Abstract
Purpose
Dry eye disease (DED) is associated with ocular surface inflammation that is thought to be primarily mediated by CD4 T cells. The purpose of this study was to investigate whether this T cell mediated immune response is generated in the lymphoid compartment, and to characterize the functional phenotype of the T cells activated in DED.
Methods
DED was induced in female C57BL/6 mice by exposure to a desiccating environment in the controlled environment chamber (CEC) and to systemic scopolamine. T cells from regional draining lymph nodes (LN) of DED mice and normal mice were analyzed for surface activation markers (CD69 and CD154), chemokine and cytokine receptors, and for their proliferation potential.
Results
Draining LN of DE mice showed increased frequencies of CD69 and CD154 expressing T cells with higher proliferative capacity. In addition, these LN T cells primarily showed a Th-1 phenotype, expressing significantly higher levels of IFN-γ and IL-12Rβ2 but not IL-4R. Similarly, the LN of DE mice showed significantly increased frequencies of T cells expressing CXCR3 and CCR5, but not CCR4, suggesting a bias toward a Th-1 phenotype.
Conclusions
Our data demonstrate that a Th-1 type immune response is induced in the regional LN of DE mice. The identification of specific cytokine/chemokine receptors overexpressed by these T cells may signify potential novel targets/strategies for the treatment of DED.
Keywords: dry eyes, immunopathology, lymphocyte subsets
Introduction
Dry eye disease (DED) is one of the most common causes for patients seeking ophthalmic care for symptoms of ocular irritation and blurred vision.1 In more severe cases DED can lead to blindness due to corneal ulceration or infection.2, 3
To date, the pathogenesis of DED has not been fully understood, although it is widely recognized that DED is associated with ocular surface inflammation.4-8 This inflammation is thought to be primarily mediated by CD4+ T cells.9-10 The significance of T cell-mediated inflammation in DED is supported by several distinct lines of observation. First, in addition to the vast majority of DED patients responding to corticosteroids, Cyclosporine-A, an immunomodulatory agent that suppresses T cell lymphokines (IL-2), has been shown to be effective in improving the signs and symptoms of patients with severe DED.9 Second, T cell infiltration of the conjunctiva is observed in both clinical and experimental DED,9-10 concomitant with expression of the CCR5 chemokine receptor which is critical for recruitment of CD4+ T cells.6 Third, adoptive transfer of CD4+ T cells from DE mice into T cell deficient nude mice, in of itself, leads to the development of DED.11 While the immune system in T cell deficient nude mice is different from wild type mice, this observation strongly suggests a pathogenic role for T cells in DED. Despite the strong evidence for T cell involvement in the pathogenesis of DED the site and mechanism of T cell activation in DED is not known.
Regional lymph nodes (LN) have been shown to be critical sites for induction of immunity to tissue antigens since they serve as large reservoirs of lymphoid cells. In corneal transplantation for example, the critical role of draining LN in induction of alloimmunity has been established.12-14 The purpose of the present study was to investigate whether T cells in the draining LN are activated in DED by detecting the expression of surface activation markers and their proliferative capacity. Moreover, we investigated whether the T cells differentiate into a primarily Th-1 or a Th-2 response by detecting their cytokine and chemokine expression profiles.
Material & Methods
Mouse Model of Dry Eye
Eight week-old female C57Bl/6 mice (Taconic Farms, and Charles River Lab) were used in these experiments. The protocol was approved by the Institutional Animal Care and Use Committee, and all animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Dry Eye was induced by placement of mice during ten days in a Controlled-Environment Chamber (CEC)15 modified with subcutaneous administration of scopolamine to maximize ocular dryness.16 Age-matched mice not placed in the CEC were used as normal controls.
Flow Cytometry
Draining cervical LN were harvested aseptically and pooled (n=3-5 mice) separately from DE and normal mice. The single-cell suspensions obtained from these nodes were blocked with anti-FcR mAb (BD Pharmingen) for 30 min at 4°C in 1% BSA/0.02% NaN3/PBS. Cells were then stained with the following Abs for 45 min at 4°C: anti-CD4 FITC or anti-CD8 FITC, anti-CD69 PE, anti-CD154 PE, anti-CCR5 PE, anti-IL-12Rβ2, anti-IL-4R PE (BD Pharmingen), anti-CXCR3 PE (R&D), anti-CCR4 (Capralogics inc.), and their isotype-matched controls. Goat anti hamster PE and donkey anti goat PE (Jackson Immunoresearch) were the secondary antibodies applied for 45 min at 4°C against anti-IL-12Rβ2, and anti-CCR4 respectively. Finally, cells were washed and analyzed on an EPICS XL flow cytometer (Beckman Coulter).
Proliferation Assay
Draining LN were harvested as described above. CD4+ effector T cells from draining LN and T cell-depleted syngeneic splenocytes were sorted by magnetic assorted cell sorting (MACS) isolation kit (Miltenyi Biotec). CD4+ effector T cells from draining LN of normal mice served as controls. In proliferation assays, CD4+ effector T cells (1×105) were cocultured with T cell-depleted syngeneic splenocytes (1×105) and 1 μg/ml anti-CD3 antibody for 3 days. Proliferation was measured using BrdU incorporation assay (Millipore).
ELISPOT
The ELISPOT assay used to detect cytokine secretion by T cells is a modification of the previously described procedure.17, 18 Briefly, 96-well ELISPOT plates (Polyfiltronics) were coated with 4 mg/ml primary anti-IFN-γ mAb or IL-4 mAb (BD Pharmingen) in sterile PBS overnight. The plates were then washed three times with PBS and blocked for 1.5 h with PBS containing 1% BSA. Next, cells were harvested aseptically from draining LN (n=3-5 mice) of DE and normal mice. T cells were sorted by magnetic assorted cell sorting (MACS) isolation kit (Miltenyi Biotec) using anti-CD90 magnetic beads. These cells are then added to wells previously loaded with CD3e mAb (BD Pharmingen) to a final volume of 200μl of AIM-V medium. Cells were incubated for 48 h. The plates were washed three times with PBS, then four times with PBS containing 0.025% Tween 20. Biotinylated anti-IFN-γ and anti-IL-4 detection mAbs were added at 2 μg/ml (BD Pharmingen) and incubated for 2 h at room temperature. The washing steps were repeated, and after 1 h of incubation with avidin-HRP, the plates were washed again three times with PBS/0.025% Tween 20 and then three times with PBS alone. The spots were developed by the addition of the aminoethylcarbazole staining solution (Sigma-Aldrich). The resulting spots were counted and analyzed on a computer-assisted ELISPOT image analyzer (C.T.L.).
Statistics
Data are expressed as the mean ± SEM of the results for at least three separate experiments. A two-tailed Student’s t-test was performed using MS-Excel. P < 0.05 was taken as indicative of statistical significance.
Results
Increased Frequency and Proliferative Capacity of Activated T cells in DED
To investigate whether T cells are activated in the regional lymphoid compartment (draining LN) of DE mice, we measured the expression of the surface activation markers CD69, and CD154 (CD40L) on CD4+ and CD8+ T cells via two-color flow cytometry (Fig. 1A, 1B). We observed a significant increase in the percentage of both CD4+ and CD8+ T cells expressing CD69 (40%; P=0.01 and P=0.002, respectively) and CD154 (200%; P=0.004 and P=0.02, respectively) in the draining LN of DE mice as compared to those from the normal controls.
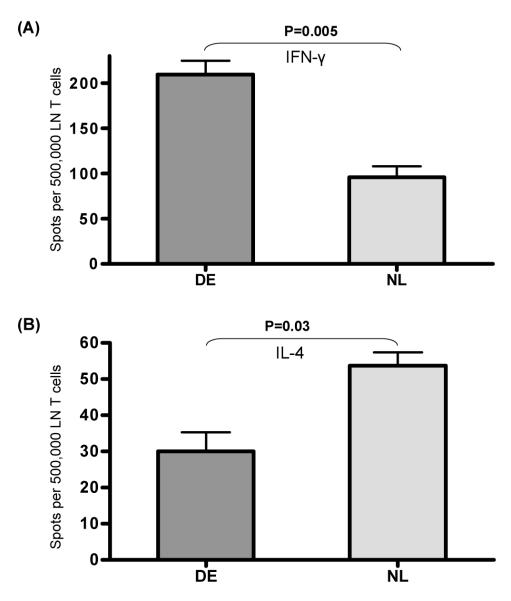
Figure 1. Analysis of T cell surface activation markers expression and its proliferative capacity.
Draining LN were harvested from DE vs. normal (NL) mice; single-cell suspensions were prepared and dual stained with anti-CD4 FITC (A) or anti-CD8 FITC (B) and either one of the activation marker antibodies anti-CD69 PE, or anti-CD154 PE. Data shown are representative of five independent experiments. (C) CD4+ T cells were sorted from the draining LN of DE vs. NL mice using the MACS cell isolation kit. CD4+ T effector cells were cocultured with T cell-depleted syngeneic splenocytes and anti-CD3 antibody for three days. Proliferative response was assessed by BrdU incorporation assay. Results are presented as optical density(OD) reading ± SEM. Data shown are representative of three independent experiments.
Antigen-stimulated T cells in the draining LN undergo proliferation to provide a larger population of antigen-specific effector T cells that mediate immune disease. To determine if T cells from DE mice undergo expansion, we compared the proliferative potential of T cells from DE versus normal mice. CD4+ T cells were magnetically sorted from harvested LN suspensions and stimulated with anti-CD3 antibody for three days in culture. Proliferation was measured using the BrdU incorporation assay (Fig. 1C). We observed a 12% increase in the proliferative capacity of CD4+ T cells isolated from the draining LN of DE mice (P=0.04) as compared to those from the normal controls.
Draining LN T cells Shift Toward a Th1 Immune Response
To determine whether the T cell response in the lymphoid compartment in DED is biased toward a Th-1 versus Th-2 phenotype, sorted T cells were stimulated with anti-CD3 antibody and ELISPOT assay was performed to quantify the frequencies of IFN-γ versus IL-4 positive T cells (Fig. 2). We found a significant 117% increase in IFN-γ (P=0.005), and a significant 79% decrease in IL-4 (P=0.03), secretion by T cells from DE mice as compared to those from the normal controls. In another set of experiments, FACS analyses for intracellular IFN-γ and BrdU incorporation showed that proliferating BrdU+CD4+T cells of DED mice also display enhanced IFN-γ expression compared to those of normal mice (data not shown).
Figure 2. Analysis of T cell cytokine secretion.
The frequencies of reactive T cells upon CD3 stimulation from draining LN of DE vs. NL mice were evaluated using the ELISPOT assay for(A) IFN-γ and (B) IL-4. The results are depicted as the mean number of spots per 0.5 million responder T cells loaded ± SEM. Data shown are representative of three independent experiments.
To further confirm the T cell immune response differentiation in the draining LN, we investigated the expression of the cytokine receptors IL-12R β2 and IL-4R on draining LN T cells via two-color flow cytometry (Fig. 3). IL-12R β2 is predominantly expressed on Th-1 cells whereas IL-4R is expressed on Th-2 cells.19, 20 We observed a significant 90% increase in the percentage of CD4+ T cells (P=0.03) (but not CD8+ T cells [P=0.40]) expressing IL-12R β2 in DE mice as compared to those from normal control. We also found that there was no change in the percentage of CD4+ T cells (P=0.60) or CD8+ T cells (P=0.49) expressing IL-4R in DE mice as compared to those from the normal controls.
Figure 3. Flow cytometric analysis of cytokine receptors by T cells.
Draining LN were harvested from DE vs. NL mice, single-cell suspensions were prepared and dual stained with anti-CD4 FITC (A) or anti-CD8 FITC (B) and either one of the cytokine receptor antibodies anti-IL-12Rβ2 PE, or anti-IL-4R PE. Data shown are representative of four independent experiments.
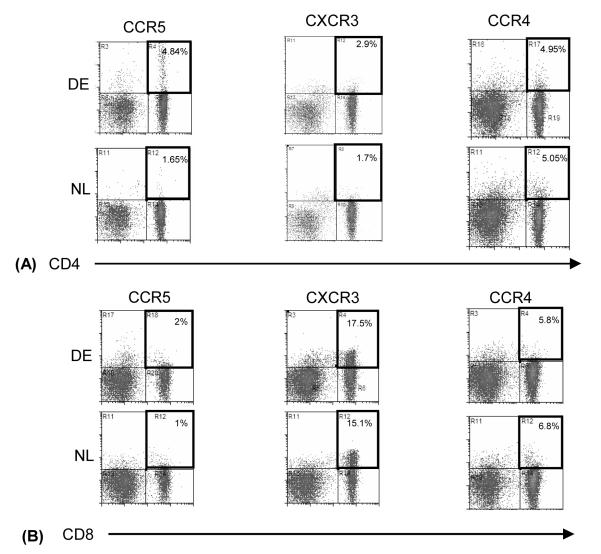
Increased Frequency of CCR5 and CXCR3 Expressing T cells
Effector T cells acquire chemokine receptors to migrate out of lymphoid compartments along chemokine gradients toward sites of inflammation in the periphery. Th-1 and Th-2 cells express distinct patterns of chemokine receptors. CCR5 and CXCR3 are predominantly expressed by Th-1 cells, while CCR4 is expressed by Th-2 cells.21 To determine the differential expression of these receptors by the draining LN T cells in DED, we performed two-color flow cytometry of CCR5, CXCR3, and CCR4 on CD4+ and CD8+ T cells (Fig. 4). We observed a significant increase in the percentage of both CD4+ and CD8+ T cells expressing CCR5 (190%; P=0.02 and 100%; P=0.005, respectively) and CXCR3 (70%; P=0.002 and 16%; P=0.01, respectively). This increase was 45% more in CD4+ T cells as compared to CD8+ T cells. We have also found that 80-85% of CXCR3 expressing CD4+ T cells co-express CCR5 both in normal and dry eye mice (data not shown). However, there was no significant change in the percentage of CD4+ (P=0.96) or CD8+ (P=0.76) T cells expressing CCR4 in the draining LN of DE mice as compared to those from the normal controls.
Figure 4. Flow cytometric analysis of chemokine receptors by T cells.
Draining LN were harvested from DE vs. NL mice, single-cell suspensions were prepared and dual stained with anti-CD4 FITC (A) or anti-CD8 FITC (B) and either one of the chemokine receptor antibodies anti-CCR5 PE, anti-CXCR3 PE, or anti-CCR4 PE. Data shown are representative of four independent experiments.
Discussion
The data presented herein demonstrate that T cells are activated in the regional LN of DE mice. These T cells have a Th-1 phenotype, as demonstrated by their ability to secrete IFN-γ and to acquire Th-1 type chemokine (CCR5 and CXCR3) and cytokine (IL-12Rβ2) receptors.
We found a significantly increased percentage of CD4+ and CD8+ T cells expressing CD69 and CD154 in DE mice as compared to normal controls. This increase was most notable among CD4+ T cells. CD69 is an early T cell activation marker and is not detected in resting lymphocytes. CD154, also known as CD40 ligand (CD40L), is predominantly expressed on activated CD4+ T cells and its expression enhances the activation and/or differentiation of these cells.22, 23 The expression of these cell surface markers is increased by activated T cells in humans with various autoimmune diseases.24-26 To confirm that the activation of the T cells is associated with their enhanced capacity to expand, we performed BrdU incorporation assay to detect the proliferative potential of these cells. We observed a higher proliferation of CD4 + T cells of DE mice as compared to those isolated from draining LN of normal controls. In the aggregate, these data point toward the generation of a T cell mediated immune response in the lymphoid compartment of DED mice. Though not a formal proof, the increased expression of CD40L suggests an immune process driven by specific antigen(s), potentially auto-antigens.23 It has been shown that autoantigens are exposed in cells undergoing apoptosis, such as the α-fodrin protein that is exposed in apoptotic lacrimal gland cells.27 Further studies are clearly needed to characterize the potential autoantigen(s) exposed by the ocular surface epithelium in DED.
Our data also demonstrate that T cells in DE mice have a significantly increased secretion of IFN- γ but not IL-4, and an increased percentage of CD4+ T cells expressing IL-12Rβ2 but not IL-4R as compared to those in normal controls. The differentiation of T cells into a Th-1 or a Th-2 response is determined by several factors, a principal one being the cytokine milieu during the interaction of antigen presenting cells (APC) with naïve T cells (Th-0). The ligation of CD154 with CD40 on the surface of APC increases the production of IL-12 by these cells.23 IL-12 is a key cytokine in Th-1-mediated autoimmune diseases,28 and promotes the secretion of IFN- γ by Th-1 cells.29-31 The primary signal transduction of IL-12 is through IL-12Rβ2,32 whose expression is believed to be confined to Th1 cells.19 Thus, these findings confirm a more notable involvement of CD4+ T cells in the initiation of a regional Th-1 immune response in DED.
Chemokine receptors are needed for the trafficking of activated T cells in vivo. Th-1 and Th-2 cells express distinct patterns of chemokine receptors. CCR5 and CXCR3 are predominantly expressed on Th-1 cells, while CCR4 is expressed by Th-2 cells.21 We found a significantly increased proportion of primarily CD4+ T cells expressing CCR5 and CXCR3 in the draining LN of DE mice as compared to normal controls. We have previously shown that CCR5 expression is increased significantly by the conjunctivae of humans with different forms of DED6 suggesting an important role for CCR5 in the ocular surface inflammation. In addition, it has been shown that the expression of CCR5, CXCR3, and their corresponding chemokine ligands, is increased by the conjunctiva and cornea of DE mice.33 Taken together, these findings suggest that the draining LN T cells acquire the chemokine receptors required for their migration and homing to the ocular surface. Recently, it has been shown that Th-17 cells play a role in autoimmune dieseases.34 In addition, Th-17 cells express CCR5 and CXCR3; therefore, their role in DED needs further investigation.35
The present study demonstrates the induction of a Th-1 immune response in the regional LN of DE mice coincident with their acquisition of specific chemokine receptors that assist their homing to the inflamed ocular surface in DED. At this time it is not known whether blockade of specific chemokine ligands/receptors may hold promise in the treatment of DED. However, it is noteworthy that several CXC and CC chemokines, and their respective receptors, have emerged as promising targets in transplantation and autoimmune disease.36-38 Future studies evaluating specific chemokine blockade, singly or in combination with cytokine systems involved in Th1 activation (e.g CD40L, IL-12), may provide novel strategies for the treatment of DED.
Footnotes
Financial interest: None
References
- 1.Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–15. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemady R, Chu W, Foster CS. Keratoconjunctivitis sicca and corneal ulcers. Cornea. 1990;9(2):170–3. [PubMed] [Google Scholar]
- 3.McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89(10):1173–80. doi: 10.1016/s0161-6420(82)34669-2. [DOI] [PubMed] [Google Scholar]
- 4.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166(1):61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol. 2006;124(5):710–6. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 7.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19(3):201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 8.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–92. [PubMed] [Google Scholar]
- 9.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43(8):2609–14. [PubMed] [Google Scholar]
- 10.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48(6):2553–60. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 11.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176(7):3950–7. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10(8):813–5. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195(2):259–68. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamagami S, Dana MR. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest Ophthalmol Vis Sci. 2001;42(6):1293–8. [PubMed] [Google Scholar]
- 15.Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46(8):2766–71. doi: 10.1167/iovs.04-1326. [DOI] [PubMed] [Google Scholar]
- 16.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–25. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 17.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352–8. [PubMed] [Google Scholar]
- 18.Shen L, Jin Y, Freeman GJ, Sharpe AH, Dana MR. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179(6):3672–9. doi: 10.4049/jimmunol.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 19.Rogge L, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185(5):825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamalainen H, Zhou H, Chou W, Hashizume H, Heller R, Lahesmaa R. Distinct gene expression profiles of human type 1 and type 2 T helper cells. Genome Biol. 2001;2(7) doi: 10.1186/gb-2001-2-7-research0022. RESEARCH0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2(2):123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 22.Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26(3):136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100(9):2404–14. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98(3):826–37. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang WX, Huang P, Hillert J. Systemic upregulation of CD40 and CD40 ligand mRNA expression in multiple sclerosis. Mult Scler. 2000;6(2):61–5. doi: 10.1177/135245850000600201. [DOI] [PubMed] [Google Scholar]
- 27.Haneji N, Nakamura T, Takio K, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science. 1997;276(5312):604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 28.Adorini L. Interleukin-12, a key cytokine in Th1-mediated autoimmune diseases. Cell Mol Life Sci. 1999;55(12):1610–25. doi: 10.1007/s000180050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 30.Manetti R, Parronchi P, Giudizi MG, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177(4):1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263(5144):235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 32.Ozenci V, Pashenkov M, Kouwenhoven M, Rinaldi L, Söderström M, Link H. IL-12/IL-12R system in multiple sclerosis. J Neuroimmunol. 2001;114(1-2):242–52. doi: 10.1016/s0165-5728(00)00449-5. [DOI] [PubMed] [Google Scholar]
- 33.Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48(6):2561–9. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 34.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18(6):670–5. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180(1):122–9. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 36.Schnickel GT, Bastani S, Hsieh GR, et al. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180(7):4714–21. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 37.Schnickel GT, Hsieh GR, Garcia C, Shefizadeh A, Fishbein MC, Ardehali A. Role of CXCR3 and CCR5 in allograft rejection. Transplant Proc. 2006;38(10):3221–4. doi: 10.1016/j.transproceed.2006.10.164. [DOI] [PubMed] [Google Scholar]
- 38.Turner JE, Steinmetz OM, Stahl RA, Panzer U. Targeting of Th1-associated chemokine receptors CXCR3 and CCR5 as therapeutic strategy for inflammatory diseases. Mini Rev Med Chem. 2007;7(11):1089–96. doi: 10.2174/138955707782331768. [DOI] [PubMed] [Google Scholar]