Abstract
Research on the radiation-induced bystander effect has been carried out mainly in 2-D tissue culture systems. This study uses a 3-D model, wherein apparently normal human diploid fibroblasts (AG1522) are grown in a carbon scaffold, to investigate the induction of a G1 checkpoint in bystander cells present alongside radiolabelled cells. Cultures were simultaneously pulse-labelled with 3H-deoxycytidine (3HdC) to selectively irradiate a minor fraction of cells, and bromodeoxyuridine (BrdU) to identify the radiolabelled cells. After thorough washing of cultures, iododeoxyuridine (IdU) was administered to detect proliferating bystander cells. The cultures were harvested at various times thereafter, and cells were reacted with two monoclonal antibodies specific to IdU/BrdU or BrdU, respectively, stained with propidium iodide, and subjected to multi-parameter flow cytometry. Cell-cycle progression was followed in radiolabelled cells (BrdU+) that were chronically irradiated by low energy beta particles emitted by DNA-incorporated 3H, and in unlabelled bystander cells (BrdU−) by a flow cytometry based cumulative labelling index assay. As expected, radiolabelled cells were delayed, in a dose-dependent manner, in G2 and subsequently G1. No delay occurred in progression of bystander cells through G1, when the labelled cells were irradiated at dose rates up to 0.32 Gy h−1.
INTRODUCTION
The radiation-induced bystander effect has been defined as the occurrence of biological effects in unirradiated cells, as a result of exposure of other cells to ionising radiation(1). Most research in this field has been carried out in two-dimensional (2-D) tissue culture systems, and mostly using alpha particles(2). Intercellular communication, via gap junctions or secreted diffusible factors, has been shown to be a major mediator of such non-targeted effects. With relevance to human radiation protection, diagnostics and therapy, it would be of particular significance to determine the existence and underlying mechanisms of radiation-induced bystander responses at the multicellular, tissue and organ levels. Human cells maintained in a three-dimensional (3-D) architecture, which mimics the way cells grow in vivo, offer an ideal study system, where experimental variables can be tightly controlled to investigate bystander effects. The use of normal human cell strains is encouraged, as it permits investigation of mechanisms in a phenotype, where signalling pathways are presumably normal. Bystander responses, including DNA damage, gene expression and alterations in cell cycle progression have been measured in AG1522 cells in 2-D culture(3). Bystander effects were also observed with V79 cells maintained in multicellular 3-D clusters consisting of cells that incorporated 3H-thymidine and unlabelled cells(4). Here, we build on those initial observations and use apparently normal human skin fibroblasts (AG1522) maintained in vitro in 2-D and in a 3-D growth model to investigate induction of cell cycle check-points in bystander cells present alongside cells that harbour 3H-deoxycytidine (3HdC) in their DNA. For these studies, the well-established cumulative labelling index (CLI) assay(5) was modified to distinguish cells labelled with 3HdC from proliferating bystander cells.
METHODS
Cell culture
Parental AG1522 were routinely cultured in 2-D in Eagle’s MEM supplemented with 2 mM L-glutamine, 12.5% foetal bovine serum, 100 U ml−1 penicillin and 100 ng ml−1 streptomycin. This growth medium is designated cMEM. For 3-D culture, 105 cells were seeded on fibronectin-coated Cytomatrix units (Cytomatrix, USA) in 1 ml cMEM, incubated at 37°C in 24 well plates and moved gently on a nutator in humidified atmosphere of 5% CO2 in air. Cells formed 3-D interactions among each other, and cultures were fed with fresh cMEM on alternate days. Experiments involving 2-D monolayer cultures were fed with cMEM on the same schedule.
Micro-irradiation and bystander environment
Bromodeoxyuridine (BrdU) and 3HdC are non-competitively incorporated into the DNA of actively advancing S-phase cells. Therefore, co-pulse-labelling cell cultures with 10 µM BrdU and 0–740 kBq ml−1 3HdC enables identification of 3H-labelled cells by anti-BrdU immunodetection methods. Hence, BrdU+ cells are 3HdC labelled, while BrdU− carry no radioactivity and may be considered bystanders. Cultures are pulse-labelled for 3 h, washed extensively in cMEM and incubated for various times. DNA-incorporated 3H is capable of delivering a high radiation absorbed dose to the cell nucleus of the labelled cells while imparting a negligible dose to the nuclei of the surrounding cells(4,6). During the pulse-label, all cells in the culture are exposed to radiation from decays occurring in the culture medium. However, the dose delivered to all cells during the pulse due to extra-cellular beta decays (<2 mGy in 3 h) is not expected to interfere with effects induced in unlabelled cells. Pulse-labelling was done 24 h after the last cMEM feeding, where the percentage of labelled cells was 3–16%. These cells incorporate 3HdC in their nuclei, although the complementary fraction constitutes unlabelled, bystander cells. Thus shortly after pulse-labelling, bystander cells are distributed among G0, G1, M and G2, and labelled cells are in S or early G2.
Cellular dosimetry
A known number of cells were subjected to liquid scintillation counting for determination of average cellular activity. As only a minor fraction of the cultured cells contain radioactivity, the measured average mBq per cell was converted to average mBq per labelled cell, upon dividing the former by the fraction of BrdU+ cells. The BrdU+ fraction was measured using flow cytometry (FCM) on parallel cultures. Assuming spherical nuclei of 3.5 µm radius, cellular doses were calculated using the cellular S-value 4.09 × 10−3 Gy (Bq·s)−1 (7).
Multi-parameter flow cytometry-based CLI assay
A modified version of the CLI assay has been developed. Briefly, the assay allows measuring the fraction of proliferating bystander cells, via immunodetection of DNA-incorporated iododeoxyuridine (IdU), specifically in BrdU− cells co-cultured with BrdU+ cells. This is facilitated by concurrently using a BrdU-specific antibody and an anti-BrdU antibody that also reacts with IdU. After pulse-labelling with 3HdC/BrdU, cultures are incubated in cMEM supplemented with 1 µM IdU and harvested with trypsin 15–40 h later. BrdU specific incorporation is associated with high levels of Alexa 633 fluorescence, while high levels of Alexa 488 fluorescence indicate incorporation of BrdU and/or IdU. Low fluorescence intensity of Alexa 633–BrdU concomitant with high levels of Alexa 488 fluorescence indicates IdU incorporation in BrdU− cells (Figure 1). Under the culture conditions used, the majority of BrdU− cells on the Cytomatrix units or monolayers were in G0 phase, due to contact growth inhibition. Thus, the procedure outlined above measures the G1 checkpoint in proliferating bystander cells. For assessment of a G1 checkpoint in, otherwise excluded, G0-phase bystander cells, 3-D-Cytomatrix cultures were pulse-labelled, held for 2, 8, or 48 h, dismantled and seeded (≤1.5 × 104 cells cm−2) in P60 dishes in cMEM medium containing 1 µM IdU. Cells were then fixed, permeabilised in EtOH, subjected to immunofluorescence procedures and analysed by FCM.
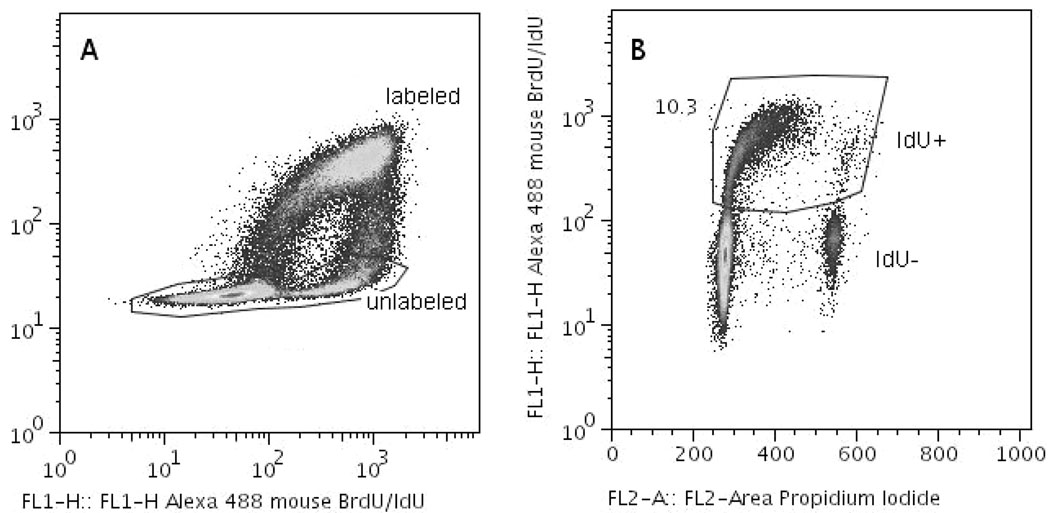
Figure 1.
Multi-parameter flow cytometric analysis of CLI in AG1522 Cytomatrix cultures pulse-labelled with 3HdC. (A) Bivariate pseudo-colour plot of BrdU specific vs. BrdU/IdU immunofluorescence. The events gated out as negative for BrdU (unlabelled bystander cells) are plotted in (B) as a bivariate plot of IdU specific fluorescence vs. DNA content (Propidium Iodide fluorescence). In this example, the number shown by the gated region in panel B is the CLI for bystander cells 18 h after exposure to IdU.
RESULTS
FCM–CLI assay validation
Preliminary tests were run to validate the FCM-based CLI assay. Cell cultures in 3-D, fed 24 h earlier, were pulse-labelled with 3HdC and BrdU, washed, harvested and subjected to FCM or autoradiography. For both techniques, the measured fraction of labelled cells was ~7%, indicating that BrdU uptake, measured by FCM, is a suitable marker of DNA-incorporated radioactivity. FCM analyses were also used to ascertain the specificity of anti-BrdU and anti-BrdU/IdU antibodies in multi-parameter FCM-CLI assay.
Cell cycle progression of 3HdC-labelled cells
Relative to sham labelled (BrdU+), cells labelled with 3HdC/BrdU experienced a dose-dependent G2 checkpoint (Figure 2). Dose rates as small as 0.02 Gy h−1 resulted in measurable G2 arrest. Moreover, cells which overcame the G2 block, to progress through M were subjected to a G1 checkpoint at the subsequent cell cycle.
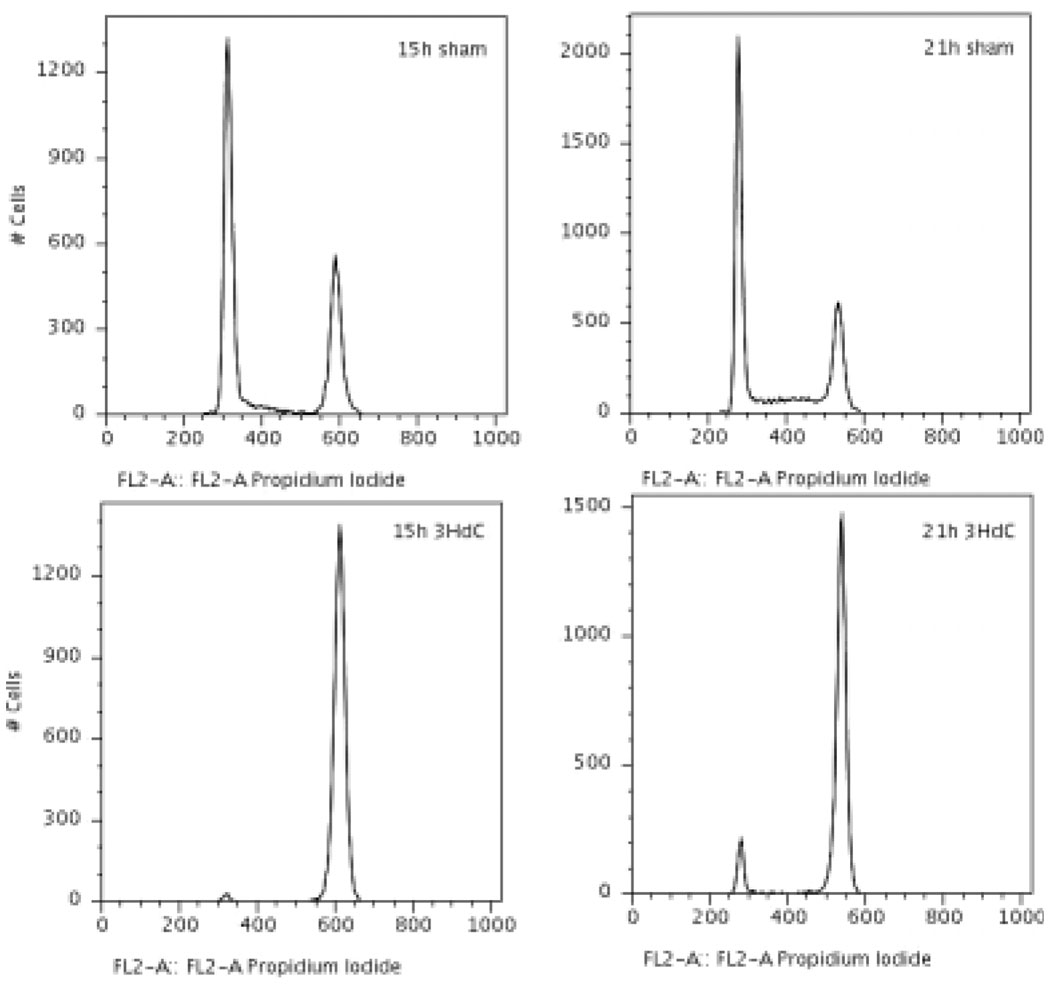
Figure 2.
DNA histograms of BrdU+ cells, sham labelled (top row) or labelled with 3HdC (bottom row, delivering 0.10 Gy h−1). At both 15 and 21 h after the end of pulse-labelling, the majority of sham labelled cells has undergone mitotic division and some have entered S-phase in the next cell cycle (top). Labelled cells, however, show a pronounced G2 arrest (bottom row).
Cell cycle progression of G1/G2/M bystanders
G1→S transition was measured using the FCM–CLI assay in cycling unlabelled bystander cells (BrdU−) (i.e. excluding G0 cells which are confluence growth inhibited). Four experiments indicated no appreciable cell cycle progression delays, relative to sham bystander cells, when labelled cells were irradiated at dose rates up to 0.11 Gy h−1.
Cell cycle progression of G0/G1/G2/M bystanders
G1→S transition was also measured in bystander cells that were mainly in G0 and bystander cells that were stimulated to proliferate following co-culture for 2, 8, or 48 h co-culture with 3HdC labelled cells. Three-dimensional populations were trypsinised, seeded at 1.5 × 104 cells cm−2 in 2-D, and progress through G1 and S monitored by CLI measurements. Relative to sham-manipulated controls, progression of bystander cells through G0→G1→S was unaffected when the labelled cells were irradiated with dose rates up to 0.32 Gy h−1 (nine experiments, representative experiment shown in Figure 3). Three experiments in confluent 2-D cultures indicated similar results (data not shown).
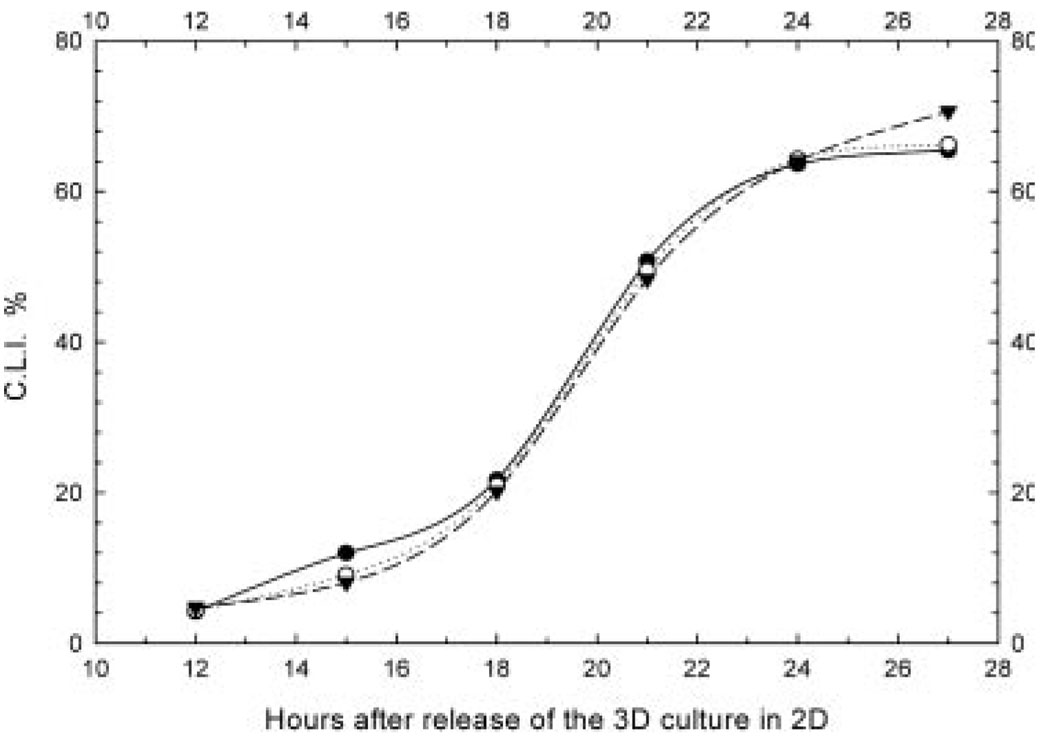
Figure 3.
CLI measured in unirradiated bystander cells (BrdU−), released in 2-D to account for bystander cells that were in G0 phase when co-cultured in 3-D. Closed circles, sham bystanders; Open circles, bystanders adjacent to cells labelled with 3HdC (0.04 Gy h−1); Closed circles, bystanders adjacent to cells labelled with 3HdC (0.23 Gy h−1). Representative data from one experiment are shown where cultures were held on Cytomatrix units for 2 h after pulse-labelling with 3HdC/BrdU.
DISCUSSION
Repeat experiments indicated that no significant G1 checkpoint is induced in bystander cells cultured in 2-D or 3-D when the dose rate in 3HdC-labelled cells ranged from 0.02 to 0.32 Gy h−1 (1.5–22 mBq per labelled cell). This was the case regardless of their cell cycle position at the beginning of irradiation. It is worth pointing out that, in this study, cells are labelled in situ. This means that the cellular activity (and dose rate) builds up slowly during pulse-labelling. Thus, any early radiation-induced intercellular signalling events are accounted for in the measured responses.
Interestingly, other 2-D co-culture studies with AG1522 cells indicate that DNA-incorporated 3H can elicit a G1 checkpoint in bystander cells only when the dose rate to the labelled cells (10% labelled, 90% bystanders) exceeds ~1 Gy h−1 (Azzam et al., manuscript in preparation). This may explain the lack of effect in the present 2-D work at lower dose rates. In other 2-D co-culture studies with WB-F344 rat liver epithelial cells, DNA-incorporated 3H was found to accelerate proliferation of co-cultured bystander cells when the dose rates to labelled cells ranged from 0.004 to 0.05 Gy h−1 (8). The percentage of labelled cells had to be at least 50% to observe a proliferative response(9). In another 3-D culture model using the V79 cell line, cells were held at 10.5°C for 72 h while accumulating beta decays from DNA-incorporated 3H (≤250 mBq per cell). In these studies, pronounced bystander killing was observed(4) and its intensity also appeared to be modulated by the fraction of cells labelled(6). When CHO and AL cells were 3-D co-cultured at 11°C in a ratio of 1:5, 3H-thymidine (12 h labelling, 100 µCi) incorporation in CHO cells yielded a significant dose-dependent increase in AL bystander killing and mutagenesis(10). It appears that 3H-induced bystander responses, within a given model, may be a function of both the fraction of labelled cells and dose or dose rate to the latter. In fact, responses may even change from pro-survival to cytotoxic depending on the concentration of the bystander signaling factors(2). Whether the propagated effect is inhibitory or proliferative may also be determined by the radionuclide, as suggested by studies with 125/123Iododeoxyuridine (presented by A. I. Kassis at this Microdosimetry Symposium).
It is apparent that comparing results from different laboratories is complicated by the diversity of experimental models, since the microenvironment may regulate intercellular signalling(11). Nevertheless, a variety of bystander responses are possible depending upon many variables, among them the radionuclide, percentage of cells labelled, dose and experimental conditions. Studies on radiation-induced bystander responses in 3-D models are emerging. In the 3-D model used in this work, dose rates up to 0.32 Gy h−1 from DNA-incorporated 3H do not induce significant alterations in the G1→S transition in bystander cells. Absence of cell cycle arrest in bystander cells is suggestive of absence of stress, including DNA damage.
ACKNOWLEDGEMENTS
This study was supported by Grant No. CA83838 from the National Institutes of Health, a New Jersey Commission for Cancer Research Post Doctoral Fellowship to M.P., and a UMDNJ Foundation Scholarship to M.P.
REFERENCES
- 1.Mothersill C, Seymour C. Radiation-induced bystander effects: past history and future directions. Radiat. Res. 2001;155(6):759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Azzam EI, Little JB. The radiation-induced bystander effect: evidence and significance. Hum. Exp. Toxicol. 2004;23(2):61–65. doi: 10.1191/0960327104ht418oa. [DOI] [PubMed] [Google Scholar]
- 3.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc. Natl Acad. Sci. USA. 2001;98(2):473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishayee A, Rao DV, Howell RW. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model. Radiat. Res. 1999;152(1):88–97. [PMC free article] [PubMed] [Google Scholar]
- 5.Azzam EI, de Toledo SM, Waker AJ, Little JB. High and low fluences of alpha-particles induce a G1 checkpoint in human diploid fibroblasts. Cancer Res. 2000;60(10):2623–2631. [PubMed] [Google Scholar]
- 6.Bishayee A, Hill HZ, Stein D, Rao DV, Howell RW. Free radical-initiated and gap junction-mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three-dimensional tissue culture model. Radiat. Res. 2001;155(2):335–344. doi: 10.1667/0033-7587(2001)155[0335:friagj]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goddu SM, Howell RW, Bouchet LG, Bolch WE, Rao DV. MIRD Cellular S Values: Self-absorbed Dose Per Unit Cumulated Activity for Selected Radionuclides and Monoenergetic Electron and Alpha Particle Emitters Incorporated into Different Cell Compartments. Reston, VA: Society of Nuclear Medicine; 1997. [Google Scholar]
- 8.Gerashchenko BI, Howell RW. Proliferative response of bystander cells adjacent to cells with incorporated radioactivity. Cytometry A. 2004;60(2):155–164. doi: 10.1002/cyto.a.20029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerashchenko BI, Howell RW. Bystander cell proliferation is modulated by the number of adjacent cells that were exposed to ionizing radiation. Cytometry A. 2005;66(1):62–70. doi: 10.1002/cyto.a.20150. [DOI] [PubMed] [Google Scholar]
- 10.Persaud R, Zhou H, Baker SE, Hei TK, Hall EJ. Assessment of low linear energy transfer radiation-induced bystander mutagenesis in a three-dimensional culture model. Cancer Res. 2005;65(21):9876–9882. doi: 10.1158/0008-5472.CAN-04-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barcellos-Hoff MH, Brooks AL. Extracellular signaling through the microenvironment: a hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat. Res. 2001;156(5 Pt 2):618–627. doi: 10.1667/0033-7587(2001)156[0618:esttma]2.0.co;2. [DOI] [PubMed] [Google Scholar]