Abstract
Rationale
Chronic opiate administration induces neuro-adaptations within the nucleus accumbens (NAc) and ventral tegmental area (VTA) that can contribute to dependence. We have shown that morphine dependence shifts the behavioral consequences of D1 dopamine (DA) receptor signaling: systemic administration of a D1 receptor agonist is rewarding and blocks naloxone-precipitated withdrawal signs in morphine-dependent rats, but has minimal effects in nondependent rats. These data suggest that D1 receptors acquire the ability to regulate reward and withdrawal in morphine-dependent rats. The brain regions involved in these effects are not known.
Objective
Studies were designed to test the hypothesis that the nucleus accumbens shell (NASh) and the ventral tegmental area (VTA) are important sites for mediating the behavioral effects of D1 receptor activation in morphine-dependent rats.
Materials and methods
The effects of microinjecting the D1 receptor agonist SKF 82958 into the NASh or the VTA on place conditioning and somatic withdrawal signs were studied in morphine-dependent and nondependent rats.
Results
Intra-NASh microinjection of SKF 82958 (1 μg/side) established conditioned place preferences in morphine-dependent but not nondependent rats, but had no effect on naloxone-induced place aversions or somatic withdrawal signs. Intra-VTA microinjection of SKF 82958 (2 μg) did not establish place preferences under any conditions, but blocked naloxone-induced place aversions without effects on somatic withdrawal signs.
Conclusions
There is an anatomical dissociation between D1 receptor-mediated reward and relief of withdrawal in morphine-dependent rats. When combined, the individual effects of D1 receptor activation in the NASh and VTA on the affective signs of precipitated morphine withdrawal resemble those seen with systemic administration.
Keywords: Nucleus accumbens, Ventral tegmental area, Place conditioning, SKF 82958, SKF 81297, Somatic withdrawal
Introduction
Opiates such as morphine are initially abused because of their rewarding effects (Wise 1989). This facilitates continued drug taking, often leading to dependence (Kreek and Koob 1998; Nestler 1992; Wise 1996). Opiate dependence is characterized by a withdrawal syndrome, which includes both somatic and affective symptoms, with dysphoria, anxiety, and depression characterizing the latter (Kreek and Koob 1998). Avoidance and alleviation of aversive withdrawal symptoms may become a primary motivation for compulsive drug taking (Koob et al. 1989; Kreek and Koob 1998), making it imperative to understand the neurobiological mechanisms underlying dependence.
Morphine acts at mu-opioid receptors (MORs) found throughout the brain, including in the nucleus accumbens (NAc) and ventral tegmental area (VTA). These mesolimbic regions are particularly critical for the rewarding and dependence-inducing effects of morphine, as well as other drugs of abuse (Wise and Rompre 1989). Morphine increases DA release in the NAc (Di Chiara and Imperato 1988). Rats will self-administer opiates directly into the VTA (Bozarth and Wise 1983), which contains dopaminergic cell bodies, and into the NAc (Olds 1982), which receives dopaminergic input from the VTA. The mesolimbic system is also important for morphine dependence: microinjections of naloxone into the NAc cause conditioned place aversions (Koob et al. 1992), and administration of a DA D2 receptor agonist directly into the NAc attenuates somatic withdrawal signs (Harris and Aston-Jones 1994). Also, DA release is dramatically decreased in the NAc during morphine withdrawal (Rossetti et al. 1992).
There is increasing evidence suggesting that the roles of DA receptors in reward processes depend upon prior drug history. DA acts at D1 and D2 receptors, which are distinguished by their abilities to stimulate (D1) or inhibit (D2) adenylate cyclase (for review, see Missale et al. 1998). In the NAc, D1 receptors and MORs tend to colocalize postsynaptically (Georges et al. 1999), whereas in the VTA, D1 receptors appear to be presynaptically localized (Kalivas and Duffy 1995; Mansour et al. 1992). It has been shown that the D1 receptor agonist SKF 82958 blocks cocaine self-administration and cocaine-induced reinstatement behavior in rats that have been extensively trained to self-administer cocaine (Self et al. 1996). Thus, in cocaine-experienced animals, activation of D1 receptors either has aversive, anti-cocaine properties, or has rewarding, satiety-inducing properties that reduce the drive to self-administer cocaine. Opiate reward is DA-independent in drug-naïve animals but is DA-dependent in opiate-dependent animals, likely due to a switch in GABAA receptor function in the VTA (Bechara et al. 1998; Laviolette et al. 2004). D1 receptor activation results in increased GABA release from NAc projection neurons in the VTA in drug-naïve animals but decreased GABA release in cocaine- and morphine-dependent animals (Bonci and Williams 1996). Together, these findings suggest a dependence-mediated shift in DA receptor function and raise the possibility that DA signaling might play a critical role in drug craving and relapse, conditions that occur with dependence and withdrawal.
Consistent with the idea that prior drug history regulates the behavioral impact of DA receptor stimulation, we previously found that systemic administration of SKF 82958 elicits place preferences in morphine-dependent, but not nondependent, rats (Chartoff et al. 2006), suggesting that D1 receptor sensitivity within reward circuits is increased as a result of morphine exposure. SKF 82958 blocks somatic and affective signs of naloxone-precipitated withdrawal, suggesting that decreased D1 receptor activation contributes to the expression of the withdrawal syndrome. The neuroanatomical substrates for these effects are not known. Considering that morphine dependence triggers adaptive changes within the mesolimbic system that can contribute to anhedonia-like states upon drug cessation (Williams et al. 2001), and that morphine withdrawal results in decreased mesolimbic DA release (Rossetti et al. 1992), we hypothesized that both the NAc and VTA would be important for mediating D1 receptor agonist-induced reward and withdrawal relief in morphine-dependent rats. To test this hypothesis, we made rats morphine dependent via subcutaneous implantation of morphine pellets and tested the effects of intra-NAc or intra-VTA microinjections of SKF 82958 on affective and somatic measures of naloxone-precipitated withdrawal. We focused on the NAc shell (NASh) because our previous work has demonstrated that this region plays a critical role in DA-dependent drug reward (Carlezon et al. 1995; Carlezon and Wise 1996) and because affective signs of morphine withdrawal are associated with robust activation of c-fos in the NASh but not the core (Frenois et al. 2002; Gracy et al. 2001).
Materials and methods
Animals and surgery
A total of 355 male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used in this study. Rats weighed 325–375 g at the time of the experiments and were housed individually after surgery on a 12-h light/dark (0700–1900 hours) cycle with ad libitum access to food and water except during testing. For studies with systemic administration of D1 agonists, 129 rats were used. For implantation of intracranial cannulae, 226 rats were used. These rats were anesthetized with intraperitoneal (IP) pentobarbital (65 mg/kg) supplemented with subcutaneous atropine (.25 mg/kg) to minimize bronchial secretions. Bilateral guide cannulae (26-gauge) were aimed 1 mm above the ventral NASh (1.5 mm anterior to bregma, ±1.0 mm from the midline, and 5.9 mm below dura) and unilateral guide cannulae (26-gauge) that were angled 10° from the midline were aimed 1 mm above the VTA (final coordinates: 5.3 mm posterior to bregma, 0.5 mm lateral to the midline, and 6.7 mm below dura). For the VTA, unilateral microinjections were used due to the close proximity of the VTA to the midline, as described (Bozarth 1987). Cannulae were implanted and affixed to the skull with four skull screws and dental cement. This enabled microinjections into an area corresponding to the intermediate zone of the NASh (Todtenkopf and Stellar 2000) and throughout the rostral–caudal extent of the VTA (~4.8–6.0 mm posterior to bregma). Because drug microinjections have a tendency to travel up the guide cannula track (Wise and Hoffman 1992), we also included a control group (dorsal NASh control) in which bilateral guide cannulae were implanted along the same trajectory as those in the NASh, but aimed above in the dorsal nucleus accumbens shell (vertex) and lateral septum (1.5 mm anterior to bregma, ±1.0 mm from the midline, and 4.6 mm below dura). Likewise, the dorsal VTA control group had unilateral guide cannulae implanted at 5.3 mm posterior to bregma, 0.5 mm lateral to the midline, and 5.4 mm below dura). Stainless steel obturators (33-gauge) extended 1.0 mm beyond the guide cannula.
Drugs
Naloxone HCl, chloro-APB hydrobromide (SKF 82958), and (±)-6-Chloro-PB hydrobromide (SKF 81297) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Naloxone was dissolved in 0.9% saline (NaCl), and SKF 82958 and SKF 81297 were dissolved in ultrapure distilled water. For systemic experiments, naloxone, SKF 82958, and SKF 81297 were administered subcutaneously (SC) to rats in a volume of 1 ml/kg. Low (0.01 mg/kg) or intermediate (0.1 mg/kg) doses of naloxone were chosen based on our previous work showing a dose-dependent dissociation between affective and somatic signs of morphine withdrawal (Chartoff et al. 2006). For intracranial experiments, a total of 2 μg SKF 82958 was administered into either the NASh or VTA. SKF 82958 (2 μg/μl) was microinjected bilaterally into the NASh (1 μg/side in 0.5 μl) or unilaterally into the VTA (2 μg in 1.0 μl), and naloxone was administered SC. We used a single VTA injection because this nucleus is relatively small and is located medially (Bozarth 1987). The dose range of SKF 82958 was chosen based on our prior work with systemic SKF 82958 administration (Chartoff et al. 2006) and earlier microinjection experiments (Swanson et al. 1997). Placebo and morphine pellets (75 mg morphine base/pellet) were obtained from the National Institute of Drug Abuse (Bethesda, MD, USA). Rats were made dependent on morphine by subcutaneous implantation of two morphine pellets (or two placebo pellets for control) while under isoflurane anesthesia, as described (Gold et al. 1994). The wounds were closed with two stainless-steel skin clips, and a triple-antibiotic ointment was applied.
Place conditioning
For place conditioning studies, an unbiased, three-compartment place conditioning apparatus (Med Associates, St. Albans, VT, USA) was used in which each compartment differed in floor texture (metallic rods or mesh), wall coloring (black or white), and lighting (dim or bright intensity), as described previously (Carlezon 2003). On day 0, rats were allowed to freely explore all three compartments for 30 min to test for inherent bias for any of the three compartments (“screening”). Rats that showed strong a priori preferences (≥18 min) for a compartment during the screening period were eliminated from the study. The remaining rats (≥85%) were used for place conditioning experiments. For the study examining the effects of systemic administration of D1 receptor agonists on place conditioning, the potent D1 receptor agonist SKF 82958 and the selective agonist SKF 81297 were used (Andersen and Jansen 1990). Rats were implanted subcutaneously with either morphine or placebo pellets, and 3 days after pellet implantation, when the rats were morphine dependent (Gold et al. 1994), they were conditioned in two separate sessions, as described previously (Carlezon 2003). In the morning (10:00 A.M.), rats received a SC injection of 0.9% saline and were confined to one side of the place conditioning apparatus for 1 hr. In the afternoon (2:00 P.M.), rats received a SC injection of a D1 agonist or naloxone alone, or a cocktail of D1 agonist and naloxone and were confined to the other side chamber for 1 h. The drug-associated compartment was counterbalanced among rats to eliminate group bias in initial preferences (Carlezon 2003). For the studies examining the effects of intra-NASh and intra-VTA administration of SKF 82958, rats underwent surgery for implantation of intracranial (IC) cannulae on day 1, after screening. Rats were implanted subcutaneously with either morphine or placebo pellets 5 or 6 days after surgery. Three days after pellet implantation, they were conditioned in two separate sessions, as described above. In the morning (10:00 A.M.), rats received an IC microinjection of water followed immediately by a SC injection of 0.9% saline and were confined to one side of the place conditioning apparatus for 1 h. In the afternoon (2:00 P.M.), rats received IC microinjections of water or SKF 82958 (NASh and dorsal NASh control, 1.0 μg/side; VTA and dorsal VTA control, 2.0 μg) followed by a SC injection of saline or naloxone (0.01 mg/kg) and were confined to the other side of the chamber for 1 h.
IC microinjections were administered using a syringe pump and 10-μL Hamilton syringes connected to polyethylene (PE) tubing, which was fitted to an injector stylette (33-gauge; Plastics One) extending 1.0 mm beyond the end of the cannula. All animals received infusions at a rate of 0.2 μl/min. Rats with bilateral cannulae in the NASh or dorsal NASh control received 0.5 μl/side over 2.5 min. Rats with unilateral cannulae in the VTA or dorsal VTA control received 1.0 μl over 5.0 min. Each infusion was followed by a 3-min period to allow for diffusion of drug before removing injector stylettes.
For both the systemic and IC D1 agonist studies, the rats were allowed to freely explore all three compartments in a 30-min “test” session (12:00 P.M.) the day after conditioning. Data are expressed as the following: [time spent in the drug-paired side minus time spent in the saline side after conditioning (testing)] minus [time spent in the drug-paired side minus time spent in the saline side before conditioning (screening)]. Data were analyzed using a three-way analysis of variance (ANOVA) [dependence×treatment×conditioning] with repeated measures on conditioning (before vs after conditioning). Significant effects were followed by simple main effects tests and post hoc Fisher’s protected t tests.
Somatic withdrawal
Two days after place conditioning testing (6 days after morphine or placebo pellet implantation), a subset of rats was used for somatic withdrawal testing. Each rat was tested multiple times over a 6-day period in response to one of the following treatment conditions: IC water and SC naloxone (0.1 mg/kg), IC SKF 82958 (NASh, 1.0 μg/side; VTA, 2 μg) and SC saline, or IC SKF 82958 and SC naloxone, administered in random order. This experimental design is similar to that used previously for examination of the effects of systemically administered SKF 82958 on somatic withdrawal (Chartoff et al. 2006). The last somatic withdrawal test was conducted 11 days after pellet implantation. Prior studies have shown stable plasma levels of morphine between 3 and 12 days after SC implantation of two morphine pellets (Gold et al. 1994). On the first day of the somatic withdrawal procedure, rats were habituated to the scoring environment; clear, 65-cm tall by 25-cm diameter Plexiglas cylinders that contained a small amount of bedding in a quiet, temperature-maintained (20°C) room. Rats were placed in the cylinders for 15 min, injected SC with vehicle (0.9% saline) and returned to the cylinders for another 15 min. The next day, an experimental treatment was administered. The rats were placed in the cylinders for 15 min and then given an intracranial microinjection of either SKF 82598 or ultrapure water (as described above). Rats were placed back in the cylinders, 5 min later, injected SC with either saline or naloxone (0.1 mg/kg), and returned to the cylinder during which time somatic withdrawal behaviors were scored for 15 min by an observer who was unaware of the treatments. The following behaviors were marked as either present or absent: diarrhea, ptosis, chromodacyrrhea (reddish-brown discharge from the eyes or nose), penile erection, and irritability to touch. The frequency of the following behaviors was quantified: jumping, cage crossing, rearing, digging, flat posture, wet dog shakes, grooming, and teeth chattering. This protocol is a modification of that described previously (Punch et al. 1997). Somatic withdrawal behaviors are reported for individual behaviors and for the total of all behaviors. Data are reported as the sum of the presence of, or the total occurrences of, the behaviors during the 15-min scoring period. The goal of our experimental design was to have a within-subjects design so that each rat received every treatment. However, we were occasionally forced to deviate from this design due to cannulae becoming blocked. Data were analyzed using a three-way ANOVA (dependence×treatment×withdrawal sign) with repeated measures on withdrawal sign. Significant effects were followed by simple main effect tests and post hoc Fisher’s protected t tests.
Histology
After testing, rats were overdosed with pentobarbital (130 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The brains were removed from the skull and immersed in the fixative for 20 h at 4°C. After fixation, the brains were stored for 4 days in 20% glycerol, 0.1 M phosphate-buffered saline (pH 7.4), and then, 40-μm sections were cut through the NAc and VTA for histological analysis of cannula placements. Sections through the NAc were cresyl violet stained, and sections through the VTA were processed for immunohistochemistry as described (Chartoff et al. 2008) with an antibody directed against tyrosine hydroxylase (TH; rabbit anti-TH, Chemicon, 1:5,000) to visualize the extent of the VTA. Only the rats in which the microinjections were targeted appropriately were included in analyses.
Results
D1 receptor agonists induce place preferences and block naloxone-induced place aversions in morphine-dependent rats
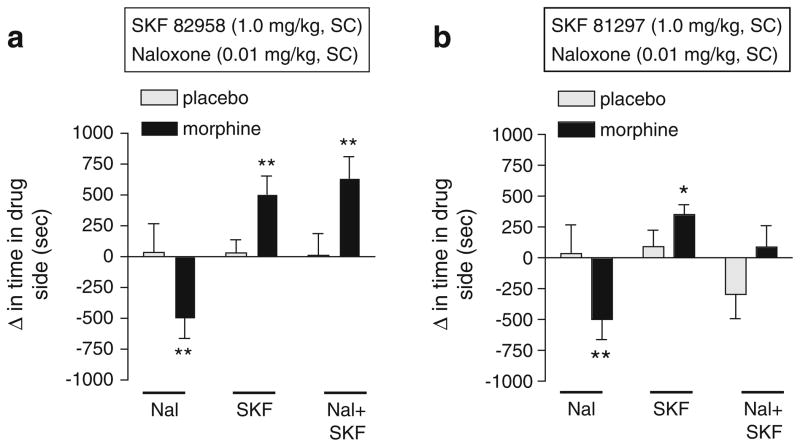
We previously demonstrated that systemic administration of SKF 82958 dose-dependently induces conditioned place preferences in morphine-dependent rats, with no significant effect in nondependent rats (Chartoff et al. 2006). In that study, all doses of SKF 82958 (0.1–1.0 mg/kg, IP) blocked naloxone-induced place aversions in morphine-dependent rats. To examine whether these effects are specific to SKF 82958 or can be generalized to other D1 receptor agonists, we compared the effects of SKF 82958 with SKF 81297, a more selective but slightly less potent D1 receptor agonist (Andersen and Jansen 1990), on place conditioning in morphine-dependent and withdrawn rats. The effects of SKF 82958 (1.0 mg/kg) on place conditioning depended on interactions among treatment, dependence, and conditioning (F(2,58) =6.39, p <0.005; Fig. 1a). Simple main effects tests reveal that a low dose of naloxone (0.01 mg/kg), which elicits affective but not somatic withdrawal signs (Chartoff et al. 2006), induced significant place aversions (p<0.01). Furthermore, SKF 82958 induced significant place preferences in morphine-dependent rats, and combined administration of SKF 82958 with naloxone induced significant place preferences (ps<0.01). Both naloxone and SKF 82958, as well as their combination, were motivationally neutral in nondependent rats. The effects of SKF 81297 on place conditioning depended on interactions among treatment, dependence, and conditioning (F(2,54) =5.18, p< 0.01; Fig. 1b). Simple main effects tests reveal that SKF 81297 induced significant place preferences in morphine-dependent rats (p<0.05), and combined administration of SKF 81297 with naloxone was motivationally neutral, suggesting that activation of D1 receptors blocked naloxone-induced place aversions. We tested higher doses of SKF 81297 (3 and 10 mg/kg) in combination with naloxone in a small number of morphine-dependent rats and did not observe place preferences (data not shown), suggesting that the difference in the effects of SKF 82958 and SKF 81297 are not due to drug dose.
Fig. 1.
The effects of the D1 receptor agonists SKF 82958 and SKF 81297 on place conditioning in morphine-dependent (black bars) and nondependent (gray bars) rats. Rats were implanted with placebo or morphine pellets and, 3 days later, underwent place conditioning with either a naloxone (0.01 mg/kg, SC), SKF 82958 (1.0 mg/kg, SC), or a mixture of naloxone and SKF 82958; or b naloxone (0.01 mg/kg, SC), SKF 81297 (1.0 mg/kg, SC), or a mixture of naloxone and SKF 81297. Data are expressed as the change in time spent in the drug-paired side after conditioning minus the time spent in the drug-paired side before conditioning (means+SEM). Significant changes in time spent in the drug-paired chamber after conditioning compared to before conditioning are: *p<0.05, **p<0.01, Fisher’s protected t tests, 8–13 rats per group. Nal naloxone, SKF SKF 82958 or SKF 81297 (see panel heading)
Intra-NASh microinjections of SKF 82958 induce place preferences in morphine-dependent rats but do not affect naloxone-induced place aversions or somatic withdrawal signs
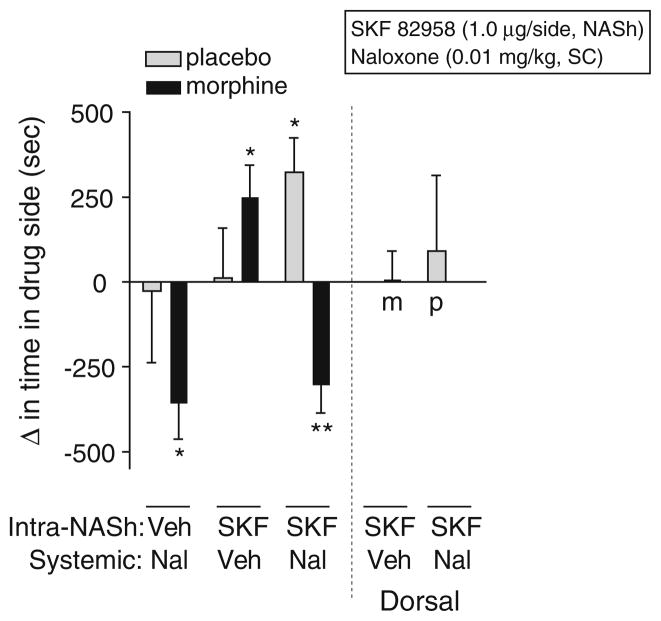
To test the hypothesis that the NASh is a substrate for D1 receptor-mediated reward and withdrawal relief in morphine-dependent rats, we conditioned rats in the place-conditioning apparatus with a single intra-NASh microinjection of SKF 82958 (1 μg/side) just prior to systemically administering a low dose of naloxone (0.01 mg/kg, IP) known to selectively precipitate affective signs of morphine withdrawal. We found that the effects of intra-NASh SKF 82958 depended on interactions among treatment, dependence, and conditioning (F(2,87) =7.08, p<0.002; Fig. 2). Simple main effects tests reveal that SKF 82958 induced significant place preferences in morphine-dependent (p< 0.05), but not nondependent, rats. Intra-NASh microinjections of SKF 82958 did not affect naloxone-induced place aversions in morphine-dependent rats (p<0.01). However, co-administration of intra-NASh SKF 82958 and systemic naloxone induced significant place preferences in nondependent rats (p<0.05). To ensure that the effects of SKF 82958 on place conditioning were due to drug action in the NASh rather than to nonspecific spread of drug up the cannula track, we performed dorsal control microinjections of SKF 82958 and found no effect on place conditioning in morphine-dependent rats and no effect in nondependent rats treated with naloxone (0.01 mg/kg, IP; Fig. 2).
Fig. 2.
Effects of intra-nucleus accumbens shell (NASh) microinjections of SKF 82958 on place conditioning in morphine-dependent (black bars) and nondependent (gray bars) rats. Rats with bilateral cannulae in the NASh or just dorsal to the NASh were implanted with placebo or morphine pellets and, 3 days later, underwent place conditioning with systemic naloxone (0.01 mg/kg, SC), intracranial SKF 82958 (1 μg/side, intra-NASh or dorsal control), or a combination of intracranial SKF 82958 and systemic naloxone. Data from bilateral microinjections of SKF 82958 into dorsal control regions are shown to the right of the dashed line. Data are expressed as the change in time spent in the drug-paired side after conditioning minus the time spent in the drug-paired side before conditioning (means+SEM). Significant changes in time spent in the drug-paired chamber after conditioning compared to before conditioning are: *p< 0.05, **p<0.01, Fisher’s protected t tests, 7–21 rats per group. Nal naloxone, SKF SKF 82958, Veh vehicle, m morphine, p placebo
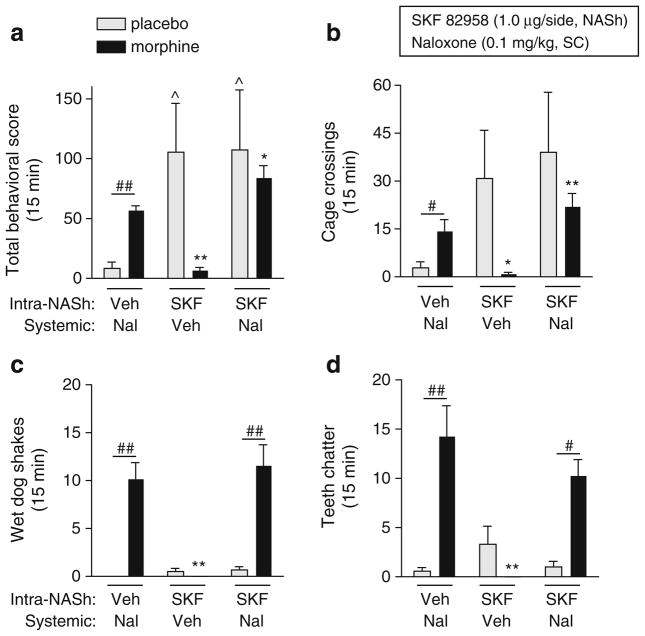
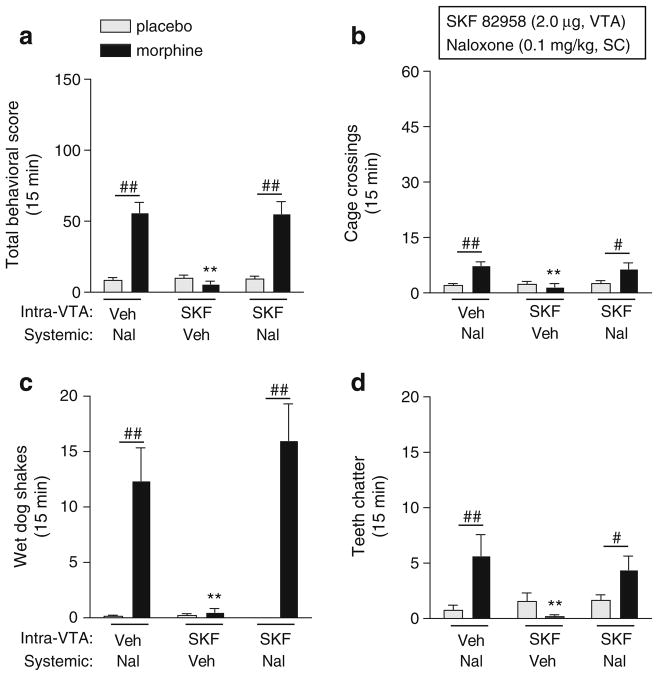
We found that the effects of SKF 82958 on somatic withdrawal signs depended on interactions among treatment, dependence, and withdrawal sign (F(6,120)=5.98, p<0.0001; Fig. 3a–d). Simple main effects tests reveal that a moderate dose of naloxone (0.1 mg/kg) significantly increased total withdrawal signs (sum of all measured signs; p<0.01), cage crossings (p<0.05), wet dog shakes (p<0.01), and teeth chatter (p<0.01) in morphine-dependent rats compared to nondependent rats. In nondependent rats, SKF 82958 increased total withdrawal signs (p<0.05; Fig. 3a) and tended to increase cage crossings (Fig. 3b) and teeth chatters (Fig. 3d). In morphine-dependent rats, SKF 82958 on its own did not induce any withdrawal signs, but significantly potentiated naloxone-induced total withdrawal signs (p< 0.05; Fig. 3a). This effect can be attributed to a D1 receptor-mediated increase in motor activity, since SKF 82958 potentiated naloxone-induced cage crossings (p<0.01; Fig. 3b), but not wet dog shakes (Fig. 3c), teeth chatters (Fig. 3d), or other nonmotor withdrawal signs (data not shown) in morphine-dependent rats. Furthermore, SKF 82958 increased cage crossings (p<0.05) but not wet dog shakes or teeth chatters in nondependent rats. Although the differences in the effects of SKF 82958 on somatic withdrawal behaviors between nondependent and morphine-dependent rats were not significant, it is evident that activation of D1 receptors in the NASh of morphine-dependent rats has a general inhibitory (rate-decreasing) effect on somatic behaviors.
Fig. 3.
Effects of intra-nucleus accumbens shell (NASh) microinjections of SKF 82958 on naloxone-induced somatic withdrawal signs in morphine-dependent (black bars) and nondependent (gray bars) rats. On test days, rats were administered bilateral intra-NASh microinjections of vehicle (water) or SKF 82958 (1 μg/side in 0.5 μl) followed 5 min later by systemic naloxone (0.1 mg/kg, SC) or vehicle (0.9% saline), and somatic withdrawal signs were scored for 15 min. a Total incidence of withdrawal behaviors, b cage crossings, c wet dog shakes, d teeth chattering were scored. Data are expressed as the mean number of incidences of the respective behavior (+SEM). Significant effects are *p<0.05, **p<0.01 compared to morphine-dependent plus naloxone, ^p<0.05 compared to placebo plus naloxone, #p<0.05, ##p< 0.01 comparing groups under the bars, Fisher’s protected t tests, 4–13 rats per group
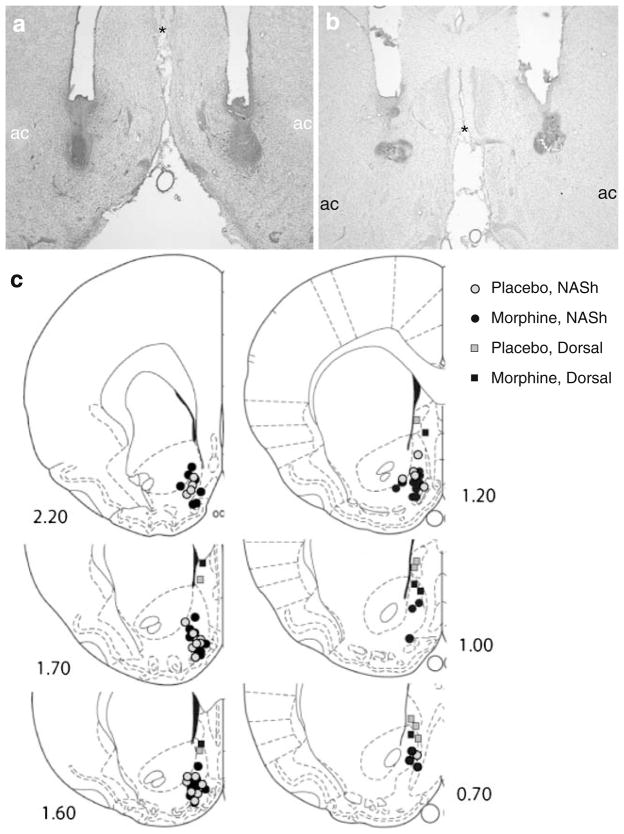
Injection sites targeting the ventral NASh were localized in the intermediate zone, at or below the level of the anterior commissure (AC; Fig. 4a, c). Injection sites targeting dorsal regions were localized along the same trajectory, but above the AC, in an area that includes the vertex of the NASh and the lateral septum (Fig. 4b, c). Nissl staining reveals minimal tissue damage at the injection sites (Fig. 4a, b).
Fig. 4.
Histological assessment of cannula placements within the nucleus accumbens. Representative Nissl-stained coronal sections from rats that received microinjections of SKF 82958 into the ventral NASh (a) and into more dorsal regions along the same trajectory (vertex of the nucleus accumbens/lateral septum; b). Individual cannula placements are mapped out on plates from the rat brain atlas that span the NASh (Paxinos and Watson 1986; c). ac anterior commissure, NASh nucleus accumbens shell; asterisk indicates common reference point between (a) and (b)
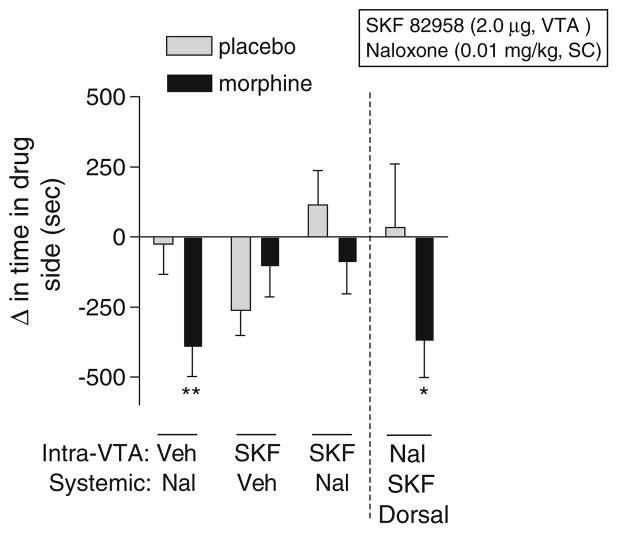
Intra-VTA microinjections of SKF 82958 block naloxone-induced place aversions but do not induce place preferences or modulate somatic withdrawal signs in morphine-dependent rats
To test the hypothesis that the VTA is a substrate for D1 receptor-mediated reward and withdrawal relief in morphine-dependent rats, we conditioned rats in the place conditioning apparatus with a single intra-VTA microinjection of SKF 82958 (2 μg) just prior to systemic administration of a low dose of naloxone (0.01 mg/kg, IP). We found that the effects of SKF 82958 in the VTA depended on interactions between dependence and treatment (F(3,101) =4.30, p<0.008) and between dependence and conditioning (F(1,101)=7.76, p<0.008; Fig. 5). Simple main effects tests reveal that naloxone induced significant place aversions in morphine-dependent rats (p<0.01), whereas rats treated with a combination of SKF 82958 and naloxone did not show place aversions, suggesting that activation of D1 receptors in the VTA blocks naloxone-induced place aversions. Placebo-implanted rats spent less time in environments previously paired with intra-VTA SKF 82958, although this effect was not statistically significant. To ensure that SKF 82958-induced affective withdrawal relief in morphine-dependent rats was due to drug action in the VTA rather than to nonspecific spread of drug up the cannula track, we performed dorsal control microinjections of SKF 82958 and found no effect on naloxone-induced place aversions in either morphine-dependent (p<0.05) or nondependent rats.
Fig. 5.
Effects of intra-ventral tegmental area (VTA) microinjections of SKF 82958 on place conditioning in morphine-dependent (black bars) and nondependent (gray bars) rats. Rats with unilateral cannulae in the VTA or just dorsal to the VTA were implanted with placebo or morphine pellets and, 3 days, later underwent place conditioning with systemic naloxone (0.01 mg/kg, SC), intracranial SKF 82958 (2 μg, intra-VTA or dorsal control), or a combination of intracranial SKF 82958 and systemic naloxone. Data from microinjections of SKF 82958 into dorsal control regions are shown to the right of the dashed line. Data are expressed as the change in time spent in the drug-paired side after conditioning minus the time spent in the drug-paired side before conditioning (means+SEM). Significant changes in time spent in the drug-paired chamber after conditioning compared to before conditioning are: *p<0.05, **p<0.01, Fisher’s protected t tests, 7–20 rats per group
To determine whether the VTA plays a role in D1 receptor-mediated attenuation of somatic withdrawal signs in morphine-dependent rats, SKF 82958 (2 μg) was microinjected into the VTA just prior to systemic administration of a moderate dose of naloxone (0.1 mg/kg, IP). Intra-VTA SKF 82958 had no effects on its own in nondependent or morphine-dependent rats and no effect on any naloxone-induced somatic withdrawal signs in morphine-dependent rats (Fig. 6).
Fig. 6.
Effects of intra-ventral tegmental area (VTA) microinjections of SKF 82958 on naloxone-induced somatic withdrawal signs in morphine-dependent (black bars) and nondependent (gray bars) rats. On test days, rats were administered unilateral intra-VTA microinjections of vehicle or SKF 82958 (2 μg in 1.0 μl) followed 5 min later by systemic naloxone (0.1 mg/kg, SC) or vehicle, and somatic withdrawal signs were scored for 15 min. a Total incidence of withdrawal behaviors, b cage crossings, c wet dog shakes, d teeth chattering were scored. Data are expressed as the mean number of incidences of the respective behavior (+SEM). Significant effects are **p<0.01 compared to morphine-dependent plus naloxone, #p<0.05, ##p<0.01 comparing groups under the bars, Fisher’s protected t tests, 6–15 rats per group
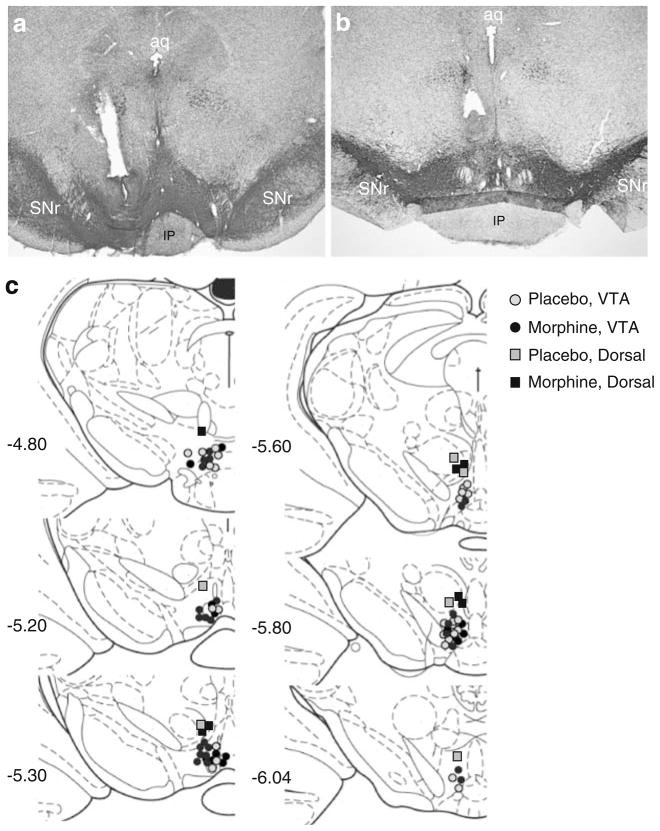
Numerous studies have reported rostral–caudal differences in the VTA with regard to neural connectivity, behavior, and gene expression (McBride et al. 1999; Olson et al. 2005; Swanson 1982). To determine whether the effects of D1 receptor activation depended on rostral–caudal distribution, we divided our place conditioning data into rostral (−4.8 to ≥−5.4 from Bregma) and caudal (≤−5.4 to −6.04 mm from Bregma) groups. We observed no differences in the behavioral effects of SKF 82958 (data not shown). Therefore, we used −4.80 to −6.04 mm from Bregma as the rostral–caudal boundaries of the VTA and called all injection sites outside this range “misses” (animals were not included in data analysis; Fig. 7).
Fig. 7.
Histological assessment of cannula placements within the ventral tegmental area (VTA). Representative coronal sections stained for tyrosine hydroxylase (TH) immunoreactivity and Nissl counter-stained are shown from rats that received microinjections of SKF 82958 into the VTA (a) and into more dorsal regions along the same trajectory (b). Individual cannula placements are mapped out on plates from the rat brain atlas that span the VTA (Paxinos and Watson 1986; c). aq aqueduct, IP interpeduncular nucleus, SNr substantia nigra pars reticulata
Discussion
Chronic drug administration can alter the molecular and behavioral effects of D1 receptor stimulation (Bonci and Williams 1996; Chartoff et al. 2006; Chartoff et al. 2003; Self et al. 1996). We have shown previously that systemic administration of a D1 receptor agonist is rewarding only in morphine-dependent rats and blocks the expression of both affective and somatic withdrawal signs (i.e., relieves withdrawal; Chartoff et al. 2006). Here, we extend these findings and demonstrate that, in morphine-dependent rats, stimulation of D1 receptors in the NASh is rewarding without reducing the aversive effects of precipitated withdrawal, whereas stimulation of D1 receptors in the VTA reduces the aversive effects of withdrawal without being rewarding. Activation of D1 receptors in either the NASh or the VTA did not have rewarding effects in nondependent rats. Furthermore, activation of D1 receptors in the NASh and VTA does not affect expression of somatic withdrawal signs. These findings suggest that DA-mediated reward and relief of affective withdrawal signs in morphine-dependent rats are anatomically dissociated from one another and may be mediated by distinct mechanisms within mesolimbic circuitry. They also confirm that opiate dependence induces a fundamental shift in D1 receptor signaling.
Dependence-associated effects of systemic D1 receptor activation
Both SKF 82958 and the more selective D1 receptor agonist SKF 81297 induce place preferences when administered systemically to morphine-dependent, but not nondependent, rats. Although extremely potent at D1 receptors, SKF 82958 can also have activity at D2 receptors, particularly at higher doses. SKF 81297, however, is highly specific for D1 receptors (Andersen and Jansen 1990; Ruskin et al. 1998). Therefore, the current finding that SKF 82958 and SKF 81297 have similar, rewarding, effects in morphine-dependent rats suggests a common role for D1 rather than D2, receptors.
The finding that activation of D1 receptors is rewarding in morphine-dependent rats is consistent with increasing evidence that prior drug experience alters DA receptor signaling. Nondependent animals can learn to self-administer some, but not all, D1 receptor agonists (Self and Stein 1992; Woolverton et al. 1984), although this depends on several sessions and repeated exposures to the drug. Other studies have shown that D1 agonists can, under some circumstances, produce place preferences when animals are exposed to the agonist multiple times over several conditioning sessions (Abrahams et al. 1998; Graham et al. 2007; White et al. 1991). This is in contrast to the current study where rats were conditioned with SKF 82958 or SKF 81297 only once and raises the possibility that repeated drug exposure is necessary to induce sensitivity to D1 receptor-mediated reward.
We also found that both SKF 82958 and SKF 81297 blocked naloxone-induced place aversions in morphine-dependent rats. There is a marked decrease in activity of DA neurons in the VTA and DA release in the NAc in response to spontaneous or naloxone-precipitated morphine withdrawal (Diana et al. 1995; Rossetti et al. 1992). A parsimonious explanation for our findings is that activation of D1 receptors partially restores DA signaling and prevents establishment of naloxone-induced place aversions. However, combined administration of naloxone and a high dose of SKF 82958, but not SKF 81297, resulted in place preferences, raising the possibility that this effect may be due, at least in part, to activation of D2 receptors. It also suggests that two separate processes are engaged: withdrawal relief through restoration of DA signaling and reward through activation of dependence-modified D1 receptor signaling.
Dependence-associated effects of D1 receptor activation in the NASh
Activation of D1 receptors in the NASh was motivationally neutral in nondependent rats but elicited significant place preferences in morphine-dependent rats, suggesting that the NASh is a substrate for morphine-induced alterations in D1 receptor reward signaling. This effect was specific to the NASh because we did not observe place preferences in morphine-dependent rats that received dorsal microinjections of SKF 82958. However, intra-NASh SKF 82958 had no effect on naloxone-induced place aversions in morphine-dependent rats, suggesting that restoration of D1 signaling in the NASh is not sufficient to relieve the aversive aspects of precipitated opiate withdrawal.
Although the neurobiological mechanisms underlying dependence-mediated shifts in DA signaling are not known, it is possible that interactions between MORs and D1 receptors are critical. It has been shown that chronic morphine causes an upregulation of cAMP-mediated signaling pathways in vitro (Chartoff et al. 2003; Sharma et al. 1977) and in several brain regions, including the NAc (Chartoff et al. 2006; Nestler and Aghajanian 1997). The cAMP-dependent protein kinase (PKA) phosphorylates several downstream substrates, which can rapidly influence neuronal excitability in the NAc (Nicola et al. 2000) and influence hedonic state. Consistent with this idea, drugs of abuse and natural rewards have been shown to transiently inhibit NAc neuronal firing (Peoples and West 1996; Roitman et al. 2005), which has led to a growing consensus that decreases in NAc neuronal activity are correlated with reward (Carlezon and Thomas 2009). D1 receptor-mediated stimulation of PKA has been shown to suppress evoked activity in NAc neurons when they are in a hyperpolarized, “down” state (Nicola et al. 2000; Surmeier et al. 1995; Zhang et al. 1998), which is consistent with our finding that activation of D1 receptors in the NASh elicited place preferences in morphine-dependent rats. Although the underlying mechanisms of these effects are unknown, it is possible that morphine dependence increases sensitivity to the rewarding effects of D1 receptor agonists by modulating depolarization state (e.g., decreasing transitions to a depolarized “up” state).
Intra-NASh SKF 82958 had no effect on naloxone-induced place aversions in morphine-dependent rats, suggesting that the rewarding effects of D1 receptor stimulation are anatomically dissociated from its ability to relieve withdrawal. There is substantial evidence that the NAc plays an important role in the aversive properties of morphine withdrawal (Harris and Aston-Jones 1994; Koob et al. 1992; Stinus et al. 1990). Microinjections of a D2 agonist into the NASh decrease somatic withdrawal signs (Harris and Aston-Jones 1994), and intra-NAc inhibition of PKA blocks place aversions associated with morphine withdrawal (Valverde et al. 1996). Considered together with the current study, D2, but not D1, receptor-containing NAc neuronal populations might be a critical substrate for morphine withdrawal.
Interestingly, SKF 82958 elicited significant place preferences in placebo-implanted control rats treated with naloxone. The mechanisms for this effect are not known but might involve naloxone-mediated blockade of the endogenous inhibitory tone of MORs on adenylate cyclase activity, which could increase cAMP levels and activation of PKA. This effect was also specific to the NASh because we did not observe place preferences in nondependent rats that received dorsal microinjections of SKF 82958 concomitant with systemic naloxone.
Dependence-associated effects of D1 receptor activation in the VTA
In morphine-dependent rats, activation of D1 receptors in the VTA was motivationally neutral on its own but blocked naloxone-elicited place aversions induced by a low dose of naloxone, suggesting that decreased D1 receptor signaling in the VTA contributes to expression of affective withdrawal signs. This effect was specific to the VTA because dorsal microinjections of SKF 82958 failed to provide withdrawal relief. Despite the fact that we microinjected an equivalent total dosage into each of these brain areas (2 μg), direct comparisons of the magnitudes of D1 receptor-mediated place conditioning are difficult because of regional differences in D1 receptor number and distribution (Georges et al. 1999; Mansour et al. 1992). Regardless, activation of D1 receptors in these regions clearly has qualitatively different effects.
In the VTA, D1 receptors are found on presynaptic afferent terminals that contain either GABA or glutamate (Cameron and Williams 1993; Kalivas and Duffy 1995). Therefore, the effect of intra-VTA SKF 82958 is most likely due to modulation of transmitter release within the VTA. In drug-naïve rats, activation of D1 receptors in the VTA facilitates GABA release in a PKA-dependent manner (Cameron and Williams 1993), which can, in turn, inhibit VTA DA neurons. This is consistent with our finding that intra-VTA SKF 82958 caused a trend toward place aversions in placebo-implanted control rats. In morphine-withdrawn rats, activation of D1 receptors in the VTA inhibits GABA release, an effect opposite to that in nondependent rats (Bonci and Williams 1996, 1997). It is possible that intra-VTA microinjections of SKF 82958 block naloxone-induced place aversions by reducing GABA release onto VTA DA neurons thus disinhibiting DA neuron firing.
One caveat to our study is that we did not test the effects of a D2 receptor agonist or the ability of a D1 receptor antagonist to block the effects of SKF 82958 and SKF 81297. It is conceivable that D2 receptor agonists would have similar behavioral effects to those of the D1 agonists, or that the ability of the D1 agonists to induce reward and provide relief of affective withdrawal signs in morphine-dependent rats is due, in part, to nonspecific actions at D2 receptors. In support of the first possibility, it has been shown that intra-NASh delivery of a D2 receptor agonist reduces somatic withdrawal signs in morphine-dependent rats (Harris and Aston-Jones 1994). In support of the second possibility, it has been shown that a 1-mg/kg dose of SKF 82958, but not SKF 81297, reduces the firing rate of substantia nigra DA neurons in a D2 autoreceptor-dependent manner (Ruskin et al. 1998), suggesting that SKF 82958 can have effects at both D1 and D2 receptors. Given our finding that systemic administration of both SKF 82958 and SKF 81297 induces place preferences and blocks naloxone-induced place aversions in morphine-dependent rats, it seems likely that those effects are D1 receptor-specific. Importantly, our finding that DA-mediated reward and withdrawal relief in morphine-dependent rats are anatomically dissociated is valid regardless of the DA receptor subtype involved.
D1 receptor modulation of somatic withdrawal
Systemic administration of SKF 82958 significantly reduced somatic withdrawal signs (Chartoff et al. 2006), suggesting that decreased D1 receptor activation contributes to expression of somatic withdrawal. In the current study, neither intra-NASh nor intra-VTA microinjections of SKF 82958 reduced somatic withdrawal signs. In fact, activation of D1 receptors in the NASh potentiated the total behavioral score for somatic withdrawal signs although this was due to a D1 receptor-mediated increase in locomotor activity, with no effects on wet dog shakes or teeth chattering. This is in contrast to previous work in which intra-NAc injection of the D1 agonist SKF 38393 potentiated wet dog shakes in morphine-dependent rats (Harris and Aston-Jones 1994). However, in addition to the slight differences in pharmacology between SKF 82958 and SKF 38393, this prior study used escalating doses of systemic morphine injections to achieve dependence. We also found that intra-NASh SKF 82958 dramatically increased cage crossings in placebo-implanted control, but not morphine-dependent rats. This suggests that chronic activation of MORs in the NASh inhibits D1 receptor-induced locomotor activity.
The neuroanatomical substrates important for D1 receptor-mediated attenuation of somatic withdrawal signs are not known. Although not tested in this study, it has been established that the periaqueductal grey (PAG) is involved in opiate withdrawal (Koob et al. 1992) and is important for autonomic and somatic components of defensive behaviors (Bandler and Shipley 1994). The PAG expresses D1 receptors (Mansour et al. 1992) and has been previously implicated in the dependence-inducing effects of opiates (Bozarth and Wise 1984). It also has connectivity with limbic brain regions that mediate affective withdrawal, such as the bed nucleus of the stria terminalis (BNST), and with brainstem medullary regions that mediate somatic withdrawal (Meloni et al. 2006; Williams et al. 2001). Interestingly, our finding that regulation of naloxone-induced place aversions and somatic withdrawal signs can be dissociated suggests that withdrawal relief does not depend on alleviation of somatic withdrawal signs. Rather, distinct neural circuitries appear to regulate reward, withdrawal relief, and somatic withdrawal. Future studies based on these findings will address cellular and molecular mechanisms that might underlie these effects.
Acknowledgments
This work was supported by National Institutes of Health grants DA012736 (to WC) and DA017524 (to EC) and conducted in a facility constructed with support from Research Facilities Improvement Program (RR11213) from the National Center for Research Resources.
References
- Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ. Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. Eur J Pharmacol. 1998;343:111–118. doi: 10.1016/s0014-2999(97)01531-8. [DOI] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nader K, van der Kooy D. A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol Biochem Behav. 1998;59:1–17. doi: 10.1016/s0091-3057(97)00047-6. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA. Neuroanatomical boundaries of the reward-relevant opiate-receptor field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res. 1987;414:77–84. doi: 10.1016/0006-8993(87)91327-8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Neural substrates of opiate reinforcement. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:569–575. doi: 10.1016/0278-5846(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods in Molecular Medicine. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacologia. 1996;128:413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacologia. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Konradi C, Carlezon WA., Jr Dopamine-dependent increase in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J Neurochem. 2003;87:107–118. doi: 10.1046/j.1471-4159.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- Frenois F, Cador M, Caille S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–490. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Graham DL, Hoppenot R, Hendryx A, Self DW. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology (Berl) 2007;191:719–730. doi: 10.1007/s00213-006-0473-5. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature. 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–5388. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46:959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1986. pp. 407–431. [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci. 1996;16:3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punch LJ, Self DW, Nestler EJ, Taylor JR. Opposite modulation of opiate withdrawal behaviors on microinfusion of a protein kinase A inhibitor versus activator into the locus coeruleus or periaqueductal gray. J Neurosci. 1997;17:8520–8527. doi: 10.1523/JNEUROSCI.17-21-08520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus Accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Rawji SS, Walters JR. Effects of full D1 dopamine receptor agonists on firing rates in the globus pallidus and substantia nigra pars compacta in vivo: tests for D1 receptor selectivity and comparisons to the partial agonist SKF 38393. J Pharmacol Exp Ther. 1998;286:272–281. [PubMed] [Google Scholar]
- Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci USA. 1977;74:3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Stellar JR. Assessment of tyrosine hydroxylase immunoreactive innervation in five subregions of the nucleus accumbens shell in rats treated with repeated cocaine. Synapse. 2000;38:261–270. doi: 10.1002/1098-2396(20001201)38:3<261::AID-SYN5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Valverde O, Tzavara E, Hanoune J, Roques B, Maldonado R. Protein kinases in the rat nucleus accumbens are involved in the aversive component of opiate withdrawal. Eur J Neurosci. 1996;8:2671–2678. doi: 10.1111/j.1460-9568.1996.tb01562.x. [DOI] [PubMed] [Google Scholar]
- White NM, Packard MG, Hiroi N. Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacologia. 1991;103:271–276. doi: 10.1007/BF02244216. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10(3):247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos JZ. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]