Abstract
Background:
Dry powder inhalers (DPIs) are inspiratory flow driven and hence flow dependent. Most patients with chronic obstructive pulmonary disease (COPD) are elderly and have poor lung function. The factors affecting their inspiratory flows through inhalers are unclear.
Objective:
To study peak inspiratory flows (PIFs) and their determinants through a DPI in COPD patients of varying age and severity.
Methods:
Flow-volume spirometry was performed in 93 COPD patients. Maximum PIF rates were recorded through an empty Easyhaler® (PIFEH; Orion Corporation, Espoo, Finland), a DPI that provides consistent dose delivery at inhalation rates through the inhaler of 28 L/min or higher.
Results:
The mean PIFEH was 54 L/min (range 26–95 L/min) with a coefficient of variation of 7%. All but two patients were able to generate a flow of ≥28 L/min. In a general linear model, the independent determinants for PIFEH were age (P = 0.02) and gender (P = 0.01), and forced expiratory volume in 1 s (FEV1) expressed as percent predicted was not a significant factor. The regression model accounted only for 18% of the variation in PIFEH.
Conclusion:
In patients with COPD, age and gender are more important determinants of inspiratory flow through DPIs than the degree of expiratory airway obstruction. Most COPD patients with varying age and severity are able to generate inspiratory flows through the test inhaler that is sufficient for optimal drug delivery to the lower airways.
Keywords: COPD, forced expiratory volume, peak inspiratory flow
Introduction
Chronic obstructive pulmonary disease (COPD) represents a global burden with increasing prevalence, morbidity, and mortality.1 Patients with diagnosed COPD are preferably treated with inhaled medications on a regular daily basis.1 Adherence and the correct use of an inhaler are important for successful treatment.2 Inability to use the inhaler correctly may result in failure to get the intended dose of medication to the airways. Therefore, knowledge of the requirements for specific inhalers is necessary when prescribing inhalation therapy.
The choice of the inhaler device is an important part of the management of patients with COPD. Two types of portable inhalers are available: pressurized metered dose inhalers (pMDI) and dry powder inhalers (DPI). DPIs are inspiratory flow driven and easier to use compared with pMDIs as no coordination is required between actuation and inhalation. In contrast to MDIs, DPIs have varying internal resistances, require sufficient inspiratory flows, and are consequently dependent, in various degrees, on the inspiratory effort of the patient.3,4 Easyhaler® (Orion Corporation, Espoo, Finland) is a device-metered DPI with a relatively high internal resistance. In vitro tests have shown that the dose delivery is consistent and accurate starting from a minimum inspiratory flow of 28 L/min through the inhaler.5,6 Easyhaler containing salbutamol, formoterol, budesonide, or beclometasone dipropionate is marketed in a number of countries, drug substances that are often prescribed for patients with COPD.
A few studies have assessed the ability of patients with COPD to use various inhaler devices.7–10 It appears that patients with severe COPD may have problems in achieving the minimum required inspiratory flow rate through DPIs to get the dose to the airways.8 Many patients with COPD are also elderly and may have muscle fatigue. Regardless of the absence or presence of disease, a study in the elderly showed that their ability to generate sufficiently high inspiratory flows through DPIs was clearly compromised.9 The main factors that affect inspiratory flows through inhalers in patients with COPD, and whether any subgroups not capable of efficient use of specific DPIs could be identified are unclear.
The aim of this study was to investigate the peak inspiratory flow through the Easyhaler (PIFEH) inhaler in patients with COPD of various degrees of airway obstruction in order to assess the ability of the patients to effectively use the device, and to investigate the determinants of the PIFEH, using other lung function variables and demographic factors of the patients as explanatory variables.
Materials and methods
A total of 104 consecutive patients with previously diagnosed COPD treated at the Skin and Allergy Hospital, Helsinki University Central Hospital, were invited to take part in the study. A total of 93 patients gave their written informed consent. Patients with a current COPD exacerbation were not allowed to participate. The patients were allowed to use their regular medication on the day of the test. All patients had typical respiratory symptoms and fulfilled the physiological criterion for COPD with a postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio of less than 70%.1 The majority of patients were current or exsmokers. The demography and baseline characteristics of the patients are shown in Table 1. The study protocol was investigator-initiated and approved by the Ethics committee of the Department of Medicine of the Helsinki University Central Hospital.
Table 1.
Demography and baseline characteristics of the patients
| Variable | Result |
|---|---|
| Number of patients | 93 |
| Sex (female/male) | 44/49 |
| Mean age, years (range) | 65 (47–84) |
| Smoking history | |
| Current smokers | 48 |
| Exsmokers | 44 |
| Nonsmokers | 1 |
| Mean pack years (range) | 40 (0–110) |
| FEV1, L (range) | 1.5 (0.49–3.30) |
| FEV1, percent predicted normal (range) | 51 (18–96) |
| FVC, L (range) | 3.0 (1.4–5.9) |
| FVC, percent predicted normal (range) | 81 (37–130) |
| FEV1/FVC (%) | 44 (21–69) |
| Current medication, number of patients (%) | |
| Inhaled corticosteroids | 89 (96) |
| Long-acting β2-agonist | 68 (73) |
| Short-acting β2-agonist | 63 (68) |
| Anticholinergic | 60 (65) |
| Theophylline | 22 (24) |
Abbreviations: FEV, forced expiratory volume; FVC, forced vital capacity.
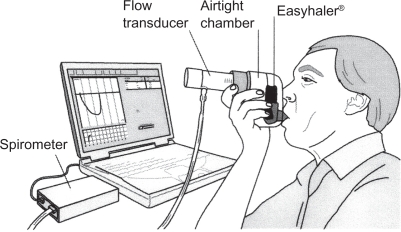
Flow-volume spirometry was performed according to ATS/ERS guidelines11 including the recording of native peak inspiratory flow (PIF) rate. Maximum PIF rates were recorded through an empty Easyhaler (PIFEH) connected to a pneumotachograph (Spiromaster MX, Medikro Ltd, Kuopio, Finland; Figure 1). The patients were instructed to exhale gently (to functional residual capacity) and then to inhale as fast and long as possible through the inhaler. They practiced this inhalation maneuver three times and thereafter three PIFEH measurements were recorded consecutively. Spirometry and PIFEH measurements were performed in random order to compensate for the possible confounding effect of muscle fatigue.
Figure 1.
The Easyhaler inhaler connected in series to a pneumotachograph.
The primary outcome variable of the study was PIFEH. The proportion of patients with PIFEH exceeding the minimum flow of 28 L/min for effective use of Easyhaler was calculated. The reproducibility of PIFEH was estimated with an analysis of variance. General linear models were used to investigate independent determinants of PIFEH and their regression coefficients, in a linear model of PIFEH = b0 + b1x1 + b2x2 + ... bkxk, where x1...k are the independent variables and b1...k are their coefficients.
Results
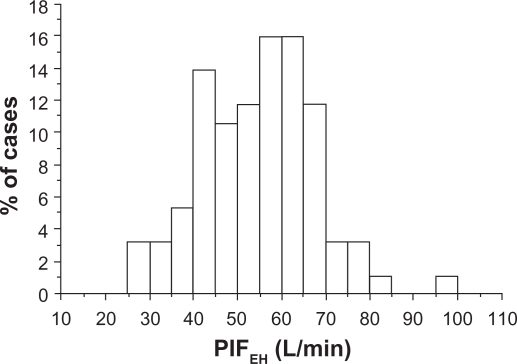
The mean PIFEH was 54 L/min (range 26–95 L/min) with a within-subject coefficient of variation of 7.4% (range 0.9%–40.7%) in three consecutive inhalations. Ninety-one of the 93 patients (98%) could generate a PIFEH ≥ 28 L/min and a total of 271 inspiratory maneuvers through the inhaler out of 279 recorded (97%) showed flow rates ≥28 L/min. The distribution of the PIFEH rates is shown in Figure 2.
Figure 2.
The distribution of the peak inspiratory flow through Easyhaler (PIFEH) in COPD patients of varying severity (n = 93).
The relationships between PIFEH and lung function data and the patients’ age, height, and weight are shown in Table 2. The native PIF had the strongest association with PIFEH both in terms of absolute values (L/min) and percent predicted normal values (r = 0.54; P < 0.0001 and r = 0.37; P = 0.0003, respectively).
Table 2.
The relationship between peak inspiratory flow Easyhaler and age, height, weight, and lung function in 93 COPD patients
| Variable | ra | P |
|---|---|---|
| Age, years | −0.30 | 0.004 |
| Height, cm | 0.26 | 0.01 |
| Weight, Kg | 0.22 | 0.03 |
| FEV1, L | 0.30 | 0.004 |
| FEV1, percent predicted | 0.11 | 0.28 |
| PEF, L/s | 0.32 | 0.002 |
| PEF, percent predicted | 0.17 | 0.11 |
| PIF, L/s | 0.54 | <0.0001 |
| PIF, percent predicted | 0.37 | 0.0003 |
Notes:
Correlation coefficient (Pearson).
Abbreviations: FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow; PIF, peak inspiratory flow.
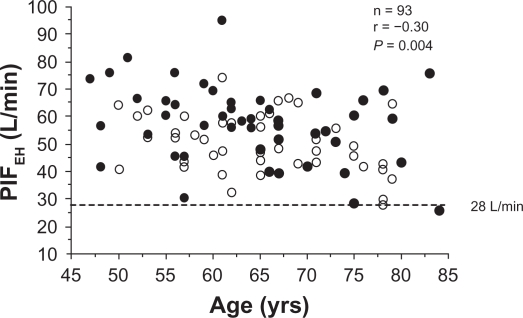
The relationship between PIFEH and the patients’ age (males and females marked separately) is shown in Figure 3. A significant correlation was seen between age and PIFEH (P = 0.004). Women had significantly lower PIFEH (P = 0.004) than men. PIFEH was also significantly related to height and weight although the correlation coefficients were small.
Figure 3.
The relationship between peak inspiratory flow through Easyhaler (PIFEH) and age in COPD patients of varying severity (n = 93). Closed circles = men; open circles = women.
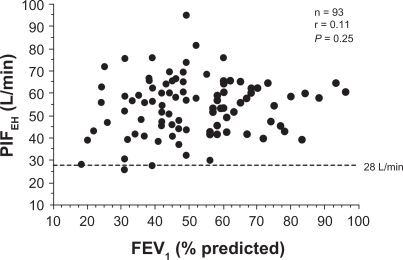
The univariate relationships of PIFEH with FEV1 and peak expiratory flow rates were statistically significant (r = 0.30; P = 0.004 and r = 0.32; P = 0.02, respectively). However, FEV1 as percent of predicted normal values, ie, FEV1 adjusted for age, gender, and height of the subject, was not significantly related to PIFEH as shown in Figure 4.
Figure 4.
The relationship between peak inspiratory flow through Easyhaler (PIFEH) and FEV1 percent predicted in COPD patients (n = 93).
To construct a model predicting PIFEH, a set of explanatory variables were introduced stepwise. Since all lung function variables showed considerable collinearity with their relationship to PIFEH, FEV1 as percent predicted was chosen to represent the level of lung function impairment in the model. Table 3 shows the results of the linear regression model for best prediction of PIFEH values. Age and gender were significant predictors of the PIF through the inhaler. Other variables introduced to the model thereafter, such as FEV1, height, and weight, had insignificant effects. The full model, however, explained only 18% of the variation in PIFEH.
Table 3.
Results of the general linear modela to predict PIFEH in 93 patients with COPD. For the full model, r2 = 0.18 (P = 0.0014)
| Parameter | Coefficient | Standard error | P |
|---|---|---|---|
| Intercept | 71.7 | 13.0 | <0.0001 |
| Age, years | −0.34 | 0.15 | 0.022 |
| Gender | |||
| female | −6.89 | 2.62 | 0.010 |
| male | 0.00 | ||
| FEV1, percent predicted | 0.089 | 0.077 | 0.25 |
| Weight, kg | 0.047 | 0.058 | 0.42 |
Notes:
y = b0 + b1x1 + b2x2 + ... bkxk, where x1...k are the explanatory parameters and b1...k are their regression coefficients; see text for an example.
Abbreviations: FEV1, forced expiratory volume in 1 s; PIFEH, peak inspiratory flow through the easyhaler.
Discussion
Our study found that almost all patients with COPD (91 of 93 patients) have an inspiratory effort sufficient to generate inspiratory flows through the test inhaler that should result in therapeutic benefit with a drug-loaded device. The series of patients represented a wide variety of disease severity and airway obstruction and the results are, therefore, applicable to most patients with COPD.
Previously, data on inspiratory flow characteristics through DPIs in COPD patients have been limited. A study using Turbuhaler®7 showed that all COPD patients were able to generate PIF through the inhaler of 28 L/min, with a variation similar to that we found (Figure 2). However, in vitro tests suggest that optimal dosing with this inhaler (Turbuhaler) would require flows of at least 40–60 L/min.6,12 Although PIF through the inhaler showed significant associations with FEV1 in liters and native PIF, this study was not aimed to investigate the effect of degree of lung function impairment (expressed as FEV1 percent predicted) or other factors such as age or gender. In an other study by Broeders et al8 the inhalation profiles through Turbuhaler and Diskus® inhalers were compared in asthmatics and COPD patients. In the severe COPD group, 7%–19% of the patients showed suboptimal flows through the inhalers. They found that PIF through the inhaler correlated with other parameters of inspiratory capacity like native PIF or maximal inspiratory pressures, which is in agreement with our results. However, the effect of other factors such as age or sex were not included in their analyses. Janssens et al9 studied inspiratory flow rates at different levels of resistance, corresponding to different types of DPIs, in elderly COPD patients. This study showed that in elderly patients, the ability to generate sufficient inspiratory flows across a DPI is compromised, but since only patients over 70 years were included, it is difficult to draw any conclusions whether age, the degree of airway obstruction, or other mechanisms are the main determinants for the inspiratory flows. The current study is the first specifically designed to clarify the relative impact of these factors, as well as the performance of Easyhaler in patients with COPD.
The present study included only patients with stable disease, and thus the results may be questioned when the inhaler has to be used in situations perceived as constrained.13 Such situations include worsening of asthma, severe COPD in general, and COPD exacerbations. Even though exhalation may be severely affected in these conditions, it is known that inspiration is much less so.14 A study of the course of inhalation profiles during an exacerbation of obstructive lung disease also showed that despite minor reductions in PIF during the acute phase, all patients were able to generate flows of 30 L/min or more through the DPIs.15 A study investigating patients with COPD of various degrees of airway obstruction did not find differences in lung deposition between subjects with mild or more severe airway obstruction.16 The current study also included a wide variety of disease severity and airway obstruction, making the results more applicable to most patients with COPD.
It is generally believed that DPIs with low internal resistance are more suitable for patients with more severe airway obstruction. However, as the turbulent energy is a product of the flow and the inhaler’s resistance, for a set energy level needed for de-aggregation of the drug particles, the flow required through a low resistance DPI will be higher than that of a high-resistance DPI.17 With a high-resistance DPI, the inspiratory flow through the inhaler will be determined mostly by the inhaler’s resistance and is less affected by varying airway resistance of the patient, which may result in a more consistent dose emission.18 The Easyhaler is an example of an inhaler with a relatively high internal resistance and consistent dose delivery over a wide range of inspiratory flows.6 The observed inspiratory flows observed in the current study (Figure 2) are well within these limits, so despite the high resistance of the inhaler, the great majority of COPD patients were able to achieve sufficient inspiratory flows through Easyhaler for optimal drug delivery to the lower airways. This finding agrees with a previous study of young children with asthma who also showed sufficient inspiratory flows through this inhaler.19 Furthermore, a study with pediatric and adult asthmatic patients has confirmed that clinical efficacy of salbutamol is maintained even at low inspiratory flow rates when using Easyhaler.20
In view of the previous literature, however, several potential factors may limit the ability of COPD patients to generate sufficient inspiratory flows through DPIs.8,9 We found significant associations with age and gender (Figure 3) which have not been specifically reported earlier, although problems with inspiratory flows in elderly patients have been shown to be common.9 The results in Figure 4 slightly contradict with those of Broeders et al8 who found suboptimal inspiratory flows more common in severe COPD patients with a mean FEV1 of 34% predicted. However, they did not analyze the effect of FEV1 as a continuous variable as in the present study. We constructed a multivariate model to predict PIFEH to clarify these factors and their relative importance, and to better identify patients with increased risk of insufficient inspiratory flows through the DPI. Factors that are generally available when treating the patients, ie, age, gender, height, weight, and lung function, were entered into the regression analysis. According to the model (Table 3), as an example, a 70-year-old female patient with FEV1 of 30% predicted and height of 160 cm should have PIFEH of 71.7 − 0.34 × 70 − 6.89 + 0.089 × 30 + 0.047 × 160 = 51.2 L/min. The regression analysis showed that age and gender had independent effects on PIFEH and were more important than the degree of airway obstruction (expressed as FEV1 percent predicted), which did not have a significant effect in the model. The low coefficient of determination suggests that additional physiological factors than those measured in this study should be considered to accurately predict PIF through inhalers. Such factors might include more direct measurements of inspiratory muscle performance or maximum inspiratory pressures.8 However, such data are seldom available when treating patients in clinical practice. The coefficient of determination was also too low for our model to be useful for clinical purposes. Therefore, it is advisable to check PIFs through the inhaler individually,17 especially in elderly patients with COPD, and whenever found unsatisfactory, alternative routes for drug administration should be considered, such as jet nebulizers or other flow-independent aerosol delivery systems.
The current results are mainly explained by the general physiological determinants of inspiratory flows. Maximum inspiratory flows are dependent on the inspiratory pressure generated by the inspiratory muscles. Since during inspiration, pleural pressure is always subatmospheric and lower than bronchial pressure, airflow limitation does not occur, although increased airway resistance in patients with obstructive pulmonary diseases may have a minor effect on flow rates. Accordingly, maximum inspiratory flows in COPD patients are significantly less affected than expiratory flows.21 Potential factors decreasing inspiratory muscle performance in COPD patients include aging,22 malnutrition, and hyperinflation,23 although in the latter factor, adaptive mechanisms may partly restore the force generating capacity of the respiratory muscles. The gender-related difference in maximum inspiratory flows has been previously shown in healthy subjects,22 but not in patients with COPD or in relation to PIF through inhalers. This difference has been attributed to the overall better strength of the skeletal muscles in men, due to hormonal or genetic differences.22
Conclusion
We conclude that age and gender in COPD patients are more important determinants of inspiratory flow through DPIs than the degree of expiratory airway obstruction. Most COPD patients with varying age and severity are able to generate inspiratory flows through Easyhaler that is sufficient for optimal drug delivery to the lower airways.
Acknowledgments
The authors wish to thank Ulla Veteläsuo, RN, for performing the lung function measurements, Olof Selroos, MD, PhD, Semeco AB, Vejbystrand, Sweden, for drafting the manuscript, Mikko Vahteristo, MSc, for statistical assistance, and Orion Pharma, Espoo, Finland, for the supply of the empty Easyhaler inhalers for the study. The study was investigator-initiated and supported by an unrestricted research grant from Orion Corporation, Espoo, Finland.
Footnotes
Disclosures
The authors report no conflicts of interest in this work. LPM has received fees for lectures in symposia sponsored by Astrazeneca, Glaxo Smith Kline, MSD, Orion Pharma and Schering Plough; PR is a full-time employee of Orion Pharma; PH is a full-time employee of Orion Pharma and holds stock in Orion Corporation; TH has received fees for lectures in symposia sponsored by Alk/Abello, AstraZeneca, Meda Oy, MSD and Orion Pharma.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease Global strategy for diagnosis, management and prevention of chronic obstructive pulmonary disease [updated 2007] Available from: www.goldcopd.com. Accessed 2009. [Google Scholar]
- 2.Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir Med. 2006;100:1479–1494. doi: 10.1016/j.rmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Clark AR, Hollingworth AM. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers – implications for in vitro testing. J Aerosol Med. 1993;6:99–110. doi: 10.1089/jam.1993.6.99. [DOI] [PubMed] [Google Scholar]
- 4.Ross DL, Schultz RK. Effect of inhalation flow rate on the dosing characteristics of dry powder inhaler (DPI) and metered dose inhaler (MDI) products. J Aerosol Med. 1996;6:215–226. doi: 10.1089/jam.1996.9.215. [DOI] [PubMed] [Google Scholar]
- 5.Vidgren M, Silvasti M, Vidgren P, Sormunen H, Laurikainen K, Korhonen P. Easyhaler multiple dose inhaler – practical and effective alternative to the pressurized MDI. Aer Sci Technol. 1995;8:335–345. [Google Scholar]
- 6.Palander A, Mattila T, Karhu M, Muttonen E. In vitro comparison of three salbutamol-containing multidose dry powder inhalers. Clin Drug Invest. 2000;20:25–33. [Google Scholar]
- 7.Dewar MH, Jamieson A, McLean A, Crompton GK. Peak inspiratory flow through Turbuhaler in chronic obstructive airways disease. Respir Med. 1999;93:342–344. doi: 10.1016/s0954-6111(99)90316-5. [DOI] [PubMed] [Google Scholar]
- 8.Broeders ME, Molema J, Hop WC, Folgering H. Inhalation profiles in asthmatics and COPD patients: reproducibility and effects of instruction. J Aerosol Med. 2003;16:131–141. doi: 10.1089/089426803321919898. [DOI] [PubMed] [Google Scholar]
- 9.Janssens W, Van den Brande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Resp J. 2008;31:78–83. doi: 10.1183/09031936.00024807. [DOI] [PubMed] [Google Scholar]
- 10.Al-Showair RAM, Tarsin WY, Khaled H, Pearson SB, Chrystyn H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help. Respir Med. 2007;101:2395–2401. doi: 10.1016/j.rmed.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V. Standardization of spirometry. ATS/ERS task force: standardization of lung function testing. Eur Resp J. 2005;26:319–338. [Google Scholar]
- 12.Prime D, Grant AC, Slater AL, Woodhouse R. A critical comparison of the dose delivery characteristics of four alternative inhalation devices delivering salbutamol: pressurized metered dose inhaler, Diskus inhaler, Diskhaler inhaler, and Turbuhaler inhaler. J Aerosol Med. 1999;12:75–84. doi: 10.1089/jam.1999.12.75. [DOI] [PubMed] [Google Scholar]
- 13.Borgström L. On the use of dry powder inhalers in situations perceived as constrained. J Aerosol Med. 2001;14:281–287. doi: 10.1089/089426801316970231. [DOI] [PubMed] [Google Scholar]
- 14.McNeill RS, Malcolm GD, Rhind Brown W. A comparison of expiratory and inspiratory flow rates in health and in chronic pulmonary disease. Thorax. 1959;14:225–231. [Google Scholar]
- 15.Broeders ME, Molema J, Hop WC, Vermue NK, Folgering H. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98:1173–1179. doi: 10.1016/j.rmed.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Derom E, Strandgarden K, Schelfhout V, Borgström L, Pauwels R. Lung deposition and efficacy of inhaled formoterol in patients with moderate to severe COPD. Respir Med. 2007;101:1931–1941. doi: 10.1016/j.rmed.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Chrystyn H. Is inhalation rate important for a dry powder inhaler? Using the In-Check Dial to identify these rates. Respir Med. 2003;97:181–187. doi: 10.1053/rmed.2003.1351. [DOI] [PubMed] [Google Scholar]
- 18.Clark A. Effect of powder inhaler resistance upon inspiratory profiles in health and disease. Respiratory Drug Delivery IV. 1994:117–123. [Google Scholar]
- 19.Malmström K, Sorva R, Silvasti M. Application and efficacy of the multi-dose powder inhaler, Easyhaler®, in children with asthma. Pediatr Allergy Immunol. 1999;10:66–70. doi: 10.1034/j.1399-3038.1999.101002.x. [DOI] [PubMed] [Google Scholar]
- 20.Koskela T, Malmström K, Sairanen U, Peltola S, Keski-Karhu J, Silvasti M. Efficacy of salbutamol via Easyhaler® unaffected by low inspiratory flow. Respir Med. 2000;94:1229–1233. doi: 10.1053/rmed.2000.0959. [DOI] [PubMed] [Google Scholar]
- 21.Stanescu D, Veriter C, van de Woestijne KP. Maximal inspiratory flow rates in patients with COPD. Chest. 2000;118:976–980. doi: 10.1378/chest.118.4.976. [DOI] [PubMed] [Google Scholar]
- 22.Chen H-I, Kuo C-S. Relationship between respiratory muscle function and age, sex, and other factors. J Appl Physiol. 1989;66:943–948. doi: 10.1152/jappl.1989.66.2.943. [DOI] [PubMed] [Google Scholar]
- 23.Decramer M. Hyperinflation and respiratory muscle interaction. Eur Resp J. 1997;10:934–941. [PubMed] [Google Scholar]