Abstract
Silent corticotrophins adenomas (SCAs) are clinically silent and non-secreting but immunostain positively for ACTH. We hypothesize that SCAs comprise both corticotroph and gonadotroph characteristics. Cohort analysis from 1994–2008 with follow-up time ranging from 1–15 years in a tertiary referral center. We compared preoperative and postoperative clinical results and tumor cytogenesis in 25 SCAs and 84 nonfunctioning adenomas in 109 consecutive patients diagnosed pre-operatively with nonfunctioning pituitary adenomas. Clinical outcomes were radiologic and hormonal measures. Pathologic outcomes were expression of relevant pituitary hormones, tissue-specific transcription factors, and electron microscopy features. Preoperative SCA presentation was similar to that observed for nonfunctioning adenomas. However, SCAs recurred postoperatively at a median of 3 years vs. 8 years for nonfunctioning adenomas (p < 0.0001). Fifty-four percent of patients with SCAs had new onset postoperative hypopituitarism vs. 17% of nonfunctioning adenomas (p < 0.025). SCAs (n = 18) were immunopositive for ACTH, cytoplasmic and nuclear SF-1, NeuroD1, DAX-1, and alpha-gonadotropin subunit, but Tpit negative, and co-expression of tumor ACTH with either SF-1 or LH was detected. In contrast, functional corticotroph adenomas (n = 11) were immunopositive for ACTH, nuclear SF-1, NeuroD1, and Tpit, but negative for DAX-1, a gonadotroph cell transcription factor. Gonadotroph adenomas (n = 23) were immunonegative for ACTH and Tpit but positive for nuclear SF-1, NeuroD1, and DAX-1. SCA electron microscopy demonstrated ultrastructural features consistent with corticotroph and gonadotroph cells. As SCAs exhibit features consistent with both corticotroph and gonadotroph cytologic origin, we propose a pathologic and clinically distinct classification of SCAs as silent corticogonadotroph adenomas.
Keywords: Pituitary adenoma, Corticotroph adenoma, Gonadotroph adenoma, Nonfunctioning adenoma
Introduction
Anterior pituitary tumors comprise ∼15% of intracranial tumors and are either hormone-secreting or functionally inactive adenomas [1, 2]. These monoclonal adenomas are usually benign and classified based on differentiated cell type origin [1]. Clinically nonfunctioning adenomas comprise mostly null cell tumors (20% of pituitary tumors) which are not immunopositive for known pituitary hormones, and gonadotroph adenomas which immunostain for but do not secrete follicle-stimulating hormone (FSH), luteinizing hormone (LH), and/or alpha-gonadotropin subunit (α-GSU) [1, 2]. Corticotroph adenomas (∼10% of pituitary tumors) are immunopositive for adrenocorticotrophic hormone (ACTH) and are associated with elevated circulating ACTH and cortisol levels leading to Cushing’s disease with features of hypercortisolism [3, 4]. However, up to 20% of corticotroph adenomas, known as silent corticotroph adenomas (SCAs), do not manifest biochemical or clinical evidence of hypercortisolism [5–7].
SCAs are usually diagnosed pre-operatively as nonfunctioning adenomas, and the definitive diagnosis of SCA is determined retrospectively after pathological examination of resected tumor tissue. These patients frequently present with tumor mass effects, including headaches, visual disturbances, and hypopituitarism likely due to tumor compression of normal pituitary tissue or parasellar structures [8]. Alternatively, SCAs may be incidentally diagnosed when brain magnetic resonance imagings (MRI) are performed for unrelated reasons [7]. SCAs therefore generally present as non-secreting macroadenomas and pre-operative laboratory studies reveal normal cortisol levels and normal to low LH/FSH and sex steroid and normal to slightly elevated prolactin (PRL) levels [5, 7, 9, 10]. These adenomas are resected usually because of mass effects [9], and patients are followed post-operatively for persistent or recurrent mass growth with serial MRIs, and monitored for development of subsequent hypopituitarism [7–11]. Rarely, SCAs transform into functional corticotroph adenomas at later stages in the natural history of the disease [9, 12, 13]. Although SCAs follow an aggressive clinical course, expression of Ki-67 and p53 growth markers is inconsistent in SCAs [9, 14–16].
World Health Organization guidelines classify SCAs as silent counterparts of functional corticotroph adenomas immunoreactive for ACTH [17]. However, biochemical profiles of the two entities differ [18]. In patients with functional corticotroph adenomas, plasma ACTH levels correlate with abundance of ACTH-immunopositive cells [18]. No such correlation is manifest in patients harboring SCAs [18]. Individual corticotroph cells in SCAs likely secrete insufficient, or bio-inactive, ACTH molecules [18]. It has been proposed that SCAs derive from proopiomelanocortin (POMC)-producing cells in the vestigial pars intermedia of the human pituitary [19] and that the clinical silence of SCAs may be due to dysregulated POMC processing [20].
Tissue-specific transcription factors are useful markers for identifying pituitary tumor cytogenesis. Anterior pituitary endocrine cell types derive from the anterior neuronal ridge [21] and cell type differentiation progresses from sequential expression of pituitary hormone genes [22]. Tpit, a tissue-specific regulator of POMC expression, is expressed in corticotrophs and melanotrophs [23, 24] and is a specific marker of POMC-producing cells in functional corticotroph adenomas [25]. Furthermore, the transcription factor NeuroD1 binds to the POMC promoter, activates POMC transcription [26], and contributes to the functional expression and differentiation of ACTH secreting adenomas as well as differentiation of nonfunctioning adenomas [27, 28]. SF-1 is localized to cells in nonfunctioning pituitary adenomas that produce the gonadotropin β-subunit [29]. DAX-1, an orphan nuclear receptor, is also a factor in gonadotroph cell differentiation [30] and is expressed in nonfunctioning adenomas [31].
Some SCAs are Tpit positive [25, 32] and express NeuroD1 [32–34], similar to observations in functional corticotroph adenomas. However, SCAs exhibit clinical behavior similar to aggressive nonfunctioning adenomas. We therefore directly compared preoperative and postoperative outcomes in SCAs and nonfunctioning adenomas and investigated pathologic features of the tumor types. Based on the findings presented here, we propose a redefinition of SCAs as a pituitary tumor subtype comprising both corticotroph and gonadotroph characteristics, i.e., a silent corticogonadotroph adenoma (SCGA), to heighten awareness of the biologic behavior of these tumors requiring closer surveillance.
Materials and Methods
Clinical Analysis
We conducted a cohort analysis of nonfunctioning adenomas treated at Cedars-Sinai Medical Center. SCAs were identified as nonfunctioning adenomas shown to exhibit immunopositive ACTH staining. Nonfunctioning adenomas were those that were immunopositive for FSH/LH or α-GSU but not immunopositive for other pituitary hormones. The study was approved by the Institutional Review Board and consent obtained from all patients. Each patient was assigned a de-identified number used for data analysis. Pre-operative MRIs evaluated for tumor size, level of invasion, and scored by the modified Hardy classification as follows: grade 1 for microadenomas, grade 2 for macroadenomas with or without suprasellar extension, grade 3 for locally invasive with bony destruction and/or into the cavernous and/or sphenoid sinus, and grade 4 for extracranial spread [35]. Post-operative MRIs were reviewed for presence of residual tumor, recurrence, or cure. Recurrence was defined as growth of residual tumor or presence of new tumor after demonstration of a negative post-operative MRI. Patient inclusion required that MRI results and/or hormonal results were recorded at least 1 year post-operatively.
Fluorescence Immunohistochemistry
We performed immunohistochemistry on functional corticotroph adenomas, SCAs, and gonadotroph adenomas. Tumor specimens were fixed in 10% formalin and embedded in paraffin. Guinea pig anti α-GSU monoclonal antibody (purchased from Dr. A.F. Parlow, National Hormone and Peptide Program, Torrance, CA, USA) was diluted 1:1,000, mouse anti-ACTH monoclonal antibody (DAKO, Carpinteria, CA, USA) was used at 1:50, rabbit anti-LH monoclonal antibody (DAKO, Carpinteria, CA, USA) was used at 1:1,000, and rabbit anti-growth hormone (GH) polyclonal prediluted antibody (Ventana, Tucson, AZ, USA), rabbit anti-PRL polyclonal prediluted antibody (Ventana, Tucson, AZ, USA), and mouse anti-TSH polyclonal prediluted antibody (Ventana, Tucson, AZ, USA) were used. Rabbit Tpit polyclonal antibody (kindly provided by Dr Jacques Drouin, Montreal, Canada) [25], rabbit anti-DAX-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-SF-1 polyclonal antibody (Affinity BioReagents, Golden, CO, USA), rabbit anti-Pit-1 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and mouse anti-NeuroD1 monoclonal antibody (Abcam, Cambridge, MA, USA) were used at 1:100–200. After deparaffinization, antigen retrieval was performed using citrate at 98.6°C and permeabilization with 0.1% Triton X. Non-specific antibody binding was blocked with 10% goat serum, primary antibody applied, and incubated overnight at 4°C. Alexa 488 conjugated goat anti-rabbit, mouse, or guinea pig secondary antibodies were applied with ToPro 3 (Invitrogen, Eugene, OR, USA) at 1:500 for 2 h, and slides mounted with Prolong Gold (Invitrogen Eugene, OR, USA). For co-staining, ACTH staining was detected using Alexa 568 conjugated anti-mouse secondary antibody and LH or SF-1 staining detected with Alexa 488 conjugated anti-rabbit secondary antibody. Antibody specificity was confirmed with gonadotroph (α-GSU, DAX-1, SF-1, LH) and functional corticotroph adenomas (Tpit, ACTH) as positive tissue controls and lung and skeletal muscle tissue as negative controls as well as specific blocking peptides.
Cells immunopositive for each respective protein were counted manually, and the sum divided by the number of nuclei immunostained for ToPro to obtain percent positive cells per captured field. Immunopositivity was classified as 3+ (50–100% positive cells), 2+ (25–50% positive cells), and 1+ (<25% positive cells), according to the incidence of positively stained cells. Cell counting was performed by ImageJ (www.rsbweb.nih.gov/ij/).
Electron Microscopy
Five SCA cases were evaluated by electron microscopy. In case no. 10, freshly dissected tissue obtained from a third surgery performed for tumor recurrences in the same patient was fixed in 2.5% glutaraldehyde in phosphate buffered saline, pH 7.4 for more than 2 h [36]. In other cases (case nos. 2, 4, 7, 18) a fragment of pituitary adenoma was deparaffinized in xylene and rehydrated in graded ethanol as previously described [37]. Subsequent steps were identical for both fresh glutaraldehyde fixed and paraffin-embedded tissues. Tissue was fixed in 1% osmium tetroxide in ddH2O for 1 h at room temperature, dehydrated through graded acetone and embedded in Eponate (Ted Pella, Redding, CA, USA). After overnight polymerization at 56°C, 1 μm sections were cut with a glass knife and stained with methylene blue to control for tumor selection area. Subsequently, 60 nm thick sections were cut with a diamond knife, counterstained with 5% uranyl acetate in methanol and in Reynold’s lead citrate for 10 min each and viewed with Jeol 100CX transmission electron microscope at 80 k. Digital images were collected with XR40 Digital Camera (Advanced Microscopy Techniques Corp., Danvers, MA, USA).
Statistical Analysis
The Fisher exact test was used to compare categorical variables and the Wilcoxon rank sum test used to compare numerical variables between two groups. Numerical results are summarized as mean ± standard deviation and categorical results summarized as percent. Time to recurrence estimates were obtained by the Kaplan-Meier method, and the log-rank test was used to compare the groups for recurrence. The hazard ratio was estimated by a Cox regression model. Logistic regression was used to analyze the association of tumor diameter and post-operative hypopituitarism. Two-sided p values were calculated and statistical significance was set at p value <0.05.
Results
One hundred and nine consecutive patients were identified as harboring nonfunctioning adenomas, and of these, 25 were identified as SCAs by evidence of positive ACTH immunostaining (23%).
Clinical Features
Of the 25 SCAs and 84 nonfunctioning adenomas studied, complete radiological data was available for 17 SCAs and 58 nonfunctioning adenomas, and complete hormonal data were available for 13 SCAs and 41 nonfunctioning adenomas, as several patients were referred from outside the state.
Patients presented on initial diagnosis with: headaches (48% in SCAs compared to 37% in nonfunctioning adenomas), visual field cut (56% vs. 67%), fatigue (24% vs. 20%), and decreased libido (24% vs. 18%; Table 1). Pre-operative endocrine abnormalities encountered are outlined in Table 2. Thirty-eight percent of SCAs presented with deficiency of at least one pituitary axis compared to 54% of nonfunctioning adenomas.
Table 1.
Preoperative clinical presentation
| SCAs n = 25% | Nonfunctioning adenomas n = 84% | |
|---|---|---|
| Male | 13 (52) | 55 (65) |
| Female | 12 (48) | 29 (35) |
| Mean age (years) | 53 ± 14.3 | 54.9 ± 14.9 |
| Mean follow-up time (years) | 3.7 ± 2.6 | 5.7 ± 3.7 |
| Headache | 12 (48) | 31 (37) |
| Visual field defect | 14 (56) | 56 (67) |
| Fatigue | 6 (24) | 17 (20) |
| Decreased libido | 6 (24) | 15 (18) |
Table 2.
Preoperative pituitary hormone deficiencies in SCAs vs nonfunctioning adenomas
| SCAs n = 13% | Nonfunctioning adenomas n = 41% | |
|---|---|---|
| Preoperative | Preoperative | |
| Overall rate of hypopituitarism | 5 (38) | 22 (54) |
| Central adrenal insufficiency | 0 (0) | 3 (7) |
| Central hypogonadism | 5 (38) | 17 (41) |
| TSH deficiency | 0 (0) | 7 (17) |
| GH deficiency | 1 (8) | 12 (29) |
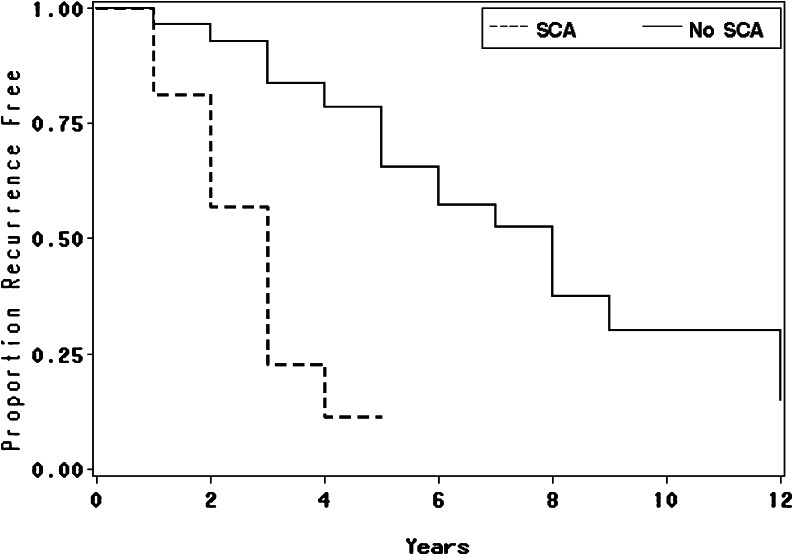
Table 3 delineates the radiologic characteristics on presentation which did not differ for the SCAs and nonfunctioning adenomas. After initial surgery, 56% of SCAs exhibited residual tumor compared to 59% of nonfunctioning adenomas. There were no differences in rates of recurrent growth from residual tissue in the two groups. However, recurrence of newly observed tumor after an initial negative postoperative MRI occurred in 25% of SCAs compared to 2% of NFAs (p < 0.007). Sixty-three percent (n = 10) of SCAs recurred with median follow-up of 3 years (1–4 years) compared to 38% (n = 23) of nonfunctioning adenomas with median follow-up of 8 years (1–15 years). The mean number of recurrences overall was 1 (±1.0) for SCAs and 0.5 (±0.7) for nonfunctioning adenomas with a mean of 1.0 in SCAs and 0.0 in nonfunctioning adenomas (Wilcoxon p value = 0.042). The time to first recurrence was significantly shorter in SCAs (median = 3 years, 95% CI = 2-3 years) compared to time of nonfunctioning adenoma recurrence (median = 8 years, 95% CI = 6–12 years), p < 0.0001. These results yielded an SCA hazard ratio of 5.6, 95% CI = 2.4–13.1 (Fig. 1).
Table 3.
Radiologic characteristics
| MRI | SCAs n = 17% | Nonfunctioning adenomas n = 58% |
|---|---|---|
| Preoperative | ||
| Initial mean tumor diameter | 30 ± 17 mm | 31 ± 13 mm |
| Hardy grade 2 | 5 (29) | 22 (41) |
| Hardy grade 3 | 11 (65) | 26 (45) |
| Hardy grade 4 | 1 (6) | 8 (14) |
| Chiasm compression | 7 (41) | 38 (66) |
| Cavernous sinus invasion | 7 (41) | 22 (38) |
| Internal carotid encasing | 4 (24) | 11 (19) |
| Sphenoid sinus invasion | 2 (12) | 9 (16) |
| Suprasellar extension | 9 (53) | 30 (52) |
| Parenchymal extension | 1 (6) | 8 (14) |
| Postoperative | ||
| Postoperative MRI | n = 16% | n = 58% |
| Postoperative residual tumor present | 9 (56) | 34 (59) |
| Postoperative no residual tumor present | 7 (44) | 24 (41) |
| Recurrent growth of residual tumor | 6 (38) | 22 (38) |
| De novo recurrence | 4 (25)* | 1 (2) |
| Stable residual | 3 (19) | 12 (21) |
| No growth with negative postoperative MRI | 3 (19) | 23 (40) |
*p < 0.007
Fig. 1.
Recurrence-free survival. Median time (years) and 95% confidence interval to first recurrence. SCA: n = 16 median = 3, 95% CI = 2-3; nonfunctioning adenoma (NFA): n = 58 median = 8, 95% CI = 6-12. P < 0.0001 (SCA vs. NFA).
Median follow-up time for post-operative hypopituitarism was 3 years (1–8 years) for SCAs and 15 years (1–15 years) for nonfunctioning adenomas. New onset post-operative hypopituitarism developed in 54% (n = 7) of patients with SCAs compared to 17% (n = 7) of those with nonfunctioning adenomas (p < 0.02; Table 4). The median time to development of hypopituitarism in SCAs was 3 years (95% CI = 1–8 years) compared to 15 years (95% of 10–15 years) for nonfunctioning adenomas, yielding a hazard ratio of 8.7 (CI = 2.4–37.8) in SCAs. Unlike SCAs, new onset post-operative hypopituitarism in nonfunctioning adenomas was associated with larger tumor diameter as assessed by logistic regression analysis (p = 0.04). While development of new onset hypopituitarism correlated with increasing tumor diameter on initial presentation in nonfunctioning adenomas, SCA recurrences did not correlate with tumor mass or invasiveness (Fisher’s exact test). None of the patients with SCAs progressed to overt Cushing’s disease during follow-up.
Table 4.
Postoperative pituitary hormone deficiencies in SCAs vs. nonfunctioning adenomas
| Hormonal axis | SCA n = 13% | Nonfunctioning adenoma n = 41% |
|---|---|---|
| New onset hypopituitarism | 7 (54)* | 7 (17) |
| Central adrenal insufficiency | 6 (46)*** | 7 (17) |
| Central hypogonadism | 3 (23) | 8 (20) |
| TSH deficiency | 8 (62)** | 11 (27) |
| GH deficiency | 4 (31) | 6 (15) |
*p < 0.02, **p < 0.04, ***p < 0.056
Immunohistochemistry
Immunohistochemistry studies were conducted on 11 functional corticotroph adenomas, 18 SCAs, and 23 gonadotroph adenomas (Table 5). Detailed results on the percent of cells immunopositive for each marker in each SCA are provided in Table 6. In contrast to functional corticotroph adenomas which all stained abundantly for nuclear Tpit, 10 SCAs were immunonegative for Tpit, and 8 SCAs were weakly immunopositive in <25% of cells. Gonadotroph adenomas were uniformly immunonegative for Tpit (Figs. 2 and 3). Testing of recurrent SCA tumor tissue showed loss of Tpit expression in one recurrent tissue sample. NeuroD1 was expressed in all three tumor types (Figs. 2 and 3). α-GSU was immunopositive in three of nine functional corticotroph adenomas, 20 of 23 gonadotroph adenomas, and in 14 of 18 SCAs. SF-1was expressed in eight of ten functional corticotroph tumors with three tumors expressing SF-1 in a majority of cells and 5 tumors expressing SF-1 in a small percentage of cells. In contrast, immunoreactive SF-1 was detected in all SCAs and gonadotroph tumors tested (Figs. 3 and 4). Importantly, SF-1 cellular localization differed between tumors, and nuclear localization was observed in gonadotroph and functional corticotroph adenomas, while both nuclear and cytoplasmic localization were observed in SCAs. Antibody specificity was confirmed using a selective blocking peptide which attenuated SF-1 nuclear staining in positive gonadotroph adenoma controls, and blocked both nuclear and cytoplasmic SCA SF-1 immunostaining (Figs. 3 and 4). As expected, SF-1 expression was undetectable in lung tissue. Single cell co-staining of ACTH and LH, or ACTH and SF-1, was demonstrated in two of five and 12 of 14 SCAs tested, respectively (Fig. 4). While DAX-1 was expressed in three of 11 functional corticotroph adenomas, all gonadotroph adenomas and all SCAs were uniformly positive for DAX-1 (Figs. 3 and 4). None of the SCAs were immunopositive for TSH, one had scant positive staining for PRL, and three had slight GH staining. All SCAs stained for Pit-1 were largely negative compared to GH-secreting adenoma positive controls.
Table 5.
Summary of positive pituitary tumor immunohistochemical staining
| Tumor type | n | αGSU n (%) | Tpit n (%) | NeuroD1 n (%) | SF-1 n (%) | DAX-1 n (%) |
|---|---|---|---|---|---|---|
| Functional corticotroph adenomas | 11 | 3/9 (33)* | 11/11 (100)** | 11/11 (100) | 8/10 (80) | 3/11 (27)**** |
| Gonadotroph adenomas | 23 | 20/23 (87) | 0/23 (0)*** | 23/23 (100) | 23/23 (100) | 23/23 (100) |
| SCAs | 18 | 14/18 (78) | 8/18 (44) | 18/18 (100) | 18/18 (100) | 18/18 (100) |
The percentage shown is the number of tumors that stain positive for a given marker of the total number of tumors for each subtype
P values vs. SCAs: * p < 0.04, ** p < 0.003, ***p < 0.0005, ****p < 0.00003
Table 6.
Immunohistochemical staining of SCAs
| SCA case no. | LH/FSH | ACTH | ACTH/LH colocalized | α-GSU | Tpit (%) | NeuroD1 (%) | SF-1 (%) | ACTH/SF-1 colocalized | DAX-1 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | n/a | + | − | +++ | +++ | + | +++ |
| 2 | − | + | n/a | − | − | +++ | +++ | n/a | +++ |
| 3 | − | + | n/a | − | − | +++ | +++ | − | +++ |
| 4 | + | + | − | + | + | +++ | +++ | + | +++ |
| 5 | − | + | n/a | + | − | +++ | ++ | n/a | +++ |
| 6 | − | + | n/a | + | + | +++ | +++ (nuc) | + | +++ |
| 7 | − | + | n/a | + | + | +++ | +++ | + | +++ |
| 8 | + | + | − | + | − | +++ | +++ | + | +++ |
| 9 | + | + | + | − | + | +++ | +++ (nuc) | + | +++ |
| 10 | − | + | n/a | + | + | +++ | ++ | + | +++ |
| 10 (1st recurrence) | − | + | n/a | n/a | − | n/a | n/a | n/a | ++ |
| 10 (2nd recurrence) | − | + | n/a | − | − | +++ | +++ | + | +++ |
| 11 | + | + | n/a | n/a | − | +++ | +++ (nuc) | + | +++ |
| 11 (1st recurrence) | + | + | + | + | − | +++ | +++ (nuc) | + | +++ |
| 12 | − | + | n/a | + | + | +++ | +++ | + | +++ |
| 13 | + | + | n/a | + | − | +++ | ++ (nuc) | n/a | +++ |
| 14 | − | + | n/a | − | − | +++ | +++ | − | +++ |
| 15 | − | + | n/a | + | + | +++ | + | n/a | +++ (cyto) |
| 16 | + | + | n/a | + | − | +++ | ++ | + | +++ |
| 17 | − | + | n/a | + | + | +++ | ++ | + | +++ |
| 18 | + | + | − | − | − | +++ | +++ | + | +++ |
Shown is scoring for each immunostained transcription factor
Staining for LH, FSH, ACTH, and α-GSU was not quantified and are scored as either present (+) or absent (−)
Staining for Tpit, NeuroD1, and DAX-1 is nuclear unless otherwise noted. Staining for SF-1 is both nuclear and cytoplasmic unless otherwise noted. For quantification of Tpit, NeuroD1, SF-1, and DAX-1, + = <25%; ++ = 25–50%; +++ = 50–100% percent positive staining according to incidence of positively stained cells
% percentage of cells, Nuc nuclear, Cyto cytoplasmic, n/a not available or not applicable
Fig. 2.
Fluorescence immunohistochemistry of corticotroph markers ACTH, Tpit, and NeuroD1 in functional corticotroph adenomas and silent corticotroph adenomas. Tumors were fixed in 10% formalin, paraffin embedded, and stained with primary antibodies, with Alexa 488 secondary antibodies and visualized with confocal immunofluorescence microscopy at ×20–40 magnification. Tumors were immunopositive for ACTH and NeuroD1. Functional corticotroph adenomas were immunopositive for Tpit, and the SCA was immunonegative for Tpit. FCA functional corticotroph adenoma.
Fig. 3.

Fluorescence immunohistochemistry of DAX-1 and SF-1in functional corticotroph adenoma, Tpit, NeuroD1, and ACTH in gonadotroph adenoma, SF-1in lung negative control, and SF-1 blocking peptide in gonadotroph adenoma. The tumors were fixed in 10% formalin, paraffin embedded, and immunostained with primary antibodies, with Alexa 488 and 588 secondary antibodies and visualized with confocal immunofluorescence microscopy at ×20–40 magnification. FCA functional corticotroph adenoma, GA gonadotroph adenoma.
Fig. 4.
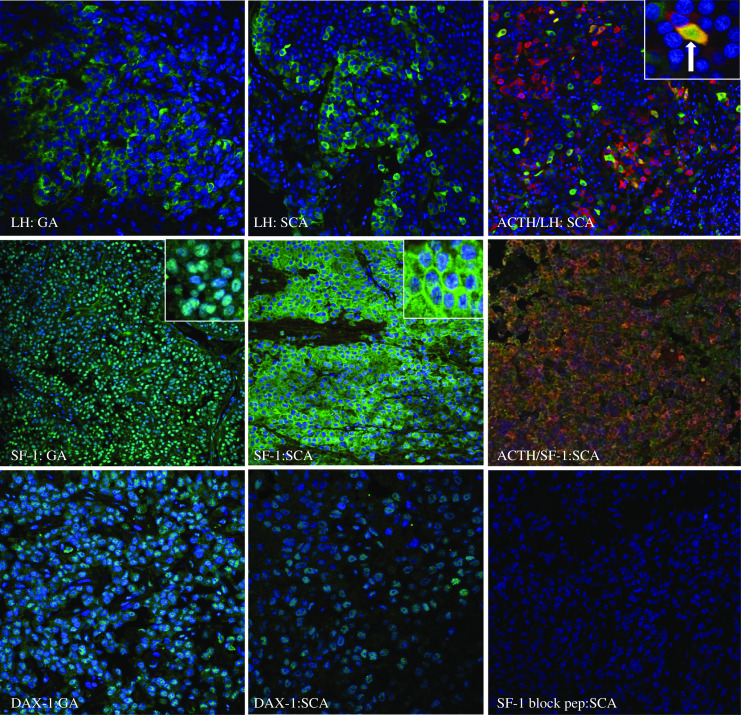
Fluorescence immunohistochemistry of gonadotroph markers LH, DAX-1, and SF-1 in gonadotroph adenomas (GA) and silent corticotroph adenomas. Tumors were fixed in 10% formalin, paraffin embedded, and immunostained with primary antibodies, with Alexa 488 and 588 secondary antibodies and visualized with confocal immunofluorescence microscopy at ×20–40 magnification. Tumors were immunopositive for LH in the gonadotroph adenoma and in this specific SCA. SCA exhibited co-localization of ACTH and LH in the same cell (white arrow), with a portion of the field enlarged for detail (×100). The gonadotroph adenoma was immunopositive for nuclear DAX-1 and SF-1. The SCA was immunopositive for nuclear DAX-1 and displayed both nuclear and cytoplasmic SF-1 immunostaining with a portion of the SF-1 field enlarged for detail (×100). GA gonadotroph adenoma.
Electron Microscopy
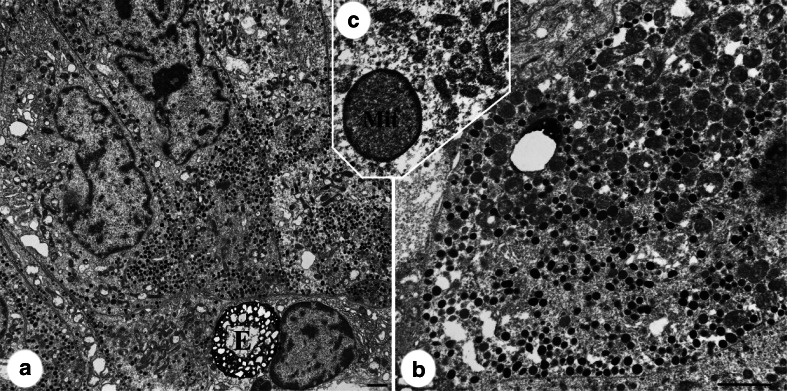
Electron microscopy of five SCA tumors showed several common features (Fig. 5). Cells had relatively increased mitochondrial density, scattered enlarged late endosomes/enigmatic bodies, and pleomorphic granule diameter (90–250 nm), shape and density, and no bundling of intermediate filaments. Two cases had enlarged mitochondria with condensation of peripheral matrix. Golgi complexes were generally large and stacks of rough endoplasmic reticulum were seen in four of five cases, but honeycomb Golgi were not noted.
Fig. 5.
Electron microscopy image of silent corticotroph adenoma. a Low power view of case 10 shows polygonal cells with numerous mitochondria, pleomorphic granules, and enigmatic body (E). Higher magnification b highlights increased mitochondrial density and variability in the size and electron density of granules. Case 18 showed mitochondrial enlargement and peripheral condensation of matrix (Mit on c). Bars 2 μm.
Discussion
We propose to refine the definition of SCAs as a tumor subtype comprising clinical and cellular characteristics of both corticotroph and gonadotroph adenoma cells and suggests naming this entity a SCGA (Fig. 6). Previous studies reported the incidence of SCAs to be up to 19% of adenomas classified preoperatively as nonfunctioning adenomas [6, 9, 14, 15, 38] and ∼20% of all corticotroph adenomas [5, 7]. While SCAs immunostained for ACTH, similar to functional corticotroph adenomas, the clinical course was more typical of nonfunctioning adenomas. SCAs presented as nonfunctioning adenomas due to local mass effects and pituitary hormone deficiencies [11]. There was a female SCA preponderance in some reports compared to nonfunctioning adenomas [7, 10, 15, 33], but not others [8–11, 14]. Up to 60% of SCAs manifested with preoperative hypopituitarism, similar to rates observed in nonfunctioning adenomas [8, 10]. However, cavernous sinus invasion as visualized by MRI was more prevalent in SCAs than in nonfunctioning adenomas [8, 9, 15, 16]. SCAs consistently demonstrated a more aggressive postoperative course compared to nonfunctioning adenomas. New onset post-operative hypopituitarism had been reported in about one-third of SCAs [8, 10] with reports of postoperative adrenal insufficiency in SCAs [5, 7, 10, 12, 39]. While SCA recurrence rates of up to 57% had been documented [8–11], these rates did not differ from those observed with nonfunctioning adenomas [6, 11, 15] nor had SCAs been shown to recur earlier [11] though one series demonstrated that SCAs frequently had multiple recurrences [6] (Table 7).
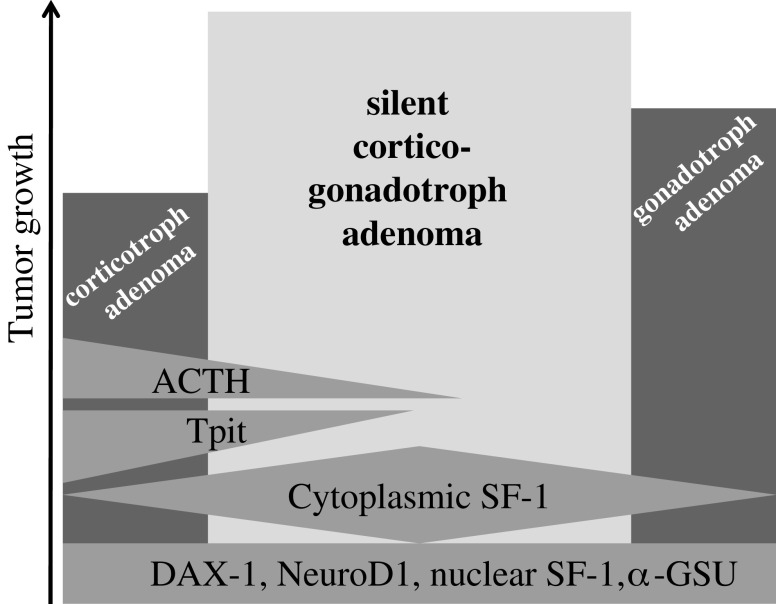
Fig. 6.
Proposed model for development of silent corticogonadotroph adenoma (SCGA). Expression of both corticotroph and gonadotroph transcription factors is observed in SCGAs. Functional corticotroph adenomas, most of which are microadenomas, express ACTH, Tpit, NeuroD1, and sometimes nuclear SF-1 and α-GSU. Gonadotroph adenomas, which vary in size from micro- to macroadenomas, express DAX-1, NeuroD1, nuclear SF-1, and α-GSU. SCGAs, which are invariably macroadenomas, express ACTH, DAX-1, NeuroD1, nuclear and cytoplasmic SF-1, and α-GSU but little to no Tpit.
Table 7.
Case series of SCAs
| Reference | SCAs (n) | Direct comparison to nonfunctioning gonadotroph adenomas | Preoperative radiological data | Preoperative hormonal data | Postoperative radiological data | Postoperative hormonal data | Significant findings |
|---|---|---|---|---|---|---|---|
| Baldewag, 2005 [9] | 22 | − | + | − | + | − | 33% recur |
| Yamada, 2007 [15] | 26 | + | + | − | + | − | Higher rates of invasion in SCAs |
| Pawlikowski, 2008 [14] | 20 | + | − | − | + | − | Higher recurrence rate in SCAs |
| Lopez, 2004 [7] | 12 | − | + | + | + | + | |
| Webb, 2003 [10] | 27 | − | + | + | + | + | 37% recur |
| Scheithauer, 2000 [8] | 23 | − | + | + | + | + | 54% recur, 55% hypopituitarism |
| Bradley, 2003 [11] | 28 | + (historical controls) | − | − | + | − | No difference in recurrence rate or time to recur between groups |
| Scheithauer, 2006 [16] | 17 | + | + | − | − | − | SCAs more invasive |
| Tateno, 2007 [33] | 8 | + | + | − | − | − | |
| Cho, 2009 [6] | 28 | + | + | + | + | + | More multiple recurrences in SCAs; younger age in SCAs |
Our study is one of the largest cohort analyses directly comparing SCAs to nonfunctioning adenomas and includes comprehensive preoperative and postoperative clinical outcomes. We demonstrate that SCAs have a 63% recurrence rate while 38% of nonfunctioning adenomas recurred. In addition, we show that SCAs recur 5 years sooner than nonfunctioning adenomas, in contrast to prior reports [11] and demonstrate that de novo recurrences are seen more frequently in patients with SCAs. A further observed difference between SCAs and nonfunctioning adenomas is development of new postoperative pituitary deficits in 54% of patients with SCAs compared to 17% of nonfunctioning adenomas, especially in the thyroid and adrenal axes. Furthermore, given that we do not find that new post-operative hypopituitarism in SCAs correlated with invasive characteristics nor with the presence of residual tumor or with recurrences, it is likely that the higher rate of post-operative hypopituitarism is reflective of a unique SCA biology or cytogenesis.
As SCAs follow a unique clinical course, they ostensibly express distinctive differentiated cellular proteins. On immunostaining, SCAs expressed ACTH to a similar degree as functional corticotroph adenomas [18]. However, examination of Tpit in SCAs have yielded conflicting reports with one study showing Tpit positive immunohistochemistry staining in three of four SCAs [25] while another demonstrated lower Tpit mRNA levels in SCAs than in functional corticotroph adenomas [33]. NeuroD1, a factor contributing to differentiation of corticotroph adenomas and α-GSU positive cells, was also expressed in SCAs [32–34]. In addition to corticotroph markers and consistent with our findings here, a single SCA showed expression of gonadotroph markers, namely DAX-1 and SF-1 [34]. Indeed, LH, FSH, and α-GSU were all expressed in SCAs and also in functional corticotroph adenomas (Table 8), often with cellular colocalization.
Table 8.
Summary of reports of ACTH/SF-1/LH expression
| Reference | N | ACTH | LH/FSH | α-GSU | SF-1 | Colocalization with ACTH | Tumor type |
|---|---|---|---|---|---|---|---|
| Suzuki et al., 2008 [34] | 5 | + | − | + | + | + for α-GSU and SF-1 | 4 CD, 1 SCA |
| Kojima et al., 2002 [18] | 2 | + | + | n/a | n/a | n/a | SCA |
| Sano et al., 1990 [48] | 2 | + | + | + | n/a | + | CD |
| Sano 1994 [49] | 1 | + | − | + | n/a | n/a | SCA |
| Skelly et al., 2000 [50] | 1 | + | − | + | n/a | n/a | CD |
| Desai et al., 1995 [51] | 9 | + | + in 3 | + | n/a | + for α-GSU | CD, 1 SCA |
| Berg et al., 1990 [52] | 9 | + | n/a | + | n/a | + in 1 CD | 8 CD, 1 SCA |
| Heshmati et al., 1988 [53] | 4 | + | + in 1 SCA | + (in 3 SCAs) | n/a | n/a | SCAs |
| Jautzke 1988 [54] | 1 | + | − | + | n/a | + | SCA |
| Landolt et al., 1988 [55] | 2 | + | n/a | + | n/a | n/a | 1 SCA, 1 CD |
| Egensperger et al., 2001 [56] | 1 | + | + | + | + | n/a | CD |
| Kamijo et al., 1991 [57] | 1 | + | + | n/a | n/a | + | CD |
| Osamura et al., 1988 [58] | 2 | + | + | + | n/a | − | SCA |
| Psaras et al., 2007 [12] | 1 | + | – | + | n/a | n/a | SCA |
| Lloyd et al., 1990 [59] | 1 | + | − | + | n/a | n/a | SCA |
| Lopez et al., 2004 [7] | 1 | + | − | + | n/a | n/a | SCA |
| Pawlikowski et al., 2008 [14] | 8 | + | + | + (in 2) | n/a | n/a | SCA |
| Webb et al., 2003 [10] | 5 | + | + | + | n/a | n/a | SCA |
| Felix et al., 1991 [60] | 1 | + | + | + | n/a | n/a | SCA |
| Black et al., 1987 [61] | 3 | + | − | + (in 1) | n/a | n/a | SCA |
The current study provides a characterization of integrated corticotroph and gonadotroph features of SCAs using immunohistochemistry and EM. SCAs incorporate corticotroph markers of NeuroD1 and ACTH and gonadotroph markers of DAX-1, α-GSU, and SF-1. On the other hand, SCAs are distinct from corticotroph and gonadotroph adenomas as evidenced by the lack of nuclear Tpit expression while maintaining the presence of cytoplasmic and nuclear SF-1, respectively. The reason for cytoplasmic localization of SF-1 is, however, unclear. Other nuclear proteins in pituitary tumors exhibit abnormal intracellular localization and change of function, for example, Brg1, a nuclear protein found to accumulate in the cytoplasm of corticotroph adenoma cells, alters pituitary tumor sensitivity to glucocorticoids [40].
SCAs had been characterized by ultrastructural morphology showing two morphologic variants of SCAs. Type 1 adenomas were similar to functional corticotroph adenomas in that they were densely granulated basophilic tumors with abundant cytokeratin filaments. Subtype 2 adenomas were chromophobic and lacked cytoplasmic intermediate filaments [41]. In addition to corticotroph features, SCAs were shown to incorporate gonadotroph elements as evidenced by the presence of honeycomb Golgi [13, 42] and increased mitochondrial density [13]. Similar findings were reported in functional corticotroph adenomas [43–45]. In this study, two SCAs tested were categorized as SCA subtype II while the remainder incorporate elements of both corticotroph and gonadotroph adenomas. In particular, oncocytic transformation seen in the three SCAs tested is typical of gonadotroph adenomas, whereas the granule pleomorphism and enlarged lysosomes/enigmatic bodies we observed in all the SCAs resemble corticotroph adenomas.
Several confounding factors may bias our results including unavailability of comprehensive clinical data on each patient. However, longer follow-up times in the NFAs (median of 8 years) compared to the SCAs (median of 3 years) further strengthens the observation of a higher number of recurrences occurring at an earlier timepoint as well as higher rates of post-operative hypopituitarism in SCAs. The immunohistochemistry technique employed may not be sufficiently sensitive to detect very low levels of Tpit expression. Although quantitative PCR would have been useful to confirm the immunohistochemistry staining, sufficient tumor tissue was not available after pathologic examination. Furthermore, micro-deposits of normal pituitary tissue contaminating these very small tumor specimens would also confound sensitive quantitative PCR measurements. Variability of immunostaining expression may be reflective of low antibody sensitivity and penetration as well as tumor heterogeneity. However, we used slides derived from the same block of tumor tissue to reduce section heterogeneity and utilized the same dilution of antibody for each batch. In addition, for the immunohistochemistry studies, we used only gonadotroph adenomas and excluded oncocytomas and null cell adenomas, for though these latter two adenomas may express low levels of gonadotroph subunits, they are not consistently positive for all gonadotroph transcription factors [46].
In summary, our results support prior findings that SCAs are benign but aggressively growing biochemically silent pituitary adenomas that combine clinical and pathological features of corticotroph and gonadotroph cells further suggesting a common corticotroph and gonadotroph pituitary progenitor cell origin. This is also supported by reports of a change of corticotroph committed cells to that of gonadotrophs in Tpit knockout mice through a possible trans-repression of Tpit and SF-1 [47].
Understanding that SCGAs comprise both cellular and clinical features of both corticotrophs and gonadotrophs has clinical implications for patients undergoing long-term SCGA follow-up. The diagnosis of this tumor subtype emphasizes the need for increased postoperative surveillance of SCGAs for earlier detection of recurrences and hypopituitarism through rigorous pituitary reserve testing thereby reducing morbidity and improving quality of life in these patients.
Acknowledgments
We thank Kolja Wawrowsky for the confocal images and Svetlana Zonis for valuable technical advice.
Grant support
NIH grant CA 075979 and T32 DK07770 and the Corrine and Lenny Sands Clinical Investigator Award
Disclosure statement
The authors have nothing to disclose
References
- 1.Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112:1603–1618. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Committee of Brain Tumor Registry of Japan Brain Tumor Registry of Japan. Neurol Med Chir (Tokyo) 1992;32:381–547. [PubMed] [Google Scholar]
- 3.Nieman LK, Beverly MKB, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier CA, Biller BM. Clinical and biochemical evaluation of Cushing's syndrome. Endocrinol Metab Clin North Am. 1997;26:741–762. doi: 10.1016/S0889-8529(05)70280-2. [DOI] [PubMed] [Google Scholar]
- 5.Sahli R, Christ ER, Seiler R, Kappeler A, Vajtai I. Clinicopathologic correlations of silent corticotroph adenomas of the pituitary: report of four cases and literature review. Pathol Res Pract. 2006;202:457–464. doi: 10.1016/j.prp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Cho HY, Cho SW, Kim SW, Shin CS, Park KS, Kim SY (2009) Silent corticotroph adenomas have unique recurrence characteristics as compared with other non-functioning pituitary adenomas. Clin Endocrinol (Oxf) (in press) [DOI] [PubMed]
- 7.Lopez JA, Kleinschmidt-Demasters BK, S Chun-I, Woodmansee WW, Lillehei KO. Silent corticotroph adenomas: further clinical and pathological observations. Hum Pathol. 2004;35:1137–1147. doi: 10.1016/j.humpath.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Scheithauer BW, Jaap AJ, Horvath E, Kovacs K, Lloyd RV, Meyer FB, Laws ER, Jr, Young WF., Jr Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery. 2000;47:723–729. doi: 10.1097/00006123-200009000-00039. [DOI] [PubMed] [Google Scholar]
- 9.Baldeweg SE, Pollock JR, Powell M, Ahlquist J. A spectrum of behaviour in silent corticotroph pituitary adenomas. Br J Neurosurg. 2005;19:38–42. doi: 10.1080/02688690500081230. [DOI] [PubMed] [Google Scholar]
- 10.Webb KM, Laurent JJ, Okonkwo DO, Beatriz Lopes M, Vance ML, Laws ER., Jr Clinical characteristics of silent corticotrophic adenomas and creation of an internet-accessible database to facilitate their multi-institutional study. Neurosurgery. 2003;53:1076–1084. doi: 10.1227/01.NEU.0000088660.16904.F7. [DOI] [PubMed] [Google Scholar]
- 11.Bradley KJ, Wass JA, Turner HE. Non-functioning pituitary adenomas with positive immunoreactivity for ACTH behave more aggressively than ACTH immunonegative tumours but do not recur more frequently. Clin Endocrinol (Oxf) 2003;58:59–64. doi: 10.1046/j.1365-2265.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- 12.Psaras T, Honegger J, Buslei R, Saeger W, Klein D, Capper D, Meyermann R, Mittelbronn M. Atypical type II silent corticotrophic adenoma developing into Cushing's disease upon second recurrence. Exp Clin Endocrinol Diabetes. 2007;115:610–615. doi: 10.1055/s-2007-984437. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama S, Kawahara Y, Sano T, Nakayama M, Kitajima S-I, Kuratsu J-I. A case of non-functioning pituitary adenoma with Cushing's syndrome upon recurrence. Neuropathology. 2001;21:288–293. doi: 10.1046/j.1440-1789.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- 14.Pawlikowski M, Kunert-Radek J, Radek M. “Silent”corticotropinoma. Neuro Endocrinol Lett. 2008;29:347–350. [PubMed] [Google Scholar]
- 15.Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T. A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery. 2007;61:580–584. doi: 10.1227/01.NEU.0000290906.53685.79. [DOI] [PubMed] [Google Scholar]
- 16.Scheithauer BW, Gaffey TA, Lloyd RV, Sebo TJ, Kovacs KT, Horvath E, Yapicier O, Young WF, Jr, Meyer FB, Kuroki T, Riehle DL, Laws ER., Jr Pathobiology of pituitary adenomas and carcinomas. Neurosurgery. 2006;59:341–353. doi: 10.1227/01.NEU.0000223437.51435.6E. [DOI] [PubMed] [Google Scholar]
- 17.Delellis RA, Lloyd RV, Heitz PU, Eng C (2004) Pathology and genetics of tumors of endocrine organs
- 18.Kojima Y, Suzuki S, Yamamura K, Ohhashi G, Yamamoto I. Comparison of ACTH secretion in Cushing’s adenoma and clinically silent corticotroph adenoma by cell immunoblot assay. Endocr J. 2002;49:285–292. doi: 10.1507/endocrj.49.285. [DOI] [PubMed] [Google Scholar]
- 19.Horvath E, Kovacs K, Lloyd RV. Pars intermedia of the human pituitary revisited: morphologic aspects and frequency of hyperplasia of POMC-peptide immunoreactive cells. Endocr Pathol. 1999;10:55–64. doi: 10.1007/BF02738816. [DOI] [Google Scholar]
- 20.Ohta S, Nishizawa S, Oki Y, Yokoyama T, Namba H. Significance of absent prohormone convertase 1/3 in inducing clinically silent corticotroph pituitary adenoma of subtype I–immunohistochemical study. Pituitary. 2002;5:221–223. doi: 10.1023/A:1025321731790. [DOI] [PubMed] [Google Scholar]
- 21.Wu W, Rosenfeld MG (2006) In Endocrinology, D. L. J and J. J. Larry, 291–308. Elsevier Saunders, Philadelphia.
- 22.Burrows HL, Douglas KR, Seasholtz AF, Camper SA. Genealogy of the anterior pituitary gland: tracing a family tree. Trends Endocrinol Metab. 1999;10:343–352. doi: 10.1016/S1043-2760(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang H-P, Tsai M-J, Rosenfeld MG. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci U S A. 2001;98:8674–8679. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamolet B, Pulichino A-M, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/S0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 25.Vallette-Kasic S, Figarella-Branger D, Grino M, Pulichino A-M, Dufour H, Grisoli F, Enjalbert A, Drouin J, Brue T. Differential regulation of proopiomelanocortin and pituitary-restricted transcription factor (TPIT), a new marker of normal and adenomatous human corticotrophs. J Clin Endocrinol Metab. 2003;88:3050–3056. doi: 10.1210/jc.2002-021934. [DOI] [PubMed] [Google Scholar]
- 26.Poulin G, Turgeon B, Drouin J. NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol. 1997;17:6673–6682. doi: 10.1128/mcb.17.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavoie P-L, Budry L, Balsalobre A, Drouin J. Developmental dependence on NurRE and EboxNeuro for expression of pituitary proopiomelanocortin. Mol Endocrinol. 2008;22:1647–1657. doi: 10.1210/me.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyama K, Sanno N, Teramoto A, Yoshiyuki Osamura R. Expression of neuro D1 in human normal pituitaries and pituitary adenomas. Mod Pathol. 2001;14:892–899. doi: 10.1038/modpathol.3880408. [DOI] [PubMed] [Google Scholar]
- 29.Asa SL, Bamberger A-M, Cao B, Wong M, Parker KL, Ezzat S. The transcription activator steroidogenic factor-1 is preferentially expressed in the human pituitary gonadotroph. J Clin Endocrinol Metab. 1996;81:2165–2170. doi: 10.1210/jc.81.6.2165. [DOI] [PubMed] [Google Scholar]
- 30.Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K-I. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol. 1999;13:1267–1284. doi: 10.1210/me.13.8.1267. [DOI] [PubMed] [Google Scholar]
- 31.Ikuyama S, Mu Y-M, Ohe K, Nakagaki H, Fukushima T, Takayanagi R, Nawata H. Expression of an orphan nuclear receptor DAX-1 in human pituitary adenomas. Clin Endocrinol (Oxf) 1998;48:647–654. doi: 10.1046/j.1365-2265.1998.00477.x. [DOI] [PubMed] [Google Scholar]
- 32.Kageyama K, Ikeda H, Nigawara T, Sakihara S, Suda T. Expression of adrenocorticotropic hormone, prolactin and transcriptional factors in clinically nonfunctioning pituitary adenoma. Endocr J. 2007;54:961–968. doi: 10.1507/endocrj.K07E-030. [DOI] [PubMed] [Google Scholar]
- 33.Tateno T, Izumiyama H, Doi M, Yoshimoto T, Shichiri M, Inoshita N, Oyama K, Yamada S, Hirata Y. Differential gene expression in ACTH–secreting and non-functioning pituitary tumors. Eur J Endocrinol. 2007;157:717–724. doi: 10.1530/EJE-07-0428. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki M, Egashira N, Kajiya H, Minematsu T, Takekoshi S, Tahara S, Sanno N, Teramoto A, Osamura RY. ACTH and alpha-subunit are co-expressed in rare human pituitary corticotroph cell adenomas proposed to originate from ACTH-committed early pituitary progenitor cells. Endocr Pathol. 2008;19(1):17–26. doi: 10.1007/s12022-008-9014-6. [DOI] [PubMed] [Google Scholar]
- 35.Bates AS, Farrell WE, Bicknell JE, Mcnicol A-M, Talbot AJ, Broome JC, Perrett CW, Thakker RV, Clayton RN. Allelic deletion in pituitary adenomas reflects aggressive biological activity and has potential value as a prognostic marker. J Clin Endocrinol Metab. 1997;82:818–824. doi: 10.1210/jc.82.3.818. [DOI] [PubMed] [Google Scholar]
- 36.Vidal S, Syro L, Horvath E, Uribe H, Kovacs K. Ultrastructural and immunoelectron microscopic study of three unusual plurihormonal pituitary adenomas. Ultrastruct Pathol. 1999;23:141–148. doi: 10.1080/019131299281635. [DOI] [PubMed] [Google Scholar]
- 37.Ngai HK, Chan KW, Or SB, Yau WL. A rapid method for reprocessing paraffin sections for diagnostic electron microscopy. J Pathol. 1985;145:59–62. doi: 10.1002/path.1711450106. [DOI] [PubMed] [Google Scholar]
- 38.Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W. Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Am J Pathol. 1980;98:617–638. [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi H, Koshiyama H, Sano T, Inoue D, Hashimoto N, Aoki N, Nakao K. A case of nonfunctioning pituitary adenoma resembling so-called silent corticotroph adenoma. Endocr J. 1997;44:329–333. doi: 10.1507/endocrj.44.329. [DOI] [PubMed] [Google Scholar]
- 40.Drouin J, Bilodeau S, Vallette S. Of old and new diseases: genetics of pituitary ACTH excess (Cushing) and deficiency. Clin Genet. 2007;72:175–182. doi: 10.1111/j.1399-0004.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 41.Asa SL (1998) Tumors of the pituitary gland. In Atlas of Tumor Pathology, Series 3, Fascicle 22. Washington, DC: Armed Forces Institute of Pathology
- 42.Sano T, Kovacs K, Asa SL, Yamada S, Sanno N, Yokoyama S, Takami H. Pituitary adenoma with “honeycomb Golgi” appearance showing a phenotypic change at recurrence from clinically nonfunctioning to typical Cushing disease. Endocr Pathol. 2002;13:125–130. doi: 10.1385/EP:13:2:125. [DOI] [PubMed] [Google Scholar]
- 43.Kim K, Yamada S, Usui M, Sano T. Co-localization of honeycomb golgi and ACTH granules in a giant ACTH-producing pituitary adenoma. Endocr Pathol. 2005;16:239–244. doi: 10.1385/EP:16:3:239. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda H, Yoshimoto T, Ogawa Y, Mizoi K, Murakami O. Clinico-pathological study of Cushing’s disease with large pituitary adenoma. Clin Endocrinol (Oxf) 1997;46:669–679. doi: 10.1046/j.1365-2265.1997.1741013.x. [DOI] [PubMed] [Google Scholar]
- 45.Sano T, Mader R, Asa SL, Qian ZR, Hino A, Yamada S. “Honeycomb Golgi” in pituitary adenomas: not a marker of gonadotroph adenomas. Endocr Pathol. 2003;14:363–368. doi: 10.1385/EP:14:4:363. [DOI] [PubMed] [Google Scholar]
- 46.Aylwin SJ, Welch JP, Davey CL, Geddes JF, Wood DF, Besser GM, Grossman AB, Monson JP, Burrin JM. The relationship between steroidogenic factor 1 and DAX-1 expression and in vitro gonadotropin secretion in human pituitary adenomas. J Clin Endocrinol Metab. 2001;86:2476–2483. doi: 10.1210/jc.86.6.2476. [DOI] [PubMed] [Google Scholar]
- 47.Pulichino A-M, Vallette-Kasic S, Tsai JP-Y, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev. 2003;17:738–747. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sano T, Kovacs K, Asa SL, Smyth HS. Immunoreactive luteinizing hormone in functioning corticotroph adenomas of the pituitary. Immunohistochemical and tissue culture studies of two cases. Virchows Arch A Pathol Anat Histopathol. 1990;417:361–367. doi: 10.1007/BF01605790. [DOI] [PubMed] [Google Scholar]
- 49.Sano T, Yamada S. Histologic and immunohistochemical study of clinically non-functioning pituitary adenomas: special reference to gonadotropin-positive adenomas. Pathol Int. 1994;44:697–703. doi: 10.1111/j.1440-1827.1994.tb02949.x. [DOI] [PubMed] [Google Scholar]
- 50.Skelly RH, Korbonits M, Grossman A, Besser GM, Monson JP, Geddes JF, Burrin JM. Expression of the pituitary transcription factor Ptx-1, but not that of the trans-activating factor prop-1, is reduced in human corticotroph adenomas and is associated with decreased alpha-subunit secretion. J Clin Endocrinol Metab. 2000;85:2537–2542. doi: 10.1210/jc.85.7.2537. [DOI] [PubMed] [Google Scholar]
- 51.Desai B, Burrin JM, Nott CA, Geddes JF, Lamb EJ, Aylwin SJB, Wood DF, Thakkar C, Monson JP. Glycoprotein hormone alpha-subunit production and plurihormonality in human corticotroph tumours—an in vitro and immunohistochemical study. Eur J Endocrinol. 1995;133:25–32. doi: 10.1530/eje.0.1330025. [DOI] [PubMed] [Google Scholar]
- 52.Berg KK, Scheithauer BW, Felix I, Kovacs K, Horvath E, Klee GG, Laws ER., Jr Pituitary adenomas that produce adrenocorticotropic hormone and alpha-subunit: clinicopathological, immunohistochemical, ultrastructural, and immunoelectron microscopic studies in nine cases. Neurosurgery. 1990;26:397–403. doi: 10.1097/00006123-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Heshmati HM, Turpin G, Kujas M, Lam X, Van Effenterre R, Racadot J, De Gennes JL. The immunocytochemical heterogeneity of silent pituitary adenomas. Acta Endocrinol (Copenh) 1988;118:533–537. doi: 10.1530/acta.0.1180533. [DOI] [PubMed] [Google Scholar]
- 54.Jautzke G. Simultaneous production of the alpha-subunit of glycoprotein hormones and other hormones in pituitary adenomas. Pathol Res Pract. 1988;183:601–605. doi: 10.1016/S0344-0338(88)80020-7. [DOI] [PubMed] [Google Scholar]
- 55.Landolt AM, Heitz PU, Zenklusen H-R. Production of the alpha-subunit of glycoprotein hormones by pituitary adenomas. Pathol Res Pract. 1988;183:610–612. doi: 10.1016/s0344-0338(88)80022-0. [DOI] [PubMed] [Google Scholar]
- 56.Egensperger R, Scheithauer BW, Horvath E, Kovacs K, Giannini C, Young WF, Lloyd R, Atkinson J, Nippoldt T. Cushing’s disease due to plurihormonal adrenocorticotropic hormone and gonadotropin-producing pituitary adenoma. Acta Neuropathol. 2001;102:398–403. doi: 10.1007/s004010100376. [DOI] [PubMed] [Google Scholar]
- 57.Kamijo K, Sato M, Saito T, Yachi A, Minase T, Noro H, Kawahara T. An ACTH and FSH producing invasive pituitary adenoma with Crooke’s hyalinization. Pathol Res Pract. 1991;187:637–641. doi: 10.1016/S0344-0338(11)80162-7. [DOI] [PubMed] [Google Scholar]
- 58.Osamura RY, Watanabe K. Immunohistochemical studies of human FSH producing pituitary adenomas. Virchows Arch A Pathol Anat Histopathol. 1988;413:61–68. doi: 10.1007/BF00844282. [DOI] [PubMed] [Google Scholar]
- 59.Lloyd RV, Fields K, Jin L, Horvath E, Kovacs K. Analysis of endocrine active and clinically silent corticotropic adenomas by in situ hybridization. Am J Pathol. 1990;137:479–488. [PMC free article] [PubMed] [Google Scholar]
- 60.Felix I, Asa SL, Kovacs K, Horvath E. Changes in hormone production of a recurrent silent corticotroph adenoma of the pituitary: a histologic, immunohistochemical, ultrastructural, and tissue culture study. Hum Pathol. 1991;22:719–721. doi: 10.1016/0046-8177(91)90295-Z. [DOI] [PubMed] [Google Scholar]
- 61.Black PM, Hsu DW, Klibanski A, Kliman B, Larry Jameson J, Chester Ridgway E, Hedley-Whyte ET, Zervas NT. Hormone production in clinically nonfunctioning pituitary adenomas. J Neurosurg. 1987;66:244–250. doi: 10.3171/jns.1987.66.2.0244. [DOI] [PubMed] [Google Scholar]