Abstract
Predominant frameworks for understanding plant ecology have an aboveground bias that neglects soil micro-organisms. This is inconsistent with recent work illustrating the importance of soil microbes in terrestrial ecology. Microbial effects have been incorporated into plant community dynamics using ideas of niche modification and plant-soil community feedbacks. Here, we expand and integrate qualitative conceptual models of plant niche and feedback to explore implications of microbial interactions for understanding plant community ecology. At the same time we review the empirical evidence for these processes. We also consider common mycorrhizal networks, and suggest these are best interpreted within the feedback framework. Finally, we apply our integrated model of niche and feedback to understanding plant coexistence, monodominance, and invasion ecology.
Plant Community Ecology Models Overlook Soil Microbial Interactions
Communities of competing plant species are stabilized by stronger negative intraspecific interactions relative to interspecific interactions[1]. Traditionally, strong negative intraspecific interactions have been thought to result from high resource use overlap[2, 3]. These models of resource partitioning have been developed into an influential framework for understanding plant community dynamics, but the empirical evidence supporting them is still limited. Plant competition experiments have not shown unequivocally that the strength of intraspecific competition exceeds that of interspecific competition[4] and the empirical evidence of coexistence of competing plant species through resource partitioning remains mixed[5-7].
In response to the perceived limitations of explaining species coexistence through resource partitioning, plant ecologists have increasingly looked for mechanisms that might limit the negative effect of competition on inferior competitors and thereby slow competitive exclusion. For instance, competition-colonization tradeoffs can allow inferior competitors to persist through their greater likelihood of establishing in transient gaps in vegetation[8]. Similarly, with a trade-off between competition and antagonist avoidance, generalist herbivores[9] and hemiparasites[10] might remove proportionally more biomass or resource from superior competitors, and thereby allow inferior competitors to persist. At one extreme, plant species have been argued to be ecologically equivalent with species composition resulting from dispersal and recruitment limitation[11]. However, accumulated evidence does not support the ecological equivalence of plant species, and does not always support a crucial role of equalizing mechanisms in maintaining local diversity[12, 13].
Current theory neglects the less visible organisms in the soil and this might be one reason for the limited success in finding a mechanism to explain the coexistence of competing plant species. The presence and composition of soil microbial communities has been shown to have large impacts on plant-plant interactions[14-16] and consequently plant diversity and composition[17, 18]. Therefore, to understand plant community structure and dynamics more completely, a microbial perspective needs to be integrated into our conceptual frameworks.
We identify three ways to incorporate microbial effects into concepts of plant community dynamics and coexistence. The first two are mechanisms through which microbes can modify plant resource competition. These are microbial modification of plant resource partitioning (expansion or contraction) and common mycorrhizal networks (CMNs; see Glossary) as potential pathways for resource sharing among interacting plants. The third, plant-soil community feedbacks, is a mechanism of modification of plant-plant interactions that does not depend upon competition for resources but rather involves the dynamics of soil microbes (changes in density and composition) which might have beneficial or detrimental effects on interacting plants. We first describe these mechanisms and the evidence for them, and then contrast and integrate these three frameworks with current theory of plant coexistence.
Resource Partitioning and Microbial Interactions
Prevailing models explaining species coexistence predict that competing species can coexist provided they are most limited by different resources and that they consume the resource they are most limited by at a higher rate than do other species [3]. Tests provide strong support for this mechanism of coexistence operating among aquatic organisms, but more limited support for it operating among terrestrial plants [5-7]. One limitation of these models is that they generally have not included microbial symbionts that mediate nutrient uptake of most terrestrial plant species. Symbiotic N-fixation, for example, contributes a large portion of the terrestrial nitrogen budget and the uptake of phosphorus by most plant species is facilitated by mycorrhizal fungi[19]. Since these symbionts modify nutrient uptake, they will also modify the conditions for competitive coexistence, either positively or negatively. For example, a symbiont that increases plant productivity and allows its host to persist at lower levels of a limiting nutrient could directly contribute to competitive exclusion of other plant species (Figure 1a), as has been documented for nutrient poor soils of the central grasslands in North America[20] and Australia[21]. We consider two potential scenarios in which coexistence through resource partitioning is microbially-mediated.
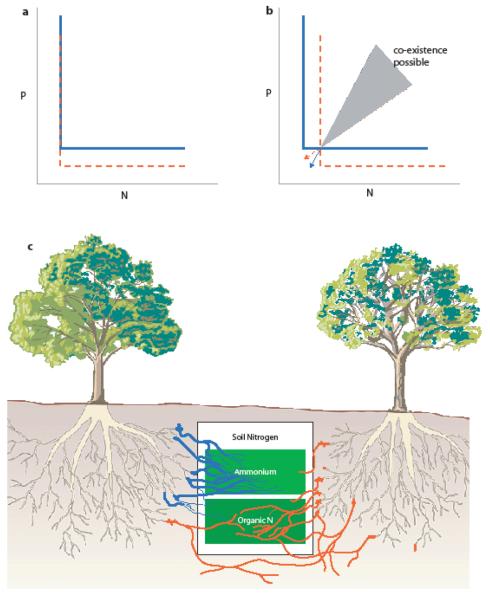
Figure 1.
Soil microbes and resource partitioning. In (a) and (b), the levels of phosphorus (P) and nitrogen (N) required for persistence are represented by the thick colored lines for a blue (solid) and orange (dashed) species of plant. (a) represents the advantage conferred to the orange species through association with an arbuscular mycorrhizal (AM) fungus that increases access to soil P, which could confer competitive superiority to the orange species in the absence of costs. If the association with the AM fungus comes at a cost in N, then this trade-off could allow coexistence of a mycorrhizal plant species (orange) with a non-mycorrhizal plant species (blue) under a range of nutrient supply points (b). The orange (dashed) and blue (solid) arrows represent the rate of consumption of the two resources of the orange and blue species, respectively. (c) presents a hypothetical example of resource partitioning among two Eucalyptus tree species mediated by specific associations with two different species of ectomycorrhizal fungi with differential access to organic and inorganic pools of N.
First we consider the situation where two plant species vary in their dependence on a symbiont for a specific soil nutrient. Such variation could contribute to resource partitioning if investment in the symbiont required greater demand for a second soil nutrient. Symbiotic nitrogen-fixation, for example, is very demanding of phosphorus due to the high ATP requirement per mole N fixed[22]. Furthermore, investment in mycorrhizal fungi, which often facilitates phosphorus uptake, can be very demanding of nitrogen as mycorrhizal fungi have a C:N ratio that is 10:1 compared to a much higher ratio in plants[23]. In these examples, nutrient uptake through the symbiosis generates a trade-off in the uptake of a second soil resource which can directly contribute to plant species coexistence (Figure 1b). Van der Heijden[24] had previously illustrated this possibility in considering interactions between a mycorrhizal dependent and a non-mycorrhizal plant species. At present there is little evidence of this mechanism allowing coexistence of plant species that vary in dependence on mycorrhizal fungi (to the contrary, see evidence of positive feedback through changes in density of mycorrhizal fungi below). There is, however, strong circumstantial evidence of symbiotic N-fixers mediating plant species coexistence between legumes and non-legumes[14, 25].
A second mechanism through which microbially-mediated resource partitioning could contribute to plant species coexistence involves plant species associating with different microbial symbionts which then provide differential access to alternate forms of particular resources[26]. At a coarse level, plants tend to associate with distinct types of mycorrhizal fungi and it is likely that association with different types of mycorrhizas could alter plant access to limiting resources in a way that allows plants to coexist. Plants that associate with arbuscular mycorrhizal fungi are expected to have increased access to P, while plants associating with ecto- or ericoid mycorrhizal fungi are expected to have increased access to N[27]. More subtly, individual species of ectomycorrhizal fungi, for example, can preferentially associate with specific hosts[28] and vary in their access to mineral and organic forms of N and P[29]. It is then possible that preferential association within this symbiosis directly contributes to resource partitioning of their hosts (Figure 1c). The few attempts to test this more subtle form of microbial mediation of resource partitioning have focused on grasslands and arbuscular mycorrhizal fungi and have failed to find support[18, 30]. Additional tests with plants in other communities and with other microbial symbionts are necessary to fully evaluate this hypothesis.
Saprophytic rhizosphere bacteria might also alter plant-plant interactions through mediation of resource partitioning[31]. Rhizosphere bacteria can alter availability of different forms of N or P in the soil through exudation of enzymes[31, 32]. Despite these microbes being less host-specific than many root symbionts, differences in microbially-produced enzymatic functions have been found in spatial association with montane grassland plant species[33, 34]. Attempts to demonstrate that these shifts in microbial community contribute to resource partitioning, however, have failed, as the plants showed similar uptake rates for different forms of N[33]. Further work is required to evaluate whether the microbial effects on resource turnover contributes to competitive exclusion in these species33.
Resource Sharing Through Common Mycorrhizal Networks
Another way in which modification of resource access can shape plant species coexistence is via transfer of resources through shared fungal symbionts, often called common mycorrhizal networks (CMNs)[35]. Given the low specificity of many species of mycorrhizal fungi, shared symbionts between plants of different species might be common in nature. It is then possible that carbon and nutrients might be transferred from plant to plant through the CMNs[35-37], and this could alter plant competitive ability. It is also possible that shared symbionts can mediate plant-plant interactions through changes in density or composition of the symbiont community. These two mechanisms (resource transfer and changes in density) have very different implications for plant communities, but are often confused within literature on CMNs.
Direct transfer of resources via mycorrhizal hyphal linkages has been suggested by experiments showing that nitrogen, phosphorus and carbon labeled in one plant can be detected in a second individual[35, 38-41]. Transfer of resources via CMNs, if supported, reflects a unique feature of the mycorrhizal symbiosis. Should the resources flow from plants with higher to plants with lower resource levels (sometimes referred to as ‘source-sink”), resource transfer could potentially contribute to plant species coexistence through minimization of differential access to resources, with linked plant species forming guilds of mutual aid[35, 37]. While there is clear evidence of net movement of carbon to mycoheterotrophic, non-photosynthetic plants which parasitize fungi for carbon, there is little evidence of ecologically meaningful exchange of resources between photosynthetic plants or that there is a significant net directional flow, as predicted by a source-sink relationship[41-43]. Where quantification of the extent of resource transfer has been achieved, quantities transferred can be very low, representing as little as 0.004% of photosynthetic carbon gain[44]. Further, defoliation of adult plants has been found to contribute to an increased mycorrhizal benefit in neighboring seedlings, the opposite of the prediction of source-sink relationships[45]. Finally, larger planted seedlings have been found to receive more carbon transfer than smaller seedlings, and the amount of carbon fixed by donor plants has been found to be unrelated to transfer[44]. Before resource transfer via CMNs can be incorporated into more general theories of plant coexistence, the ecological significance of any potential shared resources between plants must be quantitatively demonstrated to cause an increase in plant performance.
In contrast to evidence for resource sharing, there is strong evidence that plants can benefit other plants indirectly through their support of local symbiotic fungal populations and an established mycorrhizal mycelium in the soil[46-48]. This work has been interpreted as evidence of the importance of CMNs[35-37], but the implications of this for plant communities are best understood in a framework that explicitly considers consequences of changes in densities of microbes, a topic that we develop within the context of feedbacks through plants and their soil community.
Variation in Host Response to Microbes and Soil Community Feedback
In the frameworks discussed so far (microbial mediation of soil resource partitioning and the common mycorrhizal network), plant community dynamics are driven by resource competition or sharing of resources, respectively. The composition of the microbial community is critical to the process, but the dynamics of the microbes are not explicitly considered. Explicit consideration of the dynamics of the microbes (changes in density and composition) allows a third potential way in which microbes can alter plant species coexistence through indirect feedbacks on plant populations[49].
This process builds on the well established observation that plant species differ in their response to individual microbial species, as both the negative effects of individual species of soil pathogens and the positive effects of individual species of root symbionts are host-specific. As a result, the composition of the microbial community can have strong direct effects on the outcome of plant-plant interactions, as is repeatedly demonstrated in manipulative experiments[17, 18]. As soil microbial composition varies considerably within plant communities[50-52], soil communities then represent a heterogeneous environment that could contribute to plant species coexistence in an analogous way to variation in supply rates of abiotic soil resources. However, while abiotic supply rates are thought to be relatively stable over time, components of the soil community, including soil microbes, have been shown to rapidly change in response to plant identity[53-55]. This change in microbial composition will generate a feedback on plant relative performance which will define the long-term influence of soil microbes on plant species coexistence[49, 56].
Soil community feedback then involves two steps: first, the density and/or composition of the soil community changes in response to composition of the plant community, and second, the change in composition alters the relative growth rates of individual plant species (Figure 2). If the change in microbial composition increases the relative performance of the locally abundant plant species, then it would generate a positive feedback dynamic that would lead to loss of diversity at a local scale. Conversely, if the change in microbial composition decreases the relative performance of the locally abundant plant species, then it would generate a negative feedback that could contribute to plant species coexistence[49, 56]. As plant-microbe interactions likely occur at a local scale, feedbacks are commonly measured at the scale of individual plants. The influence of the soil community on plant species coexistence depends upon both the direct feedbacks on conspecifics of the host plant and on indirect feedbacks through competing species[49, 56] (Figure 2).
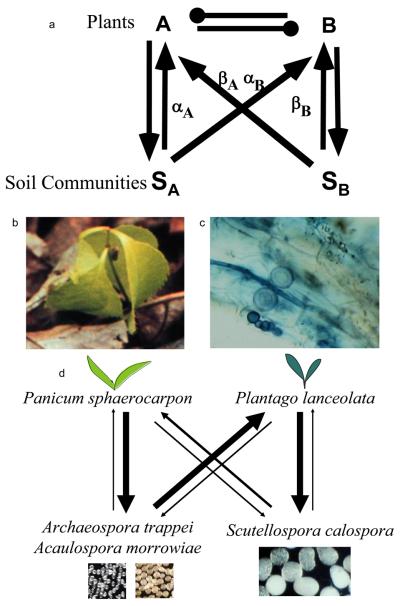
Figure 2.
Soil community feedback. (a) presents a conceptual representation of soil community feedback (modified from[49]). The presence of plant A can cause a change in the composition of the soil community, represented by SA. This change in the soil community can directly alter the population growth rate of species A (represented by αA) and it can alter the growth rate (αB) of competing plant species B (with negative effect represented by the club symbol). Similarly, the presence of plant B can cause a change in the composition of the soil community (SB) which can directly feed back (βB) on the population growth rate of plant B or indirectly feed back on the growth rate of plant B through changes in the growth rate (βA) of competing plant A. The net effect of soil community dynamics on plant species coexistence is determined by the sign and magnitude of an interaction coefficient = αA− αB− βA+ βB, which represents the net pairwise feedback[49]. (b) and (c) depict the direct negative feedback due to accumulation of pathogenic Pythium sp., as has been observed in Prunus serotina (black cherry) trees in North American forests[62]. The P. serotina seedling (b) exhibits chlorosis likely resulting from infection with Pythium sp. (c) depicts roots infected with Pythium sp. This direct negative feedback could contribute to coexistence with competing tree species when the deleterious effects of Pythium sp. are host-specific. (d) presents net pairwise negative feedback between Panicum sphaerocarpon and Plantago lanceolata generated by changes in composition of AM fungi[53]. Thickness of arrows represent the relative strengths of benefit between individual species of plants and AM fungi. Scutellospora calospora has high fitness with Plantago, but Plantago doesn't grow well with Sc. calospora. Rather, Plantago has highest growth rates in association with AM fungi, Archaeospora trappei and Acaulospora morrowiae, which themselves have high fitness in association with Panicum. The asymmetric fitness relationships generate negative feedback which can contribute to coexistence of these competing plant species. Spores of the AM fungi, Ar.trappei, Ac. morrowiae and Sc. calospora, are depicted. Photos of P. serotina seedling is credited to A. Packer, and roots and fungi are credited to J. Bever.
While positive soil community feedbacks have been measured (Box 1), soil community feedbacks have been found to be generally negative[57-61]. Moreover, the strength of measured feedbacks has been found to correlate positively with relative plant species abundance[58]. Negative soil community feedbacks have been found to result both directly through accumulation of host-specific pathogens[54, 62] and indirectly through host-specific changes in the composition of mycorrhizal fungi[53] and rhizosphere bacteria[63], as well as through changes in abundance of larger soil organisms such as nematodes[64]. The relative importance of particular microbial or larger soil organisms in driving soil community feedbacks requires additional study, but the complementarity of mechanisms likely contributes to the consistency with which negative feedbacks are observed.
BOX 1. Methodology for studies of soil community feedback.
Theory predicts that the effect of microbial community dynamics on plant species coexistence and invasion depends upon net pairwise feedbacks[49, 56, 91]. There are two qualitatively different approaches to quantifying these effects. The first would be to monitor the population dynamics of individual components of microbial communities and project the impact of these dynamics on plant-plant interactions using manipulative experiments. This approach is commonly applied to above-ground herbivores, pathogens and mutualists and has been applied to individual species of pathogens and mutualists in the soil as well[53, 54]. However, it is not possible to reconstruct the net contribution of the soil microbial community using this approach given the high diversity of soil microbes and the great difficulty in monitoring their densities.
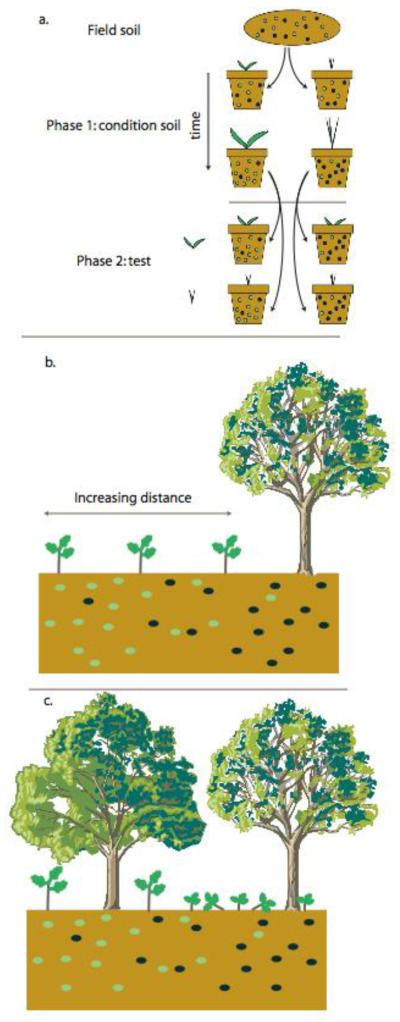
A second approach is to test plant response to entire, differentiated soil communities in order to directly estimate the net soil community feedback parameters (Figure 2). These experiments have two stages, a first in which the soil community differentiates in response to hosts and a second evaluating plant growth response. The first differentiation stage can itself be an experimental manipulation, commonly done within a greenhouse (Figure Ia). As initial soil community composition is similar in replicate pots during the first phase; the host-specific differences in microbial composition observed at the test stage can be causally attributed to host-specific differences in microbial population growth rates[49]. Alternatively, microbial community dynamics can be assumed to occur quickly compared to plant community dynamics, and therefore evidence of host-specific differentiation of soil communities can be obtained by sampling close to adult plants (Figure Ib).
Whether differentiated soil communities are obtained through experimentation or through sampling of adult plants, the test of plant response can similarly be performed in the greenhouse with inoculation of microbial communities into common background soil to isolate microbial effects[57, 80, 92]. Alternatively, in field studies, seedlings can be planted and monitored at different distances from adult plants (Figure Ib). These field experiments demonstrate potential soil community feedback under more realistic conditions than greenhouse experiments. Further isolation of microbial mechanisms can be attempted by manipulations such as biocides, trenching, or barriers[59, 74, 76]. Comparative studies analyzing the distribution and abundance of seedlings in relation to conspecific and heterospecific adults (Figure Ic) have been widely used in the context of the Janzen-Connell hypothesis[62, 70-72, 84]. This approach can be combined with manipulative experiments, described above, that more effectively isolate soil microbial effects.
Figure I.
(a) Methods used to study plant-soil community feedbacks include two-phase conditioning experiments; (b) planting seedlings at varying distances from trees, and (c) observations of seedling size-distributions in mixed species forests.
While the potential importance of soil community feedbacks to plant species coexistence receives strong support from manipulative pot experiments, Kulmatiski and colleagues[60, 65] have suggested that there is limited support for the operation of this mechanism in the field. Recent field manipulations have demonstrated negative feedback in grasslands[66, 67]. Additional evidence (Box 1) of the importance of soil community feedbacks in the field comes from tests of distance effects in mature forests, ex-situ bioassays, and in-situ plantings with barriers[62, 68, 69]. Declines in plant performance with proximity to con-specific adults can be a spatial signature of local-scale negative feedback, often referred to as the “Janzen-Connell hypothesis”. Empirical tests indicate that negative feedback is prevalent in tropical and temperate forests, particularly at germination and establishment stages[62, 70-72]. While Janzen[73] originally proposed that species-specific seed predation would be a major mechanism driving this pattern, empirical work is most consistent with host-specific soil pathogens playing a dominant role[62, 74]. Conversely, positive feedback (Box 2) has been frequently observed in both temperate and tropical ectomycorrhizal trees when comparing seedling mycorrhizal infection, growth and survival near ectomycorrhizal trees with areas without ectomycorrhizal vegetation[48, 69, 75].
Box 2. Plant soil community feedbacks in low-diversity communities.
While ecologists have historically focused on understanding plant co-existence and the maintenance of diversity, there are also plant communities of strikingly low diversity, including ectomycorrhizal (EcM) “monodominant” forests, and the establishment of monospecific stands by many invasive plants. Here we discuss three explanations for low-diversity plant communities.
| Application | ||||
|---|---|---|---|---|
| Model | Ectomycorrhizal Monodominance |
Invasive Plants | ||
| Competitive dominance | 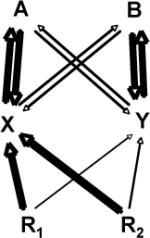 |
Soil symbionts (X) are more effective at resource acquisition and associates preferentially to EcM trees or invasive plants (A) than to other plants (B). Microbial-mediated competitive dominance is not expected in low- specificity symbioses such as arbuscular mycorrhizas. |
EcM fungi permit greater plant access to organic sources of N and some other nutrients than arbuscular mycorrhizal fungi. Monodominant stands are often characterized by an accumulation of recalcitrant litter which might favor EcM dominance [93, 94]. |
Invasive plants forming monodominant stands frequently have mutualisms novel to the invaded areas. These novel mutualisms might substantially increase the competitiveness and niche-breadth of invasive species[95], a form of the empty niche hypothesis [96]. |
| Inhibition | 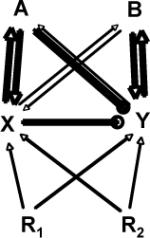 |
Inhibition might occur where the monodominant species (A) and/or its mutualists (X) inhibit the mutualists of, and hence ability of other plants (B) to acquire resources (R1, R2). |
Inhibition of soil symbionts in monodominance has not been widely considered, but competition between symbionts with consequent negative effects on plant performance[97] and incompatible symbiont interactions have been demonstrated[98]. |
The degraded mutualist hypothesis suggests invasive plants suppress native symbiont communities, indirectly reducing native plant fitness [86, 99] and appears likely where invasive plants co-invade with invasive mutualists[87, 100]. |
| Positive Feedback | 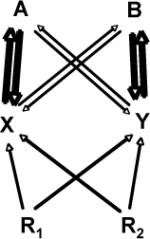 |
Positive feedback, where plants accumulate host-specific beneficial soil microbes, is inherently destabilizing, leading to single species dominance[49]. Positive feedbacks do not predict which species will dominate, but serve to amplify any small perturbation in population differences. |
EcM seedlings establishing near established EcM plants have enhanced growth and survival compared to seedlings germinating distant from established EcM plants, which represents a potential positive feedback mechanism [69, 75]. |
Positive feedback might occur through enhanced mutualisms[101] where novel, efficient combinations of alien plants and resident mutualists arise. |
Figure I.
Examples of monodominant invasive and EcM plants: invasive Pinus contorta (lodgepole pine, left) and Ulex europaeus (common gorse, middle) in New Zealand, and the tropical monodominant Dicymbe corymbosum (right) in Guyana. Photo credits: P. contorta: I. Dickie, Ulex europaeus: D. Peltzer, Dicymbe corymbosum: Krista McGuire.
Integrating, Differentiating, and Testing the Three Mechanisms
While microbially mediated resource partitioning and negative soil community feedback offer alternative mechanisms for the maintenance of diversity at a local scale (i.e. they are alternative stabilizing mechanisms[1]), resource sharing through common mycorrhizal networks represents a mechanism for minimizing fitness differences between plant species (i.e. it is a fitness equalizing mechanism[1]), perhaps making plant species within the CMNs more ecologically neutral. These three mechanisms are conceptually distinct, but they are not mutually exclusive, particularly for mycorrhizal fungi, which could conceivably influence plant-plant interactions through each of these mechanisms.
Indirect facilitation between plants, for example can be mediated by direct transfer of resources through CMNs or through changes in the density or composition of root symbionts. Recently published studies reporting support for the ecological importance of CMNs do not distinguish between these two mechanisms. For example, trenching around individual plants has been used as a field test of the consequences of breaking CMNs, thereby preventing resource sharing[76, 77], but these manipulations also reduce the density of mycorrhizal fungi available to the focal plant (and limit mycorrhizal access to soil resources). Thus this manipulation does not separate resource transfer through CMNs from indirect effects of changes in fungal density or abundance (or microbial mediation of resource partitioning). Independent approaches provide strong evidence for indirect effects through changes in density and composition of ectomycorrhizal fungi in similar systems[48, 78-80], as well as in non-fungal symbionts[81]. In fact, even the special case of mycoheterotrophic plants which derive their carbon from mycorrhizal fungi, can be most appropriately interpreted as indirect effects mediated through changes in mycorrhizal fungal density, with the photosynthetic plant host supporting the mycorrhizal fungus which is then exploited by the parasitic plants. The term “common mycorrhizal networks” creates the false impression that there is something qualitatively different about the effect of mycorrhizas on plant-plant interactions from those of other microbial mutualists (e.g. Frankia[81]), or, indeed any other mutualists (e.g. pollinators) which are often shared by coexisting plant species in nature.
Given that soil community feedbacks build on direct effects on population growth and, in the case of nutritional mutualisms, the direct effects result from improved access to resources, it is possible that microbially mediated resource partitioning and feedbacks through the soil microbial community occur simultaneously. While the few models that have looked at the joint effects of feedback and competition[56, 59, 82, 83] have not explicitly evaluated plant community dynamics with multiple resources, we can identify elements of this joint dynamic using simple diagrams (Figure 3). In particular, we can illustrate that a likely scenario of microbially mediated resource partitioning will have elements of positive feedback in microbial composition either because of differences in dependence on a single symbiont (Figure 3a) or because of the presumed best matching of plant and symbionts (Figure 3b). The conditions under which plant-plant interactions are stabilized by microbially mediated resource partitioning or destabilized by positive feedback generated by the microbial population dynamics have not been identified and remain ripe for further theoretical work. An additional complication to the process is that the change in microbial population size will be localized around individual hosts and this could generate a self-reinforcing spatial clumping: a greater concern for microbes with limited dispersal such as symbiotic and saprophytic bacteria.
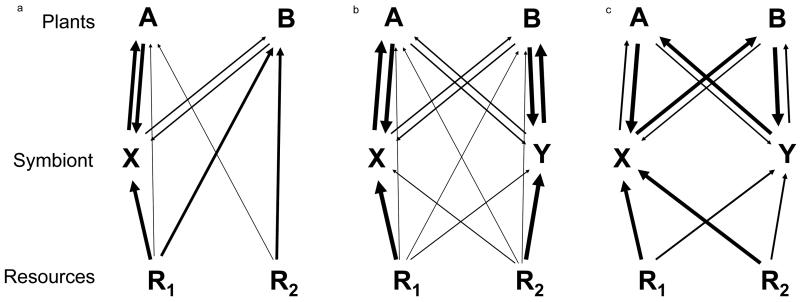
Figure 3.
(a) represents microbially-mediated resource partitioning where plant A has greater access to resource 1 through association with symbiont X (similar to Figure 1b) while (b) represents microbially-mediated resource partitioning with plants host-specific symbionts which differentially access soil resources (similar to Figure 1c). In both of these scenarios, the dynamics of plant A will be determined by the product of the stabilizing effects of the resource partitioning and the destabilizing effects of the positive feedback. (c) represents the stabilizing effect of negative feedback with the destabilizing effect of microbially-mediated competitive dominance.
Conversely, soil community dynamics within the nutritional mutualisms could stabilize plant-plant interactions even when nutritional dynamics might be destabilizing. For example, one symbiont might be a better competitor for soil resources and might more efficiently deliver these benefits to a single host species. This would be consistent with competitive dominance of the host species (Figure 3c). Yet, the plant-plant interactions could be stabilized by negative feedback generated by highly asymmetric fitness relations between the plant and symbionts (Figure 3c). Such asymmetric fitness relations resulting in negative feedback have been observed in interactions between co-occurring plants and arbuscular mycorrhizal fungi[53]. Tests of these alternative mechanisms will necessarily need to manipulate soil microbial composition while following nutrient dynamics.
Significance of Microbial Mechanisms of Plant-Plant Interactions
Of the mechanisms discussed here for microbial mediation of plant species coexistence, we suggest there is strong evidence that negative plant-soil community feedback plays a major role. This view is supported by the predominance of negative feedbacks in direct empirical tests[59, 60], the complementarity of mechanisms of negative feedback (Box 1)[56], and the evidence of positive correlation between the strength of soil community feedback and relative abundance[58]. While most of this work was done in temperate grasslands, similar processes are likely occurring in the tropical and temperate forests, where increased mortality of seedlings in proximity of adults has been commonly observed [62, 70-72, 84] and host-specific soil pathogens are a likely cause of these effects[62]. Theoretical work demonstrates the potential for negative feedback to allow coexistence of plant species that differ in competitive ability [56, 59, 82]. Moreover, as negative feedback generates oscillations in local abundance [49, 85], the storage effect [1] can increase the likelihood for coexistence. Future work needs to focus on tests of the relative importance of negative soil community feedback in plant species coexistence compared to other processes, such as resource partitioning or life-history trade-offs.
Microbial interactions can also generate strong positive feedbacks within plant communities, with these feedbacks potentially contributing directly to reduced diversity within local communities (Box 2). These positive feedbacks are often generated by changes in densities of soil microbial mutualists[48, 69, 75, 86]. In these situations, the structure and dynamics of plant communities can be strongly influenced by historical factors, including assembly and priority effects. These effects result when early occupancy of the site provides a substantial advantage to the initial coloniser, preventing or reducing the establishment of later colonists. For example the introduced plant species in California have lower dependence on mycorrhizal fungi than native plant species, and as a result initial dominance of exotic plant species following disturbance can inhibit the reestablishment of the native flora[86, 87] (Box 2). In Europe, Kardol and collaborators[61] found that early successional plants generally changed the soil community in a manner that increased the likelihood of establishment of mid-successional species. However, species dominance in the mid-successional community depended on the identity of the early successional species. The extent to which soil microbes mediate the dominance of highly invasive plant species or monodominance of tropical forests remains to be demonstrated (Box 2).
Finally, we note that disturbance, such as tillage, fire-induced plant mortality or surface mining, can severely damage soil microbial communities[88]. Given that microbial population dynamics can play a major role in plant species coexistence and can generate positive-feedback induced priority effects (Box 2), we would expect the reestablishment of the native soil community to be a limiting factor in restoration of native plant diversity and composition. Inoculation with native soil microbes has been shown to increase the rate of establishment of native plants[61, 89], though the extent to which soil microbes limit restoration success remains to be demonstrated.
Conclusions
Current plant community theory postulates that stabilizing mechanisms are essential for plant coexistence[1]. We have found evidence that microbially mediated niche modification and negative soil community feedbacks might significantly contribute to these mechanisms, but we do not find convincing evidence of ecologically meaningful resource redistribution via common mycorrhizal networks. Rather, studies claiming to show CMNs likely reflect altered densities of mycorrhizae. While this review has focused largely on plant species coexistence, we have also identified potential implications of these microbial mechanisms for plant species invasions and monodominance. We see great potential to extend the frameworks that we have developed to gain insights into the roles of soil microbial interactions in plant community response to anthropogenic environmental changes.
Acknowledgements
This collaboration was supported by the Australian Research Council-New Zealand Research Network for Vegetation Function (Working Group 61 - Plant-Soil Microbes Feedbacks). JDB was supported by NSF grants DEB-0616891, DEB-0919434 and NIH-5 R01 GM092660. IAD was supported by Ecosystem Resilience OBI of the FRST of NZ. MM and MZ were supported by ETF grants 7371, 7366 and Center of Excellence FIBIR.
Glossary
- Arbuscular mycorrizas (AM)
Symbiotic associations between plants and fungi where fungal hyphae colonize plant root cells and form arbuscules which are branching structures and site of exchange of nutrients between the plant and fungus.
- Common mycorrhizal networks (CMNs)
Extraradical hyphae from mycorrhizal fungi that grow in symbiosis with more than one plant, forming a belowground network linking numerous plant roots.
- Ectomycorrhizas (EM)
Plant-/fungal symbiosis where a fungal mantle encloses short lateral roots of plants with limited hyphal penetration of the root.
- Janzen-Connell hypothesis
Janzen[73] and Connell[90] suggested that negative feedback between trees and seedlings (, driven by increased species-specific herbivores and fungi near trees), is an important mechanism contributing to tropical rainforest diversity. This pattern is the spatial signature of negative feedback and maymight frequently be driven by soil microbes.
- Mycoheterotrophic
Symbiotic relationship between plants and fungi in which the plant obtains its food by parasitism of the fungus rather than from photosynthesis.
- Niche differentiation
Classic niche differentiation suggests that two species can co-exist when they utilize resources differently, thereby reducing interspecific competition.
- Plant-soil feedback
Process where a plant alters soil conditions (physical, (bio-)chemical, or biological), which in turn affects that plant's growth and fitness [32]. This paper focuses on soil community feedbacks where differences in direct intraspecific feedbacks versus indirect, interspecific feedbacks determines plant community outcomes. Net negative feedbacks lead to coexistence while positive feedbacks result in monodominance[49].
- Rhizosphere
Zone surrounding the roots of plants in which root secretions are common and the abundance of microorganisms is high.
- Source-sink relationships
Term borrowed from plant physiology to conceptualize the movement of resources through common mycorrhizal networks from plants with higher resource levels to plants with lower resource levels.
- Stabilizing and equalizing mechanisms of coexistence
Stabilizing mechanisms[1] increase negative intraspecific interactions relative to interspecific interactions thereby preventing local extinction of species due to interspecific competition. Equalizing mechanisms minimise average fitness differences between species which slows competitive exclusion of inferior competitors.
- Storage Effect
A mechanism proposed to explain species coexistence[1] provided three conditions are met. These are that i) plants are able to store the benefits of favourable years to provide a buffer against extinction, ii) species benefit from different combinations of environmental conditions, either as a consequence of temporal or spatial variability, and iii) the various species are subject to density dependent effects that covary with environmental conditions.
- Tropical Monodominance
Forests in the tropics in which one tree species comprises more than half of the canopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics. 2000;31:343–366. [Google Scholar]
- 2.Macarthur R, Levins R. The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist. 1967;101:377–385. [Google Scholar]
- 3.Tilman D. Resource competition and community structure. Princeton University Press; 1982. [PubMed] [Google Scholar]
- 4.Goldberg DE, Barton AM. Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. American Naturalist. 1992;139:771–801. [Google Scholar]
- 5.Dybzinski R, Tilman D. Resource use patterns predict long-term outcomes of plant competition for nutrients and light. American Naturalist. 2007;170:305–318. doi: 10.1086/519857. [DOI] [PubMed] [Google Scholar]
- 6.Miller TE, et al. A critical review of twenty years' use of the resource-ratio theory. The American Naturalist. 2005;165:439–448. doi: 10.1086/428681. [DOI] [PubMed] [Google Scholar]
- 7.Schamp BS, et al. Dispersion of traits related to competitive ability in an old-field plant community. Journal of Ecology. 2008;96:204–212. [Google Scholar]
- 8.Hastings A. Disturbance, coexistence, history, and competition for space. Theoretical Population Biology. 1980;18:363–373. [Google Scholar]
- 9.Hillebrand H. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10904–10909. doi: 10.1073/pnas.0701918104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westbury DB, Dunnett NP. The promotion of grassland forb abundance: A chemical or biological solution? Basic and Applied Ecology. 2008;9:653–662. [Google Scholar]
- 11.Hubbell SP. Neutral theory and the evolution of ecological equivalence. Ecology. 2006;87:1387–1398. doi: 10.1890/0012-9658(2006)87[1387:ntateo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Chase JM. The interaction between predation and competition: a review and synthesis. Ecol Letters. 2002;5:302–315. [Google Scholar]
- 13.Kuang JJ, Chesson P. Coexistence of annual plants: Generalist seed predation weakens the storage effect. Ecology. 2009;90:170–182. doi: 10.1890/08-0207.1. [DOI] [PubMed] [Google Scholar]
- 14.Schwinning S, Parsons AJ. Analysis of the coexistence mechanisms for grasses and legumes in grazing systems. Journal of Ecology. 1996;84:799–813. [Google Scholar]
- 15.VanderPutten WH, Peters BAM. How soil-borne pathogens may affect plant competition. Ecology. 1997;78:1785–1795. [Google Scholar]
- 16.Moora M, Zobel M. Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia. 1996;108:79–84. doi: 10.1007/BF00333217. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijden MGA. Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiology Ecology. 2006;56:178–187. doi: 10.1111/j.1574-6941.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- 18.Vogelsang KM, et al. Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytologist. 2006;172:554–562. doi: 10.1111/j.1469-8137.2006.01854.x. [DOI] [PubMed] [Google Scholar]
- 19.Vance CP. Symbiotic nitrogen fixation and phosphorus acquisition: plant nutrition in a world of declining renewable resources. Plant Physiology. 2001;127:390–397. [PMC free article] [PubMed] [Google Scholar]
- 20.Hartnett DC, Wilson GWT. Mycorrhizae influence plant community structure in tallgrass prairie. Ecology. 1999;80:1187–1195. [Google Scholar]
- 21.O'connor PJ, et al. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytologist. 2002;154:209–218. [Google Scholar]
- 22.Vance CP, et al. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 23.Johnson NC. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist. 2010;185:631–647. doi: 10.1111/j.1469-8137.2009.03110.x. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden MGA. Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search of underlying mechanisms and general principles. In: van der Heijden MGA, Sanders IR, editors. Mycorrhizal Ecology. Springer Verlag; 2002. pp. 243–265. [Google Scholar]
- 25.Cramer MD, et al. Growth of N2-fixing African savanna Acacia species is constrained by below-ground competition with grass. Journal of Ecology. 2010;98:156–167. [Google Scholar]
- 26.Reynolds H, et al. Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology. 2003;84:2281–2291. [Google Scholar]
- 27.Aerts R. The role of various types of mycorrhizal fungi in nutrient cycling and plant competition. In: van der Heijden MGA, Sanders IR, editors. Mycorrhizal Ecology. Springer Verlag; 2002. pp. 117–133. [Google Scholar]
- 28.Tedersoo L, et al. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytologist. 2008;180:479–490. doi: 10.1111/j.1469-8137.2008.02561.x. [DOI] [PubMed] [Google Scholar]
- 29.Tibbett M, Sanders FE. Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Annals of Botany. 2002;89:783–789. doi: 10.1093/aob/mcf129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds HL, et al. Variable responses of old-field perennials to arbuscular mycorrhizal fungi and phosphorus source. Oecologia. 2006;147:348–358. doi: 10.1007/s00442-005-0270-6. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds HL, et al. Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology. 2003;84:2281–2291. [Google Scholar]
- 32.Ehrenfeld JG, et al. Feedback in the plant-soil system. Annual Review of Environment and Resources. 2005;30:75–115. [Google Scholar]
- 33.Ashton I, et al. Nitrogen preferences and plant-soil feedbacks as influenced by neighbors in the alpine tundra. Oecologia. 2008;156:625–636. doi: 10.1007/s00442-008-1006-1. [DOI] [PubMed] [Google Scholar]
- 34.Suding KN, et al. Plant and microbe contribution to community resilience in a directionally changing environment. Ecological Monographs. 2008;78:313–329. [Google Scholar]
- 35.Simard SW, Durall DM. Mycorrhizal networks: a review of their extent, function, and importance. Canadian Journal of Botany. 2004;82:1140–1165. [Google Scholar]
- 36.Selosse MA, et al. Mycorrhizal networks: des liaisons dangereuses? Trends in Ecology & Evolution. 2006;21:621–628. doi: 10.1016/j.tree.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.van der Heijden MGA, Horton TR. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. Journal of Ecology. 2009;97:1139–1150. [Google Scholar]
- 38.Fitter AH, et al. Carbon transfer between plants and its control in networks of arbuscular mycorrhizas. Functional Ecology. 1998;12:406–412. [Google Scholar]
- 39.He XH, et al. Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs) Critical Reviews in Plant Sciences. 2003;22:531–567. [Google Scholar]
- 40.Simard SW, et al. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature. 1997;388:579–582. [Google Scholar]
- 41.Wilson GWT, et al. Mycorrhizal-mediated phosphorus transfer between tallgrass prairie plants Sorghastrum nutans and Artemisia ludoviciana. Functional Ecology. 2006;20:427–435. [Google Scholar]
- 42.Nakano-Hylander A, Olsson PA. Carbon allocation in mycelia of arbuscular mycorrhizal fungi during colonisation of plant seedlings. Soil Biology & Biochemistry. 2007;39:1450–1458. [Google Scholar]
- 43.Robinson D, Fitter A. The magnitude and control of carbon transfer between plants linked by a common mycorrhizal network. Journal of Experimental Botany. 1999;50:9–13. [Google Scholar]
- 44.Teste FP, et al. Net carbon transfer between Pseudotsuga menziesii var. glauca seedlings in the field is influenced by soil disturbance. Journal of Ecology. 2010;98:429–439. [Google Scholar]
- 45.Pietikäinen A, Kytöviita MM. Defoliation changes mycorrhizal benefit and competitive interactions between seedlings and adult plants. Journal of Ecology. 2007;95:639–647. [Google Scholar]
- 46.Kytöviita MM, et al. A test of mutual aid in common mycorrhizal networks: Established vegetation negates benefit in seedlings. Ecology. 2003;84:898–906. [Google Scholar]
- 47.van der Heijden MGA. Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecology Letters. 2004;7:293–303. [Google Scholar]
- 48.Nara K, Hogetsu T. Ectomycorrhizal fungi on established shrubs facilitate subsequent seedling establishment of successional plant species. Ecology. 2004;85:1700–1707. [Google Scholar]
- 49.Bever JD, et al. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. Journal of Ecology. 1997;85:561–573. [Google Scholar]
- 50.Eom AH, et al. Host plant species effects on arbuscular mycorrhizal fungal communities in tallgrass prairie. Oecologia. 2000;122:435–444. doi: 10.1007/s004420050050. [DOI] [PubMed] [Google Scholar]
- 51.Mummey DL, Rillig MC. Spatial characterization of arbuscular mycorrhizal fungal molecular diversity at the submetre scale in a temperate grassland. Fems Microbiology Ecology. 2008;64:260–270. doi: 10.1111/j.1574-6941.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho LM, et al. Spatial variability of arbuscular mycorrhizal fungal spores in two natural plant communities. Plant Soil. 2003;251:227–236. [Google Scholar]
- 53.Bever JD. Negative feedback within a mutualism: Host-specific growth of mycorrhizal fungi reduces plant benefit. Proceedings of the Royal Society of London, B. 2002;269:2595–2601. doi: 10.1098/rspb.2002.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills KE, Bever JD. Maintenance of diversity within plant communities: Soil pathogens as agents of negative feedback. Ecology. 1998;79:1595–1601. [Google Scholar]
- 55.Mitchell RJ, et al. The ecological engineering impact of a single tree species on the soil microbial community. Journal of Ecology. 2010;98:50–61. [Google Scholar]
- 56.Bever JD. Soil Community dynamics and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytologist. 2003;157:465–473. doi: 10.1046/j.1469-8137.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- 57.Bever JD. Feedback between plants and their soil communities in an old field community. Ecology. 1994;75:1965–1978. [Google Scholar]
- 58.Klironomos JN. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. [DOI] [PubMed] [Google Scholar]
- 59.Petermann JS, et al. Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology. 2008;89:2399–2406. doi: 10.1890/07-2056.1. [DOI] [PubMed] [Google Scholar]
- 60.Kulmatiski A, et al. Plant-soil feedbacks: a meta-analytical review. Ecology Letters. 2008;11:980–992. doi: 10.1111/j.1461-0248.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 61.Kardol P, et al. Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecological Monographs. 2007;77:147–162. [Google Scholar]
- 62.Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000;404:278–281. doi: 10.1038/35005072. [DOI] [PubMed] [Google Scholar]
- 63.Westover KM, Bever JD. Mechanisms of plant species coexistence: Roles of rhizosphere bacteria and root fungal pathogens. Ecology. 2001;82:3285–3294. [Google Scholar]
- 64.Van der Putten WH, et al. Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature. 1993;362:53–56. [Google Scholar]
- 65.Kulmatiski A, Kardol P. Getting plant—soil feedbacks out of the greenhouse: experimental and conceptual approaches. Progress in Botany. 2008;69:449–472. [Google Scholar]
- 66.Brandt AJ, et al. Phylogeny and provenance affect plant-soil feedbacks in invaded California grasslands. Ecology. 2009;90:1063–1072. doi: 10.1890/08-0054.1. [DOI] [PubMed] [Google Scholar]
- 67.Harrison KA, Bardgett RD. Influence of plant species and soil conditions on plant-soil feedback in mixed grassland communities. Journal of Ecology. 2010;98:384–395. [Google Scholar]
- 68.Casper BB, Castelli JP. Evaluating plant-soil feedback together with competition in a serpentine grassland. Ecology Letters. 2007;10:394–400. doi: 10.1111/j.1461-0248.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- 69.Dickie IA, et al. Spatially disjunct effects of co-occurring competition and facilitation. Ecology Letters. 2005;8:1191–1200. doi: 10.1111/j.1461-0248.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 70.Harms KE, et al. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature. 2000;404:493–495. doi: 10.1038/35006630. [DOI] [PubMed] [Google Scholar]
- 71.HilleRisLambers J, et al. Density-dependent mortality and the latitudinal gradient in species diversity. Nature. 2002;417:732–735. doi: 10.1038/nature00809. [DOI] [PubMed] [Google Scholar]
- 72.Wills C, et al. Nonrandom processes maintain diversity in tropical forests. Science. 2006;311:527–531. doi: 10.1126/science.1117715. [DOI] [PubMed] [Google Scholar]
- 73.Janzen DH. Herbivores and the number of tree species in tropical forests. The American Naturalist. 1970;104:592–595. [Google Scholar]
- 74.Bell T, et al. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecology Letters. 2006;9:569–574. doi: 10.1111/j.1461-0248.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 75.McGuire KL. Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology. 2007;88:567–574. doi: 10.1890/05-1173. [DOI] [PubMed] [Google Scholar]
- 76.Teste FP, Simard SW. Mycorrhizal networks and distance from mature trees alter patterns of competition and facilitation in dry Douglas-fir forests. Oecologia. 2008;158:193–203. doi: 10.1007/s00442-008-1136-5. [DOI] [PubMed] [Google Scholar]
- 77.Booth MG. Mycorrhizal networks mediate overstorey-understorey competition in a temperate forest. Ecology Letters. 2004;7:538–546. [Google Scholar]
- 78.Dickie IA, et al. Soil modification by different tree species influences the extent of seedling ectomycorrhizal infection. Mycorrhiza. 2006;16:73–79. doi: 10.1007/s00572-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 79.Gehring CA, Connell JH. Arbuscular mycorrhizal fungi in the tree seedlings of two Australian rain forests: occurrence, colonization, and relationships with plant performance. Mycorrhiza. 2006;16:89–98. doi: 10.1007/s00572-005-0018-5. [DOI] [PubMed] [Google Scholar]
- 80.Nuñez MA, et al. Lack of belowground mutualisms hinders Pinaceae invasions. Ecology. 2009;90:2352–2359. doi: 10.1890/08-2139.1. [DOI] [PubMed] [Google Scholar]
- 81.Zimpfer JF, et al. Localization of Casuarina-infective Frankia near Casuarina cunninghamiana trees in Jamaica. Canadian Journal of Botany. 1999;77:1248–1256. [Google Scholar]
- 82.Bonanomi G, et al. Negative plant-soil feedback and species coexistence. Oikos. 2005;111:311–321. [Google Scholar]
- 83.Umbanhowar J, McCann K. Simple rules for the coexistence and competitive dominance of plants mediated by mycorrhizal fungi. Ecology Letters. 2005;8:247–252. [Google Scholar]
- 84.Yamazaki M, et al. Distance- and density-dependent seedling mortality caused by several diseases in eight tree species co-occurring in a temperate forest. Plant Ecology. 2009;201:181–196. [Google Scholar]
- 85.Bever JD, et al. Prairie mycorrhizal fungal inoculant may increase native prairie plant diversity on restored sites. Ecological Restoration. 2003;21:311–312. [Google Scholar]
- 86.Vogelsang KM, Bever JD. Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology. 2009;90:399–407. doi: 10.1890/07-2144.1. [DOI] [PubMed] [Google Scholar]
- 87.Pringle A, et al. Mycorrhizal symbioses and plant invasions. Annual Review of Ecology, Evolution, and Systematics. 2009;40:699–715. [Google Scholar]
- 88.Alguacil MM, et al. The impact of tillage practices on arbuscular mycorrhizal fungal diversity in subtropical crops. Ecological Applications. 2008;18:527–536. doi: 10.1890/07-0521.1. [DOI] [PubMed] [Google Scholar]
- 89.Thrall PH, et al. Studies on land restoration: seed inoculation with effective root-nodule bacteria enhances the establishment, survival and growth of Acacia species. Journal of Applied Ecology. 2005;42:740–751. [Google Scholar]
- 90.Connell JH. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: den Boer PJ, Gradwell GR, editors. Dynamics of populations. Proceedings of the Advanced Study Institute. Centre for Agricultural Publishing and Documentation; 1971. pp. 298–312. [Google Scholar]
- 91.Eppstein MJ, Molofsky J. Invasiveness in plant communities with feedbacks. Ecology Letters. 2007;10:253–263. doi: 10.1111/j.1461-0248.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 92.Dickie IA, et al. Shared ectomycorrhizal fungi between a herbaceous perennial (Helianthemum bicknellii) and oak (Quercus) seedlings. New Phytologist. 2004;164:375–382. doi: 10.1111/j.1469-8137.2004.01177.x. [DOI] [PubMed] [Google Scholar]
- 93.Alexander I, Lee S. Mycorrhizas and ecosystem processes in tropical rain forest: implications for diversity. In: Burslem D, et al., editors. Biotic interactions in the tropics: their role in the maintenance of biotic diversity. Cambridge University Press; 2005. pp. 165–203. [Google Scholar]
- 94.Torti SD, et al. Causes and consequences of monodominance in tropical lowland forests. American Naturalist. 2001;157:141–153. doi: 10.1086/318629. [DOI] [PubMed] [Google Scholar]
- 95.Richardson DM, et al. Plant invasions - the role of mutualisms. Biological Review. 2000;75:65–93. doi: 10.1017/s0006323199005435. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell CE, et al. Biotic interactions and plant invasions. Ecology Letters. 2006;9:726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 97.Kennedy PG, Peay KG. Different soil moisture conditions change the outcome of the ectomycorrhizal symbiosis between Rhizopogon species and Pinus muricata. Plant and Soil. 2007;291:155–165. [Google Scholar]
- 98.Byrne K, Mitchell D. Responses of mycorrhizal and nonmycorrhizal Erica cinerea and Vaccinium macrocarpon to Glomus mosseae. Mycorrhiza. 2004;14:31–36. doi: 10.1007/s00572-003-0273-2. [DOI] [PubMed] [Google Scholar]
- 99.Stinson K, et al. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLOS Biology. 2006;4:1–5. doi: 10.1371/journal.pbio.0040140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolfe BE, et al. Distribution and abundance of the introduced ectomycorrhizal fungus Amanita phalloides in North America. New Phytologist. 2010 doi: 10.1111/j.1469-8137.2009.03097.x. [DOI] [PubMed] [Google Scholar]
- 101.Reinhart KO, Callaway RM. Soil biota and invasive plants. New Phytologist. 2006;170:445–457. doi: 10.1111/j.1469-8137.2006.01715.x. [DOI] [PubMed] [Google Scholar]