Abstract
Although IL-1 is a known inflammatory cytokine during pathogen infection, the role of IL-1 in skin graft rejection, particularly where foreign antigen is expressed exclusively in keratinocytes, is less understood. Here, we use a syngeneic skin graft system, where antigens are expressed in epithelial cells via either a keratin 14 or keratin 5 promoter, to explore the role of IL-1 in graft rejection and induction of epithelial antigen-specific effector CD8+ T-cell function. Keratin 5 ovalbumin (K5mOVA) transgenic skin grafts destined for rejection demonstrated increased expression of IL-1β and its receptors compared to K14 HPV16 E7 transgenic grafts that do not reject spontaneously. Rejection of OVA grafts lacking the IL-1 receptor (IL-1R1) was delayed and associated with decreased numbers of antigen-specific CD8 T cells. In contrast, K14E7 grafts survived on immunocompetent, syngeneic recipients with decreased graft levels of IL-1β and IL-1R1 and 2. However, in the absence of the IL-1 receptor antagonist, IL-1Ra, skin grafts were spontaneously rejected and an E7-specific CD8 T-cell response was primed. Thus, expression of the HPV16E7 oncoprotein in epithelial cells prevents IL-1β-associated skin graft rejection and induction of antigen-specific CD8 T-cell responses. Enhancing IL-1β signalling, via blocking of the IL-1 receptor antagonist, may represent an alternative strategy for treatment of HPV16E7-associated cancers.
Keywords: IL-1, IL-1Ra, skin, skin grafts, T cells
Introduction
The IL-1 family of cytokines represent a key control point in the innate immune response, including both potentiators, e.g. IL-1β and IL-1α, and inhibitors, e.g. IL-1Ra, of inflammation (1). While the biologically active IL-1α precursor is constitutively produced in the skin, generation of active IL-1β is tightly controlled by inflammatory events leading to caspase-1-dependent and caspase-1-independent cleavages of the IL-1 precursor (1,2). Mice deficient in IL-1α, IL-1β or both develop normally but have impaired responses to inflammatory stimuli (3). Both IL-1 proteins deliver activating signals through the IL-1R1/IL-1RAcP heterodimeric receptor. IL-1R2 acts as a decoy receptor with no apparent signal transduction (4,5). IL-1 signal transduction induces transcription of genes, including adhesion molecules, secondary cytokines and chemokines that underlie inflammation in the skin (6–8). Both the NFkB and the MAPK signalling pathways have been implicated in signal transduction from the IL-1R1 (1,9). Binding of IL-1 proteins to the activating receptors can also be blocked by the naturally occurring receptor antagonist, IL-1Ra. Recombinant IL-1Ra has been used to treat type 2 diabetes and specific IL-1-associated pro-inflammatory states (10,11). A deficiency in IL-1Ra leads to chronic inflammation and autoimmunity in some animal models (12).
We have previously shown a critical role for local pro-inflammatory signalling, resulting from tissue damage, TLR4 or TLR7, in the elimination by primed antigen-specific CD8+ T cells of epithelium where antigen expression is driven from a keratinocyte-specific promoter (13,14). IL-1 is secreted as the initial signal after injury or infection. IL-1 signalling induces expression of endothelial cell adhesion molecules and chemokine receptors on T cells in the dermis, facilitating amplification of the immune response and effector cell trafficking to the target site (15–17). Thus, IL-1β controls immune responses by linking innate and adaptive immunity through the induction of soluble factors (8) and may be a key local factor in enabling the function of antigen-specific CD8+ T cells to eliminate antigen-expressing epithelial cells.
To examine IL-1 function in the rejection of skin grafts expressing antigen exclusively in epithelial cells, we used transgenic animal models where antigen expression is driven from a keratin 14 (K14) or keratin 5 (K5) promoter. Both the keratin 14 and keratin 5 promoters direct antigen expression to the basal keratinocytes of the skin, although differences in the level of antigen expression cannot be excluded (18). Epithelial grafts expressing ovalbumin protein (K5mOVA) are rejected spontaneously, whereas grafts expressing the human papillomavirus type 16 (HPV16) E7 oncoprotein (K14E7) are not rejected, although they invoke a measurable immune response (19–21). K14E7 grafts mimic the observed immune response to anogenital epithelium infected with HPV16 and expressing E7 protein, which invoke weak E7-specific immune responses. HPV16-infected lesions are cleared over months to years from immunocompetent individuals, with significant persisting infection leading to anogenital cancer (22). Infections are rarely cleared in immuno-incompetent hosts, and immunisation with E7 does not enhance lesion clearance despite induction of E7-specific effector CD8+ T cells, suggesting that local determinants of immune effector function are critical to enable elimination of infected epithelial cells.
We therefore examined the role of IL-1 and IL-1 receptor signalling in elimination of epithelial cells expressing E7 and OVA using real-time PCR, skin grafting and assessment of antigen-specific T-cell responses. The data suggest that IL-1R1 signalling and subsequent induction of antigen-specific CD8+ T cells was important for ovalbumin skin graft rejection. HPVE7-expressing skin cells fail to induce IL-1β, IL-1R1 and E7-specific CD8+ T cells but were able to reject skin grafts in the absence of the IL-1Ra inhibitory protein.
Materials and methods
Mice and skin grafting
C57BL/6J (H-2b) mice (C57) were obtained from the Animal Resources Centre (Perth, WA, Australia). K14E7 and K5mOVA mice were bred at the Princess Alexandra Hospital Biological Resources Facility (BRF, PA Hospital, Brisbane, Qld, Australia) and have been described previously (19,23). IL-1 receptor 1 (IL-1R1−/−)-deficient mice (24), which had been backcrossed onto the C57BL/6 genetic background for eight generations, were obtained from Dr Helen Thomas (St Vincent’s Institute, Melbourne, Vic., Australia). IL-1Ra−/− mice have been previously described (3) and were obtained from Professor Iwakura (University of Tokyo, Tokyo, Japan). All mice were maintained under conventional, specific, pathogen-free conditions at the Biological Research Facility, Princess Alexandra Hospital. All animal protocols were approved by the University of Queensland Animal Ethics Committee (Brisbane, Qld, Australia).
Whole-thickness ear skin grafting has been described previously (20). Grafts were examined twice weekly for evidence of rejection. If grafts were intact 100 days after grafting, they were classified as not rejected.
Microarray
RNA samples were isolated from K14E7 skin grafts and K5mOVA skin grafts 9 days (new grafts) or 60 days after grafting (well-healed skin grafts). Control was skin that was taken from the flank of a naïve C57BL/6J mouse. All samples were collected and snap-frozen in liquid nitrogen before RNA isolation using Trizol (Invitrogen, Eugene, OR, USA). RNA was cleaned using an ArrayGrade™ Total RNA Isolation Kit (SABiosciences, Frederick, MD, USA). cRNA was oligo-dT primed from 1 μg of total RNA using the TrueLabeling–AMP™ 2.0 kit and biotinylated as per the manufacturer’s instructions (SABiosciences, Frederick, MD, USA). The resultant labelled cRNA was purified with the ArrayGrade cRNA cleanup kit (SABiosciences, Frederick, MD, USA) and hybridised to an Oligo GEArray Mouse Inflammatory Cytokines and Receptors Microarray (OMM-011; SABiosciences, Frederick, MD, USA). Luminescent spots were detected via a Typhoon scientific scanner (GE Health-care, Piscataway, NJ, USA). The GEArray Expression Analysis suite (SABiosciences, Frederick, MD, USA) was used to quantify signal intensities from the generated 8- or 16-bit TIFF images. Data were corrected for background and normalised to an internal control, peptidylprolyl isomerase A (Ppia).
Quantitative real-time PCR (qRTPCR)
FastStart SYBR green (Roche Applied Science, Indianapolis, IN, USA) qRTPCR reactions were performed using the Rotor Gene 6000 machine (Corbett Research, Concord, NSW, Australia) and standardised against the peptidylprolyl isomerase A (Ppia) housekeeping gene. Primers were as follows: Ppia forward 5′-ATAAGGGTTCCTCCTTTCAC-3′ and reverse primer 5′-TCCTCAAATTTCTCTCCGTA-3′; IL-1α forward 5′-CAACTGAAAAGCACACTCAA-3′ and reverse primer 5′-TCCTACTCAACTGGCATTTT-3′; IL-β forward 5′-GTT GATTCAAGGGGACATTA-3′ and reverse primer 5′-AGCT TCAATGAAAGACCTCA-3′; IL-1Ra forward 5′-AGGTGTC TTCTGCTCTACCA-3′ and reverse primer 5′-AAGTGACTT GATTGGTCTGG-3′; IL-1R1 forward 5′-ACAGCCAGTGTT TATTTGCT-3′ and reverse primer 5′-TTCTCCATTTGTGT TGTTCA-3′; IL-1R2 forward 5′-AAAGAATTCATCTCCAG CAA-3′ and reverse primer 5′-TTCCTTATTGCCTTTAT CCA-3′.
Immunohistochemistry
Cellular infiltrates were detected in 10% neutral-buffered, formalin-fixed, paraffin-embedded 0.3-um sections of excised grafts stained with haematoxylin and eosin (H&E).
IFN-γ ELISPOT (Enzyme-linked immunosorbent spot) assay
Spleens were removed from mice 21 days after grafting and mixed splenocyte populations prepared. IFN-γ secretion of effector cells was determined based on a previously published protocol (25). A total of 5 × 105 splenocytes were incubated overnight in the presence of IL-2 and the appropriate CD8 epitope (SIINFEKL for K5mOVA; RAHYNIVTF for K14E7) in nitrocellulose ELISPOT plates (Millipore Multiscreen-HA, Danvers, MA, USA) coated with IFN-γ capture antibodies (0.8 μg/ml; BD Biosciences, North Ryde, NSW, Australia) and blocked with foetal calf serum (FCS). IFN-γ was detected by 3-h incubation with biotinylated anti-mouse IFN-γ detection antibody (0.1 μg/ml; BD Biosciences), followed by incubation with avidin horseradish peroxidase solution (Sigma Aldrich, St. Louis, MO, USA). Spots were developed with 3,3′-diaminobenzidine tetra-hydrochloride dehydrate reagent (Sigma Aldrich) and quantitated digitally.
Statistics
Expression and ELISPOT data were analysed using a Student’s t-test (unpaired, one tailed), while graft survival data were analysed using a log-rank test.
Results
IL-1β-associated inflammation is associated with rejection of skin grafts where antigen expression is driven from a keratinocyte-specific promoter
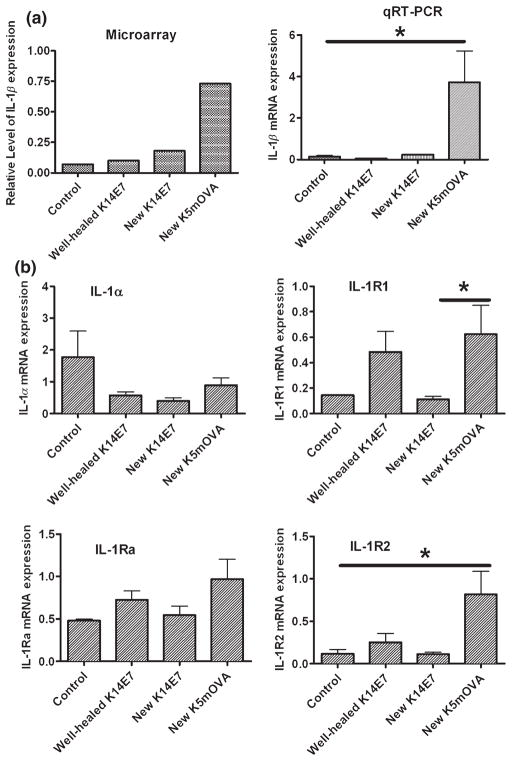
Skin grafts that express OVA or hGH driven by a keratin promoter are spontaneously rejected, while grafts expressing HPV16 E6 or E7 protein are not (19,21). We used grafts expressing OVA or E7 (K5mOVA and K14E7, respectively) to determine whether inflammation-induced signals promote rejection of grafts where antigen expression is driven from a keratinocyte-specific promoter. K5mOVA skin grafts were spontaneously rejected from naïve C57BL6/J recipients 17–27 days after surgery (mean retention time = 21 days; Fig. 1), while control, non-transgenic skin was accepted for at least 100 days when grafted onto identical recipients (Fig. 1). In contrast to OVA-expressing grafts, K14E7 skin grafts were not rejected from syngeneic, C57BL6/J recipients (Fig. 1), as has been described previously for this tumor antigen (26). Histological examination 21 days after grafting, at a time when histologically visible effects of inflammation because of the grafting process itself had resolved, demonstrated a substantial cell infiltrate into rejecting K5mOVA skin grafts (Fig. 1; middle panel). A less-intense cellular infiltrate was observed in K14E7 grafts and control, non-transgenic grafts (Fig. 1; bottom and top panels, respectively). These findings support the idea that inflammation enables rejection of skin grafts where antigen expression is driven from a keratinocyte-specific promoter. We therefore examined the prototypical, inflammatory gene, IL-1β, and its family members 9 days after grafting. IL-1β mRNA expression was markedly increased in newly placed skin grafts expressing ovalbumin relative to newly placed or well-healed K14E7 skin grafts or control grafts (Fig. 2a). IL-1α and IL-1Ra expression levels in control, OVA and E7 grafts were similar (Fig. 2b, left panels). IL-1R1 and IL-1R2 were over-expressed in grafts expressing OVA, relative to grafts expressing E7 and to control grafts (Fig. 2b, right panels). These results indicate that a primary mediator of inflammation, IL-1β, and its two receptors are expressed at higher levels in grafts destined for rejection than in those expressing antigen destined for acceptance.
Figure 1.
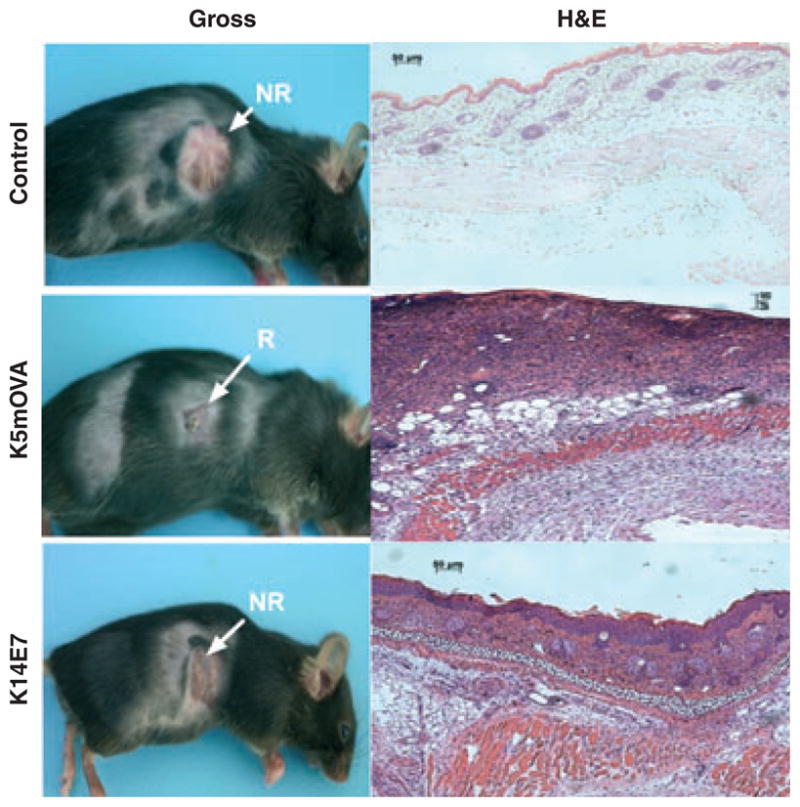
Rejecting skin comprising transgenic keratinocytes has increased cellular infiltrate. Ear skin transgenic for OVA (K5mOVA) or E7 (K14E7) expressed from a keratin promoter or control, non-transgenic skin (C57BL/6) was grafted to C57BL/6 naïve recipients. Gross morphology (left panels) of rejected (R) K5mOVA grafts and non-rejected (NR) K14E7 or control grafts was assessed 21 days after grafting. Haematoxylin and eosin (H&E) staining of paraffin-embedded skin tissue at day 21 postgrafting (right panels).
Figure 2.
Rejecting skin grafts have increased levels of IL-1β. (a) Expression of IL-1β in full skin thickness (dermis + epidermis) samples by microarray analysis and semi-quantitative real-time PCR. Microarray data are the average relative expression [relative to the housekeeping gene, peptidylprolyl isomerase A (Ppia)] from two individual samples while the real-time PCR data are based on four individual skin samples for each group (qRTPCR: K5mOVA versus control P = 0.028; new K14E7 and well-healed K14E7 versus control P > 0.05, K5mOVA versus new K14E7 P = 0.031; K5mOVA versus well-healed K14E7 P = 0.026). (b) qRTPCR analysis of IL-1 family members in full skin thickness tissue samples. Expression data are relative to a housekeeping gene, Ppia, and represent four individual skin samples in each group. Significant difference *P < 0.050.
Local IL-1 signalling is necessary for K5mOVA graft rejection
To establish a role for IL-1 signalling in K5mOVA skin graft rejection, we utilised mice deficient in the activating receptor, IL-1R1. K5mOVA skin grafted onto IL-1R1−/− recipients unresponsive to IL-1 (27) was rejected without significant delay, whereas rejection of IL-1R1-deficient K5mOVA skin grafts placed on IL-1R1 sufficient recipient mice was delayed (Fig. 3a; P = 0.012). When both the donor and the recipient lacked IL-1R1, 73% of grafts were retained for at least 100 days ((Fig. 3a; P = 0.001). These data suggest that K5mOVA graft rejection in the majority of mice depends on IL-1 signal transduction by cells that originate from both the recipient and the graft. We next tested whether induction of OVA-specific CD8+ T cells was influenced by a lack of IL-1 signalling (Fig. 3b). Significantly fewer IFNγ-secreting, OVA-specific CD8+ T cells were observed when IL-1R1 expression was absent from the donor and recipient (Fig. 3b; P = 0.041). These results suggest that for efficient rejection of a graft where antigen expression is driven from a keratinocyte-specific promoter, IL-1 signalling to graft- and recipient-associated cells is required. This property associates with the ability to prime a naïve, antigen-specific CD8+ T-cell response.
Figure 3.
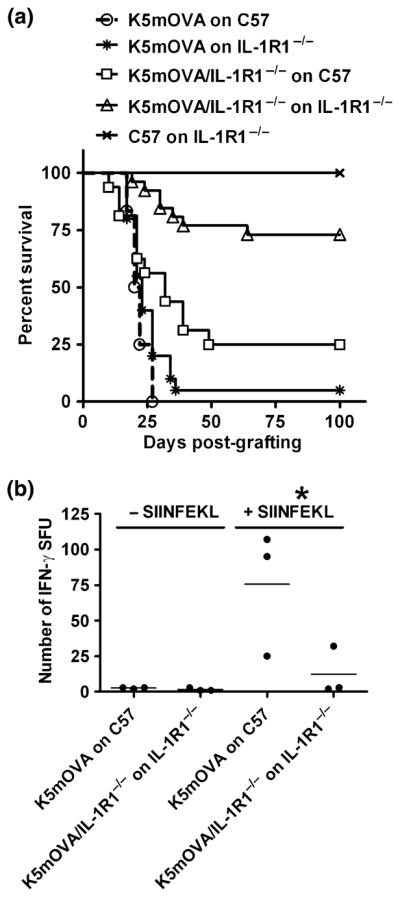
IL-1R signalling is required for rejection of OVA-expressing skin. (a) Mice were grafted with wild-type K5mOVA ear skin grafts onto non-transgenic recipients (○; n = 12) or recipients deficient in IL-1R1−/− ( ; n = 20, P > 0.05 compared with wild-type K5mOVA grafts). Alternatively, K5mOVA grafts deficient in IL-1R1 were grafted onto non-transgenic recipients (□; n = 16, P = 0.012) or recipients deficient in IL-1R1 (△; n = 26, P = 0.001). Non-transgenic ear skin grafted onto IL-1R1−/− recipient mice acted as a negative control for graft rejection (X; n = 8). A Kaplan–Meier analysis of graft survival over time is plotted. (b) OVA-specific CD8+ T cells in OVA-grafted animals with or without IL-1R1. Splenocytes from graft-primed animals were analysed at day 21 postgrafting for the ability to respond to an ovalbumin peptide (SIINFEKL) by overnight IFN-γ ELISPOT with (+SIINFEKL) and without (−SIINFEKL) peptide. Pooled data from three independent experiments are shown.
; n = 20, P > 0.05 compared with wild-type K5mOVA grafts). Alternatively, K5mOVA grafts deficient in IL-1R1 were grafted onto non-transgenic recipients (□; n = 16, P = 0.012) or recipients deficient in IL-1R1 (△; n = 26, P = 0.001). Non-transgenic ear skin grafted onto IL-1R1−/− recipient mice acted as a negative control for graft rejection (X; n = 8). A Kaplan–Meier analysis of graft survival over time is plotted. (b) OVA-specific CD8+ T cells in OVA-grafted animals with or without IL-1R1. Splenocytes from graft-primed animals were analysed at day 21 postgrafting for the ability to respond to an ovalbumin peptide (SIINFEKL) by overnight IFN-γ ELISPOT with (+SIINFEKL) and without (−SIINFEKL) peptide. Pooled data from three independent experiments are shown.
Removal of an antagonist of IL-1 signalling precipitates rejection of K14E7 skin grafts
IL-1Ra−/− mice have a higher availability of IL-1 than wild-type mice because of the lack of the endogenous antagonist, and as such, increased IL-1 signalling capacity (28). We hypothesised that low levels of IL-1β protein in K14E7 skin grafts might induce functional IL-1 signalling if the antagonist of IL-1 was removed. E7-expressing skin grafts, accepted by IL-1Ra+/+ mice, were rejected if both the donor skin graft and recipient mouse lacked IL-1Ra (Fig. 4a; P = 0.030). E7 graft rejection was also observed when the recipient mouse lacked IL-1Ra, but the skin graft was wild type (Fig. 4a; P = 0.020). However, when the donor graft lacked IL-1Ra and the recipient mouse was wild type, the rate of spontaneous rejection was not significantly different from the control. These data suggest that IL-1Ra inhibits induction of the systemic immune response to prevent rejection of K14E7 grafts. To confirm whether generation of E7-specific, CD8+ T cells was increased in graft-primed IL-1Ra−/− mice, spleens from grafted mice were examined for E7-specific, IFN-γ secreting T cells (Fig. 4b). Splenocytes isolated from E7 graft-primed IL-1Ra−/− mice demonstrated significantly more E7-specific, IFN-γ producing cells than E7 graft-primed controls and C57Bl/6J recipients (Fig. 4b; P = 0.020). Thus, removal of the IL-1 receptor antagonist, IL-1Ra, enables rejection of K14E7 skin grafts associated with increased numbers of E7-specific CD8+ T cells.
Figure 4.
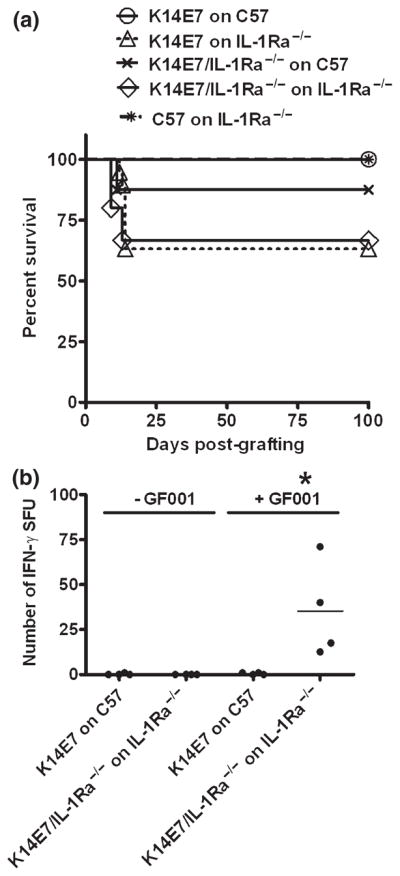
Loss of IL-1Ra leads to spontaneous K14E7 graft rejection (a) Wild-type K14E7 skin grafts were placed on naïve C57BL/6 recipients (○; n = 12) or IL1-Ra−/− recipients (△; n = 19, P = 0.020 compared to wild-type graft). Alternatively, K14E7 grafts deficient in IL-1Ra were placed on naïve C57BL/6 recipients (x; n = 8, P > 0.05) or IL1-Ra−/− recipients (◇; n = 15, P = 0.030). Non-transgenic C57BL/6 skin was placed on IL-1Ra−/− as a negative control graft ( ; n = 8). A Kaplan–Meier analysis of graft survival over time is plotted. (b) E7-specific CD8+ T cells in K14E7-grafted animals with or without IL-1Ra. Splenocytes from graft-primed animals were analysed at day 21 postgrafting for the ability to respond to E7 peptide (GF001) by overnight IFN-γ ELISPOT in the presence (+GF001) or absence (−GF001) of peptide. Data represent individual animals assessed in independent experiments.
; n = 8). A Kaplan–Meier analysis of graft survival over time is plotted. (b) E7-specific CD8+ T cells in K14E7-grafted animals with or without IL-1Ra. Splenocytes from graft-primed animals were analysed at day 21 postgrafting for the ability to respond to E7 peptide (GF001) by overnight IFN-γ ELISPOT in the presence (+GF001) or absence (−GF001) of peptide. Data represent individual animals assessed in independent experiments.
Discussion
We present evidence that the rejection of skin grafts containing antigen-bearing keratinocytes depends upon IL-1 signalling and associates with IL-1β production and the generation of an effector CD8+ T cell.
K5mOVA grafts that were spontaneously rejected from naïve recipients showed cellular infiltration into the graft bed associated with local inflammation. The cellular infiltrate consisted predominantly of mononuclear cells and coincided with locally increased IL-1β. IL-1β is capable of stimulating inflammation through activation of stromal cells, resulting in leucocyte infiltration to potentiate and sustain the local inflammatory response (29,30). Dermal injection of this cytokine has been shown to increase the infiltration of predominantly monocytic cells into human skin (31). Although keratinocytes express large amounts of IL-1α (32), no significant change in IL-1α mRNA levels was observed in new K5mOVA grafts, suggesting that this gene is regulated differently from IL-1β during grafting. Upregulation of the activating, IL-1 receptor type 1 was consistent with the observed inflammation while parallel increases in the inhibitory receptor, IL-1R2, was somewhat unexpected. Acquisition of IL-1R2 expression in the skin graft may be a late event designed to limit the damage invoked by the IL-1/IL-1R1 inflammatory axis. Kinetic studies of receptor expression would be required to resolve this issue.
IL-1R1−/− mice are unresponsive to IL-1 and fail to activate intracellular signal transduction (27). K5mOVA-expressing skin grafts that are normally rejected from naïve animals, when transplanted as IL-1R1 deficient grafts onto IL-1R1−/− recipients, did not reject. The inability of IL-1R1−/− mice to reject K5mOVA/IL-1R1−/−skin grafts was associated with a reduction in the number of antigen-specific CD8+ T cells in the spleens of recipient mice. Similarly, CD4+ T-cell priming was shown to be reduced in IL-1R1−/− mice during delayed-type hypersensitivity responses in the skin (33). IL-1 has been shown to be involved in T-cell priming by increasing expression of the co-stimulatory molecules CD40L and OX40 on T cells (34), both of which are crucial for effective T-cell priming. Our data suggest that IL-1 signalling during K5mOVA graft rejection is responsible for the priming of OVA-specific CD8+ T cells, which are associated with rejection of K5mOVA skin grafts. Interestingly, removal of IL-1 signalling from the graft tissue had a more profound effect on graft rejection than loss of IL-1 signalling in the graft recipient. This would be consistent with graft-resident Langerhan cells or dermal dendritic cells, both of which express IL-1R1 (15,35), responding to IL-1 and playing a role in cross-presentation of ovalbumin for T-cell priming after trafficking to the lymph node (36). In addition, removal of IL-1 signalling from both the recipient and donor graft tissue greatly enhanced graft survival over either IL-1R1 deficiency in recipient or graft alone, suggesting collaboration between these two compartments during graft rejection.
In contrast to the spontaneous rejection of K5mOVA grafts, skin from mice expressing a different foreign antigen, HPV16E7, failed to reject and had suppressed levels of IL-1β and its receptors. There is little evidence in the literature that HPVE7 protein can directly affect the transcription of the IL-1β gene or the signalling from the IL-1R1. One study indicated that HPV E6 may regulate the activity of the NFκB pathway which, in turn, can regulate the production of IL-1β transcript (37). In our study, the cellular source of the IL-1β is unknown given that the microarray was performed on digested, full-thickness skin grafts. HPVE7 is expressed solely in the keratinocytes of the skin in our model, and these cells can produce IL-1β particularly when given environmental signals that activate the inflammasome (38). Consequently, while it is possible that E7 expression may regulate IL-1β production from the keratinocyte, macrophage/dendritic cell populations within the skin represent a major source of IL-1β during inflam-mation. Given that E7 induces epithelial hyperplasia in the skin, we speculate that the skin microenvironment may be altered in K14E7 mice such that IL-1β production from resident macrophage/dendritic cell populations is indirectly suppressed during grafting.
IL-1 signalling in the skin is tightly regulated, suggesting that the equilibrium between agonist and antagonist harbours the inflammatory potential of IL-1 in skin (39). The E7 tumor antigen, which can modulate host gene expression (40), alters this balance to overcome the normal protective immune response mechanisms and allow the persistence of skin grafts expressing tumor antigen. Recently, additional members of the IL-1 family have been identified, having parallel functions to IL-1R1, IL-1β and IL-1Ra (41). Whether these molecules impact upon IL-1β signalling in the epidermis remains to be determined.
Given the low levels of IL-1 production in K14E7 grafts, we tested whether removal of the IL-1R antagonist would improve IL-1 signalling and lead to graft rejection. Previous studies using skin delayed-type hypersensitivity (DTH) suggested that deletion of IL-1Ra exacerbated the DTH reaction, while administration of soluble IL-1Ra in graft versus host disease lead to reduced disease (33,42). In addition, patients with cervical cancer have been found to have IL-1Ra gene polymorphisms that associate with protection from disease (43). K14E7 skin grafts were rejected from a proportion of IL-1Ra−/− recipient mice and this associated with an E7-specific CD8+ T-cell response. Removal of IL-1Ra from the recipient, rather than the graft, enabled K14E7 skin graft rejection, suggesting that graft infiltrating, host cells may be the key producers of suppressive IL-1Ra acting in the skin or that IL-1Ra acts locally in the lymph node to suppress cross-presentation of skin antigens. To determine whether IL-1Ra from the host acts predominantly in the skin, future experiments would address K14E7 × IL-1R1−/− grafts onto IL-1Ra−/− recipients to determine whether IL-1 signalling locally in the skin, via IL-1R1, is required for K14E7 graft rejection. This would also help resolve whether OVA and E7 skin graft rejections have a similar requirement for IL-1 signalling in graft-derived cells.
Failure of the majority of IL-1Ra−/− mice to reject K14E7 skin grafts may reflect quantitative differences in the IL-1β production (and thus positive signalling) between individual mice after grafting. It should also be noted that all graft-primed IL-1Ra−/− mice induced E7-specific CD8+ T cells despite the fact that the majority of mice did not reject their K14E7 grafts. One possibility is that the magnitude or quality of the immune response also has an influence on graft rejection.
Both skin graft models provide strong evidence that adaptive immunity is controlled by IL-1 signalling. Previous work investigating the role of IL-1 in the induction of adaptive immunity using IL-1 mutant mice showed that the priming of antigen-specific T cells in delayed-type hypersensitivity is initiated by IL-1β signal transduction (33), as is antigen-specific T-cell activation in experimental autoimmune encephalomyelitis (44). Additionally, IL-1β has been shown necessary for T-cell-dependent antibody production and the promotion of antigen-specific T-cell helper function (45). In fact, IL-1 signalling has been shown to be important for helper T-cell diversity by both promoting cell-mediated Th1 development and augmenting Th2 development (46,47). Our data extend these studies and suggest a role for IL-1 signalling in the development of CD8+ T cells for the rejection of transgenic skin grafts.
Our data, derived from a mouse tumor antigen model, indicate that the pro-inflammatory cytokine IL-1β regulates priming and activation of effector cells that reject skin grafts. We present evidence that local inflammation and pro-inflammatory signals are required for the recruitment, activation and function of effector cells for the killing of keratinocytes that express foreign antigens. Our study highlights the necessity for the development of combinational tumor immunotherapies that increase local inflammation and augment the development of functional antigen-specific effector cells. It also suggests that blocking the activity of IL-1Ra, through localised application of IL-1Ra inhibitors, may be a practical treatment for some epithelial cancers.
Acknowledgments
This work was supported by funding from the National Health and Medical Research Council (Program Grant No. 351439), Cancer Council Australia, Cancer Research Institute (New York). UH was supported by an AUSAID M.Sc. scholarship. GRL was supported by a Lions Medical Research Fellowship. IHF is a recipient of a Queensland Government Premiers Fellowship. RT is supported by Arthritis Queensland and an ARC Future fellowship. The support of Megan Bathurst and the animal technical staff of the BRF animal facility was very much appreciated. We also thank Dr Helen Thomas (St Vincents Institute, Melbourne, Australia) and Professor Y. Iwakura (University of Tokyo, Tokyo, Japan) for provision of mice for our studies.
References
- 1.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 3.Horai R, Asano M, Sudo K, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill LA. The interleukin-1 receptor/toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 5.Colotta F, Dower SK, Sims JE, Mantovani A. The type II ‘decoy’ receptor: a novel regulatory pathway for interleukin 1. Immunol Today. 1994;15:562–566. doi: 10.1016/0167-5699(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JE, Robert C, Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol. 2000;114:602–608. doi: 10.1046/j.1523-1747.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- 7.Homey B, Zlotnik A. Chemokines in allergy. Curr Opin Immunol. 1999;11:626–634. doi: 10.1016/s0952-7915(99)00028-x. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 9.Kjellerup RB, Kragballe K, Iversen L, Johansen C. Pro-inflammatory cytokine release in keratinocytes is mediated through the MAPK signal-integrating kinases. Exp Dermatol. 2008;17:498–504. doi: 10.1111/j.1600-0625.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 10.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 11.Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 12.Horai R, Saijo S, Tanioka H, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong J, Hadis U, De Kluyver R, Leggatt GR, Fernando GJ, Frazer IH. TLR7 stimulation augments T effector-mediated rejection of skin expressing neo-self antigen in keratinocytes. Eur J Immunol. 2008;38:73–81. doi: 10.1002/eji.200737599. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Browne M, Bluhm R. Imiquimod: a review. J Cutan Med Surg. 2002;6:554–560. doi: 10.1007/s10227-001-0134-6. [DOI] [PubMed] [Google Scholar]
- 15.Groves RW, Ross E, Barker JN, Ross JS, Camp RD, MacDonald DM. Effect of in vivo interleukin-1 on adhesion molecule expression in normal human skin. J Invest Dermatol. 1992;98:384–387. doi: 10.1111/1523-1747.ep12499816. [DOI] [PubMed] [Google Scholar]
- 16.Kupper TS, Groves RW. The interleukin-1 axis and cutaneous inflammation. J Invest Dermatol. 1995;105:62S–66S. doi: 10.1111/1523-1747.ep12316087. [DOI] [PubMed] [Google Scholar]
- 17.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–1068. [PMC free article] [PubMed] [Google Scholar]
- 18.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azukizawa H, Kosaka H, Sano S, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 20.Frazer IH, De Kluyver R, Leggatt GR, et al. Tolerance or immunity to a tumor antigen expressed in somatic cells can be determined by systemic proinflammatory signals at the time of first antigen exposure. J Immunol. 2001;167:6180–6187. doi: 10.4049/jimmunol.167.11.6180. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Leggatt GR, Zhong J, et al. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J Natl Cancer Inst. 2004;96:1611–1619. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- 22.Leggatt GR, Frazer IH. HPV vaccines: the beginning of the end for cervical cancer. Curr Opin Immunol. 2007;19:232–238. doi: 10.1016/j.coi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labow M, Shuster D, Zetterstrom M, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 25.Carvalho LH, Hafalla JC, Zavala F. ELISPOT assay to measure antigen-specific murine CD8(+) T cell responses. J Immunol Methods. 2001;252:207–218. doi: 10.1016/s0022-1759(01)00331-3. [DOI] [PubMed] [Google Scholar]
- 26.Dunn LA, Evander M, Tindle RW, et al. Presentation of the HPV16E7 protein by skin grafts is insufficient to allow graft rejection in an E7-primed animal. Virology. 1997;235:94–103. doi: 10.1006/viro.1997.8650. [DOI] [PubMed] [Google Scholar]
- 27.Glaccum MB, Stocking KL, Charrier K, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 28.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apte RN, Voronov E. Interleukin-1 – a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 2002;12:277–290. doi: 10.1016/s1044-579x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 31.Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani H, Black R, Kupper TS. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Invest. 1991;87:1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nambu A, Nakae S, Iwakura Y. IL-1beta, but not IL-1alpha, is required for antigen-specific T cell activation and the induction of local inflammation in the delayed-type hypersensitivity responses. Int Immunol. 2006;18:701–712. doi: 10.1093/intimm/dxl007. [DOI] [PubMed] [Google Scholar]
- 34.Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J Immunol. 2001;167:90–97. doi: 10.4049/jimmunol.167.1.90. [DOI] [PubMed] [Google Scholar]
- 35.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cell migration in mice requires intact type I interleukin 1 receptor (IL-1RI) function. Arch Dermatol Res. 1999;291:357–361. doi: 10.1007/s004030050422. [DOI] [PubMed] [Google Scholar]
- 36.Bedoui S, Whitney PG, Waithman J, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 37.Havard L, Delvenne P, Frare P, Boniver J, Giannini SL. Differential production of cytokines and activation of NF-kappaB in HPV-transformed keratinocytes. Virology. 2002;298:271–285. doi: 10.1006/viro.2002.1468. [DOI] [PubMed] [Google Scholar]
- 38.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflamma-some mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 39.Mee JB, Antonopoulos C, Poole S, Kupper TS, Groves RW. Counter-regulation of interleukin-1alpha (IL-1alpha) and IL-1 receptor antagonist in murine keratinocytes. J Invest Dermatol. 2005;124:1267–1274. doi: 10.1111/j.0022-202X.2005.23684.x. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti O, Krishna S. Molecular interactions of ‘high risk’ human papillomaviruses E6 and E7 oncoproteins: implications for tumour progression. J Biosci. 2003;28:337–348. doi: 10.1007/BF02970152. [DOI] [PubMed] [Google Scholar]
- 41.Dunn E, Sims JE, Nicklin MJ, O’Neill LA. Annotating genes with potential roles in the immune system: six new members of the IL-1 family. Trends Immunol. 2001;22:533–536. doi: 10.1016/s1471-4906(01)02034-8. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy PL, Jr, Abhyankar S, Neben S, et al. Inhibition of interleukin-1 by an interleukin-1 receptor antagonist prevents graft-versus-host disease. Blood. 1991;78:915–918. [PubMed] [Google Scholar]
- 43.Tamandani DM, Sobti RC, Shekari M, Kaur S, Huria A. Impact of polymorphism in IL-1RA gene on the risk of cervical cancer. Arch Gynecol Obstet. 2008;277:527–533. doi: 10.1007/s00404-007-0504-4. [DOI] [PubMed] [Google Scholar]
- 44.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 45.Nakae S, Asano M, Horai R, Iwakura Y. Interleukin-1 beta, but not interleukin-1 alpha, is required for T-cell-dependent antibody production. Immunology. 2001;104:402–409. doi: 10.1046/j.1365-2567.2001.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz N, Kurrer M, Kopf M. The IL-1 receptor 1 is critical for Th2 cell type airway immune responses in a mild but not in a more severe asthma model. Eur J Immunol. 2003;33:991–1000. doi: 10.1002/eji.200323801. [DOI] [PubMed] [Google Scholar]