Abstract
Experimental autoimmune encephalomyelitis (EAE) is a model of human multiple sclerosis (MS) induced by auto-reactive T helper cells that mediate tissue inflammation and demyelination in the CNS. Initially, IFN-γ-producing Th1 cells and, more recently, IL-17-producing Th17 cells with specificity for myelin antigens have been implicated in EAE induction, but whether Th17 cells are encephalitogenic has been controversial. Moreover, a new effector T cell subset, Th9 cells, has been identified, however, the ability of this T cell subset to induce EAE has not been investigated.
Here, we have developed protocols to generate myelin oligodendrocyte glycoprotein (MOG)-specific Th17, Th1, Th2, and Th9 cells in vitro, so that we could directly compare and characterize the encephalitogenic activity of each of these subsets upon adoptive transfer. We show that MOG-specific Th1, Th17, and Th9 cells but not Th2 cells induce EAE upon adoptive transfer. Importantly, each T cell subset induced disease with a different pathological phenotype.
These data demonstrate that different effector T cell subsets with specificity for myelin antigens can induce CNS autoimmunity and that the pathological heterogeneity in MS lesions might in part be due to multiple distinct myelin-reactive effector T cells.
Introduction
Experimental autoimmune encephalomyelitis (EAE)3 has long served as an animal model for human multiple sclerosis (MS). Both diseases are characterized by inflammation and demyelination of the central nervous system (CNS). The histopathologic patterns of MS lesions are heterogeneous and vary among patients and during different stages of the disease. In acute and relapsing-remitting MS, lymphocytic infiltrates form perivascular cuffs that consist of T cells, activated macrophages and microglia. In these lesions, emerging classical focal plaques are characterized by demyelination, axonal injury and axonal loss in the white matter of the CNS. In some patients there is complement deposition in the lesions suggesting the involvement of B cells and Abs in disease pathogenesis. Moreover, in some patients with very severe and rapidly progressive disease, lymphoid follicle-like structures that contain B cells, plasma cells, T cells and dendritic cells have been observed in the leptomeninges (1). These ectopic germinal centers might orchestrate ongoing inflammation and tissue destruction within the target tissue (2). The mechanisms leading to the formation of the different types of inflammatory MS lesions are not clear.
It is well established that myelin-reactive T cells are crucial for the induction of EAE (3). Earlier studies showed that myelin-antigen specific T cells with a Th1 phenotype transfer EAE, whereas Th2 cells were found to be incapable of transferring EAE (4–6). Driven by the cytokine IL-12, Th1 cells secrete large amounts of IFN-γ, a cytokine that has been detected in inflammatory CNS lesions and is known to activate macrophages (4). These studies suggest that IFN-γ and Th1 cells are essential initiators of EAE development; other studies, however, showed that IFN-γ-deficient mice were not resistant but were in fact more susceptible to EAE (7), thus questioning the role of IFN-γ and Th1 cells in EAE pathogenesis.
More recently, another subset of T cells, called Th17 cells, has been identified. Distinct from Th1 and Th2 cells, Th17 cells are generated from naïve T cells by TGF-β and IL-6 (8, 9) and are expanded and stabilized further by IL-23. Th17 cells produce IL-17A, IL-17F, IL-21 and IL-22 and have been shown to transfer EAE. While IL-17-producing T cells expanded with IL-23 were originally reported to be more pathogenic than IFN-γ-producing T cells expanded with IL-12 (10), another report found that only Th1 cells, but not Th17 cells, induce EAE (11). These conflicting observations might be due to differences in the capacity of Th1 versus Th17 cells to induce EAE. However, since each of these studies used a different T cell differentiation protocol and T cells of different TCR specificity, the conflicting data might be due to variations in the cytokine profiles of the T cell subsets or to different antigen specificities of the effector T cells.
In addition to Th1, Th2 and Th17 cells, another effector T cell subset, Th9 cells, has recently been described (12, 13). Driven by the combined effects of TGF-β and IL-4, Th9 cells produce large amounts of IL-9 and IL-10. It has been shown that IL-9 together with TGF-β can contribute to Th17 cell differentiation and Th17 cells themselves can produce IL-9 (14). In addition, we have shown previously that Th9 cells are capable of inducing tissue inflammation in a colitis model (12); however, whether Th9 cells can induce EAE has not been investigated.
To characterize the T cell subsets that can induce EAE, we generated Th1, Th2, Th17, and Th9 cells from MOG-specific TCR transgenic T cells in vitro and transferred them into naïve syngeneic wild type recipients. Using different protocols and either one or two rounds of in vitro polarization, we could evaluate the pathogenic capacity of different types of in vitro derived T cell subsets. In this report we show that not only Th1, but also Th17 and Th9 cells are capable of inducing EAE upon adoptive transfer. While clinical severity of the disease was comparable, each subset induced disease with different albeit overlapping pathologic features that may reflect their distinct effector functions.
Materials and Methods
Animals
C57Bl/6 mice were purchased from The Jackson Laboratory; all mice used were between 7–10 weeks old. C57Bl/6 mice with a TCR specific for the peptide MOG35-55 are referred to as 2D2 mice and have been described previously (15). Mice were housed in a specific pathogen-free, viral Ab-free animal facility at the Harvard Institutes of Medicine. All breeding and experiments were reviewed and approved by the Institutional Animal Care and Use Committee of Harvard Medical School.
Antibodies
Abs to CD4 (clone RM4-5) conjugated to PerCP or FITC or allophycocyanin-Cy7, CD62L (clone MEL-14) conjugated to PE, IFN-γ (clone XMG1.2) conjugated to PE, IL-17 (clone TC11-18H10.1) conjugated to allophycocyanin or PE, IL-10 (clone JES5-16E3) conjugated to allophycocyanin and IL-9 (clone RM9A4) conjugated to PE or Alexa Fluor 647 were purchased from BioLegend. The Ab to IL-17F (clone eBio18F10) conjugated to Alexa Fluor 647 was purchased from eBioscience. Abs to IL-4 (clone 11B11) conjugated to allophycocyanin and Vα3.2 TCR (clone RR3-16) conjugated to biotin or FITC were purchased from BD Pharmingen. Streptavidin conjugated to Pacific Blue was purchased from Invitrogen.
ELISA
Cytokine secretion was measured in supernatants collected from re-stimulated cells just before transfer. Secretion of IL-9, IL-17, IFN-γ and IL-10 was measured using matching Abs (BD Pharmingen) in a sandwich ELISA. Secretion of IL-21 was measured with a DuoSet ELISA Development kit according to the manufacturer’s instructions (R&D Systems). Secretion of IL-22 was measured with an ELISA construction kit according to the manufacturer’s instructions (ANTIGENIX AMERICA Inc.). In some experiments cytokine secretion was measured using a cytokine bead array according to the manufacturer’s instructions (BD Biosciences).
Differentiation of T effector cells
Naïve T cells were isolated from spleen and lymph nodes of 7–10 week old 2D2 mice. To prepare a single cell suspension spleens and lymph nodes were mashed and passed through a 70 µm mesh. After erythrocyte lysis, CD4+ T cells were purified using magnetic beads coated with anti-CD4 Ab (clone L3T4) according to the manufacturer’s instructions (Miltenyi Biotech). Subsequently, the CD4+ cells were stained with anti-CD4 and anti-CD62L Abs and sorted into naïve CD4+CD62Lhi T cells with a BD FACS Aria. The naïve cells were cultured at a concentration of 2x106 ml−1 in RPMI 1640 medium supplemented with 10% FBS, L-glutamine, HEPES, penicillin/streptomycin, gentamicin sulfate and β-mercaptoethanol. Cells were stimulated in the presence of 107 ml−1 irradiated splenocytes (3400 rad) and either 2.5 µg/ml anti-CD3 Ab (clone 145-2C11, BioXCell) or 20 µg/ml of MOG35-55. Th1 cells were generated by addition of IL-12 at a concentration of 10 ng/ml and anti-IL-4 Ab (clone 11B11) at a concentration of 20 µg/ml into the culture (see also Figure 1). For the generation of Th17 cells naïve T cells were cultured with IL-6 at a concentration of 30 ng/ml, TGF-β at a concentration of 3 ng/ml, anti-IFN-γ (clone XMG1.2) and anti IL-4 Ab at a concentration of 20 µg/ml. Polarization of Th9 cells was induced by addition of IL-4 at a concentration of 20 ng/ml and TGF-β at a concentration of 3 ng/ml into the culture. Th2 cells were generated via addition of IL-4 at a concentration of 10 ng/ml and anti-IL-12 Ab (clone C17.8) at a concentration of 20 µg/ml.
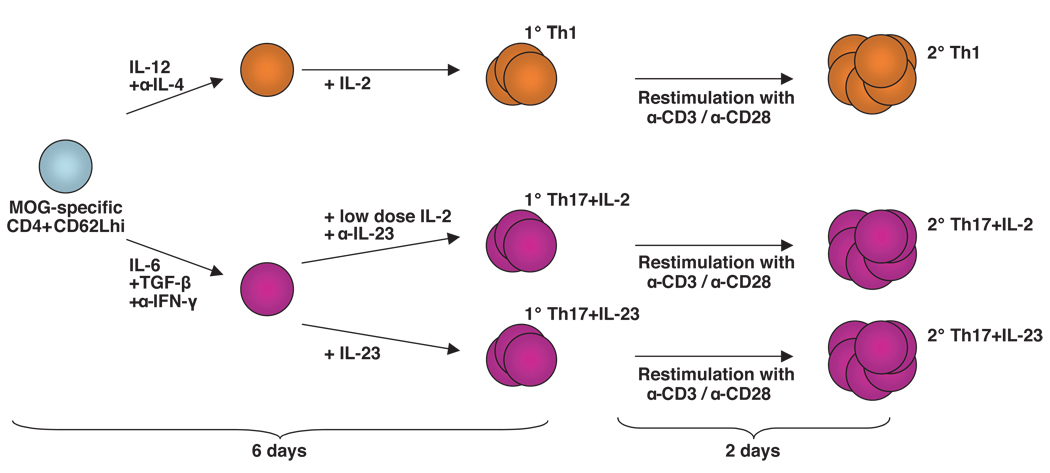
Figure 1. Generation of T cell subsets in vitro.
MOG-specific CD4+CD62Lhi T cells were differentiated into Th1 and Th17 cells. After initial activation in the presence of the indicated polarizing cytokines and neutralizing Abs for 2 days, Th1 cells were further cultured with IL-2, while Th17 cells were further supplemented either with IL-23 or with low doses of IL-2. After 6 days of culture primary (1°) Th1, Th17 plus IL-2, and Th17 plus IL-23 cells were either collected for transfer, or re-stimulated with Abs to CD3 and CD28 for 2 days to generate highly activated secondary (2°) Th1, Th17 plus IL-2, and Th17 plus IL-23 cells.
After 48h Th1, Th2, and Th9 cells were split with medium containing 20 U/ml of IL-2. Th17 cells were split after 48h using either medium containing 10 ng/ml of IL-23 or RPMI 1640 medium containing 2 U/ml of IL-2. All cytokines were purchased from R&D.
The differentiation process was monitored daily by microscopy. After an initial phase of proliferation and activation, cells reached the resting stage after 6–7 days of culture. After resting, the polarized T cells were re-stimulated by culturing them at a concentration of 2x106 ml−1 for 48h in the presence of plate-bound anti-CD3 and anti-CD28 (clone PV-1, BioXCell) Abs at a concentration of 2 µg/ml in fresh medium without any cytokines.
Induction of EAE by transfer of T effector cells
Re-stimulated cells were collected and extensively washed with PBS. 3–5 × 106 cytokine producing cells were injected i.v. into C57Bl/6 recipients. In some of the experiments recipient mice were injected i.p. with 150 ng pertussis toxin (PT) (List Biological Laboratories) on day 0 and day 2 after T cell transfer. Animals were monitored daily for development of EAE according to the following criteria: 0, no disease; 1, decreased tail tone; 2, hind limb weakness or partial paralysis; 3, complete hind limb paralysis; 4, front and hind limb paralysis; 5, moribund state. Mice were sacrificed for histopathological analysis at day 40 after transfer.
Recovery of transferred cells
Recipient mice were sacrificed at the peak of disease and perfused through the left cardiac ventricle with PBS. Brain and spinal cord were cut into pieces and digested for 30 minutes at 37°C with Collagenase D at a concentration of 2.5 mg/ml (Roche) and Deoxyribonuclease I (Sigma) at a concentration of 1 mg/ml. To prepare a single cell suspension the tissues were mashed and passed trough a 70 µm mesh. Mononuclear cells were isolated over a 37%/70% Percoll gradient (GE healthcare) as described previously (16). Transferred T cells were distinguished from endogenous T cells by the expression of Vα3.2, which is expressed on all 2D2 T cells, but only on about 0.5 - 0.7% of the endogenous T cell repertoire of C57Bl/6 mice.
Intracellular cytokine staining
Cells were stimulated for 4.5 h with 50 ng/ml phorbol 12-myristate 13-acetate (Sigma) and 1 µM ionomycin (Sigma) in the presence of monensin (GolgiStop, BD Pharmingen). Cells were then fixed with 0.4% para-formaldehyde (Electron Microscopy Sciences) and permeabilized with PBS containing 2% FCS and 0.1% Saponin (Sigma). Cells were analyzed for the production of cytokines by staining with anti-cytokine Abs and subsequent flow cytometry on a BD FACS Calibur or on a BD LSRII.
Histopathologic analysis
Mice were sacrificed 40 days after transfer and brains and spinal cords were fixed in 10% neutral buffered formalin and processed routinely for paraffin embedment. Slides were stained with Luxol fast blue-hematoxylin and eosin stains. Inflammatory foci (>10 mononuclear cells) were counted in leptomeninges and parenchyma in a blinded fashion in that the pathologist was unaware of the clinical status and T cell subset that the mice had received.
Results
Induction of EAE with primary in vitro generated T cell subsets
While some studies show that Th1 cells but not Th17 cells induce EAE, others describe Th17 cells to be more pathogenic than Th1 cells. Both Langrish et al. (10) and Kroenke et al. (17) show that IL-17 producing T cells obtained from immunized mice and expanded in vitro with IL-23 transfer EAE to naïve recipients. On the other hand, O’Connor et al. report that Th17 cells generated in vitro from naïve myelin basic protein (MBP) TCR transgenic T cells induce very little or no disease in recipients treated with pertussis toxin (PT) (11). The reasons behind these discrepancies are not clear. To address these issues we compared the ability of Th1 and Th17 cells, derived from TCR transgenic mice with the same myelin specificity, to transfer EAE after differentiation/activation in vitro.
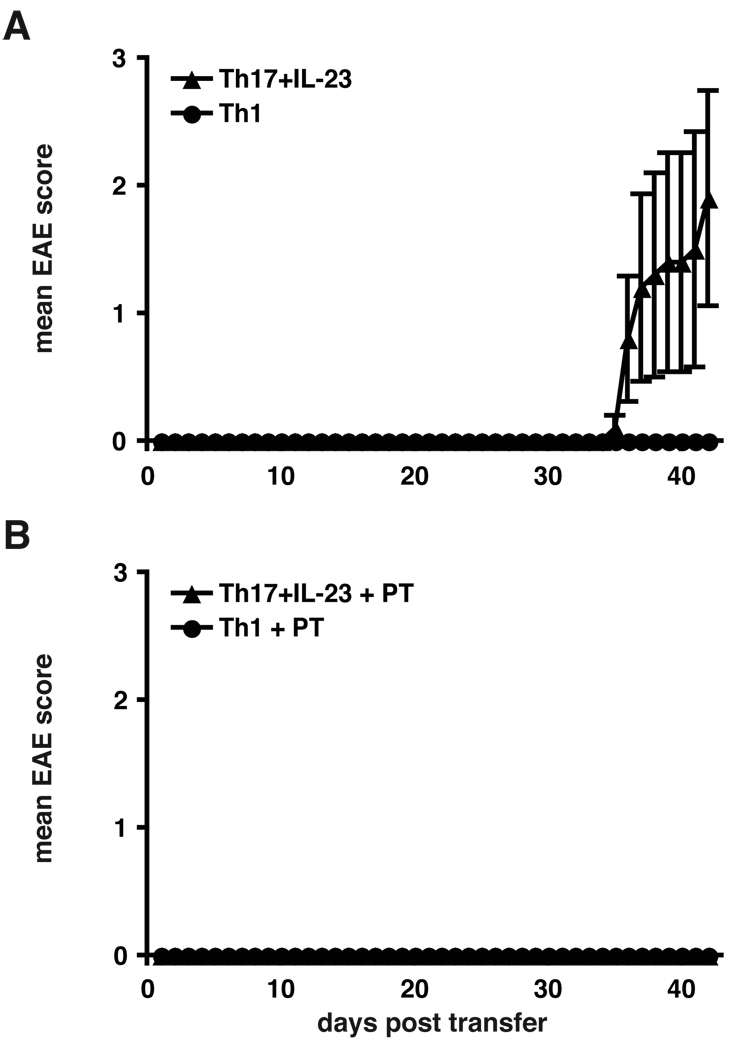
To generate T cell subsets devoid of any precursors of previously differentiated T cell subsets, we sorted naïve CD4+CD62Lhi T cells isolated from MOG-specific TCR transgenic mice (2D2 mice). These naïve T helper cells were activated in vitro in the presence of anti-CD3 Ab and irradiated antigen-presenting cells (APCs) and differentiated into Th1 and Th17 cells with polarizing cytokines (Figure 1). Th1 cells were generated in the presence of IL-12 and a neutralizing Ab to IL-4 (1°Th1). Th17 cells were generated with IL-6 and TGF-β in the presence of neutralizing Abs to IFN-γ and IL-4. In addition, Th17 cells were further cultured in the presence of IL-23 (1°Th17 plus IL-23). After 4 days, the extent of differentiation was determined by intracellular cytokine staining, and the differentiated T cells were injected into C57Bl/6 recipients. Interestingly, only animals that had received Th17 cells cultured in the presence of IL-23 (1°Th17 plus IL-23) developed clinical EAE with a very late onset (Figure 2A and Table I). In contrast, recipients of Th1 cells remained completely healthy throughout the observation period. Consistent with this observation, no lesions could be found upon histopathological examination in the brain and spinal cord of 1°Th1 cell recipients. On the contrary, recipients of 1°Th17 plus IL-23 cells had large numbers of inflammatory lesions in the meninges and the parenchyma (Table I). These data show that after a single round of differentiation/activation in vitro, MOG-specific Th17 cells but not Th1 cells induced EAE.
Figure 2. Transfer of primary Th1 and Th17 cells.
A, primary Th17 cells but not Th1 cells can transfer EAE. MOG-specific CD4+CD62Lhi T cells obtained from 2D2 TCR transgenic mice were stimulated with irradiated APCs and anti-CD3 and differentiated into Th1 or Th17 cells with polarizing cytokines. Th17 cells were additionally supplemented with IL-23. After 5 days 4×106 cytokine producing cells were injected i.v. into C57Bl/6 recipients. Recipient animals were observed for the development of clinical signs of EAE for 42 days. Error bars represent SEM. The data are representative of 2 independent experiments. B, recipients of primary Th1 and Th17 cells were administered pertussis toxin at the time of T cell transfer to study the effects of pertussis toxin on the induction of EAE by Th1 versus Th17 cells. Experiments were performed as in A, but recipient mice received 150 ng pertussis toxin i.p. on day 0 and 2 after transfer.
Table I.
Transfer of primary MOG-specific Th1 and Th17 cells into C57Bl/6 recipients
| Clinical disease | Histological diseaseA | ||||||
|---|---|---|---|---|---|---|---|
| Incidence | Mortality | Mean day of onsetA |
Mean maximum scoreA |
Number of lesions in meninges |
Number of lesions in parenchyma |
Total number of lesions |
|
| 1° Th1 | 0/5 | 0/5 | N/A | N/A | N/A | N/A | N/A |
| 1° Th17+IL-23 | 3/5 | 0/5 | 37.7 ± 3.8 | 3.2 ± 1.0 | 69 ± 42 | 121 ± 59 | 190 ± 101 |
Averages calculated only from mice that developed clinical signs of EAE
N/A – not applicable
To determine whether co-administration of PT would increase the ability of Th1 or Th17 cells to induce EAE, recipients were treated with PT at the time of T cell transfer. Surprisingly, administration of PT did not enhance but suppressed the development of EAE induced by Th17 cells (Figure 2B). This experiment indicated that the administration of PT following the injection of 1°Th17 plus IL-23 cells could abrogate the ability of Th17 cells to induce EAE.
Differentiation of effector T cell subsets (Th1, Th2, Th17 and Th9) in vitro
To improve the ability of Th1 and Th17 cells to induce EAE, we tested different protocols for in vitro differentiation. Thus, we determined whether re-activation after initial differentiation in vitro could provide highly committed and activated T cell subsets capable of transferring more consistent disease than the Th1 and Th17 cells that were activated only once in vitro. In addition to Th1 and Th17 cells, we wanted to test the ability of Th9 and Th2 cells to transfer EAE in parallel. According to this new protocol, we sorted MOG-specific CD4+CD62Lhi naive cells from 2D2 TCR transgenic mice and differentiated them in vitro into Th1, Th2, Th17 and Th9 cells by adding the various polarizing cytokines required for each T cell subsets. After the initial differentiation with polarizing cytokines, T cells were reactivated in the presence of anti-CD3 / anti-CD28 Abs for 48h to generate highly activated T cells. The protocols used to generate the various effector T cell subsets are shown in Figure 1.
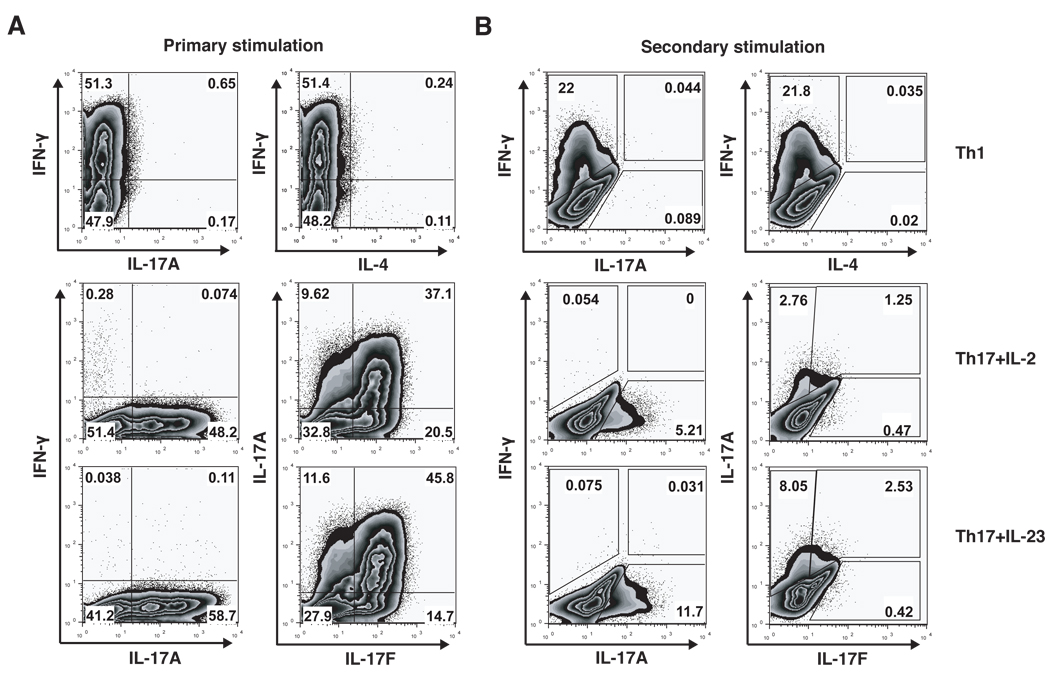
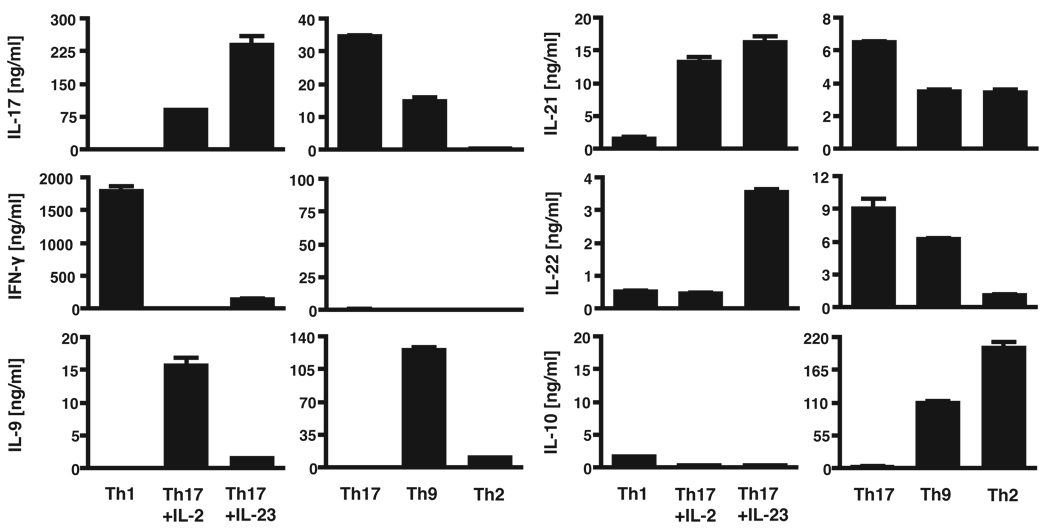
After in vitro differentiation, we performed intracellular cytokine staining and/or cytokine ELISA during both the primary (1°) and secondary rounds (2°) of polarization to confirm that the T cell subsets were differentiated appropriately (Figures 3 and 4). As expected, Th1 cells produced mainly IFN-γ and no IL-17 or IL-4. Th17 cells produced massive amounts of IL-17A and IL-17F, as well as IL-21 and IL-22. We did not observe more than 0.3% of Th17 cells producing IFN-γ even after the secondary stimulation. Overall, the percentage of IL-17-producing cells and the total amount of secreted IL-17 and IL-22 measured by ELISA were increased when Th17 cells were further cultured in the presence of IL-23; however, secretion of IL-9 by Th17 cells was decreased in the presence of IL-23. Th2 cells produced primarily IL-4, IL-10 and small amounts of IL-9 and IL-21, whereas production of IL-17, IFN-γ and IL-22 was not detected in Th2 cells. Th9 cells, generated by activation in the presence of TGF-β and IL-4, produced large amounts of IL-9 and IL-10 as well as small amounts of IL-17, IL-21, IL-22 and IFN-γ. These cytokine profiles show that all T cell effector subsets are differentiated appropriately in vitro as indicated by the analyses of their signature cytokines.
Figure 3. Intracellular cytokine profile of in vitro differentiated T cell subsets.
In vitro generated Th1 cells and Th17 cells are highly differentiated and produce only subset-specific cytokines. MOG-specific CD4+CD62Lhi T cells were stimulated with irradiated APCs and anti-CD3 and differentiated into Th1 or Th17 cells with polarizing cytokines. After 2 days Th17 cells were supplemented either with low doses of IL-2 or with IL-23 for additional 4 days. A, in the primary stimulation protocol T cells were analyzed for the production of cytokines by intracellular cytokine staining after 4 days of in vitro differentiation. B, in the secondary stimulation protocol T cells were analyzed for the production of cytokines by intracellular cytokine staining after 2 days of re-stimulation with anti-CD3 and anti-CD28 Abs.
Figure 4. Cytokine profiles of in vitro differentiated T cell subsets.
In vitro generated secondary Th1, Th17, Th2 and Th9 cells are highly differentiated and produce subset-specific cytokines. MOG-specific CD4+CD62Lhi T cells were differentiated into Th1, Th17, Th9 and Th2 cells with polarizing cytokines. 48 h after re-stimulation the amounts of IL-17, IFN-γ, IL-9, IL-21, IL-22 and IL-10 secreted into the cell culture medium were determined by ELISA.
Induction of EAE with secondary in vitro generated T effector cells
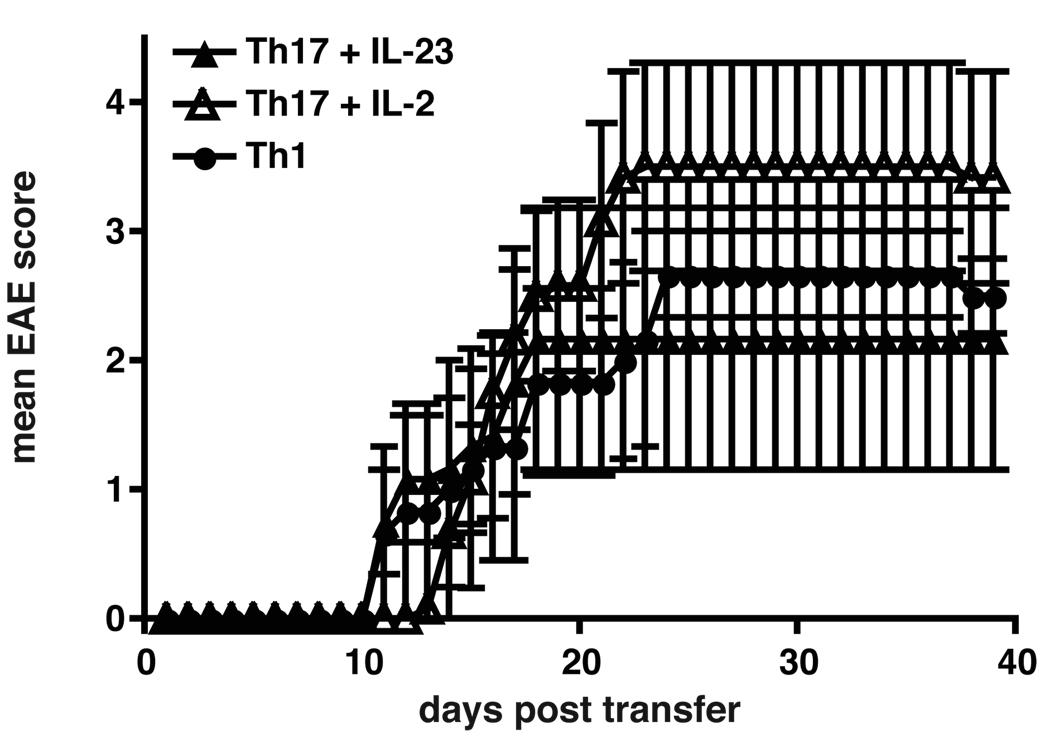
First, we wanted to compare the pathogenic capacity of Th1 and Th17 cells after a second re-stimulation in vitro. 48h after secondary activation T cells were injected into naïve syngeneic WT mice without co-administration of PT and the recipients were observed for the development of EAE. The transfer of T cells following secondary activation resulted in the induction of EAE in Th1 and Th17 cell recipients (Figure 5). Th1 cell recipients developed classical signs of EAE within 12 days after transfer and reached a mean maximum score of 2.9 around day 24 (Figure 5 and Table II). Th17 cells generated either in the presence or absence of IL-23 (2°Th17 plus IL-23 and 2°Th17 plus IL-2) both induced EAE albeit with subtle differences. Both 2°Th17 plus IL-23 and 2°Th17 plus IL-2 cells transferred EAE with a very high mean maximal score of 3.6 and 4.4, respectively. While some Th17 cell recipients showed classical signs of EAE, others developed atypical signs of EAE, such as ataxia, severe imbalance, and weight loss associated with high mortality, and some Th17 recipients showed a combination of both classical and atypical signs of EAE. The recipients of 2°Th17 plus IL-23 cells had massive meningeal and parenchymal infiltrates and had almost twice as many lesions as 2°Th1 cell recipients. 2°Th17 cells cultured without IL-23 also induced more lesions than Th1 cells, although to a lesser extent than 2°Th17 plus IL-23 cells (Table II).
Figure 5. Induction of EAE with secondary Th1 and Th17 cells.
Secondary Th1, Th17 plus IL-2 and Th17 plus IL-23 cells induce EAE with similar severity and onset upon adoptive transfer. MOG-specific CD4+CD62Lhi T cells were stimulated with irradiated APCs and anti-CD3 and differentiated into Th1 or Th17 cells with polarizing cytokines. Th17 cells were supplemented either with low doses of IL-2 or with IL-23. After 7 days cells were re-stimulated in the presence of anti-CD3 and anti-CD28 Abs for 48h. 3×106 cytokine producing cells were injected i.v. into C57Bl/6 recipients. Recipient animals were observed for the development of clinical signs of EAE for 40 days. Shown are the mean clinical scores of one experiment, error bars represent SEM. Similar results were obtained in 3 independent experiments.
Table II.
Transfer of secondary MOG-specific Th1 and Th17 cells into C57Bl/6 recipients
| Clinical disease | Histological diseaseC | ||||||
|---|---|---|---|---|---|---|---|
| Incidence | Mortality | Mean day of onsetB |
Mean maximum scoreB |
Number of lesions in meninges |
Number of lesions in parenchyma |
Total number of lesions |
|
| 2° Th1 | 7/7 | 0/7 | 12.1 ± 3.3 | 2.9 ± 0.5 | 38 ± 11 | 61 ± 23 | 99 ± 26 |
| 2° Th17+IL-2 | 9/11 | 6/11 | 14.0 ± 2.1 | 4.4 ± 0.9 | 59 ± 24 | 91 ± 33 | 150 ± 54 |
| 2° Th17+IL-23 | 6/10 | 2/10 | 13.5 ± 3.6 | 3.6 ± 1.1 | 83 ± 14 | 112 ± 13 | 195 ± 21 |
Averages calculated only from mice that developed clinical signs of EAE
Averages calculated only from mice that developed clinical signs of EAE and survived
This experiment demonstrated that both Th1 and Th17 cells, generated from naïve precursors of the same TCR specificity, induced very severe EAE independently of each other and that 2°Th17 cells induced more massive infiltration and increased numbers of CNS lesions than 2°Th1 cells.
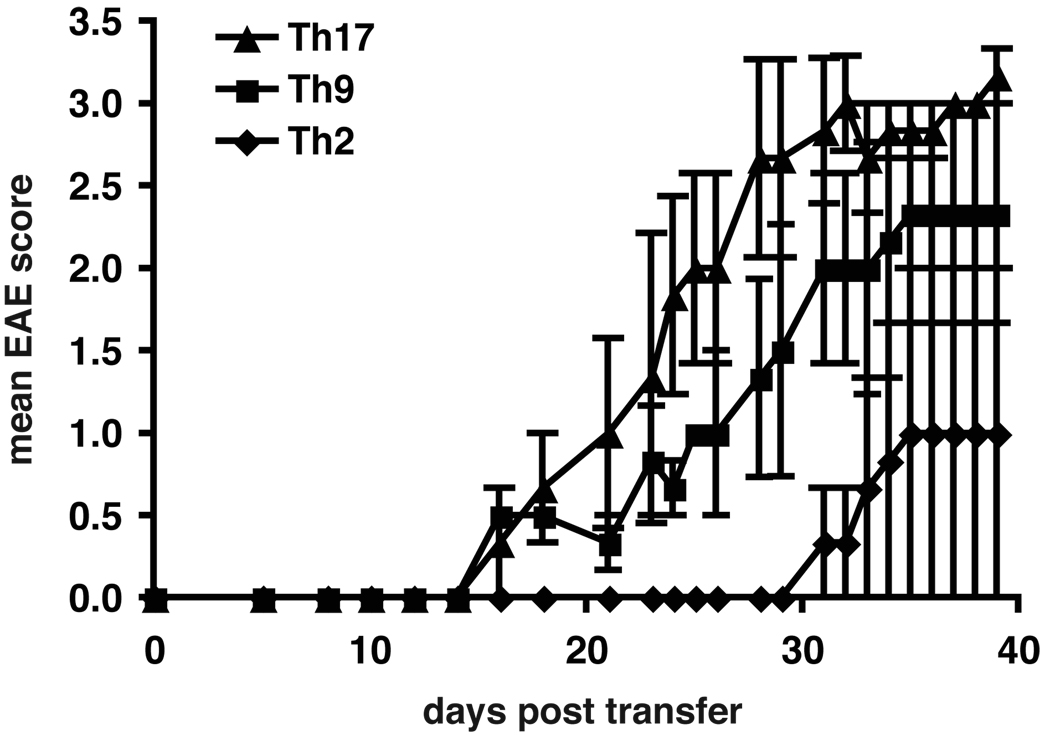
We next evaluated the capacity of 2°Th2 and Th9 cells to induce EAE using 2°Th17 cells as a positive control for comparison. For this set of experiments we stimulated naïve MOG-specific T cells in the presence of MOG35-55 and antigen-presenting cells together with polarizing cytokines. Th2 cells were differentiated in the presence of IL-4 and IL-12-neutralizing Ab, while Th9 cells were generated by the addition of IL-4 and TGF-β to the culture medium. After the first round of activation, the subsets were reactivated for 48h in the presence of Abs to CD3 and CD28 and injected into naïve syngeneic recipients in the presence of PT. All Th9 cell recipients developed EAE after transfer and reached a mean maximum score of 2.3 (Table III and Figure 6). In contrast, most Th2 cell recipients did not develop EAE; only very few of the Th2 cell recipients showed clinical signs of EAE with a very late onset. Th17 cells used as a positive control in these experiments induced more severe disease than both Th2 and Th9 cells. Both Th17 and Th9 cell recipients had a similar frequency of CNS-lesions. These data showed that both secondary Th17 and Th9 cells could induce EAE with inflammatory lesions in the CNS, whereas Th2 cells generated from the same TCR transgenic T cells induced no or only very late and mild disease.
Table III.
Transfer of secondary MOG-specific Th2 and Th9 cells into C57Bl/6 recipients
| Clinical disease | ||||
|---|---|---|---|---|
| Incidence | Mortality | Mean day of onsetD |
Mean maximum scoreD |
|
| 2° Th2 | 2/6 | 0/6 | 21 ± 11 | 1.8 ± 1.3 |
| 2° Th9 | 8/8 | 0/8 | 12 ± 3.4 | 2.3 ± 1.3 |
| 2° Th17 | 3/3 | 0/3 | 19.3 ± 4.2 | 3.3 ± 0.3 |
Averages calculated only from mice that developed clinical signs of EAE
Figure 6. Induction of EAE with secondary Th17, Th9 and Th2 cells.
Secondary Th9 and Th17 cells induce EAE upon adoptive transfer, while secondary Th2 cells induce no or mild/delayed disease. MOG-specific CD4+CD62Lhi T cells were stimulated with irradiated APCs and MOG35-55 and differentiated in vitro into Th17, Th9 or Th2 cells with polarizing cytokines. Th17 cells were supplemented with IL-23. Th9 and Th2 cells were supplemented with IL-2. After 6 days cells were re-stimulated with anti-CD3 and anti-CD28 Abs for 48h without cytokines. 5×106 cells were injected i.v. into C57Bl/6 recipients that received pertussis toxin. Recipient animals were observed for the development of clinical signs of EAE for 40 days. Shown are the mean clinical scores of one experiment, error bars represent SEM. Similar results were obtained in 2 independent experiments.
In vivo cytokine profile of transferred cells
To determine whether the transferred effector cells maintain their cytokine profile in vivo, we recovered the transferred T cells from the CNS at the peak of disease and determined their cytokine profile by intracellular cytokine staining. Transferred T cells were distinguished from endogenous T cells by the expression of the Vα3.2 chain; Vα3.2 is part of the MOG-specific TCR and thus expressed on all cells derived from 2D2 mice. However, it is rarely expressed on endogenous T cells from C57Bl/6 mice.
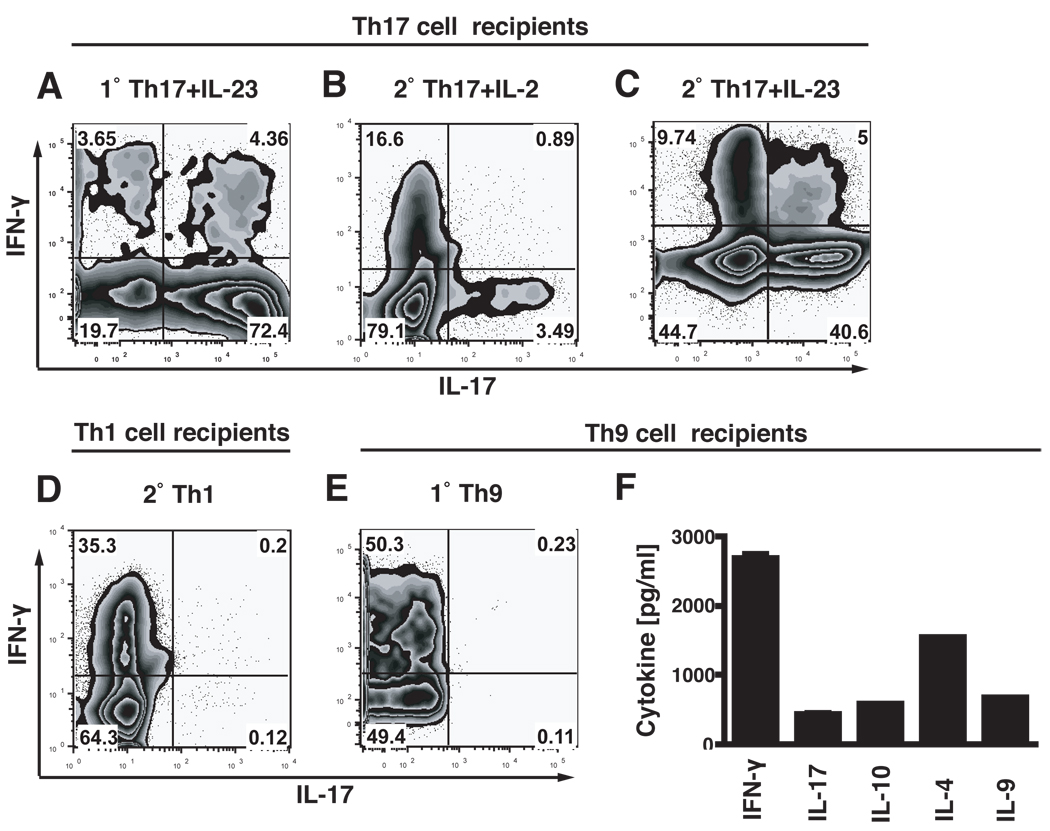
When we recovered the 1°Th17 cells cultured with IL-23 from the CNS of recipient mice, we observed a very stable Th17 phenotype, with 72.4% IL-17 producers and only 3.7% IFN-γ and 4.4% IL-17/IFN-γ double producers, respectively (Figure 7A). Since only about 50% of these Th17 cells had produced IL-17 in vitro before transfer, this experiment showed that Th17 cells activated in the presence of IL-23 in the primary culture were relatively stable in vivo.
Figure 7. Cytokine profiles of transferred cells recovered from the CNS.
At the peak of disease infiltrating cells were isolated from brain and spinal cord of recipient mice. Cells were stimulated with PMA/ionomycin for 4.5 h in the presence of monensin and transferred T cells, identified by their expression of Vα-3.2 TCR, were analyzed for the production of IL-17 and IFN-γ by intracellular cytokine staining. Cells recovered from recipients of 1°Th17 cells cultured with IL-23 produced mostly IL-17 und only little IFN-γ (A). Cells recovered from recipients of 2°Th17 cells cultured with low doses of IL-2 had decreased their IL-17 production and produced IFN-γ (B), whereas cells recovered from 2°Th17 plus IL-23 recipients maintained their IL-17 production stable but also produced some IFN-γ (C). Cells recovered from 2°Th1 cell recipients produced only IFN-γ and no IL-17 (D). Cells recovered from 2°Th9 cell recipients produced IFN-γ as was determined by intracellular cytokine staining (E). However, when infiltrating cells from Th9 cell recipients were stimulated for 12h with PMA/ionomycin besides IFN-γ also IL-17, IL-10, IL-4 and IL-9 were detected in the culture medium with a cytokine bead array (F). All graphs are representative of at least 2 independent experiments.
In contrast, 2°Th17 cells that were cultured in the presence of IL-2 (without any IL-23) in vitro had decreased their IL-17 production in vivo to 3.5% and, in addition, about 16% of transferred cells in the CNS now produced IFN-γ (Figure 7B). On the contrary, 2°Th17 cells that were cultured in the presence of IL-23 in vitro maintained their IL-17 production stable at 40% in vivo. In addition to IL-17, the 2°Th17 cells activated in the presence of IL-23 also began to produce IFN-γ either alone (9.7%) or together with IL-17 (5%) in vivo (Figure 7C). It should be noted that only about 11.6% of input Th17 cells (2°Th17 plus IL-23) were producing IL-17 in vitro just before transfer. These data further highlighted the importance of IL-23 for the development of a stable phenotype of Th17 cells even over long periods after in vivo transfer.
Transferred 2°Th1 cells recovered from the CNS of the recipients had maintained their original cytokine profile and produced only large amounts of IFN-γ but no IL-17 in vivo (Figure 7D), indicating that the Th1 phenotype is very stable in vivo and that, while Th17 cells can reshape to produce IFN-γ in vivo, Th1 cells do not start to produce IL-17 in vivo.
When we recovered cells from the CNS of Th9 cell recipients, we found that 50% of the transferred 2°Th9 cells produced IFN-γ in vivo (Figure 7E). We determined whether the 2°Th9 cells produced IL-9 in vivo by stimulating cells recovered from the CNS of Th9 cell recipients and measuring the levels of IL-9, IL-10, IL-4, IFN-γ and IL-17 in a cytokine bead array (Figure 7F). As seen in the intracellular cytokine staining, the cells produced IFN-γ, but also IL-9, IL-10, IL-4 and small amounts of IL-17. These data suggest that Th9 cells maintained the production of their original cytokines IL-9, IL-10 and IL-4 in vivo, but that they also started to produce IFN-γ and IL-17 upon transfer in vivo.
CNS-pathology in Th1, Th17 and Th9 recipients
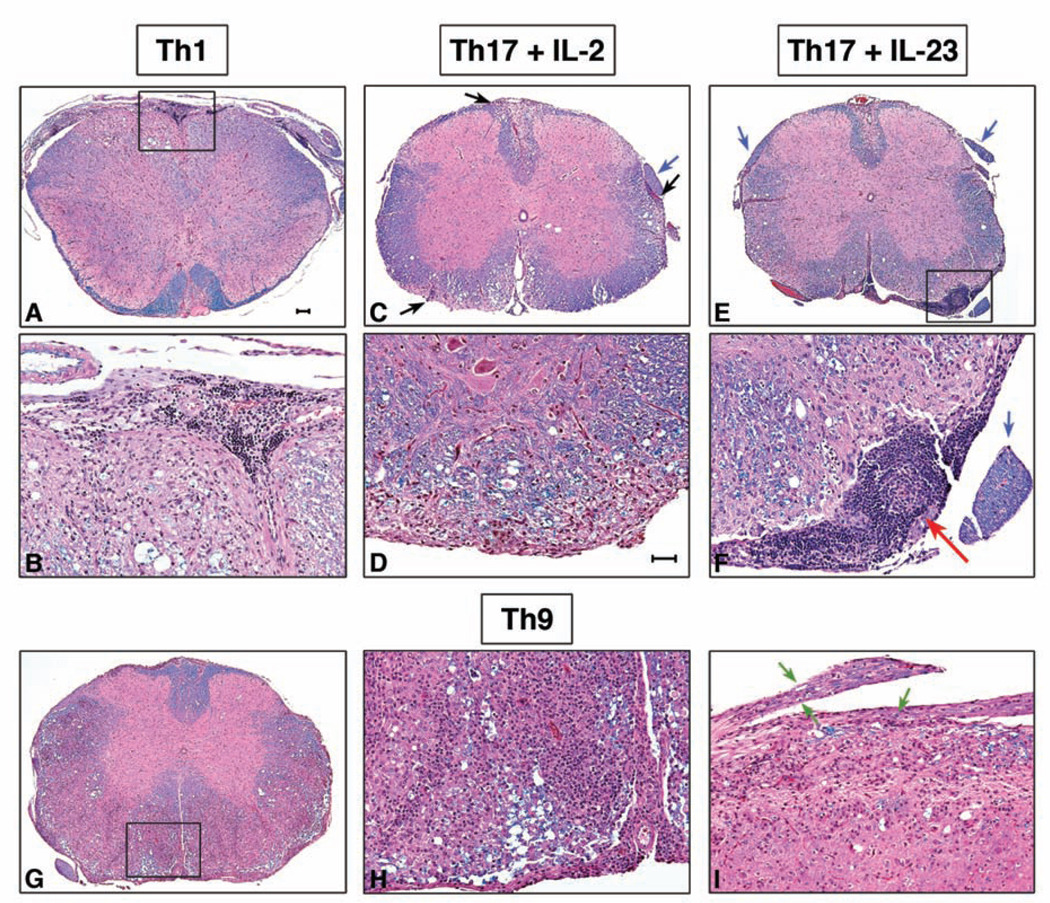
In Th1 and Th17 plus IL-2 recipients there were typical meningeal and parenchymal perivascular mononuclear cell infiltrates associated with demyelination (Figure 8 A–D). The Th17 plus IL-23 cell recipients, on the other hand, showed inflammation and demyelination together with aggregation of lymphocytes in the leptomeninges and occasionally in Virchow-Robin spaces (Figure 8E, F). Importantly, only Th17 cells cultured with IL-23 induced these aggregates that we did not observe in recipients of Th17 cells cultured in the absence of IL-23 or in recipients of any other T cell subset. These data suggest that IL-23 shapes the effector functions of Th17 cells probably by inducing a certain pattern of cytokines and cell surface molecules conducive to the formation of ectopic lymphoid follicle-like structures.
Figure 8. Histopathology of representative spinal cord sections from Th1, Th17, and Th9 cell recipient mice.
A, B: 2°Th1 cell recipients have typical meningeal and parenchymal mononuclear cell infiltrates predominantly in the white matter. Boxed area in A is shown at 20X magnification in B. There are large numbers of small lymphocytes in the subarachnoid space. Parenchymal demyelination is indicated by loss of blue staining associated with vacuolation and foamy macrophages. C, D: 2°Th17 plus IL-2 cell recipient mice show multiple foci of demyelination in white matter tracts (black arrows) and an intact spinal nerve root (blue arrow). D, 20X magnification of a subpial parenchymal lesion. E, F: 2°Th17 plus IL-23 cell recipient mice have demyelinating lesions in the white matter and large accumulations of small lymphocytes in the leptomeninges (boxed area), but intact spinal nerve roots (blue arrows). F, 20X magnification of boxed area in E shows a lymphoid follicle-like area (red arrow) in the leptomeninges. G–I: 2°Th9 cell recipient mice. G, Large areas of the anterior and lateral white matter are diffusely pink indicating loss of myelin. H, 20X magnification of boxed area in G demonstrates diffuse infiltration of sheets of mononuclear cells in the parenchyma and fewer lymphocytes in the subarachnoid space compared to other groups. I, longitudinal section of spinal cord with two nerve roots showing marked diffuse inflammatory cell infiltration and digestion chambers of Cajal indicative of myelin breakdown and Wallerian degeneration (green arrows). Luxol fast blue-hematoxylin and eosin. Bar in A = 100 µm, also for C, E, G; Bar in D = 50 µm, also for B, D, F, H and I.
The CNS lesions in Th9 recipients were characterized by massive parenchymal mononuclear cell infiltrates and extensive demyelination (Figure 8G, H). There were also some peripheral nervous system (PNS) inflammatory lesions and Wallerian degeneration in the dorsal nerve roots in Th9 recipients (Figure 8I). Th9 cell recipients also tended to have fewer infiltrates of small lymphocytes in the meninges compared to the other groups that developed EAE. This distinct lesion pattern suggests that the mechanism of EAE induction by Th9 cells differs from the mechanisms of Th1 and Th17 cells.
The histopathologic observations support our hypothesis that each subset of T cells induces EAE via a different mechanism resulting in different lesion patterns.
Discussion
Starting from the same TCR transgenic T cell population with specificity for MOG35-55, we generated various effector T cell subsets in vitro that induced EAE upon adoptive transfer. Independently of each other, Th1, Th17 and Th9 cells each could transfer EAE with similar severity and overlapping but distinct pathological phenotypes. Thus, we have begun to address some of the controversies that exist in the literature regarding the effector T cells that induce EAE. We also provide detailed protocols for generating various effector T cell populations, so that their functions can be dissected.
One of the major differences between the present study and those of other investigators is the starting population utilized for the generation of Th1 and Th17 cells in vitro. In previous studies CNS antigen-specific T cell subsets were generated by isolating T cells from immunized mice and expanding them in vitro with either Th1 or Th17 polarizing cytokines (11, 17). Since those cells have already been primed and potentially differentiated in vivo, they contain precursors of multiple T cell subsets and are thus not ideally suited to study the pathogenicity of specific subsets. To overcome this confounding problem, we utilized naïve T cells from a MOG-specific TCR transgenic line (2D2 mice) for all in vitro differentiation assays. After testing multiple protocols, we established methods for generating pure MOG-specific effector T cell subsets in vitro that can induce EAE upon adoptive transfer in vivo.
Consistent with previous studies from our laboratory and from others, we demonstrated that in vitro differentiated Th1 cells stimulated with anti-CD3 were able to induce typical clinical and histological EAE and that the disease was independent of Th17 cells. Analogous to what we showed for Th17 cells, Th1 cells were also capable of inducing EAE when stimulated with MOG35-55 in vitro (data not shown). These data are consistent with the observations of O’Connor et al., who showed that MBP–specific Th1 cells transferred EAE to B10.PL mice (11). Thus, in vitro differentiated Th1 cells can induce EAE independently of Th17 cells. In contrast to Stromnes et al. we did not observe a preferential infiltration of the spinal cord versus the brain of Th1 recipients (18). Instead, MOG-TCR transgenic Th1 cells induced lesions that were also numerous in the brain. Whether the specificity of the TCR or genetic background is responsible for differential infiltration of Th17 cells into brain versus spinal cord could not be addressed in the present study.
In contrast to the studies of O’Connor et al. (11), all Th17 cells generated by multiple different protocols were capable of inducing EAE independently of other T cell subsets. Interestingly, 2°Th17 cells cultured with low doses of IL-2 produced large amounts of IL-17 and no detectable IFN-γ in vitro, but when they were transferred in vivo their cytokine pattern had shifted, i.e. they partly lost their ability to produce IL-17 and started to produce IFN-γ. Only Th17 cells that were exposed to IL-23 during their differentiation in vitro maintained their IL-17 production also in vivo. These data revealed the importance of IL-23 for sustained IL-17 production and the stabilization of the Th17 phenotype. Nevertheless, a small percentage of Th17 cells did produce IFN-γ when they were recovered from the CNS of the recipient. These IFN-γ producers could originate from previously uncommitted cells present within the transferred cells that then differentiated into Th1 cells in vivo. On the other hand, due to an inherent plasticity of Th17 cells, these IFN-γ producers could originate from cells that were once IL-17 producers in vitro and acquired the ability to produce IFN-γ or IFN-γ together with IL-17 when transferred in vivo. It has been shown that IL-17 producing T cells can begin to produce IFN-γ when cultured with IL-12 in vitro (19), and a recent study in the diabetes model has demonstrated that Th17 cells can become IFN-γ producers in vivo (20). Our data illustrate the one-way plasticity of Th17 cells in that Th17 cells can begin to produce IFN-γ, whereas IFN-γ-producing Th1 cells are relatively stable and do not start to produce IL-17. Our data suggest that IL-23 plays an important role in the stability of Th17 cells; however, the mechanism by which IL-23 stabilizes the Th17 response is unclear. Since IL-23 can induce up-regulation of its own receptor (21), we speculate that prolonged in vitro exposure to IL-23 induces enough IL-23R expression on the surface of Th17 cells so that IL-23R effectively co-opts the IL-12Rβ1 receptor and thus assembly of a functional IL-12R is inhibited. With this mechanism IL-23 would prevent IL-12 from de-differentiating Th17 cells into Th1 cells.
Our data also clearly show that Th17 cells do transfer EAE but whether the disease is dependent on IL-17 produced by the Th17 cells is not known. A recent report indicated that IL-17A and IL-17F are not critical for the development of EAE in immunized mice (22), while others have found that IL-17A significantly contributes to the induction of EAE in immunized mice (23). It should be noted that the pathogenic capacity of Th17 cells cannot be reduced to the effects of IL-17 alone but is composed of the effects of all cytokines secreted by the Th17 cell subset.
Our results are in contrast to the findings of O’Connor et al. who reported that in vitro differentiated primary MBP-specific Th17 cells cause only very mild and delayed disease in B10.PL recipients (11). One possibility is that primary MBP-specific Th17 cells are less pathogenic than primary MOG-specific Th17 cells due to inherent differences in their TCR-specificity and availability of the encephalitogenic epitope in the CNS. In addition to these inherent differences in the T cell specificity, the protocols used to generate Th1 and Th17 cells are different. The primary Th1 and Th17 cells utilized in the O’Connor study were transferred 72h after the initial activation and the recipients were treated with pertussis toxin. We did not transfer Th1 or Th17 cells 72h after activation because T cell commitment as determined by intracellular cytokine staining and quantitative PCR of specific cytokines and transcription factors is not complete at this time point. Although we cannot exclude that the difference between the two studies results from the timing of T cell transfer, it is more likely that the injection of PT along with T cells in the O’Connor et al. study accounts for contrasting results with our study. We demonstrate that primary Th17 plus IL-23 cells lose their ability to induce EAE when the recipient mice received PT. Indeed, an early study reports that treatment of primed PLP-specific T cells with PT in vitro impairs their ability to transfer EAE (24). Since the enzymatic A subunit of PT can disrupt signaling of G-protein coupled receptors, it has been suggested that PT specifically affects the migration of T cells by interfering with chemokine-receptor signals. In fact, in an adoptive transfer model of experimental autoimmune uveitis it was shown that administration of PT after transfer of pathogenic T cells can completely inhibit the migration of the T cells to the target organ (25). Since different T cell subsets express different chemokine receptors, it is possible that PT specifically inhibits the migration of Th17 cells by targeting chemokine-receptor signaling specifically employed by Th17 cells. This would also explain why in the O’Connor study the primary Th17 cells fail to induce EAE in PT-treated recipients, whereas the Th1 cells with the same specificity transfer EAE to PT-treated recipients. We did not detect any change in the expression of CCR6 on Th17 cells when Th17 cells were treated in vitro with pertussis toxin (data not shown). However, it is possible that PT preferentially blocks the signaling of CCR6 expressed on Th17 cells (26) as opposed to CXCR3, which is expressed on Th1 cells (27, 28).
The spectrum of EAE in Th9 cell recipients was very heterogeneous; some mice only developed mild EAE, whereas others developed fulminant clinical and correspondingly extensive histologic disease. Recovery of transferred cells from the CNS of Th9 recipients showed that the Th9 cells maintained the production of their original cytokines IL-9 and IL-10, but in addition they had also increased production of IFN-γ. Similarly, we had described previously that Th9 cells in the T cell transfer model of colitis also increased production of both IFN-γ and IL-17 in vivo (12). Together, these data indicate that, similar to Th17 cells, Th9 cells might have a greater plasticity compared to Th1 cells. CNS lesions in Th9 cell recipients differed from those in Th1 and Th17 cell recipients as they were characterized by massive and more evenly distributed parenchymal infiltrates associated with marked demyelination. Compared to all other groups Th9 cell recipients appeared to have fewer infiltrates of small lymphocytes in the meninges. Interestingly, Th9 cell recipients also had some inflammatory lesions and Wallerian degeneration in the dorsal nerve roots. Similar PNS lesions were also present in some of the recipients of Th17 cells cultured with low doses of IL-2 and in the few recipients of Th2 cells that developed EAE. Since both Th17 plus IL-2 and Th2 cells also produced some IL-9 during the differentiation in vitro, this observation suggests that the development of PNS lesions may be driven by IL-9, particularly since recipients of Th1 cells and Th17 plus IL-23 cells, (which do not produce significant amounts of IL-9), did not show the same extent of PNS abnormalities. This is consistent with our previous study in which we showed that Th9 cells upon adoptive transfer into Rag-/- mice not only induced colitis but also peripheral neuritis (12).
Of note, secondary Th17 cells and Th9 cells could transfer EAE even in the presence of PT in the recipient mice, whereas primary Th17 cells failed to induce EAE in the presence of PT. The secondary re-stimulation in vitro generates highly activated T cells, which express much higher levels of chemokine-receptors than T cells activated only once in vitro. We propose that secondary Th17 cells express chemokine receptors so abundantly on their surface that the presence of PT is not sufficient to block the migration of 2°Th17 cells to their target organ. Although not extensively analyzed, the 2°Th17 cells may also express different chemokine receptors compared to 1°Th17 cells, which may limit the blocking effects of PT. Consistent with this observation, a recent study showed that MOG-specific Th17 cells that were generated in vivo by immunization and then secondarily stimulated in vitro with peptide and IL-23 transfer EAE even in PT-treated recipients (29). In addition, it is also possible that in vivo generated Th17 cells express a different combination of chemokine receptors and therefore may show different susceptibility to the effects of PT.
Overall, our data show that multiple different effector T cell subsets (Th1, Th17 and Th9) can induce EAE independently of each other. Since they all produce different cytokines, it is likely that they induce disease by overlapping but distinct mechanisms. Once the inflammatory process advances, however, the clinical disease manifestations are indistinguishable. Thus, the differences between the T cell subsets are mainly reflected in the different patterns of tissue pathology induced by each T cell subset as exemplified by ectopic lymphoid follicle formation in Th17 plus IL-23 cell recipients and more extensive PNS lesions and extensive demyelination in Th9 cell recipients. By inducing EAE with highly pure in vitro generated T cell subsets we have demonstrated that there is not only one effector T cell subset that can induce EAE, but that there are in fact several effector T cell subsets and different mechanisms by which each T cell subset can induce EAE. In view of the abundance of different types of polarizing factors present in vivo, it is likely that different autoreactive T cell subsets are generated in patients with MS and that this mixture of subsets similarly contributes to the heterogeneity of lesions.
Acknowledgements
We thank D. Kozoriz for cell sorting. A.J. is a graduate student jointly supervised by Professor Dr. Rolf Heumann (Ruhr-University Bochum, Germany) and Professor Vijay K. Kuchroo (Brigham and Women’s Hospital, Harvard Medical School, Boston, USA).
Footnotes
This work was supported by grants from the NIH (1R01NS059996 to E.B. and R01NS045937, R01NS035685, R37NS030843, R01A1044880, P01A1039671, P01NS038037 and a Javits Neuroscience Investigator Award to V.K.K.) and the National Multiple Sclerosis Society (NMSS Transition Award TA3014A1/1) to E.B. and FG1642-A-1 to V.D. and RG-2571 to V.K.K.). A.J. is the recipient of a Ph.D. scholarship by the Boehringer Ingelheim Fonds.
Abbreviations used in this paper: EAE, experimental autoimmune encephalomyelitis; GC, germinal center; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; PNS, peripheral nervous system; PT, pertussis toxin.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 4.Merrill JE, Kono DH, Clayton J, Ando DG, Hinton DR, Hofman FM. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci U S A. 1992;89:574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 6.Baron J, Madri J, Ruddle N, Hashim G, Janeway C., Jr. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF-β in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 14.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin Oligodendrocyte Glycoprotein-specific T cell Receptor Transgenic mice Develop Spontaneous Autoimmune Optic Neuritis. J. Exp. Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. Myelinspecific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lexberg MH, Taubner A, Forster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang HD. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 20.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Robbinson D, Cockle S, Singh B, Strejan GH. Native, but not genetically inactivated, pertussis toxin protects mice against experimental allergic encephalomyelitis. Cell Immunol. 1996;168:165–173. doi: 10.1006/cimm.1996.0063. [DOI] [PubMed] [Google Scholar]
- 25.Su SB, Silver PB, Zhang M, Chan CC, Caspi RR. Pertussis toxin inhibits induction of tissue-specific autoimmune disease by disrupting G protein-coupled signals. J Immunol. 2001;167:250–256. doi: 10.4049/jimmunol.167.1.250. [DOI] [PubMed] [Google Scholar]
- 26.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]