Abstract
Circulating blood monocytes supply peripheral tissues with macrophage and dendritic cell (DC) precursors and, in the setting of infection, also contribute directly to immune defense against microbial pathogens. In humans and mice, monocytes are divided into two major subsets that either specifically traffic into inflamed tissues or, in the absence of overt inflammation, constitutively maintain tissue macrophage/DC populations. Inflammatory monocytes respond rapidly to microbial stimuli by secreting cytokines and antimicrobial factors, express the CCR2 chemokine receptor, and traffic to sites of microbial infection in response to monocyte chemoattractant protein (MCP)-1 (CCL2) secretion. In murine models, CCR2-mediated monocyte recruitment is essential for defense against Listeria monocytogenes, Mycobacterium tuberculosis, Toxoplasma gondii, and Cryptococcus neoformans infection, implicating inflammatory monocytes in defense against bacterial, protozoal, and fungal pathogens. Recent studies indicate that inflammatory monocyte recruitment to sites of infection is complex, involving CCR2-mediated emigration of monocytes from the bone marrow into the bloodstream, followed by trafficking into infected tissues. The in vivo mechanisms that promote chemokine secretion, monocyte differentiation and trafficking, and finally monocyte-mediated microbial killing remain active and important areas of investigation.
Keywords: inflammation, monocyte differentiation, chemokines, microbial pathogens
INTRODUCTION
The mammalian immune system defends against a spectrum of microbial pathogens that, in terms of environmental prevalence, range from common to rare. Invasion by common environmental microbes is prevented by constitutive innate immune defenses in mucosal and epithelial tissues. On the one hand, the metabolic costs of establishing and maintaining constitutive innate defenses against ubiquitous microbes are easily justified. Highly virulent pathogens, on the other hand, are generally less prevalent and have evolved mechanisms to circumvent constitutive immune barriers. Upon infection with these organisms, auxiliary innate defenses are induced to combat the pathogen. Neutrophils, macrophages, and dendritic cells (DCs) are important cellular mediators of innate immune defense. Circulating monocytes, however, are increasingly implicated as essential players in defense against a range of microbial pathogens.
Most cellular components of the mammalian immune system derive from progenitors in the bone marrow. The typical developmental pathway begins with pluripotent bone marrow stem cells that give rise to progenitors that follow a variety of differentiation pathways to become mature cells with defined effector functions. Mammalian monocytes, a pleomorphic and pleiotropic population of circulating mononuclear cells, contribute to antimicrobial defense by supplying tissues with macrophage and DC precursors (1–4). When the mammalian host is confronted with a virulent pathogen, however, the normal, homeostatic differentiation pathway of monocytes is temporarily refocused, and bone marrow and blood monocytes differentiate into a spectrum of effector cells with distinct antimicrobial activities. This review focuses on the contributions of monocyte subsets to immune defense against microbial pathogens.
HUMAN MONOCYTE SUBSETS
In humans, circulating monocytes are divided into two subsets on the basis of the expression of CD14, a component of the lipopolysaccharide (LPS) receptor complex, and CD16, the FcγRIII immunoglobulin receptor (5). These monocyte subsets express distinct chemokine, immunoglobulin, adhesion, and scavenger receptors (3) (Table 1). CD14highCD16− monocytes (henceforth referred to as CD14+ monocytes) are large, ~18 μm in diameter, and represent ~80%–90% of circulating monocytes. In contrast, CD14lowCD16+ monocytes (referred to as CD16+ monocytes) are smaller, ~14 μm in diameter, and constitute ~10% of circulating monocytes. CD16+ monocytes increase in frequency during infections (6, 7), produce high levels of tumor necrosis factor (TNF) and low levels of IL-10 upon stimulation with Toll-like receptor (TLR) agonists (8), and therefore are also referred to as proinflammatory monocytes.
Table 1.
Surface antigen expression on the principal circulating murine and human monocyte subsetsa
| Murine Ly6C+ monocytes | Murine CX3CR1+ monocytes | Human CD14+ monocytes | Human CD16+ monocytes | References | |
|---|---|---|---|---|---|
| Monocyte markers | |||||
| CD11b | + | + | + | + | 16 |
| CD14 | ND | ND | ++ | +/− | 5 |
| CD16 (FcγRIII) | ND | ND | − | + | 5 |
| CD115 | + | + | ND | ND | 21 |
| F4/80 | + | + | ND | ND | 16 |
| Gr-1 (Ly6C/Ly6G) | ++ | +/− | ND | ND | 16 |
| Ly6C | ++ | +/− | ND | ND | 21 |
| Ly6G | − | − | ND | ND | 197 |
| Chemokine receptors | |||||
| CCR1 | ND | ND | + | − | 16, 10 |
| CCR2 | + | − | + | − | 17, 9, 10 |
| CCR4 | ND | ND | +/− | − | 16 |
| CCR5 | ND | ND | +/− | +/− | 9, 16, 10, 198 |
| CCR7 | ND | ND | +/− | − | 16 |
| CXCR1 | ND | ND | +/− | − | 16, 10 |
| CXCR2 | ND | ND | + | − | 16, 10 |
| CXCR4 | ND | ND | +/− | + | 16, 10 |
| CX3CR1 | +/− | ++ | +/− | ++ | 16, 10 |
| Other receptors and lineage markers | |||||
| 7/4 | + | − | ND | ND | 18 |
| CD4 | ND | ND | + | + | 199 |
| CD11a | + | ++ | ND | ND | 16, 10 |
| CD11c | − | + | + | + | 21, 16, 198, 199 |
| CD31 | + | + | + | + | 16 |
| CD32 (FcγRII) | ND | ND | + | ++ | 13 |
| CD33 | ND | ND | ++ | + | 13 |
| CD43 | − | + | ++ | + | 21 |
| CD49b | + | − | ND | ND | 16 |
| CD62L (L-selectin) | + | − | + | − | 17, 16, 10 |
| CD64 (FcγRI) | ND | ND | − | + | 12 |
| CD86 | ND | ND | + | ++ | 13 |
| MHC class II | Inducibleb | Inducibleb | + | ++ | 16 |
| NK1.1 | − | − | ND | ND | 16 |
| Scavenger receptor | ND | ND | +/− | + | 200 |
Surface expression levels have been arbitrarily designated as undetectable (−), marginal (+/−), positive (+), and high (++) based on flow cytometric analysis; ND, not determined.
MHC class II levels are marginal under homeostatic conditions but increase rapidly with infectious or inflammatory stimulation (16).
CD14+ and CD16+ monocytes respond to distinct trafficking cues. CD14+ monocytes express high levels of CCR1, CCR2, and CXCR2 and low levels of CX3CR1, whereas CD16+ monocytes express high levels of CX3CR1 and low levels of CCR2 (9, 10). Accordingly, CD14+ monocytes respond to monocyte chemoattractant protein (MCP)-1 (CCL2), whereas CD16+ monocytes respond to fractalkine (CX3CL1) in transendothelial migration assays (10). CD14+ monocytes express higher levels of CD62L (L-selectin) and CD11b (also referred to as Mac-1 or CR3) and lower levels of MHC class II than do CD16+ monocytes (11). A detailed review describing the differences between human monocyte subsets has been published recently (3).
Additional, albeit smaller, monocyte subsets can also be distinguished by surface molecule expression. For example, a population of CD14+CD16+CD64+ monocytes is highly phagocytic, like CD14+ monocytes, but expresses high levels of MHC class II, like CD16+ monocytes (12, 13). This subset, referred to as transitional monocytes, can activate T cells. Their developmental relationship to CD14+ and CD16+ monocytes remains unclear. Another small subset, constituting ~1%–2% of mononuclear cells (14), expresses CD56, a neural cell adhesion molecule isoform. The frequency of CD16+CD56+ monocytes is increased in patients with inflammatory bowel disease (15).
MURINE MONOCYTE SUBSETS
Owing to possible species-specific differences in receptor expression and the absence of useful monoclonal antibody reagents, murine monocyte subsets are not distinguished by CD14 and CD16 expression. Murine blood monocytes express CD115 [the receptor for macrophage colony stimulating factor (CSF-1R)], CD11b, and low levels of the F4/80 antigen. Murine monocyte subsets are distinguished by differential Ly6C, CX3CR1 (16), CCR2 (17), and 7/4 (18) expression (Table 1). Engineered expression of green fluorescent protein (GFP) from the CX3CR1 locus (termed CX3CR1gfp/+ mice) (19) has enabled monocyte subset isolation and adoptive transfer studies (16). GFPdim monocytes express low levels of CX3CR1 and high levels of CCR2 and Ly6C and are most similar to human CD14+ monocytes. GFPbright monocytes express high levels of CX3CR1 and low levels of Ly6C and do not express CCR2; they are most similar to human CD16+ monocytes.
CX3CR1lowCCR2+Ly6C+ monocytes (henceforth referred to as Ly6C+ monocytes) are granular and larger than CX3CR1+CCR2− Ly6Clow monocytes (referred to herein as CX3CR1+ monocytes), with typical diameters of 10–14 μm and 8–12 μm, respectively (16). In adoptive transfer experiments, Ly6C+ monocytes home to peripheral tissues in response to inflammatory stimuli, prompting their designation as inflammatory monocytes. Following recruitment to the inflamed peritoneum, Ly6C+ monocytes upregulate CD11c and MHC class II and migrate to draining lymph nodes, where they can promote T cell proliferation, suggesting that this monocyte subset differentiates into DCs (16). Ly6C+ monocytes have a short transit time in the bloodstream and are not recovered from peripheral tissues in the absence of inflammation (16) but instead home to the bone marrow (20). CX3CR1+ monocytes remain in the circulation for longer periods and traffic into peripheral tissues under noninflammatory conditions (16). These cells reconstitute tissue macrophages and DCs and are referred to as resident monocytes.
A third subset, constituting only ~5% of circulating murine monocytes, expresses intermediate levels of Ly6C (21) and may correspond to human CD14+CD16+CD64+ monocytes. Murine Ly6Cint monocytes and human CD14+CD16+CD64+ monocytes express a broader array of chemokine receptors than do CD14+ or CD16+ monocytes (22, 23).
Although circulating human and murine monocytes have been divided into two principal and several minor subsets, there are important species-specific differences. First, the relative frequencies of the two major subsets are different in mice and humans. Under resting conditions, CD14+ monocytes predominate in the bloodstream of humans, whereas Ly6C+ and CX3CR1+ monocytes in mice are present in roughly similar proportions. Second, human CD16+ monocytes synthesize high levels of inflammatory cytokines following TLR stimulation, whereas murine Ly6C+ monocytes, which in terms of chemokine receptor expression are more similar to human CD14+ monocytes, are more responsive to TLR stimulation. Thus, the designation of human CD16+ monocytes as proinflammatory and murine Ly6C+ monocytes as inflammatory can create confusion. To some extent, identification of monocyte subsets in different species using distinct surface markers likely accounts for some of these disparities.
MONOCYTE DEVELOPMENT AND DIFFERENTIATION
Progenitors in the Bone Marrow
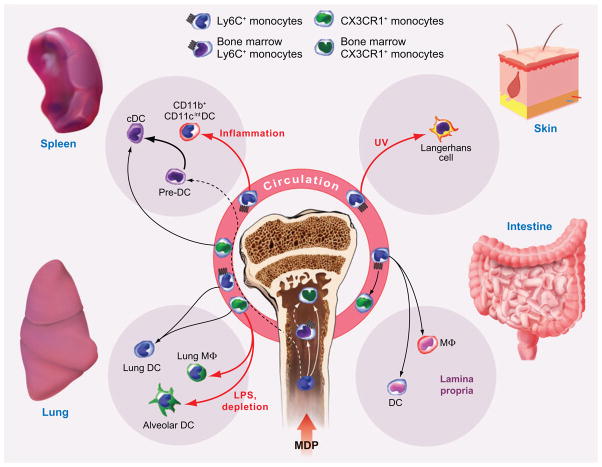
Circulating murine monocytes descend from self-renewing hematopoietic stem cells that initiate myeloid differentiation and give rise to multipotent precursors (24, 25). These multipotent cells are lineage-associated marker negative (Lin−), Sca-1+, and CD117+ (c-kit) and give rise to lineage-restricted common myeloid progenitor cells (CMPs) (26) and common lymphoid progenitor cells (CLPs) (27). Granulocyte macrophage progenitors descend from CMPs. Recently, myeloid lineage macrophage-DC progenitors (MDPs) were isolated from bone marrow suspensions of CX3CR1gfp/+ mice (28) as GFP+CD117+Lin− cells that give rise to macrophages and DCs, but not to neutrophils. When introduced into bone marrow, MDPs give rise to Ly6C+ and CX3CR1+ bone marrow monocytes, which give rise to the two principal circulating subsets (20, 28) (Figure 1).
Figure 1.
Monocyte differentiation into DCs and tissue macrophages. Macrophage-DC progenitors (MDPs) give rise to Ly6C+ bone marrow monocytes, which exit the bone marrow, in part guided by CCR2-dependent signals. Ly6C+ monocytes convert into CX3CR1+ monocytes, although the location of this event, in the circulation or bone marrow, remains incompletely understood. Black arrows indicate differentiation steps into tissue DCs and macrophages that occur under homeostatic conditions. Red arrows indicate differentiation steps that occur under inflammatory conditions (UV-induced skin injury, intratracheal LPS administration, or depletion of autologous CD11c+ cells). Dashed arrows represent steps that remain uncertain. In the case of splenic cDCs, splenic pre-DCs are the most significant upstream precursor in numeric terms (bold arrow), although MDPs and CX3CR1+ monocytes may contribute as well.
Relationship Between Murine CX3CR1+ and Ly6C+ Blood Monocytes
Exit of Ly6C+ murine monocytes from the bone marrow is driven, at least in part, by CCR2-mediated signals. The number of circulating Ly6C+ murine monocytes in CCR2−/− mice under homeostatic conditions and following systemic microbial infection (29, 30) is markedly diminished. In contrast, the frequency of circulating CX3CR1+ monocytes is similar in CCR2+/+ and CCR2−/− mice (23). Following depletion of circulating monocytes, Ly6C+ monocytes reach pretreatment levels in the bloodstream in three to four days (21). In contrast, CX3CR1+ monocytes return to the circulation seven days after depletion (21). To determine whether CX3CR1+ monocytes descend from Ly6C+ monocytes, the latter cells were labeled with fluorescent liposomes or latex microspheres following systemic depletion (21) or under steady-state conditions (31). In both cases, labeled monocytes converted from a Ly6C+ to a Ly6Clow phenotype, indicating that Ly6C+ monocytes mature into CX3CR1+ monocytes. Adoptive transfer studies in rats give similar results (32).
MONOCYTE DIFFERENTIATION IN VIVO
Circulating monocytes are precursors for tissue macrophages and many DC subsets (3). Monocytes give rise to DCs in vitro (33) and in vivo (16, 34–36), and microbial infection triggers in vivo monocyte differentiation into specialized DC populations that enhance microbial clearance (37, 38). The developmental pathways for DC sublineages in mice and humans are complex and have been reviewed comprehensively (39, 40). The contribution that circulating monocytes make to the formation and replenishment of specific DC subsets and tissue macrophages has been the focus of a number of interesting recent experiments. Many of these studies used adoptive cell transfer to investigate monocyte trafficking and differentiation. Several themes concerning the role of circulating monocytes in the repopulation of tissue macrophages and DCs are emerging.
Monocyte Differentiation into Splenic Macrophage and DC Subsets
The major splenic conventional DC (cDC) subsets (CD8+CD4−, CD8−CD4−, and CD8−CD4+) turn over rapidly, with half-lives that range from one and a half to seven days (41, 42). To maintain steady-state splenic cDC populations in mice, a daily influx of ~105 circulating progenitor cells is required (42). Previous studies have identified several candidate circulating DC precursors that appear to be distinct from monocytes (43, 44) or that are monocytic in origin (45). In addition, non-monocytic intrasplenic DC precursors with limited potential for cell division, termed pre-DCs, contribute to the maintenance of all cDC subsets (46, 47).
Under homeostatic conditions, MDPs contribute to the steady-state splenic mononuclear phagocyte pool because adoptively transferred cells give rise to CD8+ and CD8− cDCs as well as to splenic marginal zone and marginal sinus macrophages in nonirradiated recipient mice (28). In contrast, more differentiated bone marrow cell populations were much less efficient than MDPs in generating DCs (28) (20). In a separate study, splenic-resident pre-DCs gave rise to all CD8+ and CD8− DC subsets and were 50-fold more efficient in generating CD8−cDCs than were purified blood Ly6Clow monocytes (46). Thus, in the steady state, MDPs and splenic pre-DCs, rather than bone marrow and circulating monocytes, appear to reconstitute cDCs most effectively (Figure 1).
If recipient mice are irradiated, adoptively transferred Ly6C+ bone marrow monocytes readily give rise to splenic CD8+ and CD8−cDCs as well as F4/80+ splenic macrophages (45). In the setting of systemic inflammation, Ly6C+ monocytes differentiate into splenic DCs with a CD11b+CD11cintMac-3+ phenotype, distinct from the major cDC subsets (46). The phenotype of these inflammation-induced DCs is similar to TNF- and inducible nitric oxide synthase (iNOS)-producing DCs (TipDCs) that infiltrate the spleen during systemic listeriosis (37). In mice depleted of CD11c+ cells (48), adoptive transfer of MDPs or Ly6C+ bone marrow monocytes yields a similar population of CD11b+CD11cint splenic cells (20). Thus, both Ly6C+ and Ly6Clow monocytes have the capacity to differentiate into splenic DC subsets under specific host circumstances, although their contribution in the steady state may be limited (Figure 1).
Monocyte Differentiation into Intestinal and Pulmonary Mononuclear Phagocytes
The intestinal lumen and bronchoalveolar space represent major portals of entry for pathogenic microbes. Monocyte descendants play a major role in surveillance and immune defense in these tissues. In the intestinal lamina propria, intravenously transferred MDPs or Ly6C+ bone marrow monocytes differentiate into CX3CR1highCD11c+ DCs and CX3CR1lowCD11c+ macrophages (20) (Figure 1).
The respiratory tract and lung contain a number of resident macrophage and DC subsets that can be distinguished by surface antigen expression and localization (49–51). Major subsets include alveolar and lung macrophages, with a CD11c+CD11b−CX3CR1− surface phenotype, and CD11c+ CD11b+CX3CR1+ lung DCs (49, 52). Adoptively transferred CX3CR1+ monocytes traffic to the lungs of recipient mice in the steady state (16), and these cells, along with Ly6C+ monocytes, give rise to pulmonary DCs (52). In the setting of local inflammation (via intratracheal LPS administration) or depletion of autologous respiratory tract CD11c+ cells, CX3CR1+ monocytes give rise to lung macrophages and alveolar DCs as well (52) (Figure 1). In the steady state, alveolar macrophages are long-lived cells that turn over slowly (40%–60% replacement in one year) in bone marrow chimeric mice (53, 54). Although induction of local inflammation accelerates their turnover (54), the role of monocytes and local precursors in their replenishment remains unresolved.
Monocyte Differentiation into Langerhans Cells, Dermal DCs, and Lymph Node DCs
Dermal DCs and Langerhans cells (LCs) contribute to skin immunity by forming a cellular surveillance system throughout the epidermis and delivering foreign antigens to draining lymph nodes. To maintain steady-state numbers, LCs are replenished by local precursors. Upon tissue damage, such as after intense UV irradiation, circulating precursors are required for replenishment. Although both myeloid and lymphoid progenitors (particularly fetal liver kinase 2+ cells) can yield LCs, CMPs are ~20-fold more efficient in this process than are CLPs (55). In vitro, circulating human CD14+ monocytes differentiate into LCs through a CD14+CCR6+langerin+ dermal precursor (56). In a murine model of UV-induced skin injury, Ly6C+ murine monocytes labeled with latex particles enter the skin within four days, proliferate, and differentiate into MHC class II+langerin+ LCs (36) (Figure 1). F4/80+CD68+ dermal macrophages descend from infiltrating Ly6C+ monocytes as well. Inflammation in the skin also promotes Ly6C+ monocyte trafficking from the bloodstream to skin-draining lymph nodes via high endothelial venules (HEVs) (17). In this setting, MCP-1, a chemokine that triggers CCR2 signaling, is transported from the inflammatory focus in the skin to the draining lymph node, where it binds to the luminal surface of HEVs and mediates entry of CCR2-expressing Ly6C+ monocytes into the lymph node (17).
Under infectious or inflammatory conditions, skin-draining lymph nodes contain a number of non-LC DC populations that have been implicated in antigen presentation and T cell priming (57). CD11b+F4/80− monocytic cells injected subcutaneously acquire fluorescent latex particles and mature into CD11cdimMHC class II+ cells (35). In this experimental model, the Ly6Cint murine monocyte subset may represent the relevant DC precursor (23).
MONOCYTES IN MUCOSAL IMMUNITY
The mammalian intestine is home to complex microbial populations and also serves as a portal of entry for a wide range of pathogens. Many intestinal pathogens penetrate the intestinal mucosa and thereby come in direct contact with the extensive network of DCs in submucosal tissues (58). In most cases, entry of pathogens through the intestinal mucosa occurs via epithelial M cells, which overlay Peyer’s patches (59–62), and requires expression of a specialized set of bacterial virulence factors. For example, the enteric pathogen Salmonella typhimurium traverses M cells by expressing a family of genes encoded in the Salmonella pathogenicity island (SPI)-1 locus that injects virulence factors into epithelial cells (63). S. typhimurium, however, can also traverse the intestinal epithelium by an alternate route. SPI-1-deficient S. typhimurium, for example, is taken up by circulating CD18-expressing monocytes and disseminates from the intestine to spleen and liver (64). Remarkably, although CD18-deficient mice are more susceptible to splenic and hepatic infections following the intraperitoneal challenge, they are more resistant to intestinal infection by SPI-1-deficient S. typhimurium than are wild-type mice.
Although intestinal epithelial cells form a tight barrier separating bowel contents from submucosal tissues, recent studies indicate that DCs in the submucosa can extend dendrites into the bowel lumen and interact with intestinal bacteria. Rescigno et al. (65) demonstrated that CD11c-expressing cells are rapidly recruited to intestinal loops following S. typhimurium infection. Intraepithelial CD11c+ (65) and CD11b+CD8α− (66) DCs express tight junction proteins that interact with epithelial cell tight junctions, thereby allowing dendrites to pass between intestinal epithelial cells without disrupting the integrity of the barrier. Intravital microscopy of small bowel explants (67) demonstrated that formation of DC extensions requires MyD88-mediated signals in nonhematopoietic cells, presumably intestinal epithelial cells. Sampling of luminal bacteria by CD11c+ DCs can lead to direct infection of these cells (65, 67, 68) and transport of bacteria from the intestinal tract to mesenteric lymph nodes (MLNs) (68, 69). Intestinal DCs promote IgA synthesis by B cells and may prevent mucosal dissemination by commensal bacteria (68, 195). Intestinal TipDCs induce mucosal IgA secretion, and TipDC-derived nitric oxide (NO) is essential for this function (196).
The lamina propria of the small and large intestine contains extensive networks of CX3CR1-expressing DCs (70), which may originate from circulating CX3CR1high monocytes (16). CX3CL1/fractalkine, the ligand for CX3CR1, is expressed by intestinal epithelial and endothelial cells (71–73) and is required for formation of transepithelial DC extensions. CX3CR1-expressing cells can engulf both nonpathogenic and pathogenic bacteria and transport them to MLNs (70). CX3CR1-deficient mice are more susceptible to infection with S. typhimurium, suggesting that CX3CR1 signaling contributes to antimicrobial responses. It remains unclear whether enhanced susceptibility results from impaired induction of systemic immune responses or whether CX3CR1-expressing cells directly exert bactericidal activity.
Microbial infection induces the recruitment of monocytes to mucosa-associated lymphoid tissues. Following oral infection with S. typhimurium, CD11c+CD11b+ monocytes accumulate in infected organs (74). A recent report demonstrated increased frequencies of monocytes in blood and their recruitment to Peyer’s patches and MLN following oral S. typhimurium innoculation (75). These recruited monocytes resemble Ly6C+ monocytes because they express F4/80, CD11b, CCR2, and CD68, and they are the main producers of TNF and iNOS during early S. typhimurium infection (Figure 2). Although protection against Salmonella requires secretion of TNF and reactive nitrogen intermediates (RNI), it is not known whether recruitment of inflammatory monocytes is critical for host survival during infection (Table 2).
Figure 2.
Effector functions of inflammatory monocytes. In the absence of inflammation, bone marrow CCR2+ monocytes have an immature phenotype and are characterized by low levels of expression of MHC class II and costimulatory molecules. Following infection, monocytes are released into the peripheral circulation and migrate to sites of inflammation, where they express distinct effector phenotypes and undergo differentiation into DCs. The effector functions of CCR2+ monocytes are dictated by the inflammatory context and by the nature of the invading pathogen. (a) Following infection with L. monocytogenes, monocytes are first present in the marginal zone area of the spleen and subsequently migrate to the white pulp area, where bacterial lesions are established. Monocytes undergo differentiation into TipDCs and surround infected cells, thus preventing bacterial dissemination from the lesion. While most CCR2+ monocytes are not infected in vivo, monocytes in the peripheral circulation may become infected and transport bacteria to the CNS. (b) In the gastrointestinal tract, infection with S. typhimurium induces influx of inflammatory monocytes and their differentiation into TipDCs. (c) Although less is known regarding the function of monocytes during M. tuberculosis infection, they are recruited to the lung and may function as a source of nitric oxide (NO). (d ) During infection with T. gondii, inflammatory monocytes become directly infected and secrete IL-12 and NO and kill parasites. T. gondii–infected monocytes may also be involved in transport of parasites to the brain.
Table 2.
Microbial pathogenesis in the murine models of infectious disease
| Microbe | Route of infection | Target cell/site and localization | Innate immune effector molecules required for resistance | Impact of CCR2 deficiency |
|---|---|---|---|---|
| Bacteria | ||||
| L. monocytogenes | Gastrointestinal | Intestinal epithelium, hepatic and splenic macrophages, hepatocytes (intracellular, cytoplasmic) | TNF, IFN-γ, RNI, ROI | ↑ susceptibilitya |
| M. tuberculosis | Inhalation | Macrophages (intracellular, vacuolar) | TNF, IFN-γ, IL-12, RNI | ↑ susceptibility (high-dose iv infection)a |
| S. typhimurium | Gastrointestinal | Intestinal epithelium, macrophages (intracellular, vacuolar) | TNF, IFN-γ, IL-12, ROI, RNI | Not determined |
| Protozoa | ||||
| T. gondii | Gastrointestinal | Macrophages and many nucleated cells (intracellular, vacuolar) | TNF, IFN-γ, IL-12 | ↑ susceptibilitya |
| Fungi | ||||
| A. fumigatus | Inhalation | Alveolar macrophages (intracellular conidia) | ROI | ↑ pulmonary allergic responses |
| Alveolar spaces and lung tissue (extracellular conidia and hyphae) | ↓ fungal clearanceb | |||
monocytes implicated.
role of monocytes not known.
MONOCYTES IN THE IMMUNE RESPONSE TO LISTERIA MONOCYTOGENES
Innate Immune Responses to Listeria monocytogenes
Listeria monocytogenes is a Gram-positive facultative intracellular bacterium that infects a wide range of invertebrate and vertebrate hosts. One of the first manuscripts describing infection with this pathogen noted an increased number of monocytes in tissues of infected rabbits, leading to the species name “monocytogenes” (76). L. monocytogenes is acquired via the gastrointestinal tract. Successful clearance of L. monocytogenes requires activation of innate and adaptive immune responses. Innate immunity is rapidly triggered following infection and restricts in vivo bacterial growth (77). A summary of bacterial pathogenesis and innate immune defenses is shown in Table 2. Innate immune responses to L. monocytogenes infection require synthesis of TNF, IFN-γ, IL-12, and IL-18, whereas deficiency in type I interferon (IFN) receptors or the IFN regulatory factor (IRF)-3 transcription factor renders mice more resistant to infection (77).
Monocyte Function During Innate Immune Responses
Cells of myeloid lineage play a key role in defense against L. monocytogenes infection (77). Infection with L. monocytogenes induces an influx of monocytes and macrophages to sites of infection (78). Maximum recruitment of monocytes occurs 72 to 96 h following infection and thus is delayed relative to granulocyte recruitment (78). In vivo administration of RB6-8C5 antibody specific for Gr1, an epitope that is expressed on Ly6G and Ly6C antigens (79), leads to depletion of granulocytes and a subset of monocytes and renders mice highly susceptible to L. monocytogenes infection (80, 81). Gr1-expressing cells are most critical during the first 24 h of the innate immune response (81, 82).
In vivo administration of the 5C6 antibody, which blocks CD11b, renders mice highly susceptible to L. monocytogenes infection (83). Blocking CD11b abrogates monocyte and granulocyte accumulation in spleen and liver but, as with Gr1-depletion, only enhances susceptibility to L. monocytogenes infection during the first 24 h of infection. In the absence of CD11b-mediated granulocyte and monocyte recruitment, L. monocytogenes replicates within nonphagocytic cells, such as hepatocytes, and also extracellularly. Thus, CD11b-mediated recruitment of inflammatory cells is essential for bacterial containment and killing early during infection.
Role of Monocytes in Bacterial Dissemination
In humans, infection with L. monocytogenes is often associated with bacteremia and frequently results in the development of meningitis (84, 85). Although mouse models of systemic L. monocytogenes infection do not precisely replicate the course of human meningeal infection, infection of mice with very high inocula of bacteria has been used to investigate the pathogenesis of L. monocytogenes meningitis. In these settings, bloodstream L. monocytogenes is found predominantly in circulating monocytes (21, 86). The majority of infected monocytes express CD11b and high levels of Ly6C (87) and thus resemble inflammatory monocytes (16). Monocytes may also be infected in bone marrow prior to entering peripheral circulation (88).
Following infection with L. monocytogenes, Ly6C+ monocytes are recruited into the brain parenchyma, supporting the notion that these monocytes carry bacteria into the central nervous system (CNS) (87). Adoptive transfer of L. monocytogenes–infected Ly6C+CD11b+ bone marrow monocytes leads to CNS infection as early as 6 h post-transfer. Because dissemination of L. monocytogenes to the brain occurs in mice treated with gentamicin, an antibiotic that kills extracellular but not intracellular bacteria (87, 89), it has been argued that L. monocytogenes uses monocytes as a Trojan horse to enter the CNS.
Bactericidal Functions of Monocytes During Infection
Monocytes kill bacteria by producing reactive nitrogen intermediates (RNIs) and reactive oxygen intermediates (ROIs) (90) and through the action of phagolysosomal enzymes (91). Administration of iNOS inhibitor aminoguanidine and genetic deficiency in iNOS, gp91, or p47phox renders mice more susceptible to L. monocytogenes infection (92–94), implicating these mechanisms in bacterial clearance (Table 2). Signaling through TLR molecules is essential for protection during L. monocytogenes infection, and mice deficient in the TLR adaptor molecule, MyD88, are highly susceptible to infection (95, 96). MyD88 deficiency is associated with diminished IL-12, IFN-γ, TNF, and NO responses (95, 96), and thus MyD88-deficient mice are more susceptible to L. monocytogenes infection than mice lacking IFN-γ or both IL-12 and IFN-γ (96).
Although the preceding studies demonstrated the importance of TLR signaling in defense against L. monocytogenes, it remains unclear in which cell population TLR signaling is most critical. A recent study took advantage of the essential role of gp96, an endoplasmic reticulum chaperone, in the folding of TLR molecules. gp96−/− macrophages fail to respond to intracellular and cell surface TLR ligands but respond normally to activation by IFN-γ, TNF, and IL-1. Using mice with monocyte- and macrophage-specific deletion of gp96, these investigators demonstrated that TLR signaling in these cells is essential for defense against L. monocytogenes despite intact signaling via IFN-γ, TNF, and IL-1 receptors (97).
Recruitment of TNF- and iNOS-Producing Monocytes During L. monocytogenes Infection
Mice lacking CCR2 are highly susceptible to L. monocytogenes and succumb to infection within four days, a time frame that indicates failure of innate immune defenses (98). L. monocytogenes–infected, CCR2-deficient mice have normal levels of IL-12 and IFN-γ but markedly diminished levels of TNF and iNOS in infected spleens (37). During the first three days of systemic infection, TNF and iNOS are predominantly expressed by a population of monocyte-derived TipDCs that are recruited to foci of infection (Figure 2). Recruitment of TipDCs to spleen does not occur in CCR2-deficient mice. In infected spleens, TipDCs express CD11b, low or intermediate levels of CD11c, high levels of intracellular Mac-3, high levels of Ly6C, and variable levels of F4/80 (29, 37) and are distinct from conventional and plasmacytoid DCs. TipDCs express high levels of MHC class II and costimulatory molecules during L. monocytogenes infection.
TipDCs recruited to infected spleens are derived from bone marrow monocytes. Although Ly6C+ monocytes are present in the peripheral circulation and spleen of L. monocytogenes–infected wild-type mice, they are absent from the blood and spleen of infected CCR2-deficient mice but instead accumulate in the bone marrow (29). In the absence of infection, Ly6C+ cells in the bone marrow do not express MHC class II or CD11c. In vitro culture of these progenitors with listerial antigens and IFN-γ leads to upregulation of MHC class II and CD11c molecules and induces iNOS, recapitulating the TipDC phenotype seen during in vivo infections. Interestingly, Ly6C+ monocytes accumulating in the bone marrow of CCR2-deficient mice during infection express MHC class II and costimulatory molecules but not CD11c, suggesting that the bone marrow environment only induces partial monocyte differentiation. It is not clear whether Ly6C+ monocytes accumulating in the bone marrow contribute to antimicrobial responses. However, despite uncontrolled bacterial growth in spleen and liver of CCR2-deficient mice, bacterial numbers are comparable or slightly lower in bone marrow of CCR2-deficient mice compared with wild-type mice, suggesting that monocytes retained in the bone marrow of CCR2-deficient mice mediate antimicrobial effects.
Antimicrobial Function of TipDCs
Failure to recruit TipDCs to sites of infection diminishes clearance of L. monocytogenes (Table 2). TipDCs produce the highest amounts of TNF of any cell population in the L. monocytogenes–infected spleen. However, because many hematopoietic and nonhematopoietic cells can secrete TNF, it remains unclear which source of TNF is essential for bacterial clearance. One recent study demonstrated that mouse strains with selective depletion of TNF in monocytes and macrophages are highly susceptible to infection, suggesting that these cell populations are the relevant source of TNF in defense against L. monocytogenes infection (99). Interestingly, deficiency in type I IFN signaling leads to increased numbers of TNF-producing mononuclear cells (100), although the mechanisms that lead to this increase remain incompletely defined.
NO production is also diminished in spleens of infected CCR2-deficient mice, but whether iNOS expression by TipDCs contributes directly to in vivo microbial killing remains unclear. TipDCs do not appear to be directly infected in vivo, suggesting that NO produced by these cells may act on local cells to enhance microbicidal activity. Alternatively, bacteria may be very rapidly killed and degraded by infected TipDCs, so that infected cells are not readily detected. Although recruitment of TipDCs to spleen is MyD88-independent, TNF secretion by TipDCs requires MyD88-mediated signals (101). TNF and iNOS production by recruited CCR2-expressing monocytes may be important in immune protection against some but not all bacterial pathogens. During Escherichia coli–induced urinary tract infection, TipDCs are rapidly recruited to the infected bladder but do not appear to participate in protective immune responses (102).
MONOCYTE RECRUITMENT DURING MYCOBACTERIUM TUBERCULOSIS INFECTION
Mycobacterium tuberculosis is an inhaled intracellular bacterial pathogen that persists in macrophages of infected organs. Protective immunity to tuberculosis is T cell–mediated and requires secretion of IFN-γ, TNF, and IL-12 and production of RNIs (103). Successful activation of immune responses against mycobacteria requires signaling through MyD88 (104, 105). Pathogenesis of M. tuberculosis infection is summarized in Table 2.
A number of recent studies have focused on the recruitment and function of circulating monocytes during tuberculosis. Following aerosol infection of mice with M. tuberculosis, DCs, monocytes, macrophages, and granulocytes traffic into the bronchoalveolar space (49). Recruited monocytes express CD11b, F4/80, and CCR2 and thus are characterized as inflammatory monocytes. The frequency of F4/80+ monocytes is significantly reduced in CCR2−/− mice following M. tuberculosis challenge (106).
The susceptibility of CCR2-deficient mice is influenced by the dose of M. tuberculosis infection. CCR2 deficiency results in early mortality following high-dose intravenous (iv) challenge and aerosol challenge (107, 109). In this setting, CCR2 deficiency also leads to delayed T cell priming and a reduction in the number of IFN-γ-secreting CD4 T cells in the lung (107, 108). In contrast to high-dose iv infection, CCR2-deficient mice survive low-dose aerosol infection despite reduced recruitment of alveolar macrophages, diminished iNOS levels, and delayed T cell influx (109). Thus, CCR2+ monocytes may be required for protection when bacterial burdens are high, such as during systemic M. tuberculosis infection. In this setting, recruited monocytes may provide a source of iNOS (Figure 2). In contrast, the bactericidal functions of resident alveolar macrophages may be sufficient to control bacterial growth following low-dose aerosol infection with M. tuberculosis.
How much human CCR2-expressing monocytes participate in immune responses during human mycobacterial diseases is not known. CCR2-expressing cells are detected in human skin lesions caused by Mycobacterium leprae, the cause of leprosy (110). In vitro, human monocytes can be differentiated into two distinct subsets, DC-SIGN+CD16+ macrophages and CD1b+DC-SIGN− DCs. DC-SIGN+CD16+ and CD1b+DC-SIGN− cells can be detected in leprosy skin lesions (111), suggesting that monocyte recruitment and differentiation occur in the setting of human disease. A promoter polymorphism that induces hyperproduction of MCP-1 is associated with increased susceptibility to pulmonary tuberculosis (112). High levels of circulating MCP-1 may lead to desensitization of CCR2, thereby limiting the recruitment of monocytes to sites of lesions. This mechanism has been proposed as an explanation for the increased susceptibility of MCP-1 transgenic mice to infection with L. monocytogenes (113).
Generation of NO by human macrophages in vitro is difficult to demonstrate, and many different experimental strategies have rendered inconsistent results. However, iNOS expression in human monocytes can be induced in vitro in response to M. tuberculosis lipoproteins (114) and in vivo in the lungs of patients with active M. tuberculosis (115), suggesting that the RNI-mediated pathway may be operative in human infection. RNI-independent mycobacterial killing by human monocytes and macrophages has also been reported. Human monocytes can kill M. tuberculosis in a TLR-dependent and NO-independent manner (116).
MONOCYTE RECRUITMENT DURING TOXOPLASMA GONDII INFECTION
Toxoplasma gondii is a protozoan pathogen that infects a wide range of mammals including humans. T. gondii is an obligate intracellular parasite and resides in a vacuole in many different nucleated cell populations. Recent studies have demonstrated that monocyte recruitment is essential for initial restriction of T. gondii growth in the murine mouse model of toxoplasmosis (117, 118). Protection against T. gondii requires MyD88-mediated signaling and induction of IL-12, IFN-γ, and IFN-γ-inducible p47 GTPase but is independent of RNI production (119, 120). Table 2 depicts the summary of pathogenesis and innate immune responses during toxoplasmosis.
Gr-1-expressing monocytes are recruited to the peritoneum four to five days following intraperitoneal infection of mice with an attenuated strain of Toxoplasma gondii (117). Recruited monocytes express CD68, CD11b, F4/80, and iNOS and secrete IL-12p40 in vivo. T. gondii infects monocytes in the peritoneum, stimulating upregulation of MHC class II and costimulatory molecules and differentiation into DCs. Monocytes purified from peritoneum of infected mice inhibit parasite replication in vitro by NO-independent mechanisms. Recently, Ling et al. (121) demonstrated that in vivo destruction of T. gondii by peritoneal CD11b+F4/80+ macrophages occurs via stripping of the parasite plasma membrane followed by fusion with autophagosomes.
CCR2-deficient mice are more susceptible to T. gondii infection, a phenotype that correlates with diminished recruitment of Gr-1-expressing monocytes to sites of infection (118). Diminished recruitment of inflammatory monocytes to sites of T. gondii infection and increased in vivo parasite growth occur despite normal levels of IFN-γ and TNF. However, IL-12p70 levels are diminished in the serum of infected CCR2-deficient mice, suggesting that IL-12 secretion by inflammatory monocytes contributes to protection (Figure 2).
Inflammatory monocytes implicated in resistance to T. gondii infection resemble L. monocytogenes–induced TipDCs. However, they appear to mediate immune protection in a manner distinct from that of TipDCs. Monocytes elicited by T. gondii and L. monocytogenes infections may originate from the same circulating progenitor and be driven to differentiate along similar pathways, but, given the different molecular composition of these two pathogens, the inflammatory cues driving differentiation are likely distinct. Identifying the signals that drive monocyte trafficking and differentiation in these two settings will require additional experimentation.
Monocytes may be involved in the transport of T. gondii tachyzoites to the brain during infection (122). Following intragastric inoculation with T. gondii, parasites circulating in the bloodstream reside within monocytes. Adoptive transfer of T. gondii–infected monocytes results in the appearance of parasites in the brains of recipient mice.
ROLE OF MONOCYTES IN HOST DEFENSE AGAINST FUNGAL PATHOGENS
The filamentous mold Aspergillus fumigatus (see Table 2) and the encapsulated yeast Cryptococcus neoformans are ubiquitous in the environment, and mammalian infections are acquired via the respiratory route (123, 124). The fungal dimorph Candida albicans causes mucosal disease as well as systemic infections.
Tissue macrophages and neutrophils play critical roles in defense against fungal infection (123, 124). Although recruitment of monocytes to sites of fungal infection has been demonstrated in vivo (125), their role in fungal killing remains unclear. However, purified murine and human monocytes or cultured macrophages have been studied in vitro to characterize the induction of inflammatory and fungicidal mediators, rates of fungal killing (126, 127), and host cell and fungal transcriptional responses (128–130). Whether in vitro–defined mechanisms of fungal inactivation are operative in vivo and contribute to fungal clearance, however, will require further studies.
Recent studies in CCR2-deficient mice indicate that inflammation-induced monocyte recruitment contributes to host antifungal immune responses. In murine pulmonary cryptococcosis, CCR2−/− mice are unable to control fungal growth. Increased susceptibility is associated with diminished pulmonary macrophage recruitment and the induction of maladaptive Th2-biased T cell responses (131, 132). Although these defects are likely related to impaired recruitment of inflammatory monocytes to sites of C. neoformans infection, it is possible that CCR2-expressing natural killer (NK) and T cell subsets (133) contribute to the phenotype of CCR2−/− mice.
In a murine model of allergic disease associated with A. fumigatus antigen exposure, CCR2−/− mice exhibit prolonged pulmonary allergic responses, airway inflammation, and delayed clearance of fungal antigens, suggesting that CCR2 signaling restricts the development of fungus-associated asthmatic disease (134, 135). One hypothesis to explain this finding is that CCR2-mediated recruitment of monocytes sways A. fumigatus–specific CD4 T cell responses toward a Th1 as opposed to a Th2 phenotype. Alternatively, CCR2-mediated recruitment of cells other than inflammatory monocytes may be critical in defense against A. fumigatus infection. In an experimental setting of invasive aspergillosis, CCR2+ NK cells mediate protective effects (136).
MONOCYTES IN REGULATION OF ADAPTIVE IMMUNE RESPONSES
Given the capacity of monocytes to differentiate into DCs upon in vitro culture with GM-CSF and IL-4, it is reasonable to speculate that monocytes contribute to the initiation and differentiation of T cell responses (45, 137, 138). Although inflammatory stimuli generally promote monocyte differentiation into DCs and their migration to lymph nodes (35), in some settings TLR-mediated signals block monocyte differentiation into DCs (139). Nevertheless, increasing experimental evidence supports the notion that circulating monocytes impact T cell responses.
Regulation of T cell responses by monocytes in the setting of microbial infection is complex, with positive and negative contributions to T cell proliferation and differentiation. CD8 T cell responses to an attenuated strain of L. monocytogenes, for example, are enhanced in CCR2-deficient mice, suggesting that CCR2+ monocytes negatively regulate CD8 T cell proliferation or survival (37). Because NO inhibits proliferation of T cells (140, 141), iNOS expression by CCR2+ monocytes may dampen T cell responses. However, it is possible that other effector functions of CCR2+ cells inhibit T cell responses. For example, monocytes and their derivative cells secrete IL-10 in response to some microbial stimuli (142–148), and Ly6C+ monocytes purified from bone marrow secrete IL-10 in response to stimulation with heat-killed L. monocytogenes (N.V. Serbina & E.G. Pamer, unpublished results). T cell suppression by IL-10-secreting Gr-1+CD11b+ cells during polymicrobial sepsis has also been reported (149). In this experimental system, Gr-1+CD11b+ cells expand in spleen, lymph nodes, and bone marrow during sepsis in a MyD88-dependent manner.
In contrast to L. monocytogenes infection, during M. tuberculosis infection CCR2+ monocytes enhance T cell priming and Th1 differentiation (106–108). Although T cells can express CCR2 (133, 150), defects in T cell recruitment during tuberculosis in CCR2-deficient mice are attributed to impaired monocyte and DC trafficking and are independent of T cell CCR2 expression (106).
In the setting of cutaneous Leishmania major infection, CCR2+ monocytes are recruited to skin lesions (38, 151). In L. major–infected CCR2-deficient mice, protective Th1 responses are attenuated, whereas Th2 responses are enhanced, which impairs microbial clearance. Following cutaneous infection, monocytes enter draining lymph nodes via afferent lymphatics and HEVs and give rise to functionally distinct DC subsets that are not present in the steady state (38). Within the infected dermis, monocytes differentiate into dermal DCs with a CD11cintLy6ChighMHC classIIintCD86low phenotype and mature into CD11cintLy6ClowMHC class IIhighCD86high DCs upon transit to the lymph node (38). Intravenously or subcutaneously transferred monocytes differentiate into this DC subset, which exhibits high T cell stimulatory capacity ex vivo. In contrast, a second CD11cintLy6ChighMHC class IIintCD86− DC subset enters popliteal lymph nodes via HEVs because intravenous, but not subcutaneous, monocyte transfer results in their appearance. This DC subset is phenotypically less mature and primes CD4 T cells less efficiently than does the dermal-derived DC subset described above.
Direct, monocyte-mediated priming of T cell responses was demonstrated using OVA-conjugated particles. In this system, circulating B cells and neutrophils transferred antigens to immature monocytes in bone marrow (152), which then traffic to spleen and lymph nodes and prime OVA-specific T cells. Although it is unclear whether this route of antigen presentation occurs during microbial infections, circulating CD11b+Gr-1+ monocytes can internalize bacterial antigens in blood and traffic to splenic marginal zones where they interact with B cells and induce differentiation into plasmablasts and T cell–independent antibody responses (153).
CHEMOKINES AND CHEMOKINE RECEPTORS
Optimal immune responses to infection depend on chemokine networks to facilitate recruitment of specific leukocytes to sites of infection. Chemokine-mediated monocyte recruitment is pivotal for immune control of a variety of microbial infections. Chemokines are divided into four groups on the basis of the position of cysteine residues: C chemokines have one cysteine, CC chemokines have two adjacent cysteines near the amino terminus, CXC chemokines have an amino acid separating two cysteines, and CX3C chemokines have three amino acids located between two cysteines (154). CC chemokines trigger chemokine receptors on monocytes, basophils, eosinophils, T cell subsets, and DCs. The CCR2-binding chemokines MCP-1 (CCL2), MCP-2 (CCL8), MCP-3 (CCL7), and MCP-5 (CCL12) belong to this family. The only known member of the CX3C chemokine family is fractalkine (FKN, or CX3CL1). The soluble form of FKN is a potent chemoattractant for subsets of monocytes, T cells, and NK cells (155).
ROLE OF MCPs AND CCR2 IN MONOCYTE RECRUITMENT DURING INFECTION
MCP-1−/− mice are not as susceptible to L. monocytogenes infection as CCR2−/− mice (37, 101, 118), suggesting that MCP-1 is not the sole CCR2 ligand responsible for monocyte recruitment and that other CCR2 ligands are induced during infection. Recently, MCP-3−/− and MCP-2/5−/− mice have been generated and examined for monocyte trafficking (30). MCP-3−/− mice, like CCR2−/− and MCP-1−/− mice, have diminished numbers of inflammatory monocytes in peripheral blood. In contrast, MCP-2/5−/− mice have normal circulating monocyte numbers, indicating that MCP-1 and MCP-3 are the predominant CCR2 ligands maintaining homeostatic numbers of circulating inflammatory monocytes (30). The role of MCP-3, MCP-2, and MCP-5 in antimicrobial defense is not known.
Induction of MCPs by Infection and Inflammation
MCP-1 is induced during L. monocytogenes infection, with detectable levels in spleen 6 h after bacterial inoculation (98, 101). The source of MCP-1 during in vivo bacterial infection, however, remains unclear. Because γδ T cell–deficient mice have diminished MCP-1 mRNA levels in the liver following infection compared with wild-type mice, investigators have suggested that γδ T cells produce MCP-1 (156). In vitro, infection of murine macrophages and hepatocytes with L. monocytogenes rapidly induces MCP-1 expression (157). T. gondii infection also induces MCP-1 expression in vivo and increases MCP-1 mRNA expression in peritoneal exudate cells (118). In vitro, T. gondii infection induces MCP-1 secretion in human fibroblasts, epithelial cells, and astrocytes (158–161). MCP-1 secretion by fibroblasts following infection depends on the stage of the parasite (160). Infection with fungal pathogens, such as Aspergillus fumigatus, also induces in vivo MCP-1 expression, but the level of induction differs depending on the level of pre-existing immunity (134). MCP-1 production following M. tuberculosis infection has been examined in several different cell types in vitro. CD14+ blood monocytes from patients with active tuberculosis express higher levels of MCP-1 mRNA and protein than do CD14+ monocytes from healthy individuals with latent, inactive tuberculosis (162). The human A549 alveolar epithelial cell line infected with M. tuberculosis also expresses elevated MCP-1 mRNA and protein levels. Intracellular growth is necessary for M. tuberculosis to induce MCP-1 in alveolar epithelial cells, but neither mycobacterial virulence nor the rate of intracellular growth correlates with the level of chemokine production (162).
Although MCP-1 expression is commonly measured following infections or in an inflammatory setting, much less is known about the expression and regulation of the other major CCR2 ligands, MCP-2, MCP-3, and MCP-5. Whereas in some circumstances MCP-1 and MCP-3 expression are coordinately regulated, in others their expression levels differ (163, 164). MCP-3 expression by different cell types appears to be more restricted than MCP-1 expression. Murine MCP-5, the closest homolog of human MCP-1 in terms of amino acid sequence (165), is expressed in lymphoid tissues and the lung and attracts human monocytes in chemotaxis assays (165). Although its role in defense against infections is unclear, MCP-5 has been implicated in the migration of leukocytes through the lung interstitium (166) and also in the recruitment of fibrocytes to the lung in the setting of pulmonary fibrosis (167).
Induction and Regulation of MCP-1 Expression
In vitro studies characterizing MCP-1 induction have been performed in a range of different cell types [monocytes (168), fibroblasts (169), epithelial cells (169, 170), endothelial cells (171, 172), vascular smooth muscle cells (173)] under different conditions (170). These studies have generated a complex picture with many consistent themes, but they have also produced a number of contradictory findings. The contradictions likely reflect real differences in MCP-1 induction pathways in distinct cell types. A summary of surface receptors and downstream signaling molecules involved in MCP-1 production in different cell types is provided in Table 3.
Table 3.
Receptors and signaling molecules in MCP-1 production pathway
| MTECs |
|
| Mesothelial cells |
|
| Macrophages |
|
| Monocytes |
|
| HeLa cells |
|
TLRs and Nod molecules recognize bacterial ligands and initiate immune responses. Stimulation of TLR-2 and TLR-4 in mouse renal tubular epithelial cells (MTECs) and stimulation of TLR-1, TLR-2, TLR-3, TLR-4, and TLR-9 in macrophages induce MCP-1 (170). MCP-1 induction by Nod stimulation differs depending on the cell type being investigated. Although stimulation of bone marrow–derived macrophages and DCs with the synthetic Nod1 agonist KF1B does not induce MCP-1 production (174), stimulation of mouse mesothelial cells does induce MCP-1 production (175).
The contributions of innate immune signaling adaptor molecules and downstream kinases in MCP-1 regulation have been investigated in several systems. Induction of MCP-1 in bone marrow–derived macrophages following L. monocytogenes infection is MyD88-independent but requires bacterial invasion of the cytoplasm (101). In mesothelial cells, Nod1-mediated MCP-1 production, but not TLR-mediated MCP-1 production, requires RIP2-mediated signaling (175). Induction of MCP-1 in MTECs by TLR stimuli requires NF-κB activation but not p38 mitogen-activated protein kinase (MAPK) or c-Jun N-terminal kinase ( JNK) activity (170). In HeLa cells, however, induction of MCP-1 by T. gondii requires ERK1/2 and JNK MAPK activation, but it is independent of p38 MAPK (176). Induction of MCP-1 in human monocytes by M. tuberculosis requires NF-κB, ERK, and p38 MAPK signaling (177).
In addition to direct induction of MCP-1, stimulation of TLRs and Nods during infection also induces inflammatory cytokines, such as TNF and type I IFN. One interesting question is whether these inflammatory mediators also regulate MCP-1 production in vivo. In vitro, MCP-1 induction by TNF and type I and type II IFNs has been examined (169, 172, 178). TNF-mediated induction and regulation of MCP-1 have been investigated in fibroblasts and involve distal and proximal regulatory regions (178–184). The two functional κB sites located in the distal regulatory region and a GC-box in the proximal regulatory region are critical for TNF-mediated induction of MCP-1 (178). Sp1, a DNA sequence–specific transcription factor, is essential for MCP-1 promoter assembly and molecular communication between the two NF-κB-dependent sites (182). More recent studies demonstrated that Sp1 and NF-κB are required for histone acetylation within the MCP-1 promoter (179). Although TNF stimulates MCP-1 production in fibroblasts, TNF-mediated signaling is not required in vitro for MCP-1 production by macrophages or MTECs (170) or in vivo during L. monocytogenes infection (101).
Anti-inflammatory factors also modulate MCP-1 production. Glucocorticoids inhibit MCP-1 synthesis in a variety of cell types (185–187), a process that involves changes in MCP-1 mRNA stability. Steroid-induced mRNA degradation is attributed to a 224 nucleotide dexamethasone-sensitive region within the coding region of the MCP-1 message (173). Stabilization of MCP-1 mRNA is mediated by direct association with the glucocorticoid receptor (188).
MCP Structures and Functions: Oligomerization and Binding to GAGs
The structure of human MCP-1 has been extensively investigated. Two structural features of MCP-1 appear to be particularly important for its in vivo activity (189). The first relates to residues that enable dimerization. Point mutations in MCP-1 that prevent dimerization, but not association with CCR2, markedly abrogate in vivo inflammatory cell recruitment in mice (189). Interestingly, the same mutant forms of MCP-1 remain active in in vitro chemotaxis assays, indicating that the rules governing chemotaxis in vivo differ from those required for conventional in vitro chemotaxis assays. However, human MCP-3 is active in vivo despite the fact that it does not oligomerize (189), suggesting that MCP-3 and MCP-1 function differently. The second feature of MCP-1 that affects its in vivo activity relates to residues that associate with glycosaminoglycans (GAGs) (190). As with dimerization, point mutations that diminish association with GAGs abrogate in vivo inflammatory cell recruitment, whereas in vitro chemotaxis assays remain intact (191–193). In addition, the process of dimerization and GAG binding can be interdependent, as MCP-1 is induced to oligomerize when it binds to GAGs. Thus, both dimerization and association with GAGs are essential for in vivo MCP-1 activity. These two structural features are thought to facilitate the formation of highly localized foci of MCP-1, which in turn may generate chemotactic gradients that enable monocyte migration (194).
Models of MCP Function in vivo During Infection
Microbial infection induces MCP-1 production by a wide variety of cells. Whether MCP-1 is principally produced by cells that are directly infected or by bystander cells that respond to inflammatory cytokines or microbial molecules that are released into extracellular fluids is unresolved. Although many studies have measured chemokine levels in serum and in tissues such as spleen, liver, kidneys, and brain, it remains unknown whether the chemokines that are detected in these assays contribute to monocyte recruitment and antimicrobial defense. It is possible, however, that MCP-1 levels in serum promote monocyte emigration from bone marrow. Alternatively, serum MCP-1 may be irrelevant, and only MCP-1 produced within the bone marrow, potentially by uninfected cells responding to TNF, IFN-γ, or type I IFNs, may promote the emigration of monocytes from bone marrow into the circulation. Several models can be proposed to explain MCP-1-mediated recruitment of monocytes to sites of infection. In the simplest model, MCP-1 is produced and released by microbially infected cells, establishing a chemokine gradient that guides responding monocytes to the site of infected cells (Figure 3a). While attractive for its simplicity, this model does not explain how monocyte emigration from the bone marrow is induced by infection in spleen or other tissues, and it suggests that once serum chemokine levels increase to the high levels seen in many infections, MCP-1 gradients and the ability to guide monocytes to sites of infection would be lost. An alternative model for in vivo MCP-1 function is that chemokines bind to tissue-specific GAGs, possibly in bone marrow and also at sites of infection, and in this way guide monocytes out of the bone marrow into the bloodstream and then into infected tissues (Figure 3b). This model provides a mechanism by which MCP-1 produced in infected tissues can circulate to bone marrow, bind GAGs, oligomerize, and promote monocyte emigration. Although both models are supported by a number of in vitro studies and some in vivo experiments, they remain unproven. Further studies that elucidate the in vivo processes that promote monocyte recruitment in the setting of microbial infection will be exciting and may provide important opportunities to improve immune defense in the immunocompromised host.
Figure 3.
Models of in vivo MCP-1-mediated monocyte recruitment. During infection, MCP-1 is produced and secreted by microbially infected or by cytokine-stimulated uninfected cells. In the first model (a), secreted MCP-1 establishes a gradient across the distance from infection site and attracts monocytes to infection sites. In an alternative model (b), the MCP-1 gradient is established not by distance from chemokine production site but rather by chemokine binding with specific GAGs. Association with GAGs increases MCP-1 concentration in specific regions and further facilitates oligomerization of MCP-1.
SUMMARY
Inflammatory monocytes play an essential role in innate immune defense against microbial infection and also contribute to adaptive immune responses and long-term immunity. Recent investigations have started to reveal how the constitutive pathway of monocyte maturation and differentiation into tissue macrophages and DCs is redirected in the setting of microbial infection. Infections with a diversity of pathogens, including Listeria monocytogenes, Toxoplasma gondii, and Cryptococcus neoformans, require CCR2-mediated recruitment of monocytes to sites of infection, where they restrict further microbial growth and invasion. In the absence of infection, circulating inflammatory monocytes return to the bone marrow and differentiate into monocytes that constitutively supply peripheral tissues with macrophages and DCs. Much remains to be learned about the trafficking cues that finely control the numbers of macrophages and DCs in various tissues and the stimuli that redirect trafficking and monocyte differentiation in the setting of microbial infection.
Acknowledgments
The authors’ research is supported by the National Institutes of Health (AI39031, E.G.P.; K12 CA120121, N.V.S.; T32 AI055409, T.M.H.), Irvington Institute for Immunological Research (N.V.S.), and Charles H. Revson Foundation (T.M.H.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
Natalya V. Serbina, Email: serbinan@mskcc.org.
Eric G. Pamer, Email: pamere@mskcc.org.
LITERATURE CITED
- 1.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–35. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Furth R, Diesselhoffden Dulk MC, Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973;138:1314–30. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/s1074-7613(03)00178-x. [DOI] [PubMed] [Google Scholar]
- 5.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 6.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–76. [PubMed] [Google Scholar]
- 7.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 8.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 9.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 10.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–7. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–58. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 12.Grage-Griebenow E, Flad HD, Ernst M, Bzowska M, Skrzeczynska J, Pryjma J. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology. 2000;202:42–50. doi: 10.1016/S0171-2985(00)80051-0. [DOI] [PubMed] [Google Scholar]
- 13.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64+/CD16+ blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Sconocchia G, Keyvanfar K, El Ouriaghli F, Grube M, Rezvani K, et al. Phenotype and function of a CD56+ peripheral blood monocyte. Leukemia. 2005;19:69–76. doi: 10.1038/sj.leu.2403550. [DOI] [PubMed] [Google Scholar]
- 15.Grip O, Bredberg A, Lindgren S, Henriksson G. Increased subpopulations of CD16+ and CD56+ blood monocytes in patients with active Crohn’s disease. Inflamm Bowel Dis. 2007;13:566–72. doi: 10.1002/ibd.20025. [DOI] [PubMed] [Google Scholar]
- 16.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 17.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–73. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–35. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–80. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–17. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 22.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–64. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 23.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–40. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–97. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 27.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 28.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 29.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–17. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 30.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–18. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol. 2006;176:4155–62. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–83. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 35.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–61. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 36.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 38.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–31. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–50. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Kamath AT, Pooley J, O’Keeffe MA, Vremec D, Zhan Y, et al. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–70. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- 42.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–83. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 43.del Hoyo GM, Martin P, Vargas HH, Ruiz S, Arias CF, Ardavin C. Characterization of a common precursor population for dendritic cells. Nature. 2002;415:1043–47. doi: 10.1038/4151043a. [DOI] [PubMed] [Google Scholar]
- 44.O’Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, et al. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 2003;101:1453–59. doi: 10.1182/blood-2002-03-0974. [DOI] [PubMed] [Google Scholar]
- 45.Leon B, Martinez del Hoyo G, Parrillas V, Vargas HH, Sanchez-Mateos P, et al. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8− and CD8+ splenic dendritic cells. Blood. 2004;103:2668–76. doi: 10.1182/blood-2003-01-0286. [DOI] [PubMed] [Google Scholar]
- 46.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–71. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 47.Diao J, Winter E, Cantin C, Chen W, Xu L, et al. In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J Immunol. 2006;176:7196–206. doi: 10.4049/jimmunol.176.12.7196. [DOI] [PubMed] [Google Scholar]
- 48.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–35. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 50.Holt PG. Pulmonary dendritic cells in local immunity to inert and pathogenic antigens in the respiratory tract. Proc Am Thorac Soc. 2005;2:116–20. doi: 10.1513/pats.200502-017AW. [DOI] [PubMed] [Google Scholar]
- 51.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–72. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 52.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–7. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–93. [PubMed] [Google Scholar]
- 54.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, et al. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35:227–35. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 55.Mende I, Karsunky H, Weissman IL, Engleman EG, Merad M. Flk2+ myeloid progenitors are the main source of Langerhans cells. Blood. 2006;107:1383–90. doi: 10.1182/blood-2005-05-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–58. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 57.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 58.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 59.Wassef JS, Keren DF, Mailloux JL. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57:858–63. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inman LR, Cantey JR. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer’s patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983;71:1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grutzkau A, Hanski C, Hahn H, Riecken EO. Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–15. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galan JE. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 64.Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–8. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 65.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–67. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 66.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–81. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 67.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–65. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 69.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–74. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 70.Niess JH, Brand S, Gu X, Landsman L, Jung S, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–58. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 71.Muehlhoefer A, Saubermann LJ, Gu X, Luedtke-Heckenkamp K, Xavier R, et al. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol. 2000;164:3368–76. doi: 10.4049/jimmunol.164.6.3368. [DOI] [PubMed] [Google Scholar]
- 72.Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158:855–66. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brand S, Sakaguchi T, Gu X, Colgan SP, Reinecker HC. Fractalkine-mediated signals regulate cell-survival and immune-modulatory responses in intestinal epithelial cells. Gastroenterology. 2002;122:166–77. doi: 10.1053/gast.2002.30329. [DOI] [PubMed] [Google Scholar]
- 74.Sundquist M, Wick MJ. TNF-α-dependent and -independent maturation of dendritic cells and recruited CD11cintCD11b+ cells during oral Salmonella infection. J Immunol. 2005;175:3287–98. doi: 10.4049/jimmunol.175.5.3287. [DOI] [PubMed] [Google Scholar]
- 75.Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 76.Murray EGD, Webb RA, Swann MBR. A disease of rabbits characterized by a large mononuclear monocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes. J Pathol Bacteriol. 1926;29:407–39. [Google Scholar]
- 77.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–23. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 78.North RJ. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970;132:521–34. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fleming TJ, O’hUigin C, Malek TR. Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to alpha-bungarotoxin and other neurotoxins. J Immunol. 1993;150:5379–90. [PubMed] [Google Scholar]
- 80.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–68. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of antigranulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–46. [PubMed] [Google Scholar]
- 82.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–96. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–36. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 85.Marco AJ, Prats N, Ramos JA, Briones V, Blanco M, et al. A microbiological, histopathological and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J Comp Pathol. 1992;107:1–9. doi: 10.1016/0021-9975(92)90090-h. [DOI] [PubMed] [Google Scholar]
- 86.Drevets DA. Dissemination of Listeria monocytogenes by infected phagocytes. Infect Immun. 1999;67:3512–17. doi: 10.1128/iai.67.7.3512-3517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, et al. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172:4418–24. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 88.Join-Lambert OF, Ezine S, Le Monnier A, Jaubert F, Okabe M, et al. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol. 2005;7:167–80. doi: 10.1111/j.1462-5822.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- 89.Drevets DA, Jelinek TA, Freitag NE. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect Immun. 2001;69:1344–50. doi: 10.1128/IAI.69.3.1344-1350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–32. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 91.Amer AO, Swanson MS. A phagosome of one’s own: a microbial guide to life in the macrophage. Curr Opin Microbiol. 2002;5:56–61. doi: 10.1016/s1369-5274(02)00286-2. [DOI] [PubMed] [Google Scholar]
- 92.Dinauer MC, Deck MB, Unanue ER. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J Immunol. 1997;158:5581–83. [PubMed] [Google Scholar]
- 93.Endres R, Luz A, Schulze H, Neubauer H, Futterer A, et al. Listeriosis in p47phox−/− and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–32. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 94.Beckerman KP, Rogers HW, Corbett JA, Schreiber RD, McDaniel ML, Unanue ER. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–95. [PubMed] [Google Scholar]
- 95.Edelson BT, Unanue ER. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J Immunol. 2002;169:3869–75. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 96.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–68. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, et al. Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–26. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–62. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 100.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–33. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 102.Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, et al. Tumor necrosis factor α- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun. 2006;74:6100–7. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 104.Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–4. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–99. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peters W, Cyster JG, Mack M, Schlondorff D, Wolf AJ, et al. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J Immunol. 2004;172:7647–53. doi: 10.4049/jimmunol.172.12.7647. [DOI] [PubMed] [Google Scholar]
- 107.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2001;98:7958–63. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peters W, Dupuis M, Charo IF. A mechanism for the impaired IFN-γ production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J Immunol. 2000;165:7072–77. doi: 10.4049/jimmunol.165.12.7072. [DOI] [PubMed] [Google Scholar]
- 109.Scott HM, Flynn JL. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun. 2002;70:5946–54. doi: 10.1128/IAI.70.11.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirkaldy AA, Musonda AC, Khanolkhar-Young S, Suneetha S, Lockwood DN. Expression of CC and CXC chemokines and chemokine receptors in human leprosy skin lesions. Clin Exp Immunol. 2003;134:447–53. doi: 10.1111/j.1365-2249.2003.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–60. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Flores-Villanueva PO, Ruiz-Morales JA, Song CH, Flores LM, Jo EK, et al. A functional promoter polymorphism in monocyte chemoattractant protein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med. 2005;202:1649–58. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rutledge BJ, Rayburn H, Rosenberg R, North RJ, Gladue RP, et al. High level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol. 1995;155:4838–43. [PubMed] [Google Scholar]
- 114.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–36. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 115.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, Nathan C, Xie QW, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, et al. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science. 2001;291:1544–47. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 117.Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukoc Biol. 2003;74:1015–25. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 118.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–69. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yap GS, Sher A. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology. 1999;201:240–47. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 120.Yap GS, Sher A. The use of germ line-mutated mice in understanding host-pathogen interactions. Cell Microbiol. 2002;4:627–34. doi: 10.1046/j.1462-5822.2002.00224.x. [DOI] [PubMed] [Google Scholar]
- 121.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–71. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c-and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–16. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 124.Hohl TM, Rivera A, Pamer EG. Immunity to fungi. Curr Opin Immunol. 2006;18:465–72. doi: 10.1016/j.coi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Duong M, Ouellet N, Simard M, Bergeron Y, Olivier M, Bergeron MG. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J Infect Dis. 1998;178:1472–82. doi: 10.1086/314425. [DOI] [PubMed] [Google Scholar]
- 126.Schaffner A, Douglas H, Braude AI, Davis CE. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983;42:1109–15. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ibrahim-Granet O, Philippe B, Boleti H, Boisvieux-Ulrich E, Grenet D, et al. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect Immun. 2003;71:891–903. doi: 10.1128/IAI.71.2.891-903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim HS, Choi EH, Khan J, Roilides E, Francesconi A, et al. Expression of genes encoding innate host defense molecules in normal human monocytes in response to Candida albicans. Infect Immun. 2005;73:3714–24. doi: 10.1128/IAI.73.6.3714-3724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fan W, Kraus PR, Boily MJ, Heitman J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot Cell. 2005;4:1420–33. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cortez KJ, Lyman CA, Kottilil S, Kim HS, Roilides E, et al. Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus. Infect Immun. 2006;74:2353–65. doi: 10.1128/IAI.74.4.2353-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 vs T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–27. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 132.Traynor TR, Herring AC, Dorf ME, Kuziel WA, Toews GB, Huffnagle GB. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J Immunol. 2002;168:4659–66. doi: 10.4049/jimmunol.168.9.4659. [DOI] [PubMed] [Google Scholar]
- 133.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, et al. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 134.Blease K, Mehrad B, Lukacs NW, Kunkel SL, Standiford TJ, Hogaboam CM. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Asperigillus fumigatus conidia. J Immunol. 2001;166:1832–42. doi: 10.4049/jimmunol.166.3.1832. [DOI] [PubMed] [Google Scholar]
- 135.Blease K, Mehrad B, Standiford TJ, Lukacs NW, Gosling J, et al. Enhanced pulmonary allergic responses to Aspergillus in CCR2−/− mice. J Immunol. 2000;165:2603–11. doi: 10.4049/jimmunol.165.5.2603. [DOI] [PubMed] [Google Scholar]
- 136.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112:1862–70. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells. Semin Immunol. 2005;17:313–18. doi: 10.1016/j.smim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 138.Bechetoille N, Andre V, Valladeau J, Perrier E, Dezutter-Dambuyant C. Mixed Langerhans cell and interstitial/dermal dendritic cell subsets emanating from monocytes in Th2-mediated inflammatory conditions respond differently to proinflammatory stimuli. J Leukoc Biol. 2006;80:45–58. doi: 10.1189/jlb.0205109. [DOI] [PubMed] [Google Scholar]
- 139.Rotta G, Edwards EW, Sangaletti S, Bennett C, Ronzoni S, et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253–63. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Albina JE, Henry WL., Jr Suppression of lymphocyte proliferation through the nitric oxide synthesizing pathway. J Surg Res. 1991;50:403–9. doi: 10.1016/0022-4804(91)90210-d. [DOI] [PubMed] [Google Scholar]
- 141.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, et al. Induction of nitric oxide synthase in mouse dendritic cells by IFN-γ, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 142.Caspar-Bauguil S, Puissant B, Nazzal D, Lefevre JC, Thomsen M, et al. Chlamydia pneumoniae induces interleukin-10 production that down-regulates major histocompatibility complex class I expression. J Infect Dis. 2000;182:1394–401. doi: 10.1086/315856. [DOI] [PubMed] [Google Scholar]
- 143.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–86. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park DR, Skerrett SJ. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ: differential responses of blood monocytes and alveolar macrophages. J Immunol. 1996;157:2528–38. [PubMed] [Google Scholar]
- 145.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–26. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 146.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel TR. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–49. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–65. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-γ- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–93. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 149.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–56. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conrad SM, Strauss-Ayali D, Field AE, Mack M, Mosser DM. Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages. Infect Immun. 2007;75:653–65. doi: 10.1128/IAI.01314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–97. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 154.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 155.Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-α-converting enzyme mediates the inducible cleavage of fractalkine. J Biol Chem. 2001;276:44622–26. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 156.DiTirro J, Rhoades ER, Roberts AD, Burke JM, Mukasa A, et al. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun. 1998;66:2284–89. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barsig J, Flesch IE, Kaufmann SH. Macrophages and hepatocytic cells as chemokine producers in murine listeriosis. Immunobiology. 1998;199:87–104. doi: 10.1016/S0171-2985(98)80066-1. [DOI] [PubMed] [Google Scholar]
- 158.Denney CF, Eckmann L, Reed SL. Chemokine secretion of human cells in response to Toxoplasma gondii infection. Infect Immun. 1999;67:1547–52. doi: 10.1128/iai.67.4.1547-1552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brenier-Pinchart MP, Pelloux H, Simon J, Ricard J, Bosson JL, Ambroise-Thomas P. Toxoplasma gondii induces the secretion of monocyte chemotactic protein-1 in human fibroblasts, in vitro. Mol Cell Biochem. 2000;209:79–87. doi: 10.1023/a:1007075701551. [DOI] [PubMed] [Google Scholar]
- 160.Brenier-Pinchart MP, Vigan I, Jouvin-Marche E, Marche PN, Pelet E, et al. Monocyte chemotactic protein-1 secretion and expression after Toxoplasma gondii infection in vitro depend on the stage of the parasite. FEMS Microbiol Lett. 2002;214:45–49. doi: 10.1111/j.1574-6968.2002.tb11323.x. [DOI] [PubMed] [Google Scholar]
- 161.Brenier-Pinchart MP, Blanc-Gonnet E, Marche PN, Berger F, Durand F, et al. Infection of human astrocytes and glioblastoma cells with Toxoplasma gondii: monocyte chemotactic protein-1 secretion and chemokine expression in vitro. Acta Neuropathol. 2004;107:245–49. doi: 10.1007/s00401-003-0804-0. [DOI] [PubMed] [Google Scholar]
- 162.Lin Y, Gong J, Zhang M, Xue W, Barnes PF. Production of monocyte chemoattractant protein 1 in tuberculosis patients. Infect Immun. 1998;66:2319–22. doi: 10.1128/iai.66.5.2319-2322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fujita M, Furukawa Y, Nagasawa Y, Ogawa M, Nakamura Y. Down-regulation of monocyte chemotactic protein-3 by activated beta-catenin. Cancer Res. 2000;60:6683–87. [PubMed] [Google Scholar]
- 164.Toney LM, Cattoretti G, Graf JA, Merghoub T, Pandolfi PP, et al. BCL-6 regulates chemokine gene transcription in macrophages. Nat Immunol. 2000;1:214–20. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- 165.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gonzalo JA, Lloyd CM, Wen D, Albar JP, Wells TN, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Colotta F, Borre A, Wang JM, Tattanelli M, Maddalena F, et al. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992;148:760–65. [PubMed] [Google Scholar]
- 169.Struyf S, Van Collie E, Paemen L, Put W, Lenaerts JP, et al. Synergistic induction of MCP-1 and -2 by IL-1β and interferons in fibroblasts and epithelial cells. J Leukoc Biol. 1998;63:364–72. doi: 10.1002/jlb.63.3.364. [DOI] [PubMed] [Google Scholar]
- 170.Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, et al. Roles of Toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002;169:2026–33. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- 171.Rollins BJ, Pober JS. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991;138:1315–19. [PMC free article] [PubMed] [Google Scholar]
- 172.Brown Z, Gerritsen ME, Carley WW, Strieter RM, Kunkel SL, Westwick J. Chemokine gene expression and secretion by cytokine-activated human microvascular endothelial cells. Differential regulation of monocyte chemoattractant protein-1 and interleukin-8 in response to interferon-γ. Am J Pathol. 1994;145:913–21. [PMC free article] [PubMed] [Google Scholar]
- 173.Poon M, Liu B, Taubman MB. Identification of a novel dexamethasone-sensitive RNA-destabilizing region on rat monocyte chemoattractant protein 1 mRNA. Mol Cell Biol. 1999;19:6471–78. doi: 10.1128/mcb.19.10.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–13. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, et al. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007;179:514–21. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 176.Kim JY, Ahn MH, Song HO, Choi JH, Ryu JS, et al. Involvement of MAPK activation in chemokine or COX-2 productions by Toxoplasma gondii. Korean J Parasitol. 2006;44:197–207. doi: 10.3347/kjp.2006.44.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fietta AM, Morosini M, Meloni F, Bianco AM, Pozzi E. Pharmacological analysis of signal transduction pathways required for Mycobacterium tuberculosis–induced IL-8 and MCP-1 production in human peripheral monocytes. Cytokine. 2002;19:242–49. [PubMed] [Google Scholar]
- 178.Ping D, Jones PL, Boss JM. TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the MCP-1/JE gene. Immunity. 1996;4:455–69. doi: 10.1016/s1074-7613(00)80412-4. [DOI] [PubMed] [Google Scholar]
- 179.Boekhoudt GH, Guo Z, Beresford GW, Boss JM. Communication between NF-κB and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J Immunol. 2003;170:4139–47. doi: 10.4049/jimmunol.170.8.4139. [DOI] [PubMed] [Google Scholar]
- 180.Kumar SN, Boss JM. Site A of the MCP-1 distal regulatory region functions as a transcriptional modulator through the transcription factor NF1. Mol Immunol. 2000;37:623–32. doi: 10.1016/s0161-5890(00)00097-3. [DOI] [PubMed] [Google Scholar]
- 181.Ping D, Boekhoudt G, Boss JM. trans-retinoic acid blocks platelet-derived growth factor-BB-induced expression of the murine monocyte chemoattractant-1 gene by blocking the assembly of a promoter proximal Sp1 binding site. J Biol Chem. 1999;274:31909–16. doi: 10.1074/jbc.274.45.31909. [DOI] [PubMed] [Google Scholar]
- 182.Ping D, Boekhoudt G, Zhang F, Morris A, Philipsen S, et al. Sp1 binding is critical for promoter assembly and activation of the MCP-1 gene by tumor necrosis factor. J Biol Chem. 2000;275:1708–14. doi: 10.1074/jbc.275.3.1708. [DOI] [PubMed] [Google Scholar]
- 183.Ping D, Boekhoudt GH, Rogers EM, Boss JM. Nuclear factor-κB p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol. 1999;162:727–34. [PubMed] [Google Scholar]
- 184.Teferedegne B, Green MR, Guo Z, Boss JM. Mechanism of action of a distal NF-κB-dependent enhancer. Mol Cell Biol. 2006;26:5759–70. doi: 10.1128/MCB.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kawahara RS, Deng ZW, Deuel TF. Glucocorticoids inhibit the transcriptional induction of JE, a platelet-derived growth factor-inducible gene. J Biol Chem. 1991;266:13261–66. [PubMed] [Google Scholar]
- 186.Mukaida N, Zachariae CC, Gusella GL, Matsushima K. Dexamethasone inhibits the induction of monocyte chemotactic-activating factor production by IL-1 or tumor necrosis factor. J Immunol. 1991;146:1212–15. [PubMed] [Google Scholar]
- 187.Paine R, 3rd, Rolfe MW, Standiford TJ, Burdick MD, Rollins BJ, Strieter RM. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol. 1993;150:4561–70. [PubMed] [Google Scholar]
- 188.Dhawan L, Liu B, Blaxall BC, Taubman MB. A novel role for the glucocorticoid receptor in the regulation of monocyte chemoattractant protein-1 mRNA stability. J Biol Chem. 2007;282:10146–52. doi: 10.1074/jbc.M605925200. [DOI] [PubMed] [Google Scholar]
- 189.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci USA. 2003;100:1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Johnson Z, Proudfoot AE, Handel TM. Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005;16:625–36. doi: 10.1016/j.cytogfr.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 191.Lau EK, Paavola CD, Johnson Z, Gaudry JP, Geretti E, et al. Identification of the glycosaminoglycan binding site of the CC chemokine, MCP-1: implications for structure and function in vivo. J Biol Chem. 2004;279:22294–305. doi: 10.1074/jbc.M311224200. [DOI] [PubMed] [Google Scholar]
- 192.Chakravarty L, Rogers L, Quach T, Breckenridge S, Kolattukudy PE. Lysine 58 and histidine 66 at the C-terminal alpha-helix of monocyte chemoattractant protein-1 are essential for glycosaminoglycan binding. J Biol Chem. 1998;273:29641–47. doi: 10.1074/jbc.273.45.29641. [DOI] [PubMed] [Google Scholar]
- 193.Yu Y, Sweeney MD, Saad OM, Crown SE, Hsu AR, et al. Chemokine-glycosaminoglycan binding: specificity for CCR2 ligand binding to highly sulfated oligosaccharides using FTICR mass spectrometry. J Biol Chem. 2005;280:32200–8. doi: 10.1074/jbc.M505738200. [DOI] [PubMed] [Google Scholar]
- 194.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 195.Mora JR, Iwata M, Eksteen B, Song S-Y, Junt T, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 196.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–33. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 197.Taylor PR, Brown GD, Geldhof AB, Martinez-Romares LM, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–97. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- 198.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek S, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Grage-Griebenow E, Flad H, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 200.Draude G, von Hundelshausen P, Frankenberger M, Ziegler-Heitbrock HW, Weber C. Distinct scavenger receptor expression and function in the human CD14+/CD16+ monocyte subset. Am J Physiol. 1999;276:H1144–49. doi: 10.1152/ajpheart.1999.276.4.H1144. [DOI] [PubMed] [Google Scholar]