Abstract
Neonatal exposure to antidepressants, including selective serotonin reuptake inhibitors such as citalopram, induces behavioral disturbances which persist in mature rats. These disturbances have been proposed to model the symptoms of endogenous depression. However, to date there is scant evidence for the predictive validity of any of these behaviors in response to adult antidepressant treatments. In order to directly assess the predictive validity of the early antidepressant exposure paradigm, the present study examined whether the behavioral abnormalities observed in adult animals exposed as neonates to citalopram can be reversed by adult antidepressant treatment with the prototypic antidepressant, imipramine. As noted earlier, neonatal citalopram exposure robustly increased locomotor activity and impaired male sexual behavior in adult rats. These behavioral changes were reversed following chronic adult imipramine treatment. No such reversal was observed in handled, saline treated rats. The present data support the hypothesis that some of the lasting behavioral abnormalities induced by early antidepressant exposure are sensitive to clinically relevant antidepressant treatments thus adding a measure of predictive validity to this paradigm as a model of these depressive symptoms.
Keywords: Neonatal treatment, Citalopram, Imipramine, Locomotor activity, Sexual behavior
1. Introduction
Neonatal administration of the serotonin reuptake inhibitors (clomipramine, citalopram, zimeldine, LU-10-134C) as well as some other inhibitors of monoamine transport to rodents during the early life period from postnatal day 8 (PN8) to PN21 results in pattern of maladaptive behaviors that are evident long after drug discontinuation and persist into adulthood. These behavioral changes in adult rats include changes in locomotor activity (Hartley et al., 1990; Mirmiran et al., 1983) reduced male sexual activity and competence (Neill et al., 1990), and increased rapid eye movement (REM) sleep time (Mirmiran et al., 1981). In addition, increased ethanol consumption, dysregulation of the hypothalamic–pituitary–adrenal axis, reduced latency to enter the REM sleep phase, reduction of shock induced aggression and, in some cases, increased immobility in the forced swim test have been observed following early antidepressant exposure (reviewed in Maciag et al., 2006).
This constellation of behavioral disturbances following neonatal clomipramine exposure in rats has been proposed as a model of endogenous depression by Vogel and Vogel (1982). They noted that the pattern of abnormalities found in adult rats exposed as neonates to clomipramine resembled elements of the syndrome of human endogenous depression including such changes as decreased sexual, aggressive and pleasure-seeking activities, increased motor activity and REM sleep disturbances (Nelson and Charney, 1981). While neonatal antidepressant treatment appears to have some face validity in terms of the symptoms and signs displayed as a model of depression, data regarding the pharmacological responsiveness of this model (predictive validity) is minimal.
We have recently demonstrated that neonatal treatment with clomipramine and the highly selective serotonin reuptake inhibitor (SSRI), citalopram, produces increased locomotor activity and decreased sexual behavior in adult animals indicating that inhibition of the serotonin transporter is sufficient to induce the behavioral syndrome previously reported by Vogel and coworkers (Vogel and Vogel, 1982; Maciag et al., 2006). The purpose of the present study was to determine whether the lasting behavioral changes in adult animals induced by neonatal exposure to an antidepressant can be reversed or attenuated by chronic adult administration of the prototypic tricyclic antidepressant, imipramine. In the present study we employed neonatal exposure to citalopram, in order to avoid possible confounding effects of earlier generation antidepressants used in other studies.
2. Materials and methods
2.1. Animals and treatment
The male offspring of timed-pregnant Long Evans rats were used in these experiments. All procedures were approved by the UMMC Animal Care and Use Committee and complied with AAALAC and NIH standards. Shortly after delivery (PN1-3) the pups were sexed and the males selected and cross-fostered as necessary to produce litters of 4–5 pups. In order to parallel the earlier studies of Vogel et al., rat pups were injected subcutaneously (s.c.), beginning at PN8 with citalopram (Tocris, Ellisville, MO) at a dose 5 mg/kg or saline in a volume of 0.1 ml twice daily (0600 and 1200 hours). As in previous studies, exposure continued for 14 days until PN21. This dose of citalopram was chosen in order to achieve a brain concentration of citalopram comparable to adult rats and, in the case of serum, to both adult rat and human therapeutic blood levels. Although not presented in this manuscript, these concentrations can be found in Maciag et al., 2006. Each litter included pups from both treatment groups. Pups were weaned at PN28 and housed in groups of 2–3/cage under standard laboratory conditions with ad lib access to food and water. Except for weekly weighing, rats were left undisturbed until PN42. Beginning in young adulthood (PN42), imipramine at a dose 20 mg/kg (in a volume of 1 ml/kg, p.o.) was administered once daily from PN42 and throughout the behavioral testing period in order to maintain active serum and brain drug concentrations. A dose of imipramine in this range (10–20 mg/kg/day) has been shown to reliably active in other animal models of depression such as learned helplessness and chronic mild stress (Monleon et al., 1995; Papp et al., 1996; Sherman et al., 1982). Since multiple-day antidepressant treatment is required to achieve therapeutic results in humans (4–6 weeks) as well predictive activity in well-validated rat models such as learned helplessness (4–5 days) and chronic mild stress (3–5 weeks), treatment with imipramine was begun 18 days prior to the first behavioral test. Controls received an equivalent volume of saline. On days when animals were engaged in behavioral testing, drugs were administered at least 1 h after the conclusion of that day’s testing.
2.2. Behavioral testing
Behavioral testing was conducted on adult rats (≥PN60) during the dark phase of the light:dark cycle. Rats were brought to a sound-attenuated testing room to acclimate for 1 h before each test. At least 7 days were allowed between behavioral tests in order to diminish any possible carryover effects.
2.2.1. Locomotor activity
At PN60 rats were tested for locomotor activity. Rats were placed individually into locomotor activity monitoring units (transparent Plexiglas, 43 cm2 floor, 20 cm walls—Opto-Varimex, Columbus Instruments) under moderate light conditions (300 lx) for 30 min. This system employs infrared detectors at body height (4.5 cm) and at rearing height (12 cm). Each detector consists of 15 sensors in the X- and Y-coordinate planes spaced 2.54 cm apart with a maximum resolution of 1.27 cm. A computer acquisition system recorded horizontal and vertical activity in 5 min epochs. Data were analyzed for time ambulating, zone of activity, distance traveled, stereotypies and rearing.
2.2.2. Sexual behavior
At PN90, each male rat was tested for sexual behavior (Feng et al., 2001; Vogel et al., 1996). Males were placed in a clear Plexiglas observation chamber (45×25×20 cm) for a 10 min adaptation period. The test was initiated by placing a female into the arena with the male. A group of ovariectomized females (stimulus females) were brought into estrous with estradiol benzoate (5 μg, s.c.; 48 and 24 h prior to testing) and progesterone (500 μg, s.c.; 4–6 h prior to testing). Each test lasted 60 min and was conducted under dim red light. Each encounter was videotaped and analyzed for number of mounts, intromissions, ejaculations, latency to first mount and latency to first intromission.
2.3. Statistical analysis
Data analyses were performed using SPSS software (Version 11.5, Chicago, IL). Statistical significance of the motor activity and sexual activity frequency data was determined with two factor analysis of variance (neonatal treatment × adult treatment) followed by Bonferroni-corrected t-test. To analyze latencies to begin mounting and intromission in the sexual activity studies, we employed Kaplan–Meier analysis using Log–Rank statistics.
3. Results
3.1. Locomotor activity
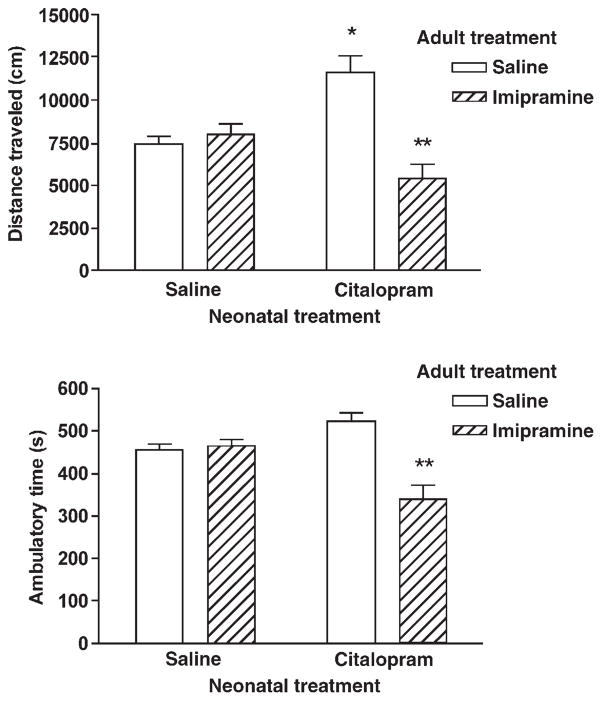
Neonatal citalopram exposure increased locomotor activity in adult rats, when compared to control group (saline). A significant increase in distance traveled and a trend to increase in ambulatory time was observed. Statistical analysis revealed a main effect of adult treatment on distance traveled (F1, 27 = 11.387, P = 0.002) and on ambulatory time (F1, 27 =13.991, P=0.001). Adult chronic imipramine treatment attenuated the locomotor activity in rats neonatally exposed to citalopram but not in control rats, neonatally exposed to saline (Fig. 1).
Fig. 1.
Effect of chronic administration of saline or imipramine on locomotor activity (upper panel-distance traveled; lower panel-ambulatory time) of rats neonatally exposed to saline or citalopram. Data represents the mean ± S.E.M. of 5–14 rats per group. Two factor analysis of variance followed by Bonferroni test: *P<0.05 vs neonatal saline + adult saline; **P<0.01 vs neonatal citalopram + adult saline treatment.
3.2. Sexual behavior
Overall analysis of variance revealed that rats neonatally exposed to citalopram exhibited lower sexual activity including significantly reduced numbers of mounts (F1, 25 = 14.83, P=0.001), intromissions (F1, 25 =8.91, P =0.006) and ejaculations (F1, 25 = 7.32, P = 0.012). Chronic administration of imipramine improved sexual activity in citalopram—but not in saline-treated rats (Fig. 2). Similarly, Kaplan–Meier analysis of mounting behavior (number of mounts and mount latency) showed that neonatal exposure to citalopram disrupted mounting behavior (Log–Rank=10.26, df=1, P=0.0014) and adult imipramine treatment partially but not completely restored this behavior in citalopram exposed rats (Log–Rank=3.70, df=1, P=0.0544). However imipramine treatment in saline exposed rats somewhat impaired mounting behavior (Table 1). Citalopram treated rats also had longer intromission latency than saline rats, but the differences were not significant (data not shown).
Fig. 2.
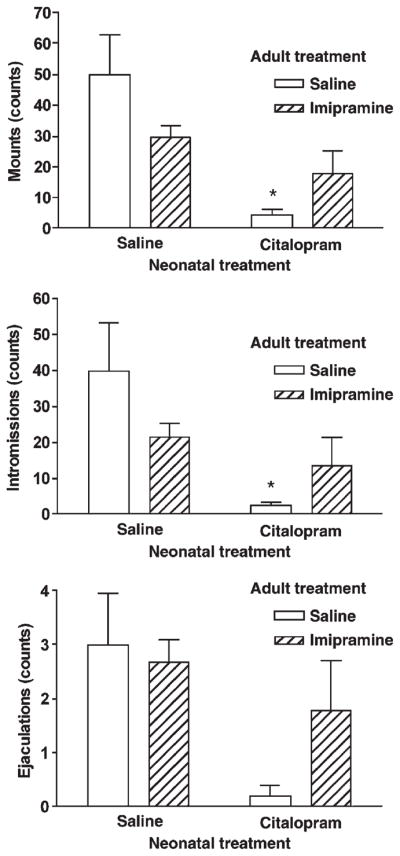
Effect of chronic administration of saline or imipramine on sexual behavior (upper panel-number of mounts; middle panel-number of intromissions; lower panel-number of ejaculations) of rats neonatally exposed to saline or citalopram. Data represents the mean ± S.E.M. of 5–13 rats per group. Two factor analysis of variance followed by Bonferroni test, *P<0.05 vs neonatal saline + adult saline treatment.
Table 1.
Effect of chronic administration of saline and imipramine on mount latency of rats neonatally exposed to saline and citalopram
| Neonatal treatment | Adult treatment | Mount latency (s)±S.E.M. | Failures (%) |
|---|---|---|---|
| Saline | Saline | 39.8±16.5 | 0 |
| Imipramine | 51.1±18.8 | 0 | |
| Citalopram | Saline* | 1532.0±755.0 | 40 |
| Imipramine | 164.4±59.8 | 0 |
Data represents the mean ± S.E.M. of 5–13 rats per group. When mount behavior did not occur during the experiment, the mount latency was considered as 3600 s (observation period). Failures represent the percentage of animals failing to display mounting behavior during the observation period.
Kaplan–Meier (Log–Rank=10.26, df=1, P=0.0014).
3.3. Other behaviors
Other behaviors related to antidepressant activity or hedonic drive (forced swim test and saccharin-sweetened fluid consumption) were not significantly affected either by neonatal antidepressant exposure or adult imipramine treatment (data not shown).
4. Discussion
Because of the face validity of certain behaviors produced by early antidepressant exposure to symptoms of depression, such as increased REM sleep duration and impaired male sexual function, this paradigm was proposed by Vogel et al. as a model of depression. However, this proposal has been criticized due to the fact that neonatal antidepressant exposure results in a large number of long term behavioral responses, not all of which appear analogous to human depressive symptoms. Inasmuch as there is no human literature linking early antidepressant exposure to later depressive symptoms, the model also lacks construct validity as a rodent model of depression. Finally, only one attempt was made by Vogel et al., to demonstrate predictive validity for this paradigm which, while positive, involved a small sample and was not replicated. Very recently, Vazquez-Palacios et al. have reported that adult fluoxetine treatment can reverse the effects of early clomipramine exposure in the forced swim test (Vazquez-Palacios et al., 2005; Vogel et al., 1990).
In our previous study, we have reproduced the findings of Vogel et al. with clomipramine and demonstrated that early exposure to the highly selective serotonin transport inhibitor, citalopram, reliably reproduces behavioral effects previously reported for the much less selective first generation antidepressants (Maciag et al., 2006). These data indicate that the behavioral effects of early antidepressant exposure are likely a consequence of their actions on serotonergic neurons.
This is further supported by our findings revealing profound and long term reductions in both the rate-limiting serotonin synthetic enzyme (tryptophan hydroxylase) in dorsal raphe and in serotonin transporter expression in cortex after neonatal exposure to citalopram and to a lesser extent to clomipramine (Maciag et al., 2006). Furthermore other investigators have reported that neonatal clomipramine exposure results in reductions in basal monoamine concentration and turnover in subcortical regions (striatum, hypothalamus, limbic structures) in adult animals (Feenstra et al., 1996; Hilakivi et al., 1987; Vijayakumar and Meti, 1999; Yannielli et al., 1999). Similarly, animals neonatally exposed to clomipramine display reduced firing of neurons in the dorsal raphe nuclei (Kinney et al., 1997).
To date, our studies have indicated that increased locomotor activity and impaired male sexual behavior are the most reliable behavioral indices of early SSRI exposure. In the present study, we have demonstrated that chronic adult treatment with the prototypic antidepressant, imipramine, can diminish or reduce these effects. These data represent the first demonstration that neonatal SSRI exposure results in lasting behavioral effects which can be reversed with chronic adult antidepressant exposure indicating that this paradigm may have predictive validity as a model of a subset of depressive symptoms.
In line with some (Yoo et al., 2000) but not other (Hansen et al., 1997; Hilakivi and Hilakivi, 1987; Velazquez-Moctezuma and Diaz-Ruiz, 1992) previous studies of the long term behavioral effects of early antidepressant exposure we did not observe reliable increases in immobility in the forced swim test. Moreover, we observed no evidence of hedonic deficits such as reduced consumption of sweetened fluids as previously reported in animal models of depressive symptomatology such as the chronic mild stress paradigm (Papp et al., 1996). Since we have been able to reliably assess these behaviors in other studies (reviewed in Paul, 1997), we do not believe that the inactivity of neonatal antidepressant exposure in these studies represents a technical failure of the studies. However, we note that, unlike earlier studies, the rats employed in the present studies were handled and gavaged on a daily basis throughout the behavioral testing period. It may be that this procedure diminished responsiveness in these two behavioral tests relative to previous studies which handled adult animals only intermittently for the tests themselves.
Several limitations to this study must be acknowledged. In this initial study, only one antidepressant, imipramine, at a single dose, 20 mg/kg/day, was used to test the hypothesis that early SSRI exposure produces behaviors which are responsive to chronic antidepressant treatment. It is not known whether these responses are selective for antidepressants between classes of psychotherapeutic agents. Similarly, the time-course for the effect of adult antidepressant treatment reversal of these behaviors remains to be determined. Finally, of course, these studies do not admit speculation as to the mechanism by which imipramine reverses the behavioral effects of early SSRI exposure. Additional studies will be needed to resolve these issues.
Nonetheless, these data indicate the neonatal exposure to SSRIs produces antidepressant-reversible behaviors in adult rats. Together with our previous data (Maciag et al., 2006), these data suggest that early SSRI exposure produces lasting neurobiological effects that may parallel some symptoms of depression. These data clearly indicate that the long term effects of early antidepressant exposure deserve additional scrutiny.
Acknowledgments
The authors gratefully acknowledge the helpful discussion of this project with Drs. Rick C.S. Lin, Kim L. Simpson and James Shaffery. This publication was supported in part by research funds from the Center for Psychiatric Neuroscience at the University of Mississippi Medical Center which is supported by NIH Grant Number RR-P20 RR17701 from the Institutional Development Award (IDeA) Program of the National Center for Research Resources.
References
- Feenstra MG, van Galen H, Te Riele PJ, Botterblom MH, Mirmiran M. Decreased hypothalamic serotonin levels in adult rats treated neonatally with clomipramine. Pharmacol Biochem Behav. 1996;55:647–652. doi: 10.1016/s0091-3057(96)00276-6. [DOI] [PubMed] [Google Scholar]
- Feng P, Ma Y, Vogel GW. The critical window of brain development from susceptive to insusceptive. Effects of clomipramine neonatal treatment on sexual behavior. Brain Res Dev Brain Res. 2001;129:107–110. doi: 10.1016/s0165-3806(01)00158-4. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Sanchez C, Meier E. Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? J Pharmacol Exp Ther. 1997;283:1333–1341. [PubMed] [Google Scholar]
- Hartley P, Neill D, Hagler M, Kors D, Vogel G. Procedure- and age-dependent hyperactivity in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990;14:69–72. doi: 10.1016/s0149-7634(05)80161-7. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Hilakivi I. Increased adult behavioral ‘despair’ in rats neonatally exposed to desipramine or zimeldine: an animal model of depression? Pharmacol Biochem Behav. 1987;28:367–369. doi: 10.1016/0091-3057(87)90454-0. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, Hilakivi I, Ahtee L, Haikala H, Attila M. Effect of neonatal nomifensine exposure on adult behavior and brain monoamines in rats. J Neural Transm. 1987;70:99–116. doi: 10.1007/BF01252512. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Vogel GW, Feng P. Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: implications for endogenous depression. Brain Res. 1997;756:68–75. doi: 10.1016/s0006-8993(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RCS, Paul IA. Neonatal antidepressant exposure has lasting effects on behaviour and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran M, van de Poll NE, Corner MA, van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Scholtens J, van de Poll NE, Uylings HB, van der Gugten J, Boer GJ. Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Brain Res. 1983;283:277–286. doi: 10.1016/0165-3806(83)90184-0. [DOI] [PubMed] [Google Scholar]
- Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- Neill D, Vogel G, Hagler M, Kors D, Hennessey A. Diminished sexual activity in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990;14:73–76. doi: 10.1016/s0149-7634(05)80162-9. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Charney DS. The symptoms of major depressive illness. Am J Psychiatry. 1981;138:1–13. doi: 10.1176/ajp.138.1.1. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl M, Willner P. Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol. 1996;296:129–136. doi: 10.1016/0014-2999(95)00697-4. [DOI] [PubMed] [Google Scholar]
- Paul IA. NMDA receptors and affective disorders. In: Skolnik P, editor. Antidepressants. New Pharmacological Strategies. Humana Press; Totowa, New Jersey: 1997. pp. 145–158. [Google Scholar]
- Sherman AD, Sacquitne JL, Petty F. Specificity of the learned helplessness model of depression. Pharmacol Biochem Behav. 1982;16:449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant effects of nicotine and fluoxetine in an animal model of depression induced by neonatal treatment with clomipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:39–46. doi: 10.1016/j.pnpbp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Velazquez-Moctezuma J, Diaz-Ruiz O. Neonatal treatment with clomipramine increased immobility in the forced swim test: an attribute of animal models of depression. Pharmacol Biochem Behav. 1992;42:737–739. doi: 10.1016/0091-3057(92)90022-8. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M, Meti BL. Alterations in the levels of monoamines in discrete brain regions of clomipramine-induced animal model of endogenous depression. Neurochem Res. 1999;24:345–349. doi: 10.1023/a:1020992314534. [DOI] [PubMed] [Google Scholar]
- Vogel G, Vogel FA. A new animal model of human endogenous depression. Sleep Res. 1982;11:222a. [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- Vogel G, Hagler M, Hennessey A, Richard C. Dose-dependent decrements in adult male rat sexual behavior after neonatal clorimipramine treatment. Pharmacol Biochem Behav. 1996;54:605–609. doi: 10.1016/0091-3057(95)02276-7. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Kargieman L, Gregoretti L, Cardinali DP. Effects of neonatal clomipramine treatment on locomotor activity, anxiety-related behavior and serotonin turnover in Syrian hamsters. Neuropsychobiology. 1999;39:200–206. doi: 10.1159/000026584. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Bunnell BN, Crabbe JB, Kalish LR, Dishman RK. Failure of neonatal clomipramine treatment to alter forced swim immobility: chronic treadmill or activity-wheel running and imipramine. Physiol Behav. 2000;70:407–411. doi: 10.1016/s0031-9384(00)00261-4. [DOI] [PubMed] [Google Scholar]