NKT cell regulation of CD8 T cell priming to protein Ag expressed in skin, we used the K5mOVA transgenic mouse, in which OVA is expressed under the keratin 5 promoter in keratinocytes as an inert, cell membrane-associated protein. A previous study has shown that K5mOVA skin is rejected when transplanted onto syngeneic immunocompetent recipients, in a CD8 T cell-dependent manner (20). In this skin graft model, OVA taken up by professional APC in skin is cross-presented to CD8 T cells in the skin-DLN, thus allowing us to specifically investigate the effects of NKT cells in cross-priming of effector CD8 T cells to Ag derived from skin. It is likely that NKT cells enhance the priming of CD8 T cells through activation of dendritic cells in the LN as recently observed in an influenza model (30).
By observing delayed rejection of K5mOVA grafts on NKT-deficient CD1dKO and Jα18KO mice, we show that invariant, type 1 NKT cells enhance cell-mediated immunity in skin. NKT cells led to greater generation of OVA-specific CD8 T cells postgrafting, even without exogenous stimulation with α-GalCer. NKT “help” was restricted to the initial cross-priming response, as primed C57B6 and CD1dKO mice were equally sufficient in mounting a rapid recall response to a second graft. Overcoming the requirement for priming of an endogenous CD8 T cell response, by transfer of in vitro activated OT-1 cells into grafted mice, led to rapid rejection of K5mOVA grafts on both NKT-replete and NKT-deficient recipients. This confirmed the finding that NKT cells promote the priming, but not effector functions of CD8 T cells. In contrast, a recent study by Hong et al. (31) showed in a tumor model that NKT cells are capable of regulating secondary Ag-specific CD8 T cell responses. They found that secondary anti-tumor CD8 T cell effector function was optimal in the presence of NKT cells. Although it is clear that they were testing a secondary CD8 T cell response, it is not possible to distinguish this from additional priming that may have occurred on transfer of CD8 T cells into the tumor-bearing host. We cannot rule out the possibility that additional CD8 T cell priming was invoked on re-grafting with K5mOVA skin in our model; however, even if this was occurring, the presence of NKT cells could not enhance this sufficiently to detect differences in the rate of graft rejection.
Because the discovery of Th17 cells, the relative roles of Th1 and Th17 effector cells and associated cytokines in inflammation and allograft rejection (reviewed in Refs. 32–35) has been controversial. There is convincing evidence that IFN-γ can synergize with, or inhibit the activity of IL-17, and vice versa (4–10). We have recently shown that skin-infiltrating NKT cells regulate local T cell effector function under inflammatory conditions by IFN-γ production (36). Independent studies have recently discovered an NK1.1− NKT cell subset capable of rapid IL-17 production on TCR engagement or PMA/ionomycin stimulation (14, 15). This subset, found preferentially in skin-DLNs, was defined by expression of CCR6, CD103, and CD121a, and shown to respond preferentially to inflammatory signals in the skin (15). The current study investigated IFN-γ and IL-17 production by NKT cells in skin-DLNs under inflammatory conditions of skin grafting and assessed the functional consequences of this cytokine production in the context of Ag-specific CD8 T cell generation and K5mOVA graft rejection. Consistent with the aforementioned study, IL-17 was the predominant cytokine produced by steady-state NKT cells in the skin-DLNs, the majority being NK1.1− with expression of CD103 (data not shown). On grafting, however, the proportion of IL-17–producing NKT cells decreased, whereas IFN-γ–producing cells remained unaltered with grafting. This created an IFN-γ bias that was enhanced by α-GalCer treatment, stimulating further IFN-γ production by NKT cells. Increased IFN-γ production was associated with faster K5mOVA graft rejection. Biased IFN-γ production was also established within the general T cell population on grafting in NKT-replete mice, suggesting that in addition to self-production, NKT cells may regulate the differentiation of IFN-γ–producing and IL-17–producing effector cells. NKT reconstitution of Jα18KO mice enhanced the rate of graft rejection, except when the transferred NKT cells were incapable of IFN-γ production (IFN-γKO NKT), confirming that IFN-γ production from NKT cells is important for skin graft rejection in this CD8 T cell-dependent model. In addition, IL-17 did not augment the response, nor inhibit IFN-γ–mediated effects. This contrasts with studies in organ allograft rejection and graft-versus- host disease where IL-17 promotes the rejection of the graft (32, 37). We speculate that cross-presentation of keratinocyte-derived Ag may be more dependent on IFN-γ than direct presentation of allogeneic MHC where IL-17 plays a role. Although the target Ag (OVA) of the CD8 T cell response is known in our model, the nature of the CD1d-binding lipid, which activates the NKT cells, is unclear. We speculate that a steady state or inflammation-associated (from grafting process) lipid is bound to CD1d. Candidate lipids include the ubiquitous isoglobotrihexosylceramide, B-galactosylceramide, and B-glucosylceramide or inflammation-induced ligands such as lysophospholipids (38). Epidermis is also a rich producer of various sphingolipids, which may bind to CD1d in the skin (39).
Overall, our data show that invariant NKT cells promote adaptive immunity to cell-associated Ag expressed in skin by regulating the IFN-γ/IL-17 cytokine balance in secondary lymphoid tissue during cross-priming of effector CD8 T cells. The bias toward IL-17 production by steady-state NKT cell subsets in skin-DLN may be associated with homeostatic regulation in skin. Understanding the inflammatory cues inducing a switch from IL-17 to IFN-γ production by NKT cells will provide insight into the regulation of Th1/Th17 immunity to a range of skin-related diseases.
Acknowledgments
We thank Prof. Dale Godfrey and Kon Kyparissoudis for supplying the α-GalCer-loaded mouse CD1d tetramer, Sean Smith for genotyping of mice, and the staff of the Biological Research Facility at Princess Alexandra Hospital for excellent technical assistance and animal care.
This work was supported by a program grant from the National Health and Medical Research Council of Australia (569938) and grants from the Cancer Council Australia, the Australian Cancer Research Foundation, and the Cancer Research Institute (New York). I.H.F. is the recipient of a Queensland Government Premier’s Fellowship. This work was also supported in part by National Institutes of Health Grant 1 UOI CA141583-01 to I.H.F.
Abbreviations used in this paper
- αGC
αGalCer
- αGalCer
α-galactosylceramide
- DLN
draining lymph node
- KO
knock-out
- LN
lymph node
- SFU
spot forming units
- Tet
tetramer
- Veh
vehicle control
- wt
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin-17 in inflammatory skin disorders. Curr. Opin. Allergy Clin. Immunol. 2007;7:374–381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 4.Awasthi A, Kuchroo VK. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat. Immunol. 2009;10:568–570. doi: 10.1038/ni0609-568. [DOI] [PubMed] [Google Scholar]
- 5.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 6.Irmler IM, Gajda M, Bräuer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J. Immunol. 2007;179:6228–6236. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 7.Kelchtermans H, Schurgers E, Geboes L, Mitera T, Van Damme J, Van Snick J, Uyttenhove C, Matthys P. Effector mechanisms of interleukin-17 in collagen-induced arthritis in the absence of interferon-gamma and counteraction by interferon-gamma. Arthritis Res. Ther. 2009;11:R122. doi: 10.1186/ar2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J. Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69:2010–2017. doi: 10.1158/0008-5472.CAN-08-3479. [DOI] [PubMed] [Google Scholar]
- 10.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 2009;183:1463–1470. doi: 10.4049/jimmunol.0804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balato A, Unutmaz D, Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J. Invest. Dermatol. 2009;129:1628–1642. doi: 10.1038/jid.2009.30. [DOI] [PubMed] [Google Scholar]
- 12.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 13.Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T. Murine NKT cells produce Th17 cytokine interleukin-22. Cell. Immunol. 2009;254:81–84. doi: 10.1016/j.cellimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doisne JM, Becourt C, Amniai L, Duarte N, Le Luduec JB, Eberl G, Benlagha K. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J. Immunol. 2009;183:2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 16.Lee KA, Kang MH, Lee YS, Kim YJ, Kim DH, Ko HJ, Kang CY. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell. Immunol. 2008;251:50–55. doi: 10.1016/j.cellimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshiga Y, Goto D, Segawa S, Ohnishi Y, Matsumoto I, Ito S, Tsutsumi A, Taniguchi M, Sumida T. Invariant NKT cells produce IL-17 through IL-23-dependent and -independent pathways with potential modulation of Th17 response in collagen-induced arthritis. Int. J. Mol. Med. 2008;22:369–374. [PubMed] [Google Scholar]
- 19.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, Sumikawa Y, Okabe M, Yoshikawa K, Itami S. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur. J. Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn LA, Evander M, Tindle RW, Bulloch AL, de Kluyver RL, Fernando GJ, Lambert PF, Frazer IH. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology. 1997;235:94–103. doi: 10.1006/viro.1997.8650. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto K, Leggatt GR, Zhong J, Liu X, de Kluyver RL, Peters T, Fernando GJ, Liem A, Lambert PF, Frazer IH. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J. Natl. Cancer Inst. 2004;96:1611–1619. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- 24.Kenna TJ, Thomas R, Steptoe RJ. Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood. 2008;111:2091–2100. doi: 10.1182/blood-2007-07-103200. [DOI] [PubMed] [Google Scholar]
- 25.Narayan S, Choyce A, Fernando GJ, Leggatt GR. Secondary immunisation with high-dose heterologous peptide leads to CD8 T cell populations with reduced functional avidity. Eur. J. Immunol. 2007;37:406–415. doi: 10.1002/eji.200535688. [DOI] [PubMed] [Google Scholar]
- 26.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol. Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillonneau C, Mintern JD, Hubert FX, Hurt AC, Besra GS, Porcelli S, Barr IG, Doherty PC, Godfrey DI, Turner SJ. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc. Natl. Acad. Sci. USA. 2009;106:3330–3335. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong C, Lee H, Park YK, Shin J, Jung S, Kim H, Hong S, Park SH. Regulation of secondary antigen-specific CD8(+) T-cell responses by natural killer T cells. Cancer Res. 2009;69:4301–4308. doi: 10.1158/0008-5472.CAN-08-1721. [DOI] [PubMed] [Google Scholar]
- 32.Atalar K, Afzali B, Lord G, Lombardi G. Relative roles of Th1 and Th17 effector cells in allograft rejection. Curr. Opin. Organ Transplant. 2009;14:23–29. doi: 10.1097/MOT.0b013e32831b70c2. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Wood KJ. Interleukin-23 and TH17 cells in transplantation immunity: does 23+17 equal rejection? Transplantation. 2007;84:1071–1074. doi: 10.1097/01.tp.0000287126.12083.48. [DOI] [PubMed] [Google Scholar]
- 35.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 36.Mattarollo SR, Rahimpour A, Choyce A, Godfrey DI, Leggatt GR, Frazer IH. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J. Immunol. 2010;184:1242–1250. doi: 10.4049/jimmunol.0902191. [DOI] [PubMed] [Google Scholar]
- 37.Dander E, Balduzzi A, Zappa G, Lucchini G, Perseghin P, Andrè V, Todisco E, Rahal D, Migliavacca M, Longoni D, et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009;88:1261–1272. doi: 10.1097/TP.0b013e3181bc267e. [DOI] [PubMed] [Google Scholar]
- 38.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]