Abstract
Navigation systems, devices and intra-procedural software are changing the way we practice interventional oncology. Prior to the development of precision navigation tools integrated with imaging systems, thermal ablation of hard-to-image lesions was highly dependent upon operator experience, spatial skills, and estimation of positron emission tomography-avid or arterial-phase targets. Numerous navigation systems for ablation bring the opportunity for standardization and accuracy that extends our ability to use imaging feedback during procedures. Existing systems and techniques are reviewed, and specific clinical applications for ablation are discussed to better define how these novel technologies address specific clinical needs, and fit into clinical practice.
Introduction
Image-guidance and minimally invasive approaches have revolutionized the management of cancer. However, diagnosis and therapy remain distinctly separate from each other in both time and space. For thermal ablation of cancer, this gap between diagnosis and therapy can be narrowed by the application of novel guidance technologies during the ablation procedure. “Medical GPS” (or multi-modality fusion-guided interventions) is enabled by electromagnetic tracking (EM tracking), and lets the physician navigate within a volume of imaging data (1–4). In this way, the precise spatial imaging information may be used when it is needed the most, during the ablation procedure. Ablations may be facilitated by real-time updated information regarding the localization of needle, ultrasound plane, and neoplastic target tissue margin during a composite ablation. In this fashion, arterial enhancing hepatomas or positron emission tomography (PET)-hot tumors may be precisely localized and targeted during a multimodality ablation with “smart” needles and “smart” ultrasound that integrates arterial phase computed tomography (CT), magnetic resonance imaging (MRI), or PET data (5)Kruecker J, Xu S, Venkatesan A, et al.: Clinical Utility of Real-Time Fusion Guidance for Biopsy and Ablation. J Vasc Interv Radiol (in press)). A treatment plan can be implemented and adjusted during the procedure by using these accessory tools that augment standard imaging. (6–11). During the same ablation session, tissue at risk for under-treatment can be identified, localized, targeted, and ablated, instead of waiting months for the residual tumor to grow in order to be identified, at which point it might prove geometrically unfavorable for local therapy. Optimization of imaging-based navigation technologies will be key to establishing the IR suite as the home for the operating room of the future, and the best location to deliver minimally-invasive, image-guided local and regional oncologic therapies. The opportunity is upon us to truly bring all the imaging information to the patient during ablation.
Background
The skills of interventional radiologists have recently been enhanced by navigation technologies with interfaces similar to video games. Image-based navigation technology closes the natural gap between radiological diagnosis and therapy. Interventional oncology and interventional radiology (IR) outcomes are highly dependent on the accuracy of needles, catheters and devices; navigation tools can help guide these devices with precision.. Guidance technology for IR procedures is constantly evolving. Interventional radiologists rely upon sophisticated manual skills (and on occasion, almost educated guesswork) to manipulate devices towards sometimes nebulous targets. Navigation technology can enhance conventional IR techniques by allowing pre-acquired images to be used during procedures, with real-time referencing of “smart” devices, enabled by tracking of needles, guidewires, and ultrasound transducers, to guide the physician’s hand. Rapid image processing technologies enable real time display of multi-planar, fused images, with the potential to improve lesion targeting. Complex spatial relations that used to be done by the imagination is now done by the computer and available for viewing during an ablation.
Navigation tools include needle-based, catheter-based, and imaging-based technologies. The development of electromagnetic (EM) tracking of needle tips and ultrasound transducers to facilitate multimodality image-guided tumor ablation may alter the paradigm for what is considered standard of care ablation. It is now possible to chisel a composite ablation piece by piece, guided by real time feedback relative to PET, MRI, or arterial phase CT data. Knowing exact locations of tumors and enabling accurate delivery of needle-based therapies should translate into improved outcomes for therapies that rely upon accuracy, such as thermal ablation (whether RF ablation, laser, cryoablation, or microwave). Multi-modality fusion guidance combines the strength of each modality. For example, the spatial resolution of CT can be combined with the temporal resolution and real time feedback of ultrasound (US) (CT is the eye, US is the hand). Metabolic and functional details can be co-registered to anatomic and morphologic data, resulting in a system which provides multi-modality fusion guidance. Sculpting a composite ablation can be facilitated by the knowledge of where the needle (and treatment zone) has been, and where the needle still needs to go. Identification of tumor tissue at risk for under-treatment is enabled by the spatial knowledge provided by tracking. Interventional oncology and IR outcomes are highly dependent on the accuracy of needle, catheter and device position and location. Navigation systems help guide these tools to where they need to be, based upon real time spatial feedback, in relation to imaging data.
Image Fusion-Guided Procedures
Image co-registration is the joining of two imaging datasets (rigid where only translation and rotation are performed or elastic where some localized stretching is performed for image correspondence)(12), whereas image fusion is the co-display of those datasets. Fusion may be accompanied by an image overlay display, or by real-time updating of 2 or more image data sets (or modalities) based upon location of a device such as a needle or an ultrasound transducer. Fusion display techniques became widespread for diagnostic applications with PET-CT, but image fusion in interventional radiology had remained only a theoretical tool until recently. Fusion allows interactive multimodality targeting by placing sensor coils on or inside biopsy and ablation needles, ultrasound transducers, guidewires, stent-grafts, catheters, scalpels, steerable endoscopes, and on the patient’s skin. In the case of tumor ablation, the sensors are often located in a guiding needle or cannula, as well as in or fixed to the ultrasound transducer itself. This enables the ultrasound transducer to act as a standard ultrasound, as well as an “MPR plane selection device”, displaying the matching imaging plane of a previously-obtained image data set (different modality - or same modality as during intervention, but different phase).
Medical GPS Technology: Optical versus Electromagnetic (EM) Tracking
Optical tracking uses passive reflectors or active light emitters on devices within view of a camera, instead of sensor coils within differential magnetic fields, as for EM tracking. The sensor coils or reflectors are like the GPS device or car and the EM field generator or infrared camera is like the “satellite”. The presence of a coil in rapidly changing magnetic fields elicits a weak electrical current (Faraday’s law of electromagnetic induction). Processing this current in the presence of the EM field generator can define a location within a Cartesian coordinate system (XYZ space). The benefit of EM (over optical) is that the device can reside out of sight and within the patient’s body, without erosion of signal. This requirement for optical tracking is termed “line-of-sight”, and necessitates a clear and direct pathway for a photon or infra-red beam from camera to device (without intervening staff or body parts). EM enabled devices can thus reside deep within the body and still report their location. However, EM tracking suffers in that the working space is somewhat limited (~40 × 40 to 70 × 70 cm2), and there may be metallic artifacts from large metal objects such as tables, x-ray sources or detectors, although metal interactions have become lesser and lesser limitations in recent years due to evolution of tracking technology. The EM field generator is typically a flat plate under the patient or a brick-like device mounted near the patient and it must reside near the working space of the patient for most EM applications. Table 1 defines key terms for tracking/navigation (Table 1).
Table 1.
Glossary
| Medical GPS | Localization of a device or image in relation to prior pre-procedural imaging. The electromagnetic field generator is the “satellite” and the needle tip is the “car” |
| Multimodality Fusion | Display of multiple modalities; useful during procedures |
| Electromagnetic Tracking | Mechanism, based on electromagnetic field generator, to locate a needle (or ultrasound plane) within a 3D volume of imaging data (like CT, MR, or PET), or to locate a 2D ultrasound plane within a 3D volume |
| “Target to Registration Error” or TRE | Difference between the real and virtual needle positions. How exact is the needle position displayed? |
| (Fiducial) Registration error” or FRE (or root mean square/RMS) | How well the images or points match up How good is the registration? Necessary, but not a guarantee for accuracy (ideally under 2mm) |
| Placement Error | How close is needle placed to point target? |
| Dynamic Reference | Patch or sensor placed on patient that corrects for patient or generator motion, or breathing. Without this, patient must remain in exact position. |
| Registration | Matching co-localized images to images, or image space to “magnetic space” “Registration error” (root mean square or RMS) “Rigid registration” matches uses fixed images vs “deformable registration” (elastic, warping) matches with flexible images, accounting for organ shift or deformation between different images or different times. |
EM Tracking Hardware and Software
Two major vendors manufacture the EM tracking equipment for the vendors (Northern Digital Inc, Waterloo, CA & Ascension Technologies, VT). Multiple vendors have EM tracking solutions for percutaneous procedures with varying degrees of complexity (Traxtal Inc., Philips Healthcare, Toronto, CA/Siemens Healthcare, Erlangen, Germany/General Electric, Milwaukee, WI/Veran Medical, St. Louis, MO/Esaote, Biosound, Indianapolis, IN/Civco, Kalona, Iowa/Hitachi Medical, Twinsburg, Ohio/Sentinelle Medical, Toronto, Canada/, State, Ultrasonix, Richmond, BC, Canada). Systems developed for surgical procedures can also be customized for percutaneous ablation (Medtronic and GE).
The location of EM field generator, PC or cart, monitor, and skin patch sensors should be well planned out and rehearsed prior to initial use. The EM tracking sensor hardware often resides within needles tips, within guidewire tips, clipped to ultrasound transducers, or within a soft foam adhesive skin patch (for semi-automatic detection and registration). EM sensors are usually described as having 5 or 6 “degrees of freedom”, with the 6th degree (if present) sensing rotation of the device around its main axis, primarily used for ultrasound probe tracking. The software is the graphical representation of the multi-modality information. This is typically ultrasound + CT or PET CT for ablation or biopsy (or CT + angio for catheter-based procedures). Occasionally data may be displayed as pre-procedural CT + intra-procedural CT, as in the case of an arterial-phase enhancing hepatocellular carcinoma that is only conspicuous briefly, during the arterial phase of enhancement. In this case, the arterial-phase CT outlines the location of the tumor, and this information is superimposed upon the unenhanced procedural CT (Fig 1). Real-time ultrasound (when tracked) can also select the plane of the enhanced CT for display (multiplanar reconstruction along the needle path).
Figure 1.
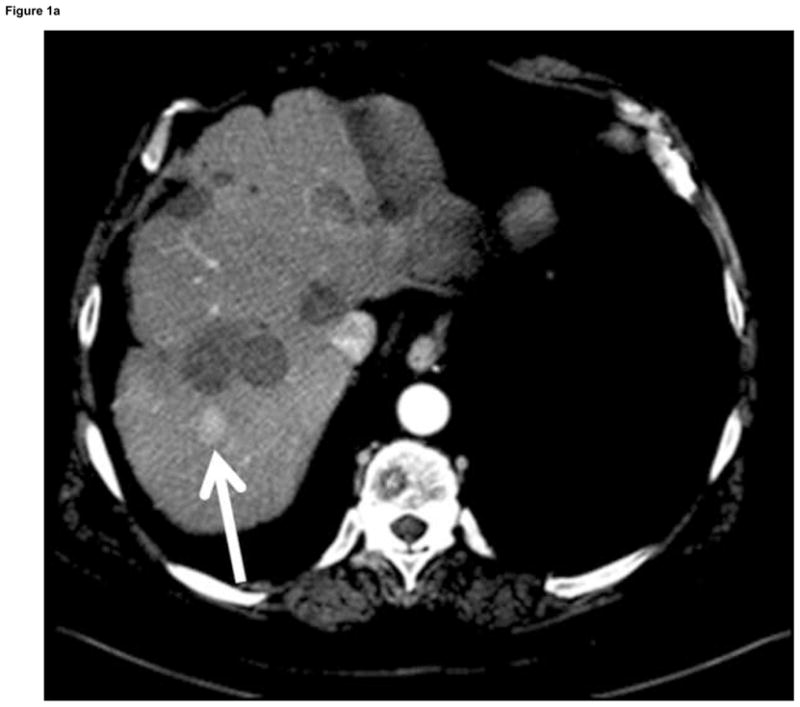
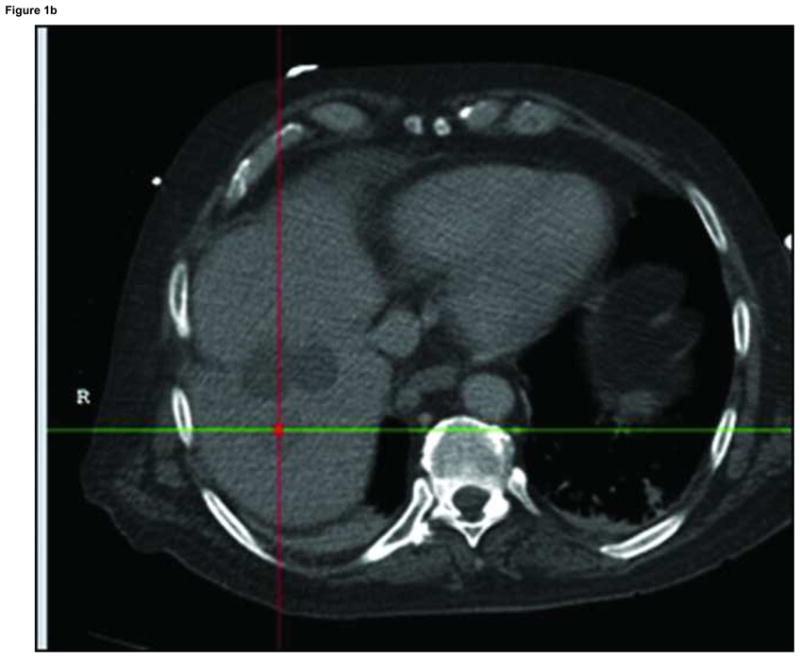
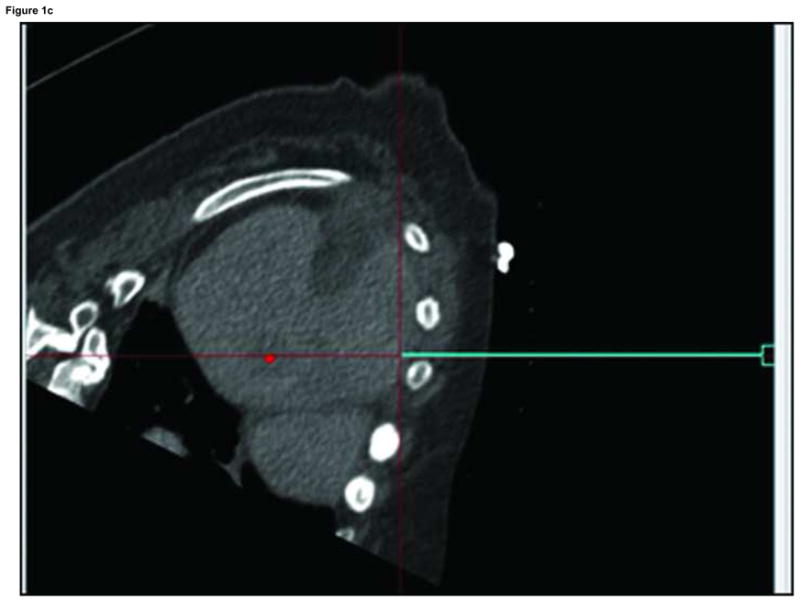
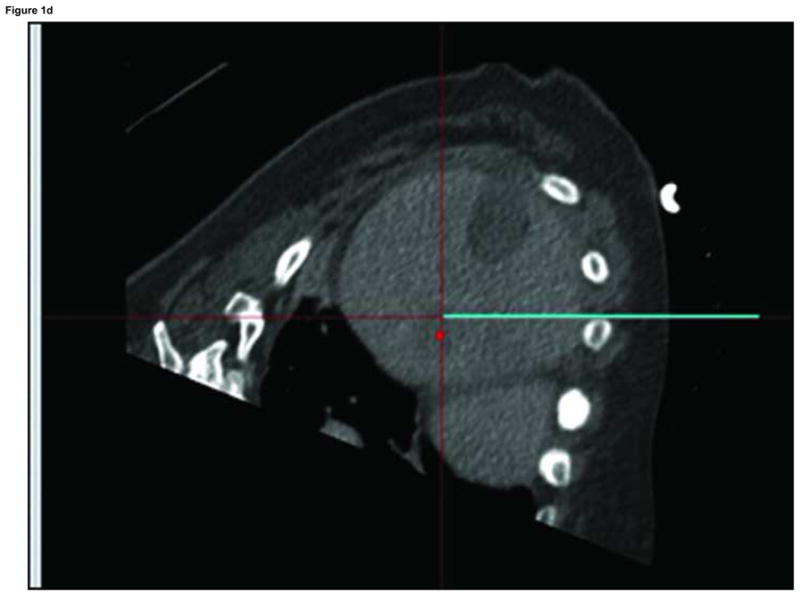
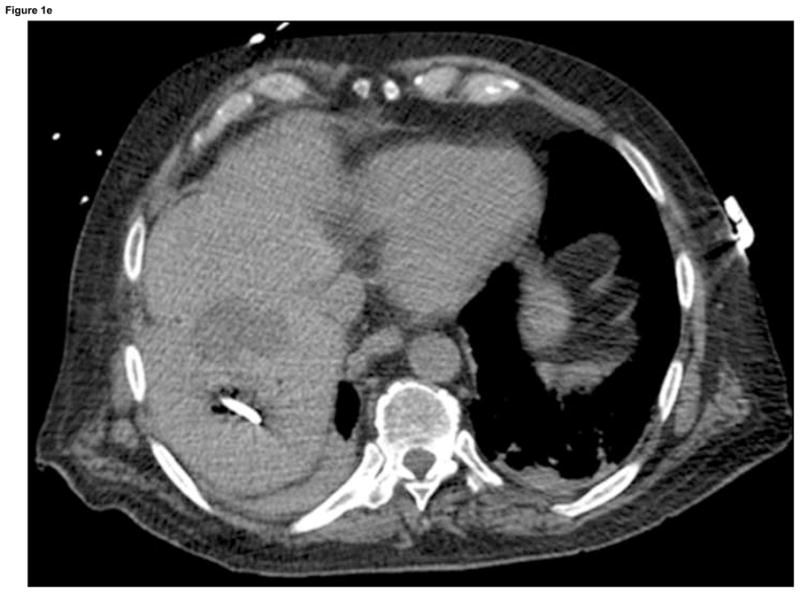
Arterial phase CT (a) showing prior non-enhancing RFA treatments and solitary enhancing hepatocellular carcinoma (arrow). Targeting interface for placement of virtual needle (blue line, b, c, d); Virtual needle in target corresponds with actual needle on CT scan (e)
Steps and Workflow
The sequence of steps and events will be dictated by which modality shows the tumor margin the best as well as operator preferences and patterns of CT and US use for biopsy and ablation. Table 2 shows one of the more common workflows for CT and US guidance (Table 2 “Steps and workflow”). The steps may differ if one is registering with automatic skin patches, or if one is using manual registration with anatomic points (landmark) or scan plane matching (umbilicus or nipples). The umbilicus or nipple level provides rough alignment and the matched anatomic points provide fine-tuning. Specific point matching in the liver often involves the porta hepatis, or identifiable shapes from vessel branching points. Automated registration methods may further simplify the process. The role for verification CT or US is also procedure-dependent. Generally speaking, the navigation tools are usually for augmentation, and often supplement, instead of replace, standard imaging guidance techniques. If using a “dynamic reference sensor” or patch, one can move the field generator box and the patient without altering the registration. If not using this special sensor on the skin, then one must maintain patient and field generator immobile during procedure, and reposition CT table to same working spot (and record Z-axis table number). Staff should be alerted to avoid dislodging the field generator and to exercise caution with regard to sterility.
Table 2.
Steps & Workflow for CT + US guided ablation/biopsy
|
= optional steps
For PET or MR-guidance, select appropriate MR sequence or attenuation-corrected PET. For PET guidance, manually register prior CT (from prior PET/CT) to intra-procedural planning CT using anatomic points (bones and landmarks), then blend in PET data and fade display between PET (or MR), US, and CT as needed (Fig. 4). Keep in mind position and breathing differences, since PET is usually acquired in shallow breathing. There is a wide variety of practice patterns that can accommodate tracking (Table 3).
Table 3.
Methods for tracking during ablation
|
Rotational angiography-based tools
Cone beam CT is the creation of a 23 or 46 cm field of view “CT-like” image from an angiography machine after rotation of the C-arm around the patient. Dyna-CT (Siemens Medical Solutions, Erlangen Germany), Innova-CT (GE Healthcare, Waukesha, Wisconsin, USA) and Xper-CT (Philips Healthcare, Eindhoven, Netherlands) are three such commercially available systems. This method then enables cone beam CT guidance, where targets and needle pathways are planned using this CT-like volumetric information (“X-per guide”, Philips Healthcare, Eindhoven, Netherlands)/“I-guide and I-Guide Cappa”, Siemens Medical Solutions, Erlangen, Germany/“Innova CT” General Electric, Milwaukee, WI). Dynamic 3D roadmapping can provide reference to prior image volumes such as CTA or rotational angiography/CT. The next generation of interventional radiology (IR) suites also integrate methods for 3D navigation either with tracking systems referencing multiple modalities or by enabling spatial information with what was once only a 2D modality (X-ray or ultrasound).. Although the systems each have different software and display formats, the concept remains the same: providing 3D imaging capabilities and information in the IR suite, in hopes of improving or facilitating needle, catheter, wire, or device placement.
For cone beam CT guidance, conventional orthogonal 2D fluoroscopy images are referenced to the rotational angiography or cone beam CT framework to provide guidance for needle based procedures such as ablation, biopsy or vertebroplasty. A skin entry and target define a pathway chosen on the CT-like image from the cone beam CT. Then the needle advancement is monitored incrementally, either in the view 90 degrees orthogonal to the needle or axial to the needle (down the needle shaft or “bird’s eye” view). Each orthogonal viewpoint has the selected pathway superimposed upon the fluoroscopic image, so needle adjustments can be made by comparing actual needle to superimposed planned pathway.
Mechanical laser pointers and needle stabilization devices
Various commercial devices exist for needle stabilization and angle selection including disposable bubble levelers with protractor-like needle guides (In-Rad), disposable adhesive arcs that stabilize the needle (Neo-Rad, Oslo, Norway and Radi) and automated laser pointers that integrate with the CT table (Neo-Rad, Oslo, Norway) or integrate directly with the CT software (NIH, Bethesda, MD & Philips Healthcare, Cleveland, OH). Many of these solutions are low cost devices to facilitate needle placement accuracy, which can be a critical determinant of outcome following ablation. Needle stabilization can also be important when heavy ablation devices are subjected to gravity, flopping down during a CT in a lateral approach.
Clinical Outcomes/Patient Impact
The accuracy, clinical utility, and impact upon patient outcomes is becoming better defined for EM tracking and multi-modality image fusion for guidance of biopsy and thermal ablation procedures. Many procedures are enabled by the technology, where targets would not have been defined without the ability to use arterial phase CT or PET or MR information during the procedure. Specifically, for biopsy and ablation, tracking can improve accuracy in terms of angle selection towards a predetermined point target (Kruecker J, Xu S, Venkatesan A, et al.: Clinical Utility of Real-Time Fusion Guidance for Biopsy and Ablation. J Vasc Interv Radiolo (in press)), and there may be advantages in terms of delivering a prescribed treatment plan or adding together multiple individual ablations in order to fully envelope a tumor with a composite ablation (multiple overlapping ablation volumes).
Clinical use of EM tracking for biopsy and ablation
It may be important to keep in mind that some systems will track the needle tip while others will track the shaft or hub. Tracking the needle tip could theoretically better account for tip deflection or bending, organ deformation, or organ motion.
The ultrasound can be tracked, with or without a tracked needle. If the ultrasound is used alone without a tracked needle, then one must look for needle images on ultrasound in order to reference the actual needle to the target location or prior imaging modality. Tracking the ultrasound and needle are most useful for the co-display of other modalities. There are a broad variety of potential clinical indications for using tracking during biopsy and ablation (Table 4).
Table 4.
When to use tracking during ablation or biopsy:
|
Future Directions
Navigation techniques have initially been applied to facilitate needle placement towards a pre-defined target. Future work will refine integrated ablation treatment plans, in a fashion similar to radiation therapy dose maps. (Figures 2–3 ) Such standardization should decrease the variability of practice patterns, and enable even less experienced operators to deliver precise ablations. The registration process will become fully automated with integrated elastic warping, tissue deformation, breathing and motion compensation. The navigation systems will become increasingly integrated with the imaging systems themselves (embedded in the ultrasound transducer, console, and angiography tables). This will in turn lead to broader ease of use and streamlined workflow. EM tracking treatment plans will identify tissue at risk for under treatment and incorporate imaging such as ultrasound contrast, elastography, MR spectroscopy, and diffusion maps. PET-guided ablation will become a standard tool for defining treatment margins, and the EM-tracked biopsy will become a tool for drug discovery and personalized medicine, selecting and verifying molecular-targeted therapies. Trainees in interventional radiology will learn needle placement skills on EM tracking simulators, instead of patients.
Figure 2.
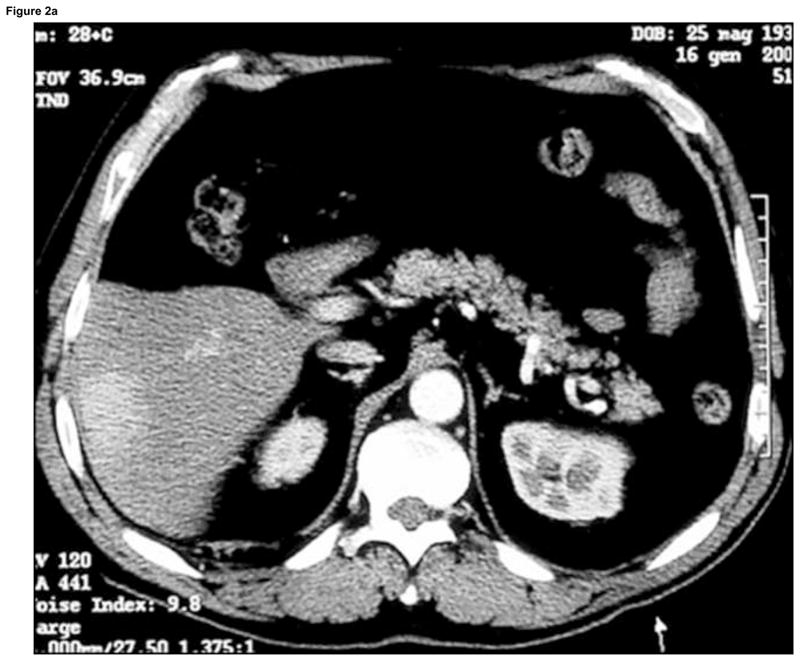
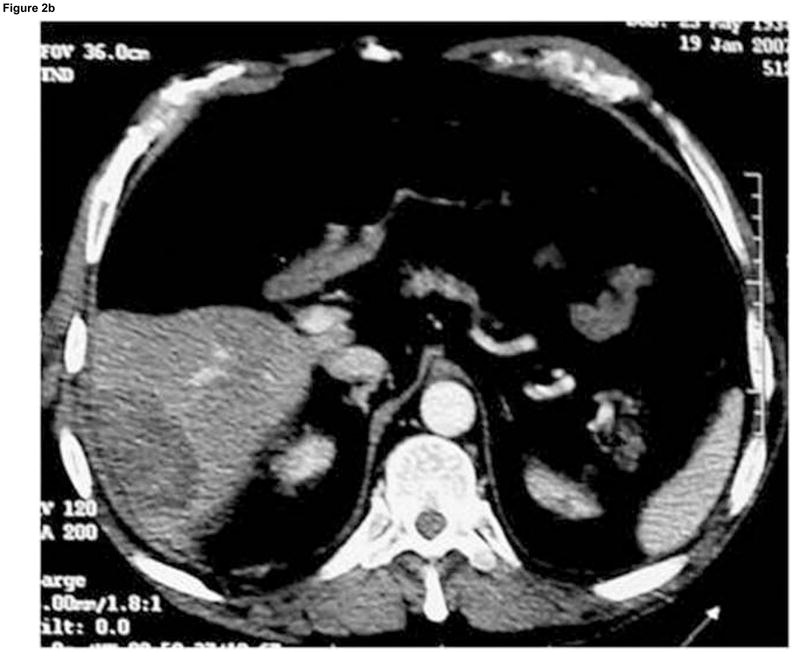
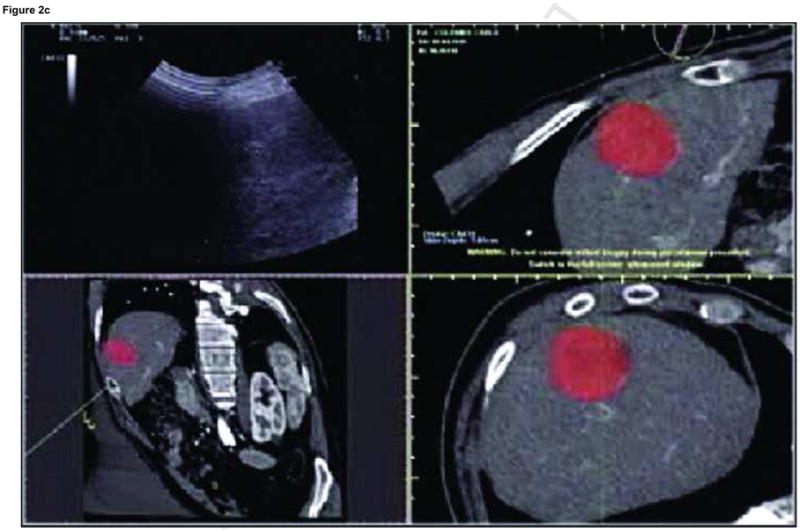
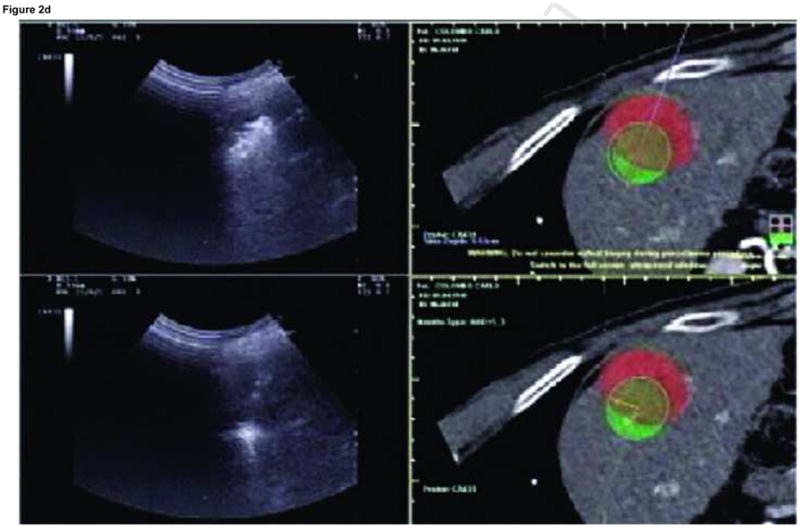
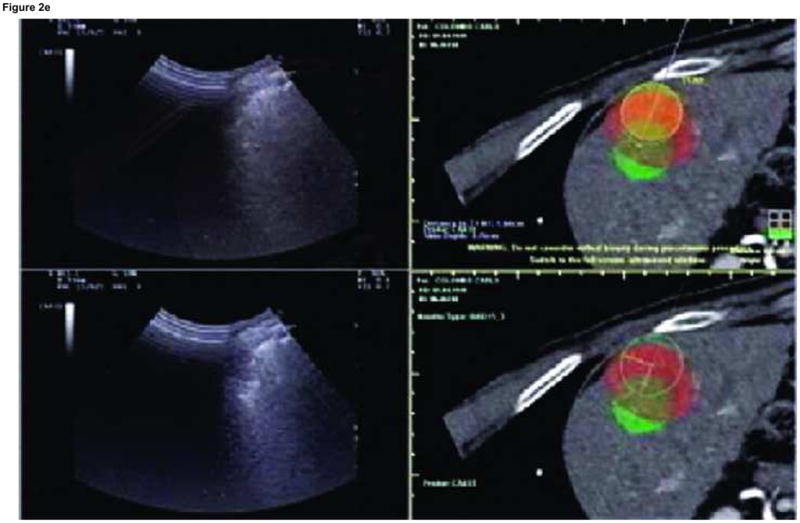
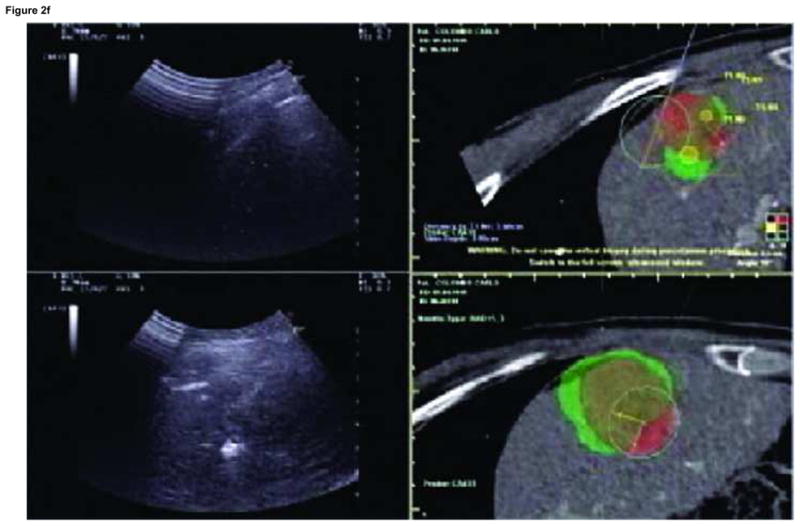
Enhanced CT scan before (a) and after (b) RFA with navigation shows complete treatment of enhancing tumor. Treatment planning sequence (c–f) with sequential overlapping of planned treatment volumes (green) superimposed on estimated tumor location (red) which was manually segmented at the beginning of the procedure. The treatment volume covers the tumor plus a margin of normal tissue (f).
Figure 3.
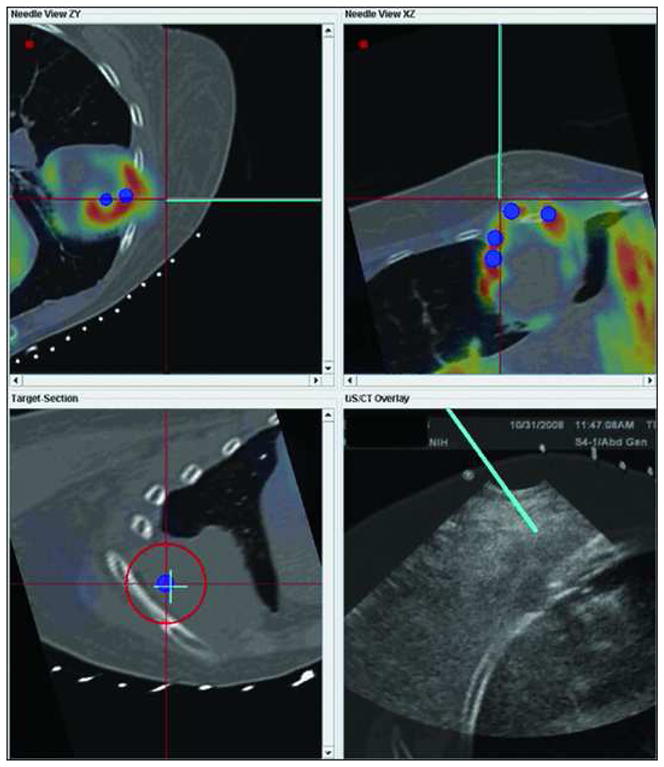
Patient with recurrent tumor and recent non-diagnostic biopsy. Graphical user interface (GUI) showing metabolic activity (blue dots) on PET scan targeted with navigation in order to sample viable part of tumor. PET data was registered to procedural multiplanar reconstructed CT and procedural ultrasound for real time feedback. Virtual needle represented by blue line. Multiplanar/multimodality navigation is displayed as well as down the needle shaft view (lower left).
Conclusion
The use of navigation technology and multi-modality image fusion represents a novel and important means by which to optimize image-guided interventions. Although speculative, improved imaging and navigation tools could potentially reduce procedure time, radiation dose, and complications while enhancing standardization. IR as a discipline is continuously finding new ways to use our foundation in imaging to improve what we do. Navigation tools at the fingertips of the physician have the potential to improve delivery of minimally-invasive image-guided local and regional cancer therapies.
Footnotes
Parts of this paper were discussed and modified from the 2010 Tracking Workshop titled “Navigation Tools & Video Games in IR & Interventional Oncology”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Banovac F, Cheng P, Campos-Nanez E, et al. Radiofrequency ablation of lung tumors in swine assisted by a navigation device with preprocedural volumetric planning. J Vasc Interv Radiol. 2010;21:122–129. doi: 10.1016/j.jvir.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood BJ, Locklin JK, Viswanathan A, et al. Technologies for guidance of radiofrequency ablation in the multimodality interventional suite of the future. J Vasc Interv Radio. 2007;18:9–24. doi: 10.1016/j.jvir.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood BJ, Zhang H, Durrani A, et al. Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol. 2005;16:493–505. doi: 10.1097/01.RVI.0000148827.62296.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaniv Z, Wilson E, Lindisch D, Cleary K. Electromagnetic tracking in the clinical environment. Med Phys. 2009;36:876–892. doi: 10.1118/1.3075829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krucker J, Xu S, Glossop N, et al. Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol. 2007;18:1141–1150. doi: 10.1016/j.jvir.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baegert C, Villard C, Schreck P, Soler L, Gangi A. Trajectory optimization for the planning of percutaneous radiofrequency ablation of hepatic tumors. Comput Aided Surg. 2007;12:82–90. doi: 10.3109/10929080701312000. [DOI] [PubMed] [Google Scholar]
- 7.Banovac F, Abeledo H, Campos-Nanez E, et al. An image-guided system for optimized volumetric treatment planning and execution for radiofrequency ablation of liver tumors. Int J CARS. 2007;2(Suppl 1):S146–S151. [Google Scholar]
- 8.Chen CR, Miga MI, Galloway RL. Optimizing Needle Placement in Treatment Planning of Radiofrequency Ablation. Proceedings SPIE 6141: Progress in Biomedical Optics and Imaging. 2006:6141. [Google Scholar]
- 9.Trovato K, Dalal S, Kruecker J, Venkatesan A, Wood BJ. Automated RFA planning for complete coverage of large tumors. Proc SPIE. 2009:7261. [Google Scholar]
- 10.Villard C, Soler L, Papier N, et al. RF-Sim: a Treatment Planning Tool for Radiogrequency Ablation of Hepatic Tumors. Seventh International Conference on Information Visualization; 2003. pp. 561–566. [Google Scholar]
- 11.Villard C, Soler L, Gangi A. Radiofrequency ablation of hepatic tumors: simulation, planning, and contribution of virtual reality and haptics. Comput Methods Biomech Biomed Engin. 2005;8:215–227. doi: 10.1080/10255840500289988. [DOI] [PubMed] [Google Scholar]
- 12.Crum WR, Hartkens T, Hill DL. Non-rigid image registration: theory and practice. Br J Radiol. 2004;77(Spec No 2):S140–S153. doi: 10.1259/bjr/25329214. [DOI] [PubMed] [Google Scholar]


