Abstract
Two 1-year studies evaluated the long-term efficacy and safety of tiotropium 5 or 10 μg versus placebo, inhaled via the Respimat® Soft Mist™ Inhaler (SMI). The two studies were combined and had 4 co-primary endpoints (trough FEV1 response, Mahler Transition Dyspnea Index [TDI] and St George’s Respiratory Questionnaire scores all at week 48, and COPD exacerbations per patient-year). A total of 1990 patients with COPD participated (mean FEV1: 1.09 L). The mean trough FEV1 response of tiotropium 5 or 10 μg relative to placebo was 127 or 150 mL, respectively (both P < 0.0001). The COPD exacerbation rate was significantly lower with tiotropium 5 μg (RR = 0.78; P = 0.002) and tiotropium 10 μg (RR = 0.73; P = 0.0008); the health-related quality of life and Mahler TDI co-primary endpoints were significantly improved with both doses (both P < 0.0001). Adverse events were generally balanced except anticholinergic class effects, which were more frequent with active treatment. Fatal events occurred in 2.4% (5 μg), 2.7% (10 μg), and 1.6% (placebo) of patients; these differences were not significant. Tiotropium Respimat® SMI 5 μg demonstrated sustained improvements in patients with COPD relative to placebo and similar to the 10 μg dose but with a lower frequency of anticholinergic adverse events.
Keywords: COPD, exacerbations, FEV1, quality of life, Respimat®, tiotropium
Introduction
Tiotropium, a long-acting anticholinergic used to treat chronic obstructive pulmonary disease (COPD), can be delivered via the Respimat® Soft Mist™ Inhaler (SMI), which uses mechanical energy (a spring) to generate a fine, slow-moving mist from an aqueous solution. The Respimat® SMI has a number of benefits; from the patient’s perspective, Respimat® SMI is a multi-dose device, simple to coordinate, and the delivered dose is independent of inspiratory effort. In terms of access to the airways, 62% of the delivered dose contains particles that are <5.8 μm, which means that this fraction is approximately 2.5 times higher than for most metered-dose inhalers (MDIs),1,2 and the mean velocity of the Soft Mist™ is approximately 5 times lower.3,4 Both of these factors contribute to a reduction in oropharyngeal deposition and to an increase in lung deposition.1,2 Dose-ranging studies have confirmed that a low dose of tiotropium (5 and 10 μg) is needed in the Respimat® SMI.5,6
The objectives of the current studies were to evaluate the long-term efficacy and safety of two doses of once-daily tiotropium versus placebo, delivered via the Respimat® SMI. Two studies identical in design were performed based on Food and Drug Administration (FDA) requirements; however, the endpoints were combined to provide the best estimation of the differences for the four co-primary endpoints and to provide adequate statistical power for COPD exacerbations.
Methods
Population
Males and females aged ≥40 years with a diagnosis of COPD7 and stable, moderate-to-severe airway obstruction (prebronchodilator forced expiratory volume in 1 second [FEV1] ≤ 60% predicted and FEV1 ≤ 70% of forced vital capacity [FVC]), and with a smoking history of ≥10 pack years were included. We used the criteria of the American Thoracic Society7 for the definition of moderate-to-severe COPD to facilitate outcome comparisons with the previously reported 1-year tiotropium HandiHaler® data. Patients with a confounding disease, including other significant respiratory conditions, were excluded, as were those who had a disease that might put them at risk because of study participation. Other exclusion criteria included known hypersensitivity to anticholinergics or any component of the Respimat® inhalation solution; drugs contraindicated with anticholinergics; prior use of Spiriva® HandiHaler®; regular use of daytime oxygen therapy, oral β-adrenergics, or long-acting β-adrenergics; or significant alcohol or drug abuse. All patients provided written, informed consent to participate. The protocol was approved by institutional ethics review boards of participating centers.
Study design
Two identical, 1-year, multicenter, multinational, randomized, double-blind, parallel-group studies (#205.254 [NCT00168844] and #205.255 [NCT00168831]) were performed comparing orally inhaled tiotropium 5 and 10 μg with placebo in patients with COPD. This paper reports the combined analyses (per protocol), and also highlights any significant differences between the individual studies.
Following an initial screening visit and 2-week run-in phase, eligible patients were randomized to receive tiotropium Respimat® SMI 5 μg (2 actuations of 2.5 μg tiotropium [Tio R5]), tiotropium Respimat® SMI 10 μg (2 actuations of 5 μg tiotropium [Tio R10]), or Respimat® placebo (2 actuations of placebo inhalation solution) once daily in the morning for 48 weeks. All drugs were supplied by Boehringer Ingelheim, Ingelheim am Rhein, Germany. Recruitment for the studies took place from January 2003 to December 2005. An additional retrospective study (#205.392) was conducted from March 2007 to January 2008 to capture data from all prematurely discontinued patients for the intended treatment period of 48 weeks to 30-day follow-up, including vital status information and changes in treatment after discontinuation of trial medication.
Allowed study medication
Oral (up to 10 mg daily of prednisone) and inhaled corticosteroids, theophylline preparations, mucolytic agents and antileukotrienes were allowed if stabilized for at least 6 weeks prior to and during the study. Patients on long-acting β-adrenergics and inhaled corticosteroids were switched to a monoproduct inhaled corticosteroid prior to run-in. Salbutamol MDI was used as rescue medication. A locally available commercial brand of salbutamol MDI was purchased by the Boehringer Ingelheim affiliate and provided to the investigator sites.
Efficacy endpoints
There were 4 co-primary endpoints for both studies:
The trough FEV1 response at week 48 (24-hour post-dose FEV1 expressed as change from study baseline predose FEV1)
St George’s Respiratory Questionnaire (SGRQ) total score at the end of the 48-week treatment period
The Mahler Transition Dyspnea Index (TDI) focal score after 48 weeks of treatment
COPD exacerbations per patient-year defined as respiratory adverse events lasting ≥3 days and requiring treatment with antibiotics and/or oral corticosteroids and/or a significant change in prescribed respiratory medication including inhaled bronchodilators.
This definition of an exacerbation is consistent with previously published trials8 in order to permit comparisons with previous studies. The exacerbation endpoints in the study included:
The patients (%) with at least 1 COPD exacerbation
The number of exacerbations per patient-year of treatment
The time to first COPD exacerbation.
The total time in exacerbations is the number of days, as determined by the physician (based on clinical judgment) that exacerbations continued, expressed as a percentage of the total time that each patient remained on treatment.
Secondary endpoints included FVC, peak expiratory flow rate (PEFR) (measured using a mechanical meter) and weekly mean number of occasions (per day as needed) that rescue medication was used. COPD symptom scores (wheezing, shortness of breath, coughing, and tightness of chest) were based on the investigator’s assessment of the patient’s condition during the week just prior to the clinic visit. The Physician’s Global Evaluation (PGE), which was based on the physician’s opinion of the patient’s overall clinical condition, and was rated from poor (1–2) to excellent (7–8), and the Patient’s Global Rating (PGR), which was performed by the patients who rated their condition as “much better” to “much worse”, were also assessed. Detailed information on exacerbations and COPD exacerbation-related hospitalizations were recorded.
Clinical efficacy measures, including spirometry and health-related quality-of-life (HRQoL), patient diary cards information (predose and evening PEFR, occasions of rescue medication use, and drug compliance, ie, whether treatment was taken or not) were measured throughout the 48-week treatment period. Some variables (diary cards, SGRQ, Mahler TDI, COPD symptom scores, PGE, PGR, and adverse events) were also measured 21 days after medication had stopped; this was defined in the protocol. Data collected during the 21-day follow-up period were used descriptively to evaluate any evidence of a rebound effect when tiotropium was stopped.
Safety endpoints
Adverse and fatal adverse events were monitored throughout the run-in, 48-week treatment period and for a 30-day follow-up. End-of-study (week 48) changes in vital signs, routine laboratory, and physical examination were also recorded. As benzalconium chloride and ethylene diamine tetra-acetic acid are excipients in the Respimat® SMI formulation that have been reported to cause dose-related bronchoconstriction,9–11 particular attention was paid to respiratory events indicative of paradoxical bronchoconstriction, such as a drop in FEV1 ≥15% from study day baseline, rescue medication use, cough, wheeze, and dyspnea within 30 minutes after treatment administration. Cardiovascular safety was monitored in a subset of patients using 12-lead electrocardiogram (ECG) and Holter monitoring; these measures were performed at randomization, week 16, and week 40. The retrospective study also collected vital status information for patients who discontinued prematurely, covering the mean treatment period plus observation period (ie, a total of 369 days).
Statistical analysis
Each study was sufficiently powered to detect differences in three co-primary endpoints (trough FEV1 response, SGRQ total score, and Mahler TDI focal score), but were not sufficiently powered individually to test for between-treatment group differences in exacerbations. This was described in the protocols and was prespecified and FDA-approved that the studies would be combined. In both individual studies, a sample of 810 patients was considered adequate to detect a difference of 0.13 L in mean trough FEV1 (5% significance level), and the combined analysis had at least 95% power to detect a difference of 0.05 L in mean trough FEV1 response (5% significance level). The combined analysis was adequate to detect a mean difference of 0.4 exacerbations per year with 76% power (5% significance level), to detect a mean difference in Mahler TDI focal score of 1 unit with 90% power and to detect a mean difference of 4 units in the SGRQ total score with 96% power (5% significance level).
Three of the co-primary endpoints (trough FEV1 response, SGRQ total score, and Mahler TDI focal score at 48 weeks) were analyzed using fixed effects analysis of covariance (ANCOVA) with terms for smoking status at study entry, center and treatment group with baseline value as a continuous covariate. Incorporating center as a fixed effect was considered a more conservative approach and was also prespecified in the analysis plan prior to unblinding. Fisher’s least significant difference procedure was applied where pair-wise comparisons between treatment groups were made. Responders were defined as those achieving a change of 4 units in the SGRQ total score and 1 unit in the Mahler TDI focal score (minimal clinically important differences [MCID]).12–14 The remaining co-primary endpoint, COPD exacerbations, was evaluated using Wilcoxon–Mann–Whitney tests for pair-wise comparisons. Kaplan–Meier estimates of the probability of no COPD exacerbation on any given test day were performed. The analysis of COPD exacerbation was performed using a pooled analysis, and as both studies were similar in design and execution, each trial was not incorporated as a random effect in the model. Pair-wise treatment comparisons of the time to the first COPD exacerbation were made using the log-rank test. Similar analyses were performed for hospitalizations.
An ANCOVA model was also used to analyze the secondary efficacy variables (FVC, PGE, COPD symptom scores, PEFR, and rescue medication). An analysis of variance model, which included terms for smoking status at study entry, center and treatment group, was performed on the PGR data. Only those patients with baseline and on-treatment data for at least one primary endpoint were included in the efficacy analyses. Randomized patients who received ≥1 dose of study medication were included in the safety analysis. Post-hoc subgroup analyses were performed on trough FEV1 response at 48 weeks to see whether patients who were taking inhaled corticosteroids at inclusion responded differently to those patients not taking inhaled corticosteroids. The model used to analyze the co-primary endpoint trough FEV1 response at 48 weeks was also used for this subgroup analysis.
Descriptive statistics were used for safety outcomes; safety was not a predefined endpoint so the trials were not powered to detect differences in incidence of adverse events between treatment groups.
Results
Patient disposition and characteristics
A combined total of 2544 patients signed informed consent and were screened for entry; however, 554 did not meet the inclusion criteria. A total of 1990 patients were randomized and received treatment; 93 patients were excluded from the FEV1 assessments; 192 patients were excluded from the SGRQ and Mahler TDI assessments and 90 patients were excluded from rescue medication and PEFR assessments. The main reasons for these exclusions were data not evaluable or patients having received less than 5 days of study treatment. The baseline characteristics were comparable between randomized treatment groups (Table 1). Adherence to medication was high; 96.1% (Tio R5), 95.4% (Tio R10), and 95.7% (placebo) took medication as prescribed during the study. More patients in the placebo groups discontinued treatment prematurely compared with the two tiotropium treatment groups (Table 2). The discontinued patients had more severe lung disease at baseline than completers, and this difference was more pronounced in the placebo groups. Tiotropium-treated patients completing the studies therefore had slightly worse lung function at baseline than placebo-treated patients who completed the study (difference in mean, placebo–Tio R5: FEV1 = 0.028 L [standard error of the mean [SEM] = 0.024 L] and placebo–Tio R10: FEV1 = 0.026 L [SEM = 0.025 L]).
Table 1.
Patient demographics and characteristics of all randomized patients
| Variables* | Tiotropium Respimat® SMI 5 μg (n = 670) | Tiotropium Respimat® SMI 10 μg (n = 667) | Placebo (n = 653) |
|---|---|---|---|
| Male:female (%) | 73.3:26.7 | 74.7:25.3 | 74.6:25.4 |
| Age (years) | 64.7 (8.6) | 65.1 (8.5) | 65.2 (8.7) |
| Current smoker (%) | 37.9 | 34.8 | 36.1 |
| Duration of COPD (years) | 8.3 (6.4) | 9.0 (7.4) | 9.5 (7.5) |
| FEV1 (L) | 1.066 (0.4) | 1.065 (0.40) | 1.058 (0.4) |
| Pre-bronchodilator FEV1 (% predicted normal) | 38.0 (11.7) | 37.7 (11.7) | 37.5 (11.6) |
| Post-bronchodilator FEV1 (% predicted normal) | 46.6 | 45.3 | 46.2 |
| FEV1/FVC (%) | 42.4 (11.5) | 42.4 (11.1) | 42.1 (11.0) |
| FEV1 (% reversibility at 30 minutes following 400 μg salbutamol)† | 20 (18) | 19 (17) | 21 (17) |
| Patients taking any pulmonary medication (%) | 80 | 86 | 85 |
| Corticosteroids, oral (%) | 3 | 3 | 3 |
| Corticosteroids, inhaled (%) | 49 | 57 | 55 |
| β-adrenergics, short-acting (%) | 52 | 57 | 53 |
| β-adrenergics, long-acting (%) | 30 | 30 | 29 |
| Anticholinergics, short-acting (%) | 45 | 44 | 45 |
| Xanthines (%) | 14 | 16 | 15 |
| Mucolytics (%) | 4 | 3 | 4 |
Notes:
Mean (SD) unless otherwise stated;
Mean (median) compared with baseline values. Predicted normals from European Community for Steel and Coal Statement.15
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SMI, Soft Mist™ Inhaler.
Table 2.
Disposition and spirometry at baseline in accordance with discontinuation status
| Variables (mean ± SEM unless otherwise stated) | Tiotropium Respimat® SMI 5 μg (n = 670) | Tiotropium Respimat® SMI 10 μg (n = 667) | Placebo (n = 653) |
|---|---|---|---|
| Completed patients (%) | 82.8 | 79.6 | 68.6 |
| Adverse events (%) | 10 | 11.8 | 18.7 |
| Worsening of disease under study (%) | 4.6 | 5.1 | 14.1 |
| FEV1, all patients (L) (% predicted normal) | 1.066 ± 0.015 [38.0] | 1.065 ± 0.016 [37.7] | 1.058 ± 0.015 [37.5] |
| FEV1, completed patients (L) (% predicted normal) | 1.081 ± 0.016* [38.2] | 1.082 ± 0.017* [38.0] | 1.108 ± 0.018 [39.1] |
| FEV1, discontinued patients (L) (% predicted normal) | 1.001 ± 0.042 [36.9] | 1.000 ± 0.039 [36.4] | 0.950 ± 0.028 [34.1] |
| Difference in FEV1, completed minus discontinued patients (L)† | 0.079 ± 0.040 | 0.082 ± 0.039 | 0.158 ± 0.032 |
Notes:
P < 0.0001; mean difference between active treatment and placebo for day 337.
Represents the mean (SEM) difference in baseline FEV1 values (by treatment group) between patients who completed the study and patients who discontinued prematurely.
Abbreviations: FEV1, forced expiratory volume in 1 second; SMI, Soft Mist™ Inhaler.
Spirometry assessments
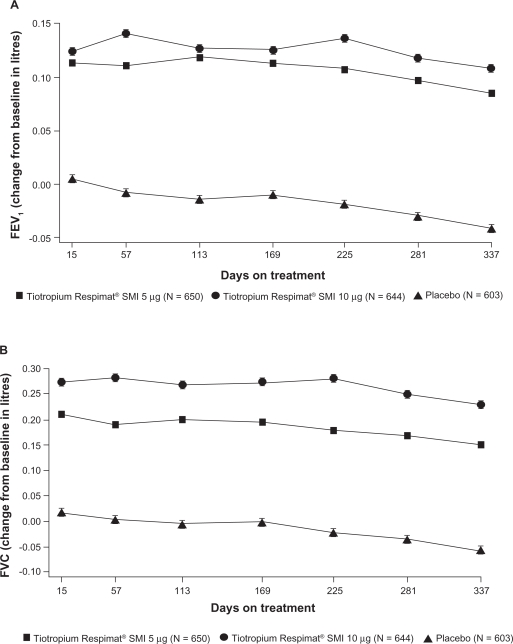
Long-term (24-hour) bronchodilation was achieved after treatment with once-daily Tio R5 and Tio R10 (Figure 1a). The mean (SEM) differences between Tio R5 and Tio R10 and placebo for the first co-primary endpoint, combined mean trough FEV1 response, was 127 mL and 150 mL, respectively (both P < 0.0001) (Table 3a). There was also a slight, nonsignificant improvement in combined mean (SEM) trough FEV1 of 23 mL after treatment with Tio R10 compared with Tio R5 (Figure 1a) (Table 3a). There was no evidence of tachyphylaxis as the improvements in FEV1 after active treatments on day 1 were comparable with those observed after 48 weeks (Figure 1a). FEV1 reversibility at baseline did not influence responsiveness to Tio R5 and Tio R10 measured as post-dose FEV1 on Test Day 337 (Table 3b). Patients treated with placebo, on the other hand, appeared to have a marked decrease in reversibility response after 48 weeks.
Figure 1.
The adjusted mean (SEM) trough (a) FEV1 (L) response and (b) FVC (L) response during 48 weeks of treatment with tiotropium 5 μg, tiotropium 10 μg, or placebo (n = 1897) (P < 0.0001 for tiotropium 5 μg–placebo and tiotropium 10 μg– placebo for mean improvement in FEV1and FVC).
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SMI, Soft Mist™ Inhaler.
Table 3.
(A) The mean (SEM) treatment differences at week 48 for primary and secondary efficacy variables and (B) the difference between post-bronchodilator FEV1 at baseline and post-dose on test day 337
| (A) Treatment differences |
Treatment† comparison |
||
|---|---|---|---|
| Tiotropium Respimat® SMI 5 μg–placebo | Tiotropium Respimat® SMI 10 μg–placebo | Tiotropium Respimat® SMI 10 μg–5 μg | |
| Primary | |||
| Trough (24-h post-dose) FEV1 (L) response (n = 1897) | 0.127* (0.013) | 0.150* (0.013) | 0.023 (0.013) |
| [95% CI] | [0.101, 0.153] | [0.124, 0.175] | [−0.002, 0.048] |
| SGRQ total score (n = 1798) | −3.5* (0.7) | −3.8* (0.7) | −0.4 (0.7) |
| [95% CI] | [−4.9, −2.1] | [−5.3, −2.4] | [−1.7, 1.0] |
| Mahler TDI focal score (n = 1798) | 1.05* (0.17) | 1.08* (0.17) | 0.02 (0.16) |
| [95% CI] | [0.73, 1.38] | [0.75, 1.40] | [−0.29, 0.34] |
| Secondary | |||
| ‡Rescue medication use (occasions per day) [n = 1900] | −0.6* (0.1) | −0.7* (0.1) | −0.1 (0.1) |
| [95% CI] | [−0.8, −0.4] | [−0.9, −0.5] | [−0.3, 0.1] |
| ‡Morning PEFR (L/min) (n = 1900) | 22* (3) | 28* (3) | 5 (3) |
| [95% CI] | [17, 28] | [22, 33] | [0, 10] |
| ‡Evening PEFR (L/min) (n = 1900) | 27* (3) | 33* (3) | 6** (3) |
| [95% CI] | [22, 32] | [28, 39] | [1, 12] |
| (B) Treatment comparison | Tiotropium Respimat®SMI 5 μg | Tiotropium Respimat®SMI 10 μg | Placebo |
| Post-bronchodilator FEV1 at baseline | 1.281 | 1.281 | 1.282 |
| Post-bronchodilator FEV1 on test day 337 (30 minutes post-dose) | 1.242 | 1.270 | 1.053 |
P < 0.0001 vs placebo;
P < 0.05.
Notes:
Adjusted for smoking status at entry, center and baseline value. A last observation carried forward approach was used for all missing data except rescue medication use and PEFR
These data reflect the overall mean.
Abbreviations: FEV1, forced expiratory volume in 1 second; PEFR, peak expiratory flow rate; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; SMI, Soft Mist™ Inhaler.
There was also a substantial increase in trough FVC on active treatment (difference in mean, Tio R5–placebo: 0.209 L [SEM = 0.027 L] and Tio R10–placebo: 0.286 L [SEM = 0.027 L]; both P < 0.0001), which was generally sustained over the 48-week period (Figure 1b). Morning and evening PEFR were also statistically significantly (P < 0.0001) improved after treatment with both doses of tiotropium compared with placebo (Table 3a).
Lung function data according to whether patients used inhaled corticosteroids or not at inclusion is shown in Table 4. Numerically, the patients who were not on inhaled corticosteroids had a slightly better lung function than those patients who were using inhaled corticosteroids. However, irrespective of whether the patients were on inhaled corticosteroids or not, both groups and both tiotropium doses showed statistically significant (P < 0.0001) improvements compared to placebo for trough FEV1, AUC(0–3 h) FEV1, and peak FEV1. The magnitude of the changes in pulmonary function indices for the active treatments compared with placebo were on average slightly smaller for the patients who were not on inhaled corticosteroids compared with those who were.
Table 4.
A comparison of lung function parameters according to whether patients used ICS at baseline
| Treatment | Tiotropium Respimat® SMI 5 μg | Tiotropium Respimat® SMI 10 μg | Placebo | |||
|---|---|---|---|---|---|---|
| ICS use at baseline | Yes | No | Yes | No | Yes | No |
| Patients (n) | 316 | 334 | 366 | 278 | 327 | 276 |
| Mean (SD) FEV1 | 36.9 | 39.0 | 37.1 | 38.5 | 36.9 | 38.6 |
| (% predicted normal) at baseline | (11.7) | (11.5) | (12.0) | (11.4) | (11.1) | (11.2) |
| Mean (SD) | 41.8 | 43.0 | 42.2 | 42.7 | 41.5 | 43.1 |
| FEV1/FVC at baseline | (11.8) | (11.2) | (10.6) | (11.8) | (10.7) | (11.1) |
| Mean (SEM) | 0.130 | 0.108 | 0.163 | 0.135 | – | – |
| Δtrough FEV1 (L) | (0.018) | (0.020) | (0.017) | (0.021) | – | – |
| vs placebo at day 337 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | – | – |
| Mean (SEM) | 0.202 | 0.192 | 0.231 | 0.216 | – | – |
| ΔFEV1 (L) AUC(0–3 h) vs | (0.019) | (0.021) | (0.018) | (0.022) | – | – |
| placebo at day 337 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | – | – |
| Mean (SEM) | 0.211 | 0.197 | 0.238 | 0.222 | – | – |
| ΔPeak FEV1 (L) | (0.020) | (0.023) | (0.020) | (0.024) | – | – |
| vs placebo at day 337 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | – | – |
Abbreviations: ICS, inhaled corticosteroid; Δ, difference; AUC, area under the curve; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SMI, Soft Mist™ Inhaler.
HRQoL assessments
The improvement in the unadjusted second co-primary endpoint, SGRQ total score, for both tiotropium doses was statistically superior to placebo (−3.5 [Tio R5–placebo] and −3.8 [Tio R10–placebo]; P < 0.0001) (Table 3a), and the adjusted mean change from baseline to week 48 exceeded the accepted MCID of 4 units in the SGRQ after active treatment in both studies: Tio R5: −5.1 units; Tio R10: −5.5 units; placebo: −1.6 units. The percentage of patients with improvements exceeding the MCID (ie, responders) at week 48 was also statistically significantly higher (P < 0.05) for Tio R5 (50.5%) and Tio R10 (51.4%) compared with placebo (40.7%).
Dyspnea scores
For the Mahler TDI focal score, a third co-primary endpoint, both tiotropium doses were statistically superior (P < 0.0001) to placebo at week 48, and the difference exceeded the quoted MCID of 1 unit (Table 3a). The percentage of responders (meeting or exceeding MCID) at week 48 was significantly higher (P < 0.0001) for both tiotropium groups compared with placebo (Tio R5 56%, Tio R10 56% vs placebo 44%).
COPD exacerbations and hospitalizations
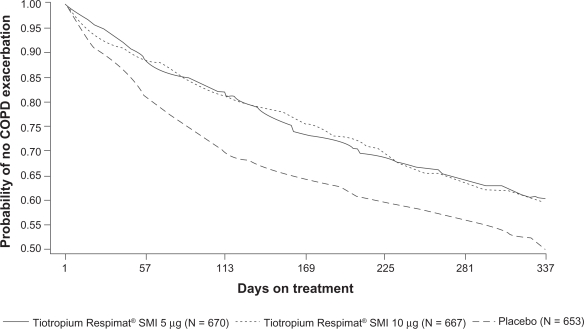
Improvements in favor of tiotropium were also seen in the fourth co-primary efficacy endpoint of COPD exacerbations (Table 5). The mean COPD exacerbation rate (per patient-year) was statistically significantly reduced on treatment with both tiotropium doses and in each of the trials; the odds ratio was 0.75 (Tio R5 vs placebo; P < 0.01) and 0.74 (Tio R10 vs placebo; P < 0.001) (Table 5). The time (lower quartile) to first exacerbation (days) was not statistically significant for the Tio R5 dose compared with placebo in trial #205.254 (P = 0.09), but when the results of both trials were combined, results were statistically significant for both tiotropium doses (P < 0.0001). The probability of remaining exacerbation-free over the 48-week treatment period was also higher in both tiotropium groups compared with placebo (Figure 2). The secondary exacerbation measures were all statistically significantly improved after active treatments compared with placebo (Table 5). Only a small percentage of patients experienced ≥1 COPD exacerbation-related hospitalization, which was lower in both tiotropium groups compared with placebo, but not statistically significant.
Table 5.
COPD exacerbation and related hospitalizations in patients treated with tiotropium 5 μg, 10 μg, or placebo
| Tiotropium Respimat® SMI 5 μg (n = 670) | Tiotropium Respimat® SMI 10 μg (n = 667) | Placebo (n = 653) | |
|---|---|---|---|
| Patients (%) with ≥1 exacerbation | 37.2* | 36.9* | 44.1 |
| Odds ratio† [95% CI] | 0.75* [0.60, 0.93] | 0.74** [0.59, 0.92] | 1.00 |
| Time (lower quartile) to first exacerbation (days) | 160** | 178** | 86 |
| COPD exacerbation rate (per patient year) | 0.93 | 1.02 | 1.91 |
| Mean time (%) in exacerbation§ | 4.0* | 3.9* | 5.6 |
| Mean hospitalization‡ per patient-year | 0.12 | 0.16 | 0.20 |
| Patients (%) with ≥1 hospitalization‡ | 5.8 | 5.8 | 6.7 |
Notes:
Versus placebo (Chi2, ie, unadjusted for extent of exposure);
Due to COPD exacerbation;
Expressed as the mean of the percentage of days each patient remained on randomized treatment.
P < 0.01;
P < 0.001 vs placebo.
Abbreviations: COPD, chronic obstructive pulmonary disease; SMI, Soft Mist™ Inhaler.
Figure 2.
Kaplan–Meier plot illustrating probability of remaining exacerbation-free over the 48-week treatment period (N = 1990) (P < 0.0001 for tiotropium 5 μg–placebo and tiotropium 10 μg–placebo for mean time [lower quartile] to first exacerbation).
Abbreviations: COPD, chronic obstructive pulmonary disease; SMI, Soft Mist™ Inhaler.
Both tiotropium doses significantly improved all four co-primary endpoints in the combined analysis. Also for each of the separate trials, the improvements were comparable between the two tiotropium doses, and were significant for both doses (individual data not shown).
Additional secondary outcomes
Over the 1-year period, active treatment was associated with, on average, a reduction of five occasions per week in rescue medication use, compared with placebo (P < 0.0001) (Table 3a). Mean PGE, PGR, and COPD symptom scores at week 48 were also statistically significantly improved (P < 0.0001 [P < 0.05 for coughing]) compared with placebo. A total of 1524 (76.6%) of the 1990 patients randomized to treatment completed the planned observation time. The post-treatment (ie, day 21) SGRQ total scores were −4.0 (Tio R5), −4.0 (Tio R10), and −3.5 (placebo); the post-treatment Mahler TDI focal scores were 1.0 (Tio R5), 1.3 (Tio R10), and 1.5 (placebo); the post-treatment PGR scores were 3.3 (Tio R5), 3.2 (Tio R10), and 3.2 (placebo); the post-treatment PGE scores were 0.4 (Tio R5), 0.4 (Tio R10), and 0.5 (placebo). There was no evidence of a rebound effect in the COPD symptom scores.
Safety assessments
The mean exposure to treatment was higher in both tiotropium groups compared with placebo, mainly because fewer patients discontinued prematurely due to worsening of disease under study (Table 2). Both tiotropium groups were associated with a higher incidence of gastrointestinal disorders than placebo, which was primarily due to dry mouth (Tio R5: 7.2%; Tio R10: 14.5%; placebo: 2.1%) and constipation (Tio R5: 2.1%; Tio R10: 2.2%; placebo: 1.5%). In addition, urinary tract infections were higher in the tiotropium groups (Tio R5: 2.5%; Tio R10: 4.2%; placebo: 1.1%).
A total of 17.2% of patients experienced at least one serious adverse event, and these were generally balanced across the treatment groups (Table 6). COPD exacerbations were the most commonly reported serious adverse event (Tio R5: 4.9%; Tio R10: 6.0%; placebo: 5.7%). Cardiac angina was more common on active treatments than placebo (Tio R5: 0.4%; Tio R10: 1.0%; placebo: 0.2%), whereas myocardial infarction was more frequent in the placebo group (Tio R5: 0.3%; Tio R10: 0.1%; placebo: 0.9%). There were no clinically relevant changes in ECG or Holter monitoring parameters for all treatment groups. Paradoxical bronchoconstriction was not observed for any treatment. The incidence of respiratory events, rescue medication use, and asymptomatic decreases in FEV1 within 30 minutes of treatment administration were small and remained unchanged throughout the study across all treatment groups.
Table 6.
The frequency (n [%]) of patients with ≥3% adverse events in any treatment group and mortality data
| Tiotropium Respimat® SMI 5 μg (n = 670) | Tiotropium Respimat® SMI 10 μg (n = 667) | Placebo (n = 653) | |
|---|---|---|---|
| Patients with any adverse event | 505 (75.4) | 525 (78.7) | 502 (76.9) |
| With serious adverse events | 108 (16.1) | 125 (18.7) | 110 (16.8) |
| Mean exposure to treatment (days) | 304.7 | 297.2 | 265.6 |
| Gastrointestinal disorders | 142 (21.2) | 193 (28.9) | 97 (14.9) |
| General disorders and administration site conditions | 54 (8.1) | 33 (4.9) | 39 (6.0) |
| Infections and infestations | 90 (13.4) | 95 (14.2) | 79 (12.1) |
| Musculoskeletal and connective tissues disorders | 94 (14.0) | 100 (15.0) | 78 (11.9) |
| Nervous system disorders | 75 (11.2) | 65 (9.7) | 66 (10.1) |
| Lower respiratory system disorders | 304 (45.4) | 299 (44.8) | 360 (55.1) |
| Upper respiratory system disorders | 208 (31.0) | 203 (30.4) | 171 (26.2) |
| Vascular disorders | 30 (4.5) | 41 (6.1) | 35 (5.4) |
| All-cause mortality on treatment plus 30-day observation period† | 12 (1.79) | 17 (2.55)* | 5 (0.77) |
| All-cause mortality including discontinued patients‡ | 16 (2.39) | 18 (2.70) | 10 (1.53) |
P = 0.0161 vs placebo.
All fatal adverse events reported until day 369; this reflects the mean exposure to treatment for study completers plus the 30-day observation period. However, four known fatal events were excluded from this analysis: 2 cases in the placebo group had unknown outcomes and 1 case in each of the active treatment groups as the patients died more than 366 days after their first dose of medication.
This also includes the retrospective study 205.392.
Notes: Classified using Medical Dictionary for Regulatory Activities (MedDRA Version 8) system organ class.
Abbreviations: SMI, Soft Mist™ Inhaler.
The occurrence of fatal adverse events during treatment plus the 30-day follow-up observation period was lower in the placebo group than active treatments. When the results from patients who discontinued treatment prematurely were included (ie, information of an additional 409 patients, covering 97.7% of the 1990 randomized patients), this difference was reduced to a nonsignificant numerical imbalance between active treatment groups and placebo (Table 6).
Discussion
The data show that once-daily tiotropium (5 and 10 μg) Respimat® SMI provides sustained (ie, 24-hour) bronchodilation, and the spirometry improvements achieved (ie, trough FEV1 response and trough FVC supported by changes in morning and evening PEFR) were sustained over 48 weeks, without evidence of tachyphylaxis. These improvements are consistent with studies using HandiHaler®.6,16–22 In our analysis, which included patients with a mean baseline FEV1 of 1.06 L, the mean trough FEV1 improved by 127 and 150 mL on Tio R5 and Tio R10, respectively. A previous 12-month study in which 550 patients with a mean baseline FEV1 of 1.04 L (39.1% predicted normal) were randomized to treatment with tiotropium 18 μg HandiHaler® showed that the mean trough FEV1 was elevated 110 mL over baseline.19
In our analysis, tiotropium (both doses) also improved the HRQoL and dyspnea measures, and there was no evidence of a post-treatment rebound effect during the 21-day follow-up period. The SGRQ total score was −3.5 (Tio R5–placebo) and −3.8 (Tio R10–placebo) and the Mahler TDI focal score >1 unit. In a previous study involving 1207 patients, the SGRQ total score and Mahler TDI focal score improved by 4.2 and 1.1 units, respectively, following 6 months of treatment with tiotropium 18 μg HandiHaler®.20
The COPD exacerbation rate (per patient-year) was significantly lower after treatment with either dose of tiotropium (37.2% in the Tio R5 group and 36.9% in the Tio R10 group experienced ≥1 exacerbation during the year). These findings are generally consistent with those observed with tiotropium 18 μg HandiHaler®,8, 21 in which, over 6 months, the percentage of patients experiencing ≥1 exacerbation was 27.9% compared with 32.3% in the placebo group.8 In the present trial, however, the exacerbation rate in the placebo group was much higher (44.1%) than in the HandiHaler® trials. Rescue medication use was significantly lower in the tiotropium groups compared with placebo during the 48-week study, which indicates that the patients were better controlled using once-daily tiotropium.
Adverse events and serious adverse events were generally well balanced between active treatment groups, and were generally comparable with those observed with tiotropium 18 μg HandiHaler®. The higher incidence of urinary tract infections and dry mouth in the tiotropium groups is a known class effect of anticholinergics. In our analysis, the incidence of dry mouth was numerically lower in the tiotropium 5 μg group compared with tiotropium 10 μg (7.2% vs 14.5%, respectively). In a previously reported study in 207 COPD patients, there was a similar systemic exposure between tiotropium 5 μg (via Respimat® SMI) and tiotropium 18 μg (HandiHaler®) whereas the 10 μg dose (Respimat® SMI) had almost double the systemic exposure.6 In the current study, there was no evidence of paradoxical (administration-related) bronchoconstriction, which is consistent with previous published findings.23–25 Cardiac angina was more common on active treatments than placebo (Tio R5: 0.4%; Tio R10: 1.0%; placebo: 0.2%). The reason for this difference is not apparent, but it could be speculated that, in patients with silent or undiagnosed ischemic heart disease, increasing mobility and exercise made possible by improved lung function might precipitate angina. The study also showed that more patients in the placebo groups discontinued treatment prematurely compared with the two tiotropium treatment groups. The discontinued patients had more severe lung disease at baseline than completers; this could have resulted in an over-estimation of treatment effects; however, low baseline FEV1 values were observed for discontinued patients across all treatment groups.
Fatal events occurred in 2.4% (Tio R5), 2.7% (Tio R10), and 1.6% (placebo) of patients; however, these differences were not significant. It was not possible to retrieve the causes of death in prematurely discontinued patients as these data were not collected for all patients during the retrospective study of vital status. Such studies are time-consuming and yield data of variable quality.
In the current study, the mortality (0.77%) in the placebo group during treatment plus the 30-day observation period is unusually low in comparison with placebo arms in selected published trials (44- to 52-week duration, ≥250 COPD patients of similar severity per study arm): 1.57%,26 1.95%,27 2.66%.28 The mortality in the Tio R5 group of 1.79% is among the lowest in the range of active treatment arms in these trials: 1.58% (roflumilast),28 1.63% (fluticasone-salmeterol),29 1.90% (salmeterol),26 1.97% (budesonide–formoterol),27 2.18% (salmeterol),29 2.33 (budesonide),27 5.10% (formoterol).27 Differential discontinuation of the most severe patients may bias against effective therapies, which could, in part, explain these findings. In the Inhaled Steroids in Obstructive Lung Disease (ISOLDE) study, withdrawal due to respiratory symptoms and rapid decline in health status were more common in placebo-treated patients than those on active treatment.30 Unfortunately, the positive impact of patients’ treatment being intensified after withdrawing from the study cannot be determined.
It is also difficult to interpret the differences in mortality rates observed in different studies. A recent publication by Hilleman et al,31 analyzing adverse outcomes associated with long-term anticholinergic use in such major trials as Investigating New Standards for Prophylaxis In Reduction of Exacerbations (INSPIRE),32 Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT®),33 and the Lung Health Study (LHS),34 draws attention to the wide variation in reported rates of serious adverse events. Kesten et al35 has drawn attention to the considerably higher incidence rate of fatal events when study drug (tiotropium administered via a Handihaler®) is discontinued at the end of the trial period, compared with the time on-treatment (23.0 vs 1.9 per 100 patient-years, respectively), which may account for some of the reported differences between studies.35 These reports and our study highlight the need for prospective standardized reporting of causes of deaths both during and following discontinuation of study drug in future COPD trials.
In summary, these data show that tiotropium (5 and 10 μg), delivered via Respimat® SMI, is effective and well tolerated in the long-term treatment of COPD, and the findings are consistent with those observed with tiotropium 18 μg HandiHaler®.16–18 Given that there was no advantage of 10 μg tiotropium over the 5 μg dose for the four co-primary efficacy endpoints, the lower dose (tiotropium 5 μg) appears to be optimal when Respimat® SMI is the preferred device. The current study was based on requirements for international registration of the Respimat® doses; however, phase IV trials are planned to compare long-term administration of tiotropium delivered by Respimat® and HandiHaler®.
Acknowledgments/disclosures
The authors would like to thank the following for their involvement:
Anne Southcott, Gary Braun, Peter Holmes, Richard Tarala, Michael Chia, Mark Hurwitz, Raffaele Scicchiatano, Phillip Thompson (Australia); Günther Ott, Otto Chris Burghuber, Mannfred Sommersgutter, Peter Hesse, Josef Eckmayr, Herbert Riemer (Austria); Alexander Legrand, Renaud Louis, Joseph Aumann, Eduard Janssens, Walter Vincken, Inge Stappaerts, Wilfried De Backer, Willy Elinck (Belgium); Michel-Y-Rouleau, Amarjit Cheema, Paul Hernandez, M Reza Maleki-Yazdi, S Lyle Malenka, David Ross, Kenneth Chapman, Tony D’Urzo, Pierre Larivée, Paolo Renzi, Satyendra Sharma, Richard Tytus (Canada); Pentti Tukiainen, Ralf Backman, Jouni Hedman, Jyrki Kotaniemi, Lauri Tammilehto (Finland); Pierre Zuck, Liliane Savary, Stéphane Beaujot, Christophe Verkindre, François Fortin, Lucien Bernabeu, Bruno Lemmens, Robért Clavel, Henri Audouin, Hervé Pegliasco (France); Ekkehard Beck, Sigrid Poßner, Winfried Schröder-Babo, Karl-Otto Steinmetz, Wolfram Feußner (Germany); Nikolaos Siafakas, Vlassios Polychronopoulos, Spyridon Tzannes, Anastasios Damianos, Konstantinos Gourgoulianis, Emanuel Ntaoukakis, Dora Orfanidou, Dimosthenes Bouros, Maria Kontouri (Greece); Stephen Lane, Walter McNicholas, Séan Gaine (Ireland); Stefano Centanni, Vito Brusasco, Roberto Dal Negro, Antonio Foresi, Luciano Gandola, Florio Innocenti, Adriano Berra, Mario Polverino, Mario Schiavina, Giovanni Schmid (Italy); Theo Bantje, Steven Gans, Reinder Aalbers, Peter Luursema, Marinius Eland, Jan Arie van Noord, J Creemers, R Stallaert, Arjan Rudolphus, Hendrik Pasma, J Westbroek, P de Bruijn (Netherlands); Harry Rea, Graham Mills (New Zealand); Finn Wammer, Sigurd Loe Steinshamm, Kjell Erik Langaker (Norway); Alexander Chuchalin, Eugen Shmelev, Alexander Sinopalnikov, Liudmila Goryachkina, Mikhail Ilkovich, Victor Kasantcev, Alexander Emelyanov (Russia); Michael Greenblatt, Leonard Herbst, James Joubert, Michelle Middle (South Africa); Julio Ancochea, Pedro Cabrera, Eusebio Chiner Vives, Xavier Farrés Fabre, Eugeni Ballester, Juan-Miguel Sanchez-Nieto, Italo Sampablo, Silvia Narejos (Spain); Tryggve Mänsson, Gunnar Johansson, Georgios Stratelis, George Granton (Sweden); Gulseren Karabiyikoglu, Erc ment Ege, Veysel Yilmaz (Turkey); Paul Anderson, Ruth Cayton, Jonathan Corne, Jon Goldman, Nicholas Harrison, Niall Keaney, Phillip Hughes, Alyn Morice, Peter Rovira, Andrew Winning (UK); Omer Abdullah, William Cale, Christopher Cooper, Fitzhugh Hamilton, Roger Guthrie, David Kukafka, Craig LaForce, Michael Mandel, Kathryn Rice, Amir Sharafkhaneh, Shari Brazinsky, David Webster, Dick Briggs, Darlene Elias, Neil Ettinger, Ricardo Gonzalez-Rothi, Nicholas Gross, Nicola Hanania, Irene Leech, Daniel Paulson, Mark Soll, Robert Zielinski, Laurence Weiss, K Scott Miller (USA). Writing assistance was provided by PAREXEL MMS.
References
- 1.Newman SP, Brown J, Steed KP, Reader SJ, Kladders H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of Respimat with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113:957–963. doi: 10.1378/chest.113.4.957. [DOI] [PubMed] [Google Scholar]
- 2.Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat® Soft Mist™ Inhaler compared to deposition by metered dose inhaler or by Turbuhaler dry powder inhaler. J Aerosol Med. 2005;18:S264–S272. doi: 10.1089/jam.2005.18.264. [DOI] [PubMed] [Google Scholar]
- 3.Zierenberg B. Optimizing the in vitro performance of Respimat. J Aerosol Med. 1999;12(Suppl 1):S19–S24. doi: 10.1089/jam.1999.12.suppl_1.s-19. [DOI] [PubMed] [Google Scholar]
- 4.Hochrainer D, Holz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurised metered dose inhalers. J Aerosol Med. 2005;18:273–282. doi: 10.1089/jam.2005.18.273. [DOI] [PubMed] [Google Scholar]
- 5.Caillaud D, Le Merre C, Martinat Y, Aguilaniu B, Pavia D. A dose-ranging study of tiotropium delivered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2:559–565. [PMC free article] [PubMed] [Google Scholar]
- 6.van Noord JA, Cornelissen PJG, Aumann JL, Platz J, Mueller A, Fogarty C. The efficacy of tiotropium administered via Respimat® Soft Mist™ inhaler or HandiHaler® in COPD patients. Respir Med. 2009;103:22–29. doi: 10.1016/j.rmed.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1995;152:S77–S120. [PubMed] [Google Scholar]
- 8.Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr, Korducki L, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 9.Miszkiel KA, Beasley R, Rafferty P, Holgate ST. The contribution of histamine release to bronchoconstriction provoked by inhaled benzalkonium chloride in asthma. Br J Pharmacol. 1988;25:157–163. doi: 10.1111/j.1365-2125.1988.tb03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YG, Wright WJ, Tam WK, Nguyen-Dang TH, Salome CM, Woolcock AJ. Effect of inhaled preservatives on asthmatic subjects. II. Benzalkonium chloride. Am Rev Respir Dis. 1990;141:1405–1408. doi: 10.1164/ajrccm/141.6.1405. [DOI] [PubMed] [Google Scholar]
- 11.Beasley R, Fishwick D, Miles JF, Hendeles L. Preservatives in nebulizer solutions: risks without benefit. Pharmacotherapy. 1998;18:130–139. [PubMed] [Google Scholar]
- 12.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21:267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- 13.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 14.Mahler DA. How should health-related quality of life be assessed in patients with COPD. Chest. 2000;117:54–57. doi: 10.1378/chest.117.2_suppl.54s. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Tammeling GJ, Cotes JE, Pederson OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;6(Suppl 16):5–40. [PubMed] [Google Scholar]
- 16.O’Donnell DE, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 17.Anzueto A, Tashkin D, Menjoge S, Kesten S. One-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulm Pharmacol Ther. 2005;18:75–81. doi: 10.1016/j.pupt.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Briggs DD, Jr, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulm Pharmacol Ther. 2005;18:397–404. doi: 10.1016/j.pupt.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Casaburi R, Mahler DA, Jones PW, Wanner A, San Pedro G, ZuWallack RL, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 20.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigo GJ, Nannini LJ. Tiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysis. Pulm Pharmacol Ther. 2007;20:495–502. doi: 10.1016/j.pupt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropium. Eur Respir J. 2002;19:209–216. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 23.Patel KR, Pavia D, Lowe L, Spiteri M. Inhaled ethanolic and aqueous solutions via Respimat Soft Mist Inhaler are well-tolerated in asthma patients. Respiration. 2005;73:434–440. doi: 10.1159/000089426. [DOI] [PubMed] [Google Scholar]
- 24.Hodder R, Pavia D, Dewberry H, Alexander K, Iacono P, Ponitz H, et al. Low incidence of paradoxical bronchoconstriction in asthma and COPD patients during chronic use of Respimat soft mist inhaler. Respir Med. 2005;99:1087–1095. doi: 10.1016/j.rmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Koehler D, Pavia D, Dewberry H, Hodder R. Low incidence of paradoxical bronchoconstriction with bronchodilator drugs administered by Respimat Soft Mist Inhaler: results of phase II single-dose crossover studies. Respiration. 2004;71:469–476. doi: 10.1159/000080631. [DOI] [PubMed] [Google Scholar]
- 26.Stockley RA, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax. 2006;61:122–128. doi: 10.1136/thx.2004.033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 28.Calverley PMA, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–161. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 29.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:144–149. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 30.Calverley PM, Spencer S, Willits L, Burge PS, Jones PW, ISOLDE Study Group. DE Premature discontinuations of patients: a potential bias in COPD clinical trials. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Eur Respir J. Chest. 2007;2003;5124:898–906. 1350–1356. [Google Scholar]
- 31.Hilleman DE, Malesker MA, Morrow LE, Schuller D. A systematic review of the cardiovascular risk of inhaled anticholinergics in patients with COPD. Int J COPD. 2009;4:253–263. doi: 10.2147/copd.s4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wedzicha JA, Calverley PMA, Seemungal TA, Hagan G, Ansari Z, Stockley RA, INSPIRE Investigators The prevention of COPD exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 33.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 34.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 35.Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE. Premature discontinuations of patients: a potential bias in COPD clinical trials. Eur Respir J. 2007;5:898–906. doi: 10.1183/09031936.00104606. [DOI] [PubMed] [Google Scholar]