Abstract
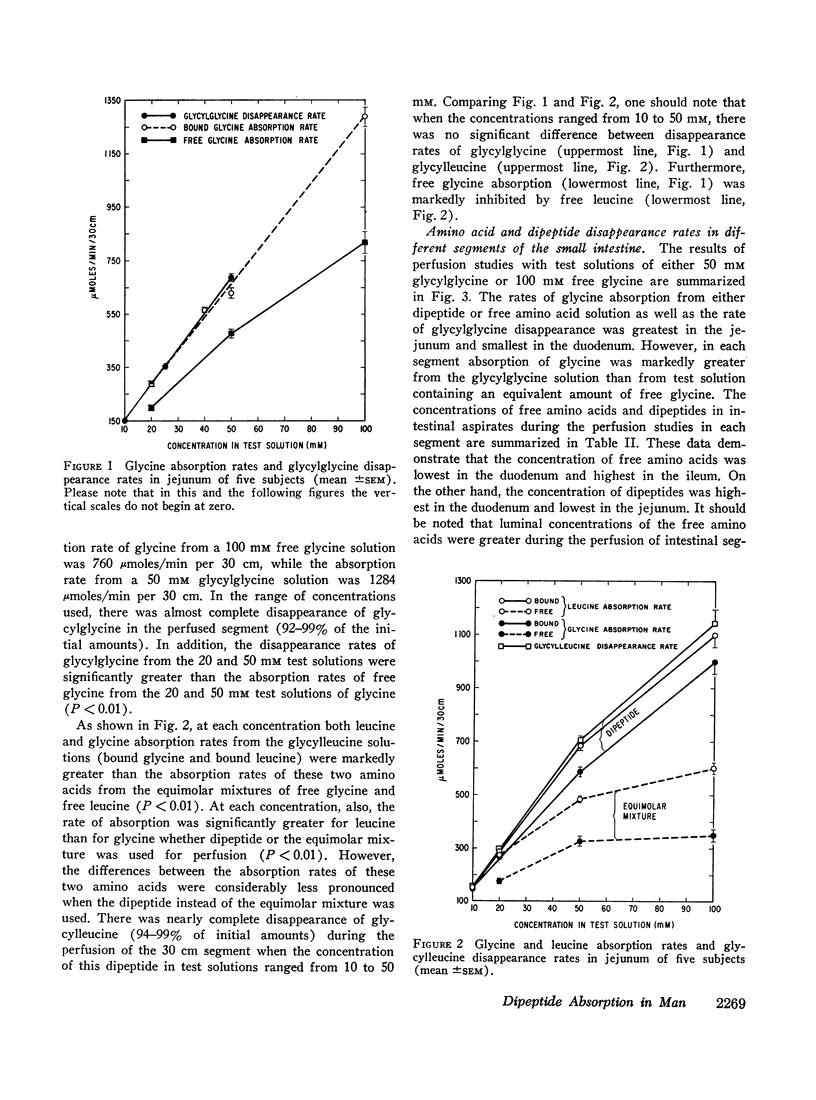
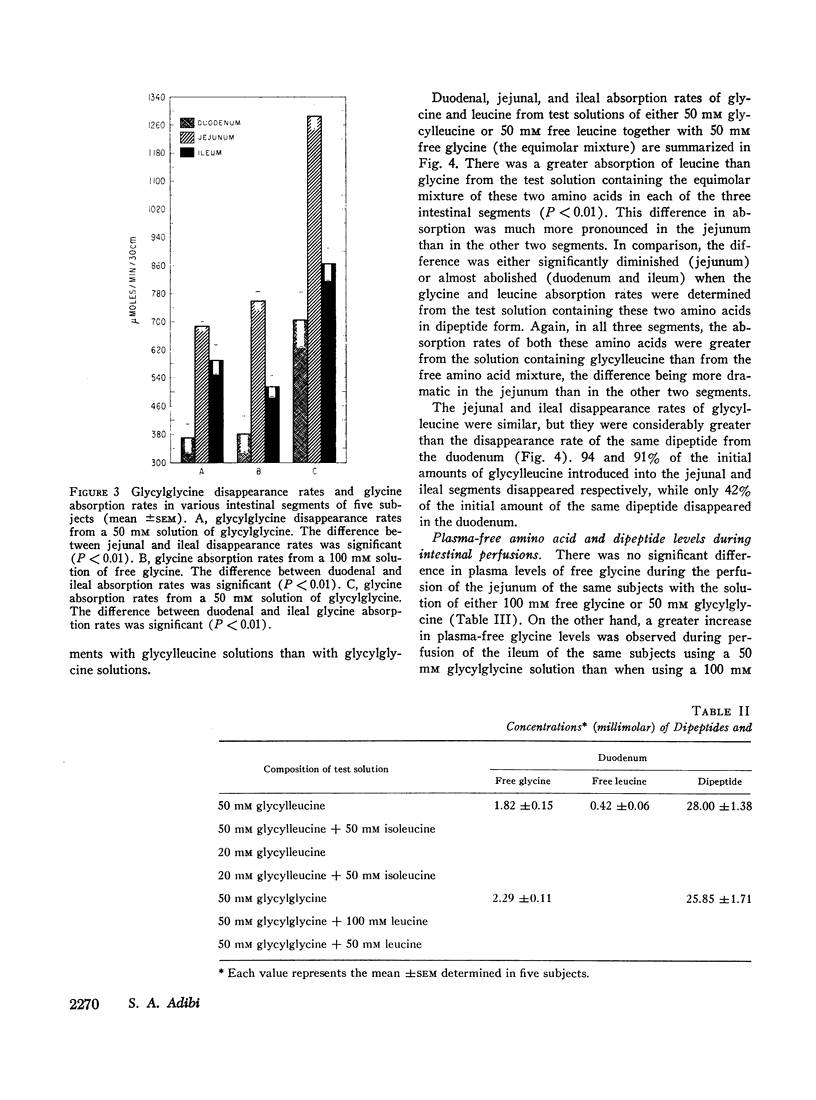
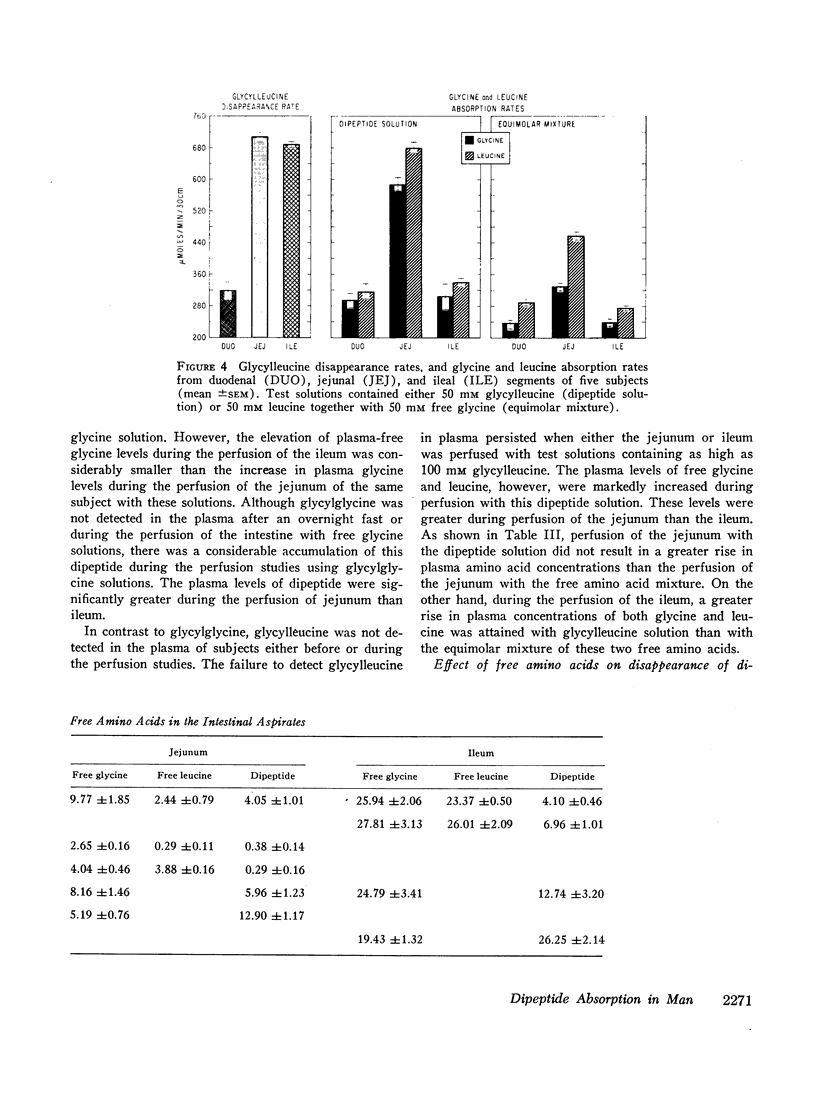
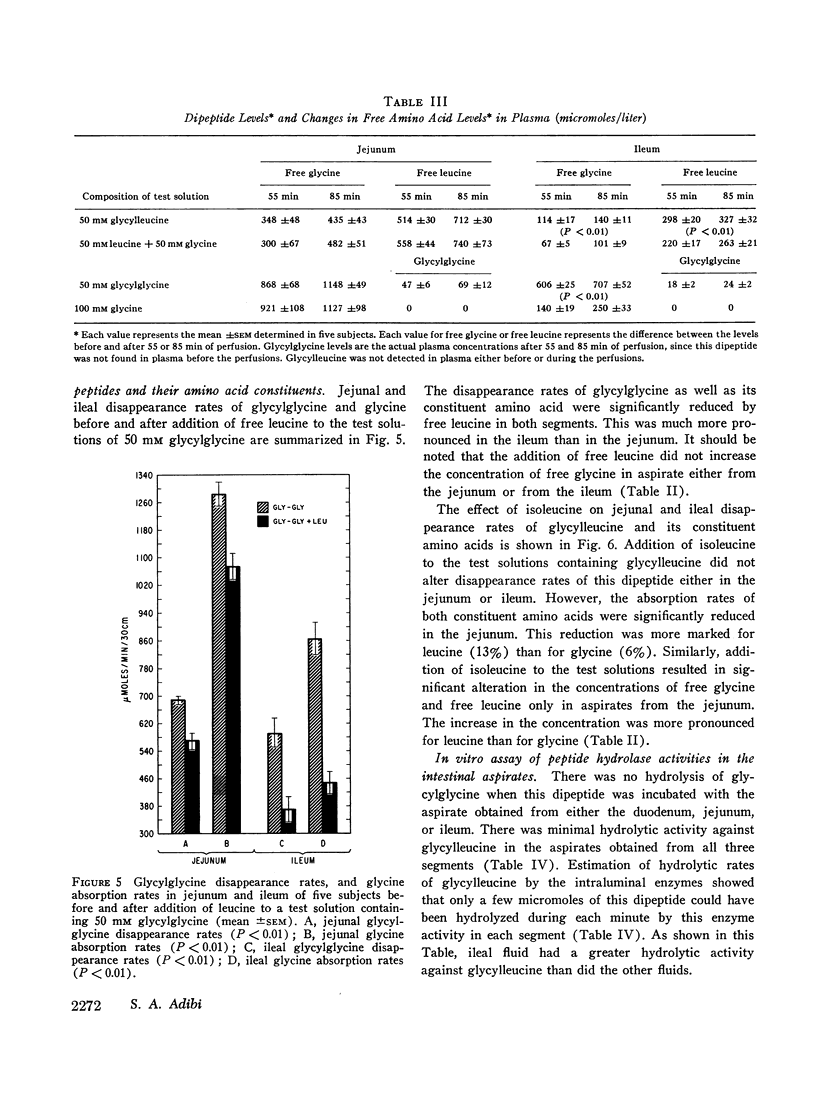
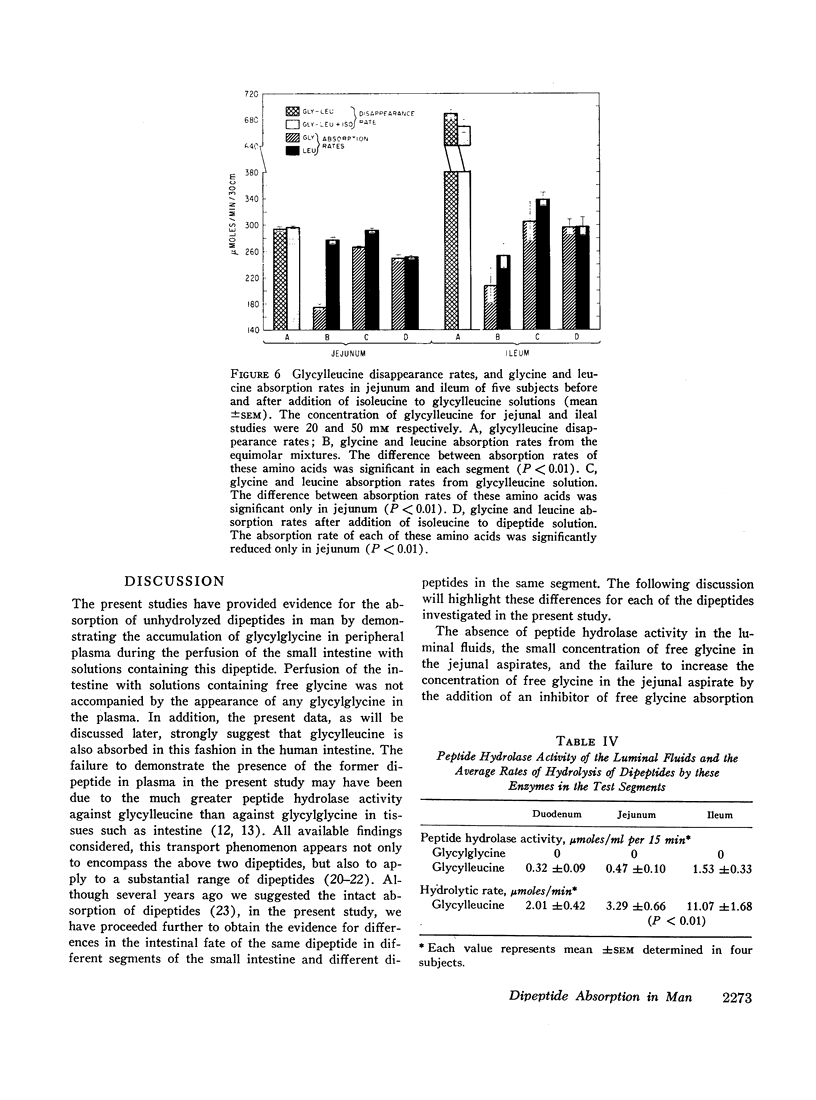
A 30 cm segment of the duodenum, jejunum, or ileum of normal human volunteers was perfused, on separate occasions, with test solutions containing either glycylglycine, free glycine, glycylleucine, or equimolar amounts of free glycine and free leucine. Luminal fluid contained no hydrolytic activity against glycylglycine and minimal activity against glycylleucine. In each intestinal segment, amino acid absorption rates were significantly greater from the test solutions containing the same amount of amino acids in dipeptide than in free form(as high as 185% increase). Perfusion of each intestinal segment with a test solution containing the equimolar mixture of free glycine and free leucine always resulted in a greater leucine than glycine absorption rate. This preferential absorption of leucine, however, was either diminished (jejunum) or almost abolished (duodenum and ileum) when the glycylleucine solution instead of the equimolar mixture was presented to the intestinal mucosa. Among the three segments, the duodenum exhibited the least potential for the disappearance of dipeptides. The jejunal and ileal dipeptide disappearance rates were either similar for glycylleucine (94% vs. 92%) or slightly different for glycylglycine (92% vs. 79%). Despite lack of a remarkable difference in the disappearance rates, absorption rates of constituent amino acids were markedly greater in the jejunum than in the ileum. This reduced amino acid absorption was brought about by a greater accumulation of free amino acids in the lumen of the ileal segment (3 to 10-fold difference). Inhibition of free glycine absorption by leucine during the perfusion of the intestine with a test solution containing glycylglycine and leucine did not result in any greater concentration of free glycine in the lumen than when the glycylglycine test solution did not contain free leucine. Similarly, inhibition of free glycine and free leucine absorption by isoleucine was not accompanied by any remarkable alteration of absorption rates of the constituent amino acids of glycylleucine. The results of these studies suggest that: (a) dipeptide disappearance in the gut lumen is principally accomplished by intact absorption and not by hydrolysis; (b) intracellular hydrolysis of dipeptides is markedly greater in the ileum than in the jejunum, while dipeptide absorption rates are either similar or only slightly different in these two segments; (c) there is no appreciable hydrolysis of glycylglycine by the membrane-bound enzymes and only a small fraction of glycylleucine is hydrolyzed by these enzymes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A., Allen E. R. Impaired jejunal absorption rates of essential amino acids induced by either dietary caloric or protein deprivation in man. Gastroenterology. 1970 Sep;59(3):404–413. [PubMed] [Google Scholar]
- Adibi S. A., Gray S. J. Intestinal absorption of essential amino acids in man. Gastroenterology. 1967 May;52(5):837–845. [PubMed] [Google Scholar]
- Adibi S. A., Gray S. J., Menden E. The kinetics of amino acid absorption and alteration of plasma composition of free amino acids after intestinal perfusion of amino acid mixtures. Am J Clin Nutr. 1967 Jan;20(1):24–33. doi: 10.1093/ajcn/20.1.24. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968 Jul;25(1):52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Leucine absorption rate and net movements of sodium and water in human jejunum. J Appl Physiol. 1970 Jun;28(6):753–757. doi: 10.1152/jappl.1970.28.6.753. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. The influence of molecular structure of neutral amino acids on their absorption kinetics in the jejunum and ileum of human intestine in vivo. Gastroenterology. 1969 May;56(5):903–913. [PubMed] [Google Scholar]
- Dent C. E. Studies on the absorption of proteins: the amino-acid pattern in the portal blood. Biochem J. 1949;44(3):318–335. [PMC free article] [PubMed] [Google Scholar]
- Fleshler B., Butt J. H., Wismar J. D. Absorption of glycine and L-alanine by the human jejunum. J Clin Invest. 1966 Sep;45(9):1433–1441. doi: 10.1172/JCI105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY G. M., INGELFINGER F. J. INTESTINAL ABSORPTION OF SUCROSE IN MAN: THE SITE OF HYDROLYSIS AND ABSORPTION. J Clin Invest. 1965 Mar;44:390–398. doi: 10.1172/JCI105152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizer W. D., Laster L. Peptide hydrolase activities of the mucosa of human small intestine. J Clin Invest. 1969 Jan;48(1):210–228. doi: 10.1172/JCI105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg T., Nordén A., Josefsson L. Intestinal dipeptidases. Dipeptidase activities in small intestinal biopsy specimens from a clinical material. Scand J Gastroenterol. 1968;3(2):177–182. doi: 10.3109/00365526809180119. [DOI] [PubMed] [Google Scholar]
- Matthews D. M., Craft I. L., Geddes D. M., Wise I. J., Hyde C. W. Absorption of glycine and glycine peptides from the small intestine of the rat. Clin Sci. 1968 Dec;35(3):415–424. [PubMed] [Google Scholar]
- Matthews D. M., Lis M. T., Cheng B., Crampton R. F. Observations on the intestinal absorption of some oligopeptides of methionine and glycine in the rat. Clin Sci. 1969 Dec;37(3):751–764. [PubMed] [Google Scholar]
- Olmsted W. W., Nasset E. S., Kelley M. L., Jr Amino acids in postprandial gut contents of man. J Nutr. 1966 Nov;90(3):291–294. doi: 10.1093/jn/90.3.291. [DOI] [PubMed] [Google Scholar]
- Peters T. J., MacMahon M. T. The absorption of glycine and glycine oligopeptides by the rat. Clin Sci. 1970 Dec;39(6):811–821. doi: 10.1042/cs0390811. [DOI] [PubMed] [Google Scholar]
- Peters T. J. The subcellular localization of di- and tri-peptide hydrolase activity in guinea-pig small intestine. Biochem J. 1970 Nov;120(1):195–203. doi: 10.1042/bj1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. B., Eichholz A., Crane R. K. Studies on the organization of the brush border in intestinal epithelial cells. IV. Aminopeptidase activity in microvillus membranes of hamster intestinal brush borders. Biochim Biophys Acta. 1967;135(5):959–965. doi: 10.1016/0005-2736(67)90065-x. [DOI] [PubMed] [Google Scholar]
- Schedl H. P., Pierce C. E., Rider A., Clifton J. A. Absorption of L-methionine from the human small intestine. J Clin Invest. 1968 Feb;47(2):417–425. doi: 10.1172/JCI105738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl H. P. Use of polyethylene glycol and phenol red as unabsorbed indicators for intestinal absorption studies in man. Gut. 1966 Apr;7(2):159–163. doi: 10.1136/gut.7.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]