Abstract
Despite the wide variety of highly potent anti-HIV drugs that have been developed and made available in clinical practice over the years, eradication of HIV infection has not been achieved. Currently, HIV infection and AIDS are thought to be chronically treatable. HIV attacks host immune cells namely macrophages and CD4+T-cells and sequesters itself into sanctuary and reservoir sites such as the lymphoid tissues, testes, and brain. Initial drug delivery efforts with prodrugs and drug conjugates focused on improving the physicochemical (i.e. solubility), biopharmaceutic (i.e. absorption, metabolism), and pharmacokinetic (i.e. blood concentrations) properties of the parent drugs. Eradicating HIV, however, will require advanced drug delivery approaches in order to access and maintain effective drug concentrations for prolonged periods of time in sanctuary sites. The current review discusses prodrug/conjugate efforts, clinical successes and describes drug delivery challenges and approaches for eradicating HIV infection.
Keywords: Anti-viral, Conjugate, Fosamprenavir, HIV, Nanocarrier, Prodrug, Targeted drug delivery, Tenofovir disoproxil fumarate
Since the first report in 1981, more than 67 million persons have been infected with HIV, and more than 27 million have died of AIDS [1, 2]. More than 40% of new infections worldwide occur among adults in the age range of 15–24 years [3]. In fact, AIDS is now the leading cause of premature death among people 15 to 59 years of age. HIV/AIDS remains both a medical and economic challenge in developing countries especially in Sub-Saharan Africa [4]. Unfortunately, after nearly 30 years of research there are still three compelling facts driving the development of new anti-AIDS drugs and delivery systems: i) there is a lack of an effective vaccine or prophylactic agent that would provide protection for the foreseeable future, ii) there is a constant need for both less expensive and more tolerable drug therapies, and iii) the development of resistant viral strains in treatment-experienced patients continue [5]. The late Dr. Paul Janssen described four ideal characteristics of a novel anti-HIV drug. The drug must possess: i) high antiviral activity against both wild-type and mutant virus, thus a drug must be effective despite the genetic flexibility of the virus and it must anticipate resistant strains that may develop once therapy is initiated, ii) high oral bioavailability and a long elimination half-life to allow for once daily dosing, which is considered the gold standard of any therapy, iii) have minimal adverse effects, which will ultimately impact patient compliance, and iv) be easy to synthesize and formulate into dosage forms, which ultimately impact the cost of the drug for both the manufacturer and the patient [6]. However, the development of drugs that meet these criteria is an arduous task. Those drugs that fall short of any of the criterion could benefit from drug delivery strategies, specifically conjugate and prodrug design to improve upon less desirable drug characteristics.
Current anti-HIV drugs include the twenty-four approved agents listed in Table I. These drugs are prescribed as part of a multiple drug regimen known as highly active antiretroviral therapy (HAART). Even though current anti-HIV drugs are effective in suppressing viral titers in infected individuals, a cure for HIV infection remains elusive. This is due to several reasons that include poor pharmacokinetics, frequent adverse drug reactions, and the lack of patient adherence to complicated drug administration regimens. Treatment compliance is critical regardless of whether a patient is treatment-naïve or treatment-experienced since poor patient compliance is often a factor in treatment failure and viral rebound. Unfortunately, once first-line drugs fail, second-line drugs are not very effective since they have less than ideal dosing parameters, which can further burden the patient [5, 7].
Table I.
FDA approved drugs in HIV therapy (drugs with abbreviations are referred to as such in the article).
| Drug | Brand name | Manufacturer |
|---|---|---|
| Nucleoside reverse transcriptase inhibitors (NRTIs) | ||
| Zidovudine (AZT) | Retrovir | GlaxoSmithKline |
| Didanosine (ddI) | Videx | Bristol Myers-Squibb |
| Zalcitabine (ddC) | Hivid | Hoffmann-La Roche |
| Stavudine (d4T) | Zerit | Bristol Myers-Squibb |
| Lamivudine (3TC) | Epivir and Zeffix | GlaxoSmithKline |
| Abacavir sulfate (ABC) | Ziagen | GlaxoSmithKline |
| Emtricitabine (FTC) | Emtriva | Gilead Sciences, Inc. |
| Nucleotide reverse transcriptase inhibitors (NtRTIs) | ||
| Tenofovir disoproxil fumarate | Viread | Gilead Sciences, Inc. |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | ||
| Nevirapine (NVP) | Viramune | Boehringer Ingelheim |
| Delavirdine (DLV) | Rescriptor | Pfizer |
| Efavirenz (EFV) | Sustiva and Stocrin | Bristol Myers-Squibb |
| Entavirine (TMC125) | Intelence | Tibotec, Inc. |
| Protease inhibitors (PIs) | ||
| Saquinavir mesylate (SQV) | Invirase and Fortovase | Hoffmann-La Roche |
| Ritonavir | Norvir | Abott Laboratories |
| Indinavir (IND) | Crixivan | Merck & Co., Inc. |
| Nelfinavir mesylate (NFV) | Viracept | Agouron Phramaceuticals |
| Amprenavir | Agenerase and Prozei | GlaxoSmithKline |
| Fosamprenavir calcium (Prodrug of amprenavir) | Lexiva and Telzir | GlaxoSmithKline |
| Atazanavir sulfate | Reyataz | Bristol Myers-Squibb |
| Lopinavir and ritonavir | Kaletra | Abott Laboratories |
| Tipranavir | Aptivus | Boehringer Ingelheim |
| Darunavir | Prezista | Tibotec, Inc. |
| Entry and fusion inhibitors | ||
| Maraviroc | Salzentry | Pfizer |
| Enfuvirtide | Fuzeon | Hoffmann-La Roche, Trimeris |
| Integrase inhibitors | ||
| Raltegravir | Isentress | Merck & Co., Inc. |
Recently anti-AIDS therapeutic development efforts have been focusing on improving drug delivery and not just on discovering new chemical entities. Initial efforts using traditional prodrug strategies have had some success resulting in two new important prodrugs, fosamprenavir and tenofovir disoproxil fumarate. These prodrugs demonstrated improved intestinal absorption by increasing water solubility and lipophilicity for fosamprenavir and tenofovir disoproxil fumarate, respectively, resulting in improved clinical efficacy over other HAART drugs [8, 9]. However, traditional prodrugs still face the same challenges as conventional anti-AIDS drugs once the prodrug is absorbed in the intestine (i.e. they are rapidly eliminated or have limited tissue distribution). Efforts to prolong the elimination half-life of anti-AIDS drugs (e.g. by PEGylation) are not expected to be successful in AIDS therapy due to the limited biodistribution of macromolecules to sanctuary sites where infected cells such as macrophages reside (e.g. the brain or lymph). Therefore, novel drug delivery concepts will be required in order to eradicate HIV infection.
In the following review, a brief overview of the molecular and cellular targets that have been identified as points of drug intervention to curtail HIV infection is presented. Prodrug strategies as well as a detailed treatment of two clinically successful prodrug examples (fosamprenavir and tenofovir disoproxil fumarate) are also described. Finally, a detailed discussion of the challenges facing targeted drug delivery for eradicating HIV infection is presented.
I. BACKGROUND
1. Viral life cycle and potential cell surface targets for drug delivery
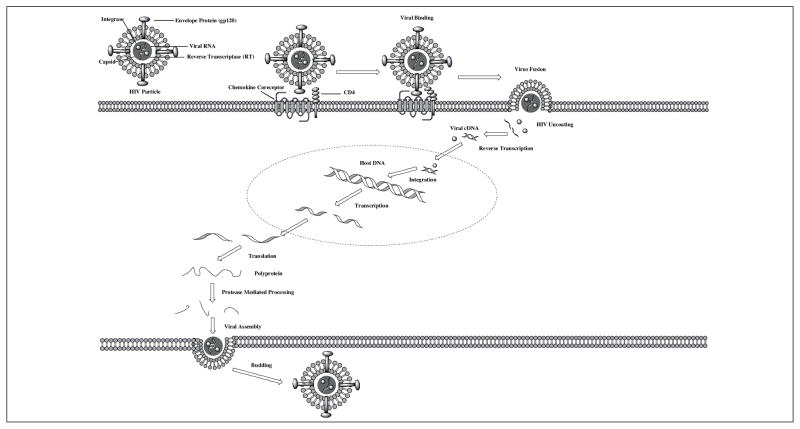
HIV is a retrovirus, whose mechanism of action involves directly integrating viral DNA into the host cells’ DNA by means of several critical steps, upon which it is replicated to produce a new progeny of virus particles (Figure 1). HIV adsorption to the cell surface is the first step of infection. This process hinges on the interaction of the negatively charged heparan sulfate molecules on the cell surface with the positively charged viral glycoprotein (gp120) [10].
Figure 1.
HIV life cycle. The viral stages include surface adsorption, binding to the cell surface receptors (CD4 and a chemokine coreceptor), fusion of the viral envelope to the cell membrane, capsid uncoating, reverse transcription, nuclear translocation of viral cDNA and integrase, integration of the viral cDNA into the host genome, transcription, translation, protease mediated cleavage, and viral assembly and budding (redrawn from [10] and [15]).
Once HIV adsorbs to the cell surface, it then binds to the CD4 receptor and a coreceptor, either CXCR4 or CCR5 via gp120 [10]. This interaction stabilizes the virus to the cell surface so that viral-cellular fusion may take place in the subsequent stages of the life cycle. Whether the virus interacts with CXCR4 or CCR5 of CD4+ cells depends upon the protein sequence of the V3 loop of gp120. Therefore, HIV is characterized as X4 tropic, R5 tropic, or dual tropic (X4R5) corresponding to the coreceptor(s) to which it binds [11]. CXCR4 and CCR5 belong to the class of receptors known as chemokine receptors. The chemokine receptors are members of the G protein coupled receptor super family, whose diverse physiological roles include host defense by chemotaxis to sites of inflammation, tumor genesis and metastasis, and embryologic development (for a review see [12]). The endogenous ligands for the chemokine receptors that act as HIV coreceptors are stromal-cell-derived factor 1 (SDF-1) for CXCR4 and regulated upon activation, normal T-cell expressed and secreted (RANTES) and macrophage inflammatory proteins 1α (MIP-1α) and 1β (MIP-1β) for CCR5 [10, 13]. All of these ligands have been shown to possess, to some extent, anti-HIV activity in vitro [13, 14].
Following the binding of the virus to CD4 and its coreceptor, a conformational change occurs that allows gp41 to insert itself into the plasma membrane of the cell and begins the process of fusing the viral particle’s envelope to the cell [15]. Upon fusion of the viral envelope with the cell membrane, the virus undergoes a process consisting of uncoating its contents within the capsid matrix into the cytosol of the infected cell. These contents include the viral RNA and the viral proteins reverse transcriptase (RT) and integrase. Reverse transcriptase converts the viral RNA into viral cDNA by utilizing the viral RNA as a template for incorporating endogenous nucleotides to elongate the cDNA [10, 16]. After reverse transcription in the cytosol, viral cDNA translocates to the nucleus and is incorporated into the host genome, this process is done by the viral protein integrase. Within each viral particle approximately 40–100 copies of integrase are released into the host cell upon viral fusion and uncoating (for a review see [17]). Viral DNA integration is a critical step in the viral life cycle, because after integration infected cells may be able to enter a period of latency, a phenomenon that will be discussed later.
New copies of viral RNA are produced as a result of host genome transcription. The proteins necessary for the assembly of virus progeny are produced in the cytosol via the translation process. This produces the Gag-Pol protein, a precursor to the other viral proteins. Gag-Pol is self-cleaved upon the folding and dimerization of the protease domain to form the active site of viral protease, thus liberating the enzyme from the Gag-Pol protein [18]. HIV protease is an aspartic-protease whose subsequent enzymatic activity upon the Gag and Gag-Pol proteins forms the components necessary for viral assembly including the structural capsid proteins and functional proteins reverse transcriptase and integrase [16, 18].
2. Eradication of HIV: target cells for therapeutic intervention
HIV infects cells that express CD4 and one of the coreceptors (i.e. CXCR4, CCR5). However these cell types, macrophages, CD4+ T-cells, and follicular dendritic cells (FDCs) occupy physiological spaces that are difficult to penetrate with drugs. Furthermore, cells that are infected can enter varying stages of latency aiding in viral persistence. Latency of the virus occurs when the viral DNA has been integrated into the host genome, but no active transcription occurs [19]. This presents a difficult challenge to eradicating HIV infection since the virus can remain dormant in cells with extended life-cycles and dormant virus cannot be killed by nearly any of currently used drugs.
CD4+ T-cells have been identified as the major contributing cell type in viral replication. Nearly 99% of all viral replication occurs in activated and productively infected CD4+ T-cells of the blood and lymphoid tissues [19]. HIV has been shown to infect several subsets of T-cells, including Th-17 cells, which secrete IL-17. Th-17 cells exist constitutively in the lamina propria of the gut and are responsible for host defense against bacterial infections in the gut [20]. The vast majority of these cells become rapidly depleted during the initial infection, leaving the gut vulnerable to bacterial infections, which results in increased activation of additional T-cells that may further assist in exposing an increased population of susceptible cells to HIV [21]. Other subsets of CD4+ T-cells are responsible for viral latency in vivo, including resting memory (CD45RO) T-cells and resting naïve (CD45RA) T-cells [19].
Macrophages and cells derived from that lineage are the other major cellular target of HIV. These cell types have longer cellular turnover (approximately 2 weeks), which contributes to the persistence of HIV. Macrophages are harbored in various organs colonizing the bone marrow, liver, thymus, spleen, lymph nodes, gut, mucosal associated lymphoid tissue, brain, lungs, and kidney. They have been implicated in carrying HIV across the blood brain barrier and establishing and maintaining HIV infection in the central nervous system (CNS) [22]. Because of their distribution to numerous organ systems, macrophages are pharmacologically difficult to reach [23, 24]. Macrophages, unlike T-cells have the ability to continually produce HIV particles with little threat of cell lysis due to viral production. Therefore, they are believed to be responsible for the secondary phase of viral decay in HAART patients and are generally believed to be an important HIV sanctuary [24, 25]. Although macrophages are not responsible for the majority of the viral production in vivo, the proportion of infected macrophages within tissues can reach up to 50% depending on the tissue in which they reside. Their physiological proximity to the other cell types involved in HIV infection compound their importance in viral infection (for a review see [26]). For these reasons macrophages play a critical role in HIV persistence.
FDCs are unable to produce HIV particles through replication, however they play a significant role in viral storage in vivo. FDCs have the ability to pool HIV and protect it from immune defenses. FDCs are able to preserve HIV infectivity even in the presence of antibodies produced by the host to neutralize the virus [27]. Also FDCs contribute to the expansion of HIV infection by migrating toward T-cells and presenting those cells with the viral particles [27]. Therefore, FDCs are a unique cellular target that has yet to be addressed by HAART approved drugs.
A model of the effect of HAART on HIV load decay in patients revealed that decreasing viral load occurs in three distinct phases: i) a rapid exponential decrease in viral load upon initiation of pharmacotherapy resulting in viral half lives of less than 6 h and virus producing cells’ half lives of approximately one day, ii) after several weeks of therapy, a decrease in the viral decay occurs with a half life of approximately two weeks, which corresponds to the slower turnover of specific cell populations such as macrophages, and iii) the final phase includes the time from non-detectable plasma levels to the elimination of virus in latently infected cells. This phase has a dramatically slower decay rate of virus of approximately 44 months, which if the population of the latently infected cells were 1.0 × 105 cells would result in viral eradication after sixty years of therapy. This reflects the inefficiency of the available pharmacotherapy in addressing viral latency and persistence in CD4+ T-cells [28].
3. Physiological barriers
If the ability to generate drug concentrations within the specific cell types did not present a great enough challenge, many physiological barriers stand in the way of drug accumulation within both latently and actively infected cells. The lymphoid tissues, brain, and testes act as HIV reservoirs or sanctuary sites since most of these drugs do not persist in these tissues at effective concentration for adequate periods of time. Each of these physiological locations poses a unique hurdle in the delivery of effective drug concentrations.
Lymphoid tissues such as the peripheral secondary lymphoid organs, the spleen, lymph nodes, and gut-associated lymphoid tissue (GALT) are the primary sites of HIV infection [24]. It is estimated that only 2% of lymphocytes are in the general circulation at any one time and the remainder are distributed among the lymphoid tissues [24]. Often the lymphoid tissues have a greater extent of infection than the peripheral blood and specifically the GALT is home to large numbers of activated T-cells, which propagate the infection [24]. For example, a few weeks after the initial infection with simian immunodeficiency virus (SIV) (a model of HIV), there is a dramatic increase in infected CD4+ T-cells in the GALT, spleen, and lymph nodes before the virus has even reached the CNS or other accessory sites of infection [23]. Also the primary lymphoid tissues, bone marrow and thymus, are said to be reservoir sites, however the extent of T-cell infection for these sites remains unknown [24]. Despite the uncertainty of the pervasiveness of the infection, the lymphoid tissue reservoir develops quickly and persists throughout the course of infection and remains the primary site of HIV production regardless of the effectiveness of HAART to diminish viral RNA levels to undetectable levels [23].
The blood-brain barrier (BBB) remains one of the most difficult physiological sites to penetrate with pharmacological agents. The barrier itself consists of brain capillary endothelial cells that are densely packed via tight junctions that prevent both endogenous substances and xenobiotics from penetrating the brain and CNS [29]. For HIV specifically, the microglial cells of the CNS are one of the main sanctuary sites of the virus, however very few antiviral drugs have the ability to enter the brain particularly AZT, 3TC, and the protease inhibitors [30, 31]. HIV infection within the CNS has led to the development of HIV associated dementia and other neurological disorders. Therefore, HIV in the brain remains unaffected by drugs that are otherwise effective at suppressing the viral replication in the rest of the body.
Besides the lack of direct penetration of drugs as a result of their charge or lipophilicity (permeability), drugs are also subjected to efflux by drug transporters like P-glycoprotein (Pgp, ABC1), that are expressed by astrocytes at the BBB [29]. Several HIV drugs have been shown to act as substrates for these efflux transporters including the protease inhibitors, ritonavir and SQV, as well as the NRTIs like AZT [29, 32]. A few methods for improving BBB include the modulation of the efflux transporters and/or utilizing drug delivery vehicles that may be conjugated to a ligand that can facilitate BBB penetration [29]. Glucose is one example of an endogenous substance that undergoes carrier-mediated transport across the BBB, whereas peptides like insulin and transferrin pass the BBB via receptor mediated transport [29]. However, in order for conjugation to become successful, a specific BBB receptor must be identified and manipulated for the translocation of a drug into the CNS. Another possibility is to utilize a cell-penetrating peptide (CPP) also known as protein transduction domains to cross the BBB [29]. This class of peptides has been shown to have the ability to act as carrier proteins for drug cargoes and remains a topic of great interest (for reviews see [33, 34]).
Other sanctuary sites include the testes of HIV infected patients. This tissue barrier limits the penetration of drugs due to its reduced vascularization. Replication-competent virus has been found in the seminal cells of men who have been receiving antiviral therapy [19]. The Sertoli cells of the testes form tight junctions with each other creating a physical barrier known as the blood-testis barrier (BTB). The presence of Pgp transporters at the BTB makes it in some ways similar to that of the BBB. The BTB also is home to HIV susceptible immune cells namely macrophages and CD4+ T-cells. Drug penetration into the testes is drug dependent, d4T shows poor penetration, whereas AZT and 3TC show efficient accumulation in the seminal plasma [35]. However, the protease inhibitors and NNRTIs concentrations are generally lower in the seminal plasma compared to blood plasma [35]. Despite the variable penetration of HAART into the BTB, it is important to note that the male reproductive organ is a route of viral transmission between individuals, so elimination of the virus from this sanctuary site is not only beneficial for the infected individual, but for susceptible individuals as well [35].
II. PRODRUG AND CONJUGATE STRATEGIES
There is incredible potential for improving anti-HIV therapy not only by increasing the potency of antiviral drugs, but also by reducing the burden of the dosing regimen. This can be achieved by modifying the physicochemical, biopharmaceutic, and pharmacokinetic properties of drugs through the development of prodrugs. Traditional prodrugs, which only include about 5–7% of marketed drugs, are classified as drugs that are activated by undergoing transformation in vivo to form the active drug [8]. Reasons for prodrug development are poor aqueous solubility, chemical instability, low oral bioavailability, lack of BBB penetration, and high first pass metabolism associated with the parent drug [8]. There has been limited prodrug development in HIV HAART despite the fact that most of the antiviral drugs developed thus far suffer from one or more of the above-mentioned limitations. Currently, only two traditional prodrugs have been developed in HIV therapy, fosamprenavir and tenofovir disoproxil fumarate. Although the NTRIs, like AZT, are metabolized intracellularly to their active phosphorylated forms, they are often regarded as active metabolites as opposed to prodrugs that are catabolized enzymatically to their active parent drug in classical prodrugs. Both fosamprenavir and tenofovir disoproxil fumarate improve the drug delivery capabilities of their parent drug by increasing intestinal absorption. A more recent trend in prodrug design has extended the prodrug platform to encompass drug delivery systems with targeting agents and releasable drugs (for a review on prodrug design strategies see [8]).
Improved therapy can be achieved using conjugation chemistry approaches for developing multicomponent and multifunctional drug delivery systems. These systems can incorporate either active or passive targeting moieties to improve distribution to the sanctuary sites in the body, for example to the lymphatics, where the majority of the viral replication and infection exists. Targeted drug delivery to macrophages and T-cells would not only improve the efficacy of antiviral drugs, but would potentially also limit the development of toxicities/adverse effects often associated with the presence of drugs in non-infected cells. Another potential auxiliary benefit of targeted drug delivery is the reduction of HIV resistance by increasing uptake into HIV infected cells. Along the same line of thinking, by accumulating drug into either those cells latently infected or not yet infected, the drug can be within the cell and interfere with early viral life cycle stages upon infection or disrupt virus replication when transitioning from a latent to active state.
1. Prodrugs
1.1. Fosamprenavir
Fosamprenavir (Figure 2) is a water-soluble, phosphate ester prodrug of the protease inhibitor drug amprenavir [36–38]. The FDA approved it for the treatment of HIV-1 protease inhibitor-naive patients in 2003 and the EMEA approved it in 2004. The drug is formulated either as a suspension or as a tablet in a dosing regimen of fosamprenavir 1400 mg twice a day or fosamprenavir 700 mg twice a day plus ritonavir 100 mg twice a day for treatment-experienced patients [37, 38].
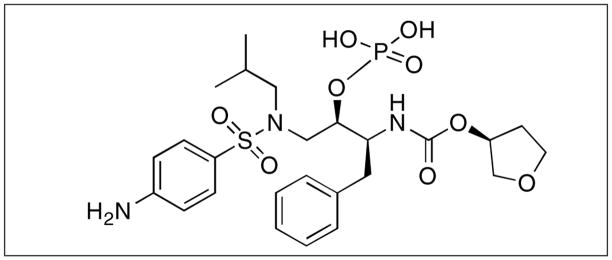
Figure 2.
Structure of fosamprenavir. IUPAC name is [(2R, 3S)-1-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-{[(3S)-oxolan-3-yl] oxycarbonylamino}-4-phenyl-butan-2-yl]oxyphosphonic acid.
Amprenavir, the active metabolite of fosamprenavir, is a protease inhibitor with demonstrated antiviral efficacy and good tolerability when used in combination with other antiretroviral agents. Fosamprenavir has no antiviral activity in vitro and any activity detected after administration is due to the presence of amprenavir [37, 39].
The oral bioavailability of amprenavir varies from 35–90% due to its low water solubility (~0.04 mg/mL) [37]. A large percentage of organic excipients were included in the original formulation to aid its dissolution, necessitating a high capsule burden (10/day with ritonavir, 16/day when used alone) [37]. The development of the prodrug, fosamprenavir, greatly enhanced the water solubility of the drug (by ~10 fold) by the addition of the phosphate group to the hydroxyl group, thus reducing the high dosage form burden [8, 37].
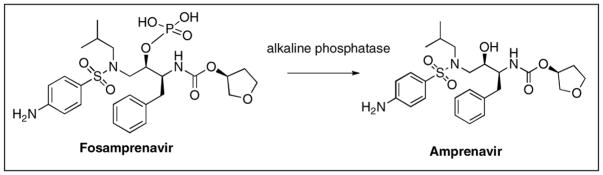
Because of fosamprenavir increased water solubility, it has poor membrane permeability and is rapidly converted to amprenavir after oral administration [38, 40]. Preclinical studies have demonstrated that fosamprenavir is converted almost entirely (99%) to amprenavir at or near the intestinal epithelium by alkaline phosphatase, the primary enzyme responsible for conversion (Figure 3) [41]. Less than 1% of the prodrug dose was detected in the portal vein of animals confirming nearly complete presystemic conversion. This observation has been further supported by measurement of plasma concentrations of fosamprenavir and amprenavir in clinical studies [42].
Figure 3.
Metabolic conversion of fosamprenavir to amprenavir by alkaline phosphatase in the intestinal lumen.
As a result of rapid conversion of fosamprenavir in the intestine to the parent drug, amprenavir is predominantly absorbed in the intestine, however it is also a substrate for P-glycoprotein (P-gp), which may play a certain role too in its absorption [38, 43]. Following administration of prodrug, the drug is rapidly absorbed as indicated by plasma drug concentrations that are measurable as early as 15 minutes post dosing, with maximum plasma concentrations occurring at 1.5–2.5 h following single and repeat dose administration [42]. Despite its rapid absorption, a secondary absorption phase appears to take place in the terminal ileum as evident from second peak in the plasma concentration curve at 10–12 h, which is less than the Cmax [44].
Co-administration of food with fosamprenavir appears to have little effect on drug absorption, therefore fosamprenavir can be taken with or without food [45].
Following oral prodrug administration to humans, the apparent volume of distribution is ~430 L suggesting drug penetration into tissues beyond the systemic circulation [38]. Amprenavir is taken up by lymphocytes with concentrations 2- to 3- fold higher than plasma concentrations [46]. Also amprenavir has been shown to penetrate human semen most likely with Pgp playing a role in the distribution into the tissues [38, 47].
1.2. Tenofovir
Tenofovir disoproxil fumarate is the first nucleotide drug to be approved for the treatment of HIV/AIDS and is classified as an acyclic nucleoside phosphonate (ANP), an analogue of adenosine monophosphate (Figure 4) [48, 49]. It has been formulated into once daily tablets (Figure 5) [50]. Therapeutically, tenofovir, (R)-9-(2-phosphonomethoxypropyl) adenine also known as bis(isopropyloxycarbonyloxymethyl)-(R)-9-(2-phosphonomethoxypropyl) adenine (bis-POC-PMPA), is administered as the oral prodrug, tenofovir disoproxil fumarate [51]. Like NRTIs, tenofovir is converted intracellularly by phosphorylation to its active form tenofovir diphosphate, which acts as a chain terminator when HIV reverse transcriptase is actively making viral DNA [52].
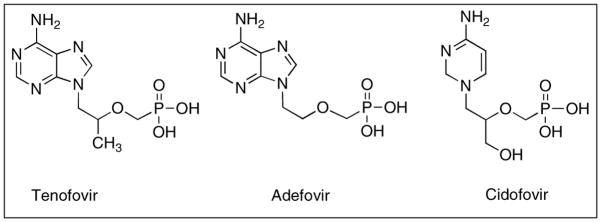
Figure 4.
Nucleoside phosphonate analogues developed for various anti-viral therapies.
Figure 5.
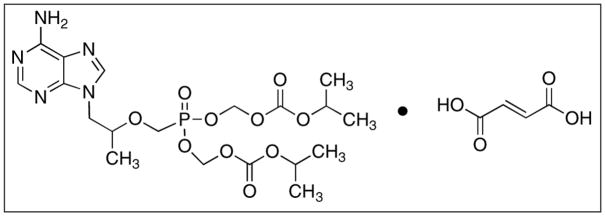
Tenofovir disoproxil fumarate or bis(isopropyloxycarbonyloxymethyl)-(R)-9-(2-phosphonomethoxypropyl) adenine. IUPAC name is 1-(6-aminopurin-9-yl) propan-2-yloxymethylphosphonic acid.
However unlike the other traditional NRTIs, tenofovir possesses a phosphonate group, which is stable in the biological milieu. The phosphonate group consists of a P-C bond protected from phosphoesterase cleavage. This is different from a phosphate group (P-O-C) that is formed upon phosphorylation of NRTIs, which is susceptible to such cleavage [53–55]. Because of the phosphonate group, tenofovir only needs to be phosphorylated twice and this allows tenofovir to avoid the initial phosphorylation step by virus-induced kinases that has been identified as the rate-limiting step in the conversion of NRTIs to their active metabolites [52, 54, 55].
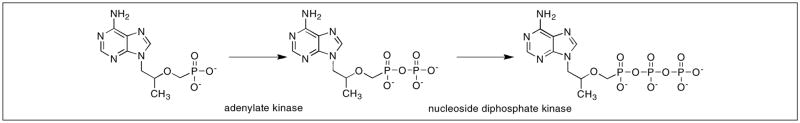
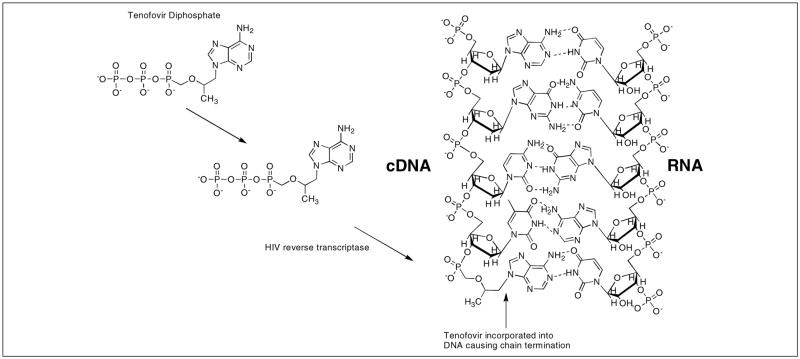
After cellular uptake of tenofovir, it is converted to its monophosphate form (TMP) by adenylate kinase found in the intermembrane space of the mitochondria, while the secondary phosphorylation takes place rapidly with the aid of nucleoside diphosphate kinase to form the active metabolite TDP (Figure 6) [51, 52, 56]. Once in the active form, TDP competes with naturally formed deoxyadenosine 5′-triphosphate (dATP) for incorporation into the viral cDNA. The DNA chain becomes terminated because TDP lacks the deoxyribose sugar moiety necessary to continue reverse-transcription (Figure 7) [49, 57, 58].
Figure 6.
Intracellular activation of tenofovir to TDP.
Figure 7.
Incorporation of tenofovir into viral cDNA and mechanism of chain termination.
Tenofovir possesses the ability to be metabolized by non-dividing cells as well as quiescent cells and can reach up to two-fold greater concentration in resting peripheral monocytes compared to stimulated monocytes [56, 61]. The potency of tenofovir in macrophages can be attributed to two factors: i) the phosphonate group that avoids initial phosphorylation as previously mentioned and ii) the low levels of the competitive substrate dATP [25]. The effective concentration of tenofovir in resting CD4+ T-cells is about ten times greater than resting macrophages, however because tenofovir does not require initial phosphorylation, it is seen to be more active than other NTRI alternatives in these cell types [25, 52]. Accumulation of drug in the resting cells means that tenofovir may possess the capability to inhibit HIV in latent cells, which is lacking in previously developed NRTIs like AZT or d4T [51].
Because of the polar nature of tenofovir, intestinal absorption is low. Therefore a prodrug was developed to increase its lipophilicity and to mask the negatively charged phosphonate group. Acyloxyalkyl esters of carboxylic acid were synthesized, since similar drugs with low bioavailability have benefited from ester linkages of phosphate groups [56]. Adefovir (Figure 4) another acyclic phosphonate drug similar to tenofovir had improved bioavailability when pivoxil groups were conjugated to the negatively charged phosphonate group [62]. For tenofovir, an isoproxil group was chosen because of its good oral bioavailability (~30%) and stability in both chemical and intestinal milieus [62, 63]. This prodrug formulation increased the lipophilicity of the drug from log P = −2.5 tenofovir to a log P = 1.3 of tenofovir disoproxil fumarate, increasing its ability to cross the intestinal membrane [64].
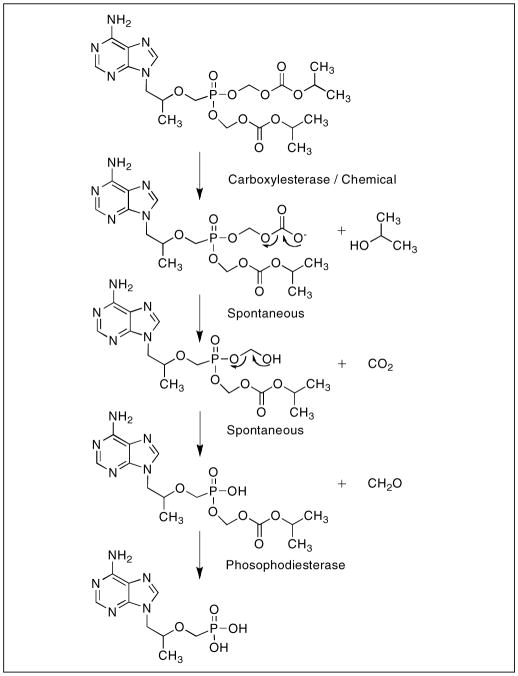
The conversion of the prodrug after absorption is mediated by enzymatic and non-enzymatic hydrolysis of the ester linkages of the phosphonate functional group [64]. The hydrolytic reactions produce carbon dioxide, formaldehyde, and the parent drug tenofovir (Figure 8). This process occurs upon absorption and is believed to be initiated by carboxylesterases, however esterases in general are relatively ubiquitous [63]. The conversion of tenofovir disoproxil fumarate to the parent drug tenofovir is rapid and complete, in vitro and in vivo studies revealed that the conversion process takes minutes to accomplish and the presence of any intermediates were undetectable by HPLC [56, 63, 65].
Figure 8.
Conversion of tenofovir disoproxil fumarate to tenofovir upon intestinal absorption.
The oral bioavailability of tenofovir can be enhanced by the consumption of high-fat meals or ester mixtures for example those derived from strawberry extracts, which prevent premature ester cleavage of the prodrug [49, 66, 67]. These ester mixtures limit the metabolism in the intestinal milieu, prior to absorption, and in vitro interfere with P-glycoprotein related efflux, while the consumption of a high-fat meal is related to the increased lipophilicity of the tenofovir prodrug [52, 66, 67].
Although tenofovir disoproxil fumarate shows increased antiviral activity in resting and activated lymphocytes, in reality very little prodrug if any will enter the systemic circulation, because of the predominance of esterases. Since it is believed that cells take up the charged parent drug, the absorption process is most likely receptor- or transporter-mediated. Cellular uptake of a similar drug, adefovir, has been shown not to use the nucleoside transporters utilized by the natural nucleosides and NRTIs [68]. Instead it is believed that tenofovir is internalized via an endocytosis pathway similar to that of adefovir. Adefovir uptake in HeLa cells is protein mediated and saturable [68]. Conflicting claims suggest that tenofovir enters cells passively by endocytosis that is neither transport mediated nor saturable [51].
Tenofovir is extensively distributed throughout the body with steady state distribution reaching approximately 1.3 L/kg from an intravenous dose. An associated volume of this magnitude is greater than the total body water [48, 50, 51, 69]. The extensive distribution throughout much of the body and tissues could be attributed to the polar nature of the parent drug tenofovir. Despite its large volume of distribution, tenofovir is virtually unable to penetrate the blood brain barrier. Although BBB penetration is limited, tenofovir does have the ability to pass the blood cerebral spinal fluid (CSF) barrier of the choroid plexus gaining access to perivascular and meningeal macrophages [70].
Intracellular elimination of tenofovir in vitro was markedly different between stimulated and resting peripheral blood mononuclear cells (PBMCs). In stimulated cells, TMP and TDP have half-lives of 15 and 11 h, respectively, which is similar to observed half-lives in T-cell lines [56, 71]. Interestingly in resting cells the half-lives of TMP and TDP reach 33 and 49 hours. The increased intracellular time could translate into increased antiviral activity especially in resting cells, a known viral sanctuary site [56]. The increased intracellular duration may be attributed to the phosphorylation of tenofovir, which locks the active metabolite within the cell.
HIV resistance to NRTIs manifests itself in two mechanisms: i) lack of incorporation and/or ii) increased excision of the drugs from the viral cDNA [72]. Because of the flexible nature of the acyclic linker and the lack of the ribose sugar, which gives the drug torsional freedom and a reduction in size respectively, tenofovir does not develop highly resistant strains of the virus [72, 73]. These properties are also advantageous against viral strains that are resistant to other NRTIs. For example M184V is a strain resistant to 3TC incorporation because its ribose ring is sterically hindered by the mutation, but the lack of the bulky side chains allows tenofovir to still be incorporated [72, 73]. The side chain features keep HIV-RT from developing a mutant that would not incorporate tenofovir, while still being able to incorporate dNTPs [72].
2. Drug conjugates and targeted nanocarrier drug delivery strategies
Traditional prodrug strategies have significantly improved HIV therapy by improving pharmacokinetics and the resulting dosing regimens. However, prodrugs can undergo non-specific activation thus potentially causing significant side effects. A prodrug strategy focusing toward the development of targeted prodrugs, which could allow for the drug release at the site of action, can address these potential problems [78, 79].
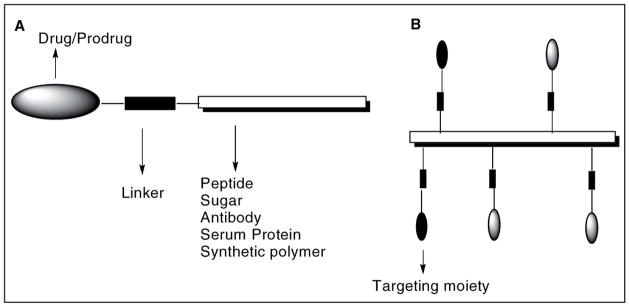
Targeting is achieved by conjugating the drug/prodrug molecules to carriers of varying molecular classes, sizes, and shapes, like sugars, growth factors, vitamins, antibodies, peptides and synthetic polymers that can transport the drugs to the target site. Site-specific release is performed by incorporating chemical linkages that are normally stable, but can be cleaved under the defined conditions at the target site. The general design of prodrug conjugates for targeted therapy is shown in Figure 9.
Figure 9.
General design of prodrug conjugates.
Design and development of target-specific prodrug/drug conjugates is challenging because the functionality of the carriers is often sensitive to subtle structural variations and therefore emphasis must be placed on the chemical aspect of preparing and characterizing them [80]. Extensive chemical experimentation and characterization should be performed to optimize the physicochemical properties (e.g. molecular weight, hydrophilicity/hydrophobicity, three-dimensional structure, and immunogenicity) of the carrier and/or conjugate before evaluating their potential biological effects. Molecular biology is currently being implemented in the design and development of targeted drug/prodrug conjugates to understand the effect of conjugation on drug activity and where relevant, to modify biologically active molecules for conjugation [80].
Our lab has used the conjugate approach to overcome several challenges associated with the physicochemical properties of SQV [4]. SQV was the first approved HIV-1 protease inhibitor used for the treatment of HIV infection. The drug suffers from limitations such as low absorptive and high secretary permeability, bioconversion to inactive metabolites, and poor solubility, all of which are commonly associated with protease inhibitors. SQV absorption in particular, is highly variable and the mean oral bioavailability of the two-marketed SQV capsule formulations range from 4 to 16%. Earlier studies have considered the first-pass intestinal hepatic metabolism by cytochrome P-450 monooxygenases (CYP) as a major determinant in the clearance of SQV, but our group, along with others, have shown that multiple membrane transporters working in concert with P-glycoprotein (P-gp) may also be responsible for this observed behavior [31, 81–84].
Prodrug conjugates of the protease inhibitors, SQV, IND, and NFV, have been developed by conjugating the drugs to fatty acids, L-valine, L-tyrosine, PEG, and D-glucose so as to improve their bioavailability [85, 86]. The only successful prodrug of a protease inhibitor to date is the phosphate-ester prodrug of amprenavir, fosamprenavir, as previously discussed. In each of these cases, the prodrugs were readily converted to active drug molecule either in the intestinal environment or on the first pass through the intestine and liver. Therefore once the prodrug is absorbed only the parent drug remains in the blood and the potential benefits of the prodrug are lost [87].
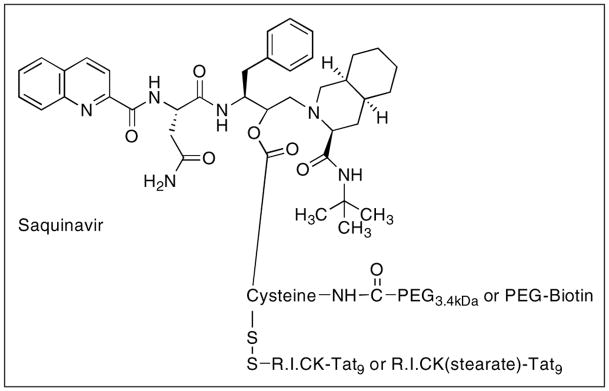
Our lab has also designed and developed SQV PEG-based nanocarriers to overcome biopharmaceutical problems associated with SQV (Figure 10) [87]. Poly(ethylene glycol) (PEG) scaffolds were covalently attached to a SQV molecule to enhance the aqueous solubility, circulation half-life, and prevent nonspecific interactions of the drug in the biological milieu. PEG was used because it is among the most versatile polymers for medical applications due to the chemical inertness of its polyether backbone, as well as its excellent solubility in aqueous media. PEG is nontoxic, non-immunogenic, and non-biodegradable, which makes it suitable for the modification of various biologically active compounds. The PEG-prodrug conjugates were further attached to either biotin or retro-inverso-cysteine-lysine-Tat9 (R.I.CK-Tat9) or R.I.CK-Tat9 (stearate) to achieve enhanced cell association and uptake. R.I.CK-Tat9 was used not only for its cell-penetrating properties but also its anti HIV-1 properties, which may be useful in combination therapy [88]. The biotin was attached because we have previously shown that conjugation to biotin or biotinylated PEG results in enhanced cellular uptake and transport of R.I.CK-Tat9 peptide via SMVT, the intestinal biotin transporter [89, 90].
Figure 10.
General design of PEG nanocarriers conjugated to SQV prodrug and biotin/retro-inverso CK-Tat9 or CK(stearate)Tat9 peptides. The prodrug is attached through a reversible ester bond, whereas the cell-penetrating peptides are attached through reducible disulfide bonds. PEG is attached through stable amide bonds and biotin is attached through PEG spacer. The nanocarrier shown here releases the SQV.
Several prototypes of these PEG nanocarriers were synthesized viz., SQV-Cys (control), SQV-Cys-PEG3.4kDa, SQV-Cys-PEG3.4kDa-biotin, SQV-Cys(R.I.CK-Tat9)-PEG3.4kDa, and SQV-Cys(R.I.CK(stearate)-Tat9)-PEG3.4kDa [87]. The PEG was bound to a cysteine residue through stable amide bonds, whereas the prodrug was attached through reversible ester bonds. Similarly, R.I.CK Tat peptides were attached through reducible disulfide bonds. In vitro studies showed that the PEG only conjugate was inactive, but further modification with biotin or the peptide restored the prodrug activity. Cytotoxicities were found in the micromolar range, but more importantly the SQV molecule was released from the prodrug conjugates. The prodrug conjugate SQV-Cys(R.I.CK-Tat9)-PEG3.4kDa was found to be most active among the conjugates investigated. We also demonstrated the combined preclinical in vitro effectiveness of R.I.CK-Tat9 alone or attached with SQV-PEG conjugates for HIV treatment [91].
Our recent efforts have focused on the design and development of PEG-nanocarriers for macrophage targeting. Macrophage targeting represents a key challenge in HIV therapy, since macrophages are not only the primary target of HIV infection, but along with CD4+ T-lymphocytes are an important source of HIV persistence during HAART [22]. They play an important role in the initial stages and throughout the course of HIV-1 infection [92]. Productively infected macrophages have been reported in both untreated patients and those receiving HAART. Also HIV-1 infection of macrophages is noncytopathic, enabling them to serve as sources of HIV production and represent major viral reservoirs, as previously mentioned [25].
Macrophages regulate their biological function in response to environmental stimuli (action of soluble factors, contact with foreign particles or cells). Hence, delivering drugs to macrophages is an extremely challenging task because of their inherent phagocytic ability and inhibition towards foreign particles even though there are drugs known that are capable of modulating macrophage activities [22]. Several liposomes, nanoparticles and microsphere-based delivery systems have been developed for delivering the drug to macrophages [22, 93]. However, another strategy that is being increasingly explored is the design and development of conjugates/nanocarriers containing ligands for specific interaction with receptors expressed on macrophage surfaces.
Macrophages possess various receptors such as formyl peptide, complement, fibronectin, lipoprotein, mannosyl, galactosyl, Fc, and many others [94, 95]. These macrophage surface receptors control the activities of the cells including; activation, recognition, endocytosis, and secretion. Conjugates/nanocarriers containing ligands specific to macrophage surface receptors may enhance uptake due to the receptor-mediated endocytosis. The advantage of using such a targeted system is that drug is delivered selectively to the HIV-infected tissues without affecting the normal tissues. As a result, the side effects are reduced, lower doses are needed, and drug administration regimens are simplified.
An ideal nanocarrier for macrophage targeting should be flexible enough in design so that different combinations of anti-HIV drugs, targeting moieties, and/or imaging agents can easily be incorporated. We have been extensively exploring the possibility of using our modular design of PEG nanocarriers (Figure 10) for macrophage targeting. A PEG-based linear copolymer, poly[poly(ethylene glycol)-alt-poly(aspartic acid)] was designed and developed [96]. The copolymer was further modified to obtain multifunctional PEG nanocarriers containing four copies of N-formyl-methionine-leucine-phenylalanine (fMLF) peptide (PEG-fMLF4) and/or four and eight copies of digoxigenin (DIG4-PEG-fMLF4 and PEG-DIG8). Branched PEG with three and four arms were used to obtain PEG nanocarriers with three and four copies of fMLF respectively. Similarly, eight-arm PEGs were used to obtain nanocarriers containing 1.1, 5.5 and 8- copies of fMLF respectively (PEG-fMLF1.1, PEG-fMLF5.5, and PEG-fMLF8) [96]. The fMLF peptides and the surrogate drug (digoxigenin) were attached through stable amide bonds. However in future work, reducible disulfide bonds would be used for the attachment of drugs/prodrugs. The fMLF peptide is recognized by formyl peptide receptors over-expressed on macrophage surfaces [97].
Nanocarriers with a single copy of fMLF were found to reduce the binding avidity for differentiated HL-60 cells when compared to the free fMLF peptide [96]. Increasing the number of fMLF peptides on PEG nanocarrier led to an increase in avidity for HL-60 cells and interestingly the geometry of the polymer backbone (linear/branched) was found to have no influence on the outcome and there was no enhancement observable when fMLF receptor negative Jurkat cells were used. Importantly, none of the nanocarriers investigated above showed an enhanced ability to activate phagocytic cells, a potential cause of various adverse side effects. The advantage of using PEG nanocarriers described herein is that multiple copies of targeting moieties and drug/prodrugs can be incorporated to obtain optimum targeting and drug payload. Furthermore, the lack of enhancement of phagocytic activation suggests that the drugs/prodrugs appended to such nanocarriers are less likely to be inactivated by degradative mechanisms induced by phagocytic activation.
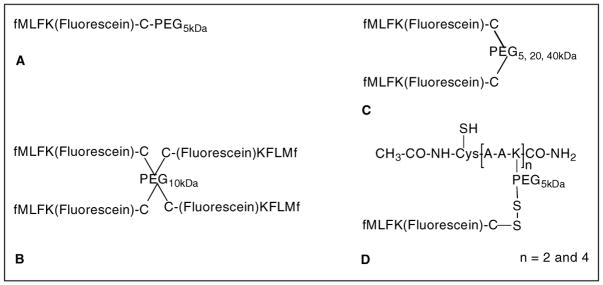
We later developed a series of PEG nanocarriers containing multiple fMLF moieties to optimize the fMLF copy number and PEG size for macrophage uptake [98]. One, two, and four-arm PEG scaffold of molecular weights 5, 10, 20, and 40 kDa were used to conjugate up to four copies of fMLFK(fluorescein)C. The following nanocarriers were synthesized: fMLFK(fluorescein)KC-PEG5kDa, [fMLFK(fluorescein)KC]2-PEG5kDa, [fMLFK(fluorescein)KC]2-PEG20k, [fMLFK(fluorescein)KC]2-PEG40kDa, and [fMLFK(fluorescein) KC]4-PEG10kDa (Figure 11). Since, the ability to produce PEGs with a defined number of anchoring moieties is limited, especially in high numbers, a modular PEGylated peptide [(acetyl-Cys-β-Ala-β-Ala-Lys)n-PEG5k] nanocarrier was designed and developed incorporating two and four copies of fMLFK(fluorescein)C [98]. The advantage of using PEG-peptide nanocarriers is that the precise copy number of drug/prodrug and targeting moieties can be attached, unlike the PEG alone nanocarriers, where only the average copy numbers were obtainable.
Figure 11.
PEG (A–C) and PEG-peptide (D) based nanocarriers developed for macrophage targeting by receptor-mediated endocytosis. The fMLF copy number was varied to obtain a nanocarrier with optimum binding properties. The molecular weight of PEG was varied to influence the molecular size of the nanocarrier whereas fluorescein molecule was attached to monitor their binding and uptake behavior.
Results from macrophage like differentiated human U937 cell-specific binding and cellular uptake studies conclusively showed that uptake is energy dependant and mediated by fMLF receptor [98]. Similar to our earlier results [96], fMLF copy number was found to influence the binding and uptake behavior. Increasing the number of fMLF moiety from one to two resulted in 4-fold enhanced uptake, but further increase in fMLF copy number to four led only to a modest increase. Molecular size was also found to influence the uptake behavior as increasing the PEG molecular weight from 5 to 20 kDa resulted in an increase in the uptake but further increase to 40 kDa led to a decreased uptake. This is consistent with earlier reports where receptor-mediated endocytosis has been shown to be strongly size dependant with optimal size requirement of ~ 25 nm or < 50 nm [99–102]. Thus, two copies of fMLF along with a molecular size of 20 kDa PEG appears to be a prerequisite for optimum macrophage targeting.
Peritoneal macrophage uptake, pharmacokinetics, and biodistribution of macrophage-targeted PEG nanocarriers for improving the HIV drug delivery were also investigated [103]. In this case the following nanocarriers were used: [fMLFK(fluorescein)C]2-PEG5kDa, [fMLFK(fluorescein)C]4-PEG20kDa, and acetyl-Cys-[β-Ala-β-Ala-Lys{PEG20kDa-CK(fluorescein)FLMf]4. Attachment of one, two or four fMLF copies increased the macrophage uptake by 3.8, 11.3 and 23.6-fold when compared to nanocarrier without the targeting moiety. Incorporation of fMLF moiety also increased the t1/2 of PEG5kDa by 3-fold, but decreased by 40% for PEG20kDa. Furthermore, the attachment of targeting moieties to the PEG nanocarriers led to an increased accumulation in liver (1.5-fold), kidney (3.2-fold), and spleen (6.9-fold). However on a molar basis, the penetration was equivalent, suggesting that the nanocarrier size and the targeting moieties are an important determinant. The PEG-peptide nanocarrier with four copies of fMLF was found to be the most promising nanocarrier tested so far. Overall, the results demonstrated the feasibility of targeting macrophages, a primary HIV reservoir site by modularly designed PEG nanocarriers. The studies also suggest a need to balance peripheral tissue penetration with target cell uptake, and we are currently working on the design and development of nanocarriers containing multiple targeting moieties and drug copies to achieve efficient targeted drug delivery [103].
III. SUMMARY AND FUTURE DIRECTIONS
In summary, the antiviral drug development process remains challenging. It has been suggested by many that drugs targeted toward viral proteins will be more specific and less toxic to the host, but they may ultimately select for mutant virus rendering the therapeutic agent ineffective. Others believe that developing drugs that target host proteins will avoid the development of viral resistance, but may have a less than desirable adverse effect profile [10]. In our opinion, a combination of these two methods is necessary to keep HIV at chronically low titers or possibly to eradicate HIV infection altogether.
Most of the antiviral drugs developed so far are viral protein inhibitors designed to disrupt the viral life cycle in HIV infected cells, namely viral DNA synthesis (reverse transcriptase), viral DNA insertion into the host genome (integrase), and viral protein cleavage (protease). Although the prodrug strategy has been successfully used to improve the physicochemical, biopharmaceutic, and pharmacokinetic properties of some anti-HIV drugs, they are unable to eradicate HIV infection because they suffer from poor specificity for the infected cell types or the physiological sites (tissues or organs) that sequester HIV. Also, these drugs do not persist in target cells for a sufficient period of time. Therefore, efforts need to shift in order to improve target cell bioavailability and persistence.
Targeted-delivery to host proteins specific to HIV susceptible cells could improve antiviral therapy because it will potentially increase either the access to the physiological reservoir sites (brain, testes, and lymphatics) or the cellular active site concentrations, unlike the conventional drug/prodrug design, where focus is on improved pharmacokinetics (i.e. blood concentration). Drug delivery to HIV-infected tissues with selectivity can be achieved by macrophage targeting. Microspheres, liposomes, and nanoparticle-based delivery systems have been developed for delivering drugs to macrophages. Macrophage targeting can also be achieved by interaction with receptors expressed on macrophage surfaces. We have shown the advantage of using modularly designed PEG-nanocarriers containing effectors (targeting ligands, drugs, and/or imaging agents) for macrophage targeting. These nanocarriers can be easily modified to incorporate various antiviral drugs/prodrugs, biologics, targeting moieties, and/or imaging agents. It has been also shown that these nanocarriers do not show an enhanced ability to activate phagocytic cells, a potential cause of various adverse side effects. We believe that the design of such nanocarriers will be a key to the development of targeted delivery systems that can accumulate into the difficult to reach physiological locations such as the brain, testes, and lymphatics.
Besides targeting macrophages, the chemokine receptors (CXCR4 and CCR5) that act as the coreceptors to HIV infection can also be used as potential targets. Targeting these receptors can have two effects: i) prophylactic protection of the cell by occupying the receptor necessary for virus-cell interaction, and/or ii) improving the specific uptake of a nanocarrier loaded with anti-viral drugs. There are several other strategies that are being explored to target viral compounds or virus-associated events [10].
Although this review has exclusively focused on anti-HIV prodrugs and conjugates that can be potentially used as chronic therapeutic agents, however new and uniquely designed pharmacological agents and strategies with specificity to the physiological and cellular targets are necessary if eradication of the virus is to become attainable.
Acknowledgments
This work was supported by grants from NIH MERIT Award (AI51214). We also wish to acknowledge our graduate students, postdoctoral fellows, and colleagues who have contributed to this work over the years. Their names appear in the references.
References
- 1.Joint United Nations Programme on HIV/AIDS and The World Health Organization-2007. [Accessed[June 11, 2008]];AIDS epidemic update. 2007 http://www.unaids.org/en/KnowledgeCentre/Resources/Publications/-2007_epiupdate_en.pdf.
- 2.Joint United Nations Programme on HIV/AIDS and The World Health Organization. [[June 11, 2008]];2006 report on the global AIDS epidemic. 2006 http://www.unaids.org/en/KnowledgeCentre/Resources/Publications/
- 3.Merson M. The HIV-AIDS pandemic at 25 - the global response. N Engl J Med. 2006;354:2414–2417. doi: 10.1056/NEJMp068074. [DOI] [PubMed] [Google Scholar]
- 4.Piot P, Bartos M, Ghys P, Walker N, Schwartlander B. The global impact of HIV/AIDS. Nature. 2001;410:968–973. doi: 10.1038/35073639. [DOI] [PubMed] [Google Scholar]
- 5.Flexner C. HIV drug development: the next 25 years. Nat Rev Drug Discov. 2007;6:959–965. doi: 10.1038/nrd2336. [DOI] [PubMed] [Google Scholar]
- 6.Janssen P, Lewi P, Arnold E, Daeyaert F, De Jong M, Heeres J, Koymans L, Vinkers M, Guillemont J, Pasquier E, Kukla M, Ludovici D, Andries K, De Bethune M, Pauwels R, Das K, Clark A, Frenkel Y, Hughes S, Medaer B, De Knaep F, Bohets H, De Clerck F, Lampo A, Williams P, Stoffels P. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethyphenyl] amino]-2-pyrimidinyl]amino]benzonitrile (R278474), Rilpivirine) J Med Chem. 2005;48:1901–1909. doi: 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- 7.Struble K, Murray J, Cheng B, Gegeny T, Miller V, Gulick R. Antiretroviral therapies for treatment-experienced patients: current status and research challenges. AIDS. 2005;19:747–756. doi: 10.1097/01.aids.0000168968.34810.ca. [DOI] [PubMed] [Google Scholar]
- 8.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–269. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 9.Hammer S, Saag M, Schechter M, Montaner J, Schooley R, Jacobsen D, Thompson M, Carpenter C, Fischl M, Gazzard B, Gatell J, Hirsch M, Katzenstein D, Richman D, Vella S, Yeni P, Volberdine P. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA Panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 10.Clercq EDE. Strategies in the design of antiviral drugs. Nat Rev Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Tang M, Zhang M, Majeed S, Montabana E, Stanfield R, Dimitrov D, Korber B, Sodroski J, Wilson I, Wyatt R, Kwong P. Structure of V3-containing HIV-1 gp120 core. Science. 2005;310:1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Pei G, Zhang W. An overall picture of chemokine receptors: basic research and drug development. Curr Pharm Des. 2004;10:1045–1055. doi: 10.2174/1381612043452749. [DOI] [PubMed] [Google Scholar]
- 13.Schols D. HIV co-receptors as targets for antiviral therapy. Curr Top Med Chem. 2004;4:883–893. doi: 10.2174/1568026043388501. [DOI] [PubMed] [Google Scholar]
- 14.Lusso P. HIV and the chemokine system: 10 years later. EMBO J. 2006;25:447–456. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazmierski W, Kenakin T, Gudmundsson K. Peptide, peptidomimetic and small-molecule drug discovery targeting HIV-1 host-cell attachment and entry through gp120, gp41, CCR5, and CXCR4. Chem Bio Drug Des. 2006;67:13–26. doi: 10.1111/j.1747-0285.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq E. The design of drugs for HIV and HCV . Nat Rev Drug Discov. 2007;6:1001–1017. doi: 10.1038/nrd2424. [DOI] [PubMed] [Google Scholar]
- 17.Pommier Y, Johnson A, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4:236–248. doi: 10.1038/nrd1660. [DOI] [PubMed] [Google Scholar]
- 18.Louis J, Clore G, Gronenborn A. Autoprocessing of HIV-1 protease is tightly coupled to protein folding. Nat Struct Biol. 1999;6:868–875. doi: 10.1038/12327. [DOI] [PubMed] [Google Scholar]
- 19.Pomerantz R. Reservoirs of human immunodeficiency virus type 1: the main obstacles to viral eradication. Clin Infect Dis. 2002;34:91–97. doi: 10.1086/338256. [DOI] [PubMed] [Google Scholar]
- 20.Reiner S. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Back-to-basics push as HIV prevention struggles. Science. 2008;319:888. doi: 10.1126/science.319.5865.888. [DOI] [PubMed] [Google Scholar]
- 22.Ahsan F, Rivas I, Khan M, Suarez A. Targeting to macrophages: role of physicochemical properties of particulate carriers – liposomes and microspheres – on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 23.Haase A. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17 doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 24.Blankson J, Persaud D, Siliciano R. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 25.Aquaro S, Calio R, Balzarini J, Bellocchi M, Garaci E, Perno C. Macrophages and HIV infection: therapeutic approaches toward this strategic virus reservoir. Antiviral Res. 2002;55:209–225. doi: 10.1016/s0166-3542(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 26.Kedzierska K, Crowe S, Turville S, Cunningham A. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 27.Burton G, Keele B, Estes J, Thacker T, Gartner S. Follicular dendritic cell contributions to HIV pathogenesis . Immunology. 2002;14:275–284. doi: 10.1016/s1044-5323(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 28.Finzi D, Blankson J, Siliciano J, Margolick J, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T, Chaisson R, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 29.Su Y, Sinko P. Drug delivery across the blood-brain barrier: why is it difficult? how to measure and improve it? . Expert Opin Drug Deliv. 2006;3:419–435. doi: 10.1517/17425247.3.3.419. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Clement J. Love blood-brain barrier permeability to azidothymidine (AZT), 3TC, and thymidine in the rat. Brain Res. 1998;791:313–316. doi: 10.1016/s0006-8993(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Sinko P. P-glycoprotein and multidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. J Pharmacol Exp Ther. 2005;312:1249–1256. doi: 10.1124/jpet.104.076216. [DOI] [PubMed] [Google Scholar]
- 32.Kim R. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34:47–54. doi: 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- 33.Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004;9:1012–1019. doi: 10.1016/S1359-6446(04)03279-9. [DOI] [PubMed] [Google Scholar]
- 34.Gump J, Dowdy S. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol Med. 2007;13:443–448. doi: 10.1016/j.molmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Shehu-Xhilaga M, De Krester D, Dejucq-Rainsford N, Hedger M. Standing in the way of eradication: HIV-1 infection and treatment in the male genital tract. Curr HIV Res. 2005;3:345–359. doi: 10.2174/157016205774370375. [DOI] [PubMed] [Google Scholar]
- 36.Thomson Healthcare Inc. . [Accessed [June 19, 2008].];Mayo Cliniccom: Fosamprenavir (Oral Route) ( http://www.mayoclinic.com/health/drug-information/DR601699.
- 37.Chapman T, Plosker G, Perry C. Fosamprenavir: a review of its use in the management of antiretroviral therapy-naive patients with HIV infection. Drugs. 2004;64:2101–2124. doi: 10.2165/00003495-200464180-00014. [DOI] [PubMed] [Google Scholar]
- 38.Wire M, Shelton M, Studenberg S. Fosamprenavir: clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet. 2006;45:137–168. doi: 10.2165/00003088-200645020-00002. [DOI] [PubMed] [Google Scholar]
- 39. [Accessed [June 19, 2008].];Glaxosmithkline - Lexvia (fosamprenavir calcium) Tablets and Oral Suspension. ( http://us.gsk.com/products/assets/us_lexiva.pdf .
- 40.Furfine E, Baker C, Hale M, Reynolds D, Salisbury J, Searle A, Studenberg S, Todd D, Tung R, Spaltenstein A. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother. 2004;48:791–798. doi: 10.1128/AAC.48.3.791-798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Studenberg S, Furfine E, Boehlert C, et al. Mechanism of absorption of GW433908, the phosphate prodrug of the HIV protease inhibitor amprenavir-HIV DART 2004. Montego Bay: Jamaica; Dec 12–16, 2004. [Google Scholar]
- 42.Wood R, Arasteh K, Stellbrink H, Teofilo E, Raffi F, Pollard R, Eron J, Yeo J, Millard J, Wire M, Naderer O. Six-week randomized controlled trial to compare the tolerabilities, pharmacokinetics, and antiviral activities of GW433908 and amprenavir in human immunodeficiency virus type 1-infected patients . Antimicrob Agents Chemother. 2004;48:124–129. doi: 10.1128/AAC.48.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polli J, Jarrett J, Studenberg S, Humphreys J, Dennis S, Brouwer K, Woolley J. Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm Res. 1999;16 doi: 10.1023/a:1018941328702. [DOI] [PubMed] [Google Scholar]
- 44.Sadler B, Chittick G, Polk R, Slain D, Kerkering T, Studenberg S, Lou Y, Moore K, Woolley J. Metabolic disposition and pharmacokinetics of [14C]-amprenavir, a human immunodefi- ciency virus type 1 (HIV-1) protease inhibitor, administered as a single dose to healthy male subjects. J Clin Pharmacol. 2001;41:386–396. doi: 10.1177/00912700122010249. [DOI] [PubMed] [Google Scholar]
- 45.Wire M, Lou Y, Shelton M, et al. Evaluation of plasma amprenavir formulations with a high fat breakfast (HFB, APV10008). 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington DC, USA. Oct 3 - Nov. 2; 2004. [Google Scholar]
- 46.Garraffo R, Lavrut T, Heripret I, Serini M, Carsenti H, Durant J, Dellmonica P. Fosamprenavir (FPV) through concentrations (Cmin) and inhibitory quotients (IQ), at steady-state, in plasma and lymphocytes of HIV infected patients receiving different dosage regimens. 6th International Workshop on Clinical Pharmacology of HIV Therapy; Quebec City, Canada. Apr 28–30; 2005. [Google Scholar]
- 47.Chaudry N, Eron J, Naderer O, Pereira A, Wire M, Fiscus S, Kashuba A. Effects of formulation and dosing strategy on amprenavir concentrations in seminal plasma of human immunodeficiency virus type-1 infected men. Clin Infect Dis. 2002;35:760–762. doi: 10.1086/342389. [DOI] [PubMed] [Google Scholar]
- 48.Fung H, Stone E, Piacenti F. Tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of HIV infection. Clin Ther. 2002;24:1515–1548. doi: 10.1016/s0149-2918(02)80058-3. [DOI] [PubMed] [Google Scholar]
- 49.Chapman T, McGavin J, Noble S. Tenofovir disoproxil fumarate. Drugs. 2003;63:1597–1608. doi: 10.2165/00003495-200363150-00006. [DOI] [PubMed] [Google Scholar]
- 50.Gilead Sciences INC. [Accessed [January 23, 2007].];Viread (tenofovir disoproxil fumarate) tablets: US package insert. ( http://www.gilead.com/pdf/viread_pi.pdf.
- 51.Kearney B, Flaherty J, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 52.Antoniou T, Park-Wyllie L, Tseng A. Tenofovir: a nucleotide analog for the management of human immunodeficiency virus infection. Pharmacotherapy. 2003;23:29–43. doi: 10.1592/phco.23.1.29.31915. [DOI] [PubMed] [Google Scholar]
- 53.Clercq EDE. Broad-spectrum anti-DNA virus and anti-retrovirus activity of phosphonylmethoxyalkylpurines and -pyrimidines. Biochem Pharmacol. 1991;42:963–972. doi: 10.1016/0006-2952(91)90276-b. [DOI] [PubMed] [Google Scholar]
- 54.Clercq EDE. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev. 2003;16 doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clercq EDE. Potential of acyclic nucleoside phosphonates in the treatment of DNA virus retrovirus infections. Expert Rev Anti infect Ther. 2003;1:21–43. doi: 10.1586/14787210.1.1.21. [DOI] [PubMed] [Google Scholar]
- 56.Robbins B, Srinivas R, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(ispropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suo Z, Johnson K. Selective inhibition of HIV-1 reverse transcriptase by an antiviral inhibitor, (R)-9-(2-phosphonylmethoxypropyl) adenine. J Biol Chem. 1998;273:27250–27258. doi: 10.1074/jbc.273.42.27250. [DOI] [PubMed] [Google Scholar]
- 58.Cherrington J, Allen S, Bischofberger N, Chen M. Kinetic interaction of the diphosphate of 9-(2-phosphonylmethoxyethyl) adenine and other anti-HIV active purine congeners with HIV reverse transcriptase and human DNA polymerases α, β and γ. Antivir Chem Chemother. 1995;6:217–221. [Google Scholar]
- 59.Birkus G, Hajek M, Kramata P, Votruba I, Holy A, Otova B. Tenofovir diphosphate is a poor substrate and a weak inhibitor of rat DNA polymerase α, δ, and ε *. Antimicrob Agents Chemother. 2002;46:1610–1613. doi: 10.1128/AAC.46.5.1610-1613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birkus G, Hitchcock M, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–723. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robbins B, Wilcox C, Fridland A, Rodman J. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharamacotherapy. 2003;23:695–701. doi: 10.1592/phco.23.6.695.32189. [DOI] [PubMed] [Google Scholar]
- 62.Holy A. Antiviral acyclic nucleoside phosphonates structure activity studies. Antiviral Res. 2006;71:248–253. doi: 10.1016/j.antiviral.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Shaw J, Suekoka C, Oliyai R, Lee W, Arimilli M, Kim C, Cundy K. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm Res. 1997;14:1824–1829. doi: 10.1023/a:1012108719462. [DOI] [PubMed] [Google Scholar]
- 64.Arimilli M, Kim C, Dougherty J, Mulato A, Oliyai R, Shaw J, Cundy K, Bischofberger N. Synthesis, in vitro biological evaluation and oral bioavailability of 9-[2-(phosphonomethoxy) propyl]adenine (PMPA) prodrugs. Antivir Chem Chemother. 1997;8:557–564. [Google Scholar]
- 65.Naesens L, Bischofberger N, Augustijns P, Annaert P, Van Den Mooter G, Arimilli M, Kim C, De Clercq E. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbon yloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob Agents Chemother. 1998;42:1568–1573. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barditch-Crovo P, Deeks S, Collier A, Safrin S, Coakley D, Miller M, Kearney B, Coleman R, Lamy P, Kahn J, Mcgowan I, Lietman P. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Gelder J, Deferme S, Naesens L, De Clercq E, Van Den Mooter G, Kinget R, Augustijns P. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab Dispos. 2002;30:924–930. doi: 10.1124/dmd.30.8.924. [DOI] [PubMed] [Google Scholar]
- 68.Cihlar T, Rosenberg I, Votruba I, Holy A. Transport of 9-(2-phosphonomethoxyethyl)adenine across plasma membrane of HeLa S3 cells is protein mediated. Antimicrob Agents Chemother. 1995;39:117–124. doi: 10.1128/aac.39.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deeks S, Barditch-Crovo P, Lietman P, Hwang F, Cundy K, Rooney J, Hellmann N, Safrin S, Kahn J. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anthonypillai C, Gibbs J, Thomas S. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006;3:1–10. doi: 10.1186/1743-8454-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robbins B, Fridland A. Metabolism and therapeutic activity of acyclic adenine phosphonate analogs. Int Antivir News. 1996;4:57–59. [Google Scholar]
- 72.Tuske S, Sarafianos S, Clark A, Ding J, Naeger L, White K, Miller M, Gibbs C, Boyer P, Clark P, Wang G, Gaffney B, Jones R, Jerina D, Hughes S, Arnold E. Structure of HIV-1 RT-DNA complexes before and after incorporation of the anti-AIDS drug tenofovir. Nat Struct Mol Biol. 2004;11:469–474. doi: 10.1038/nsmb760. [DOI] [PubMed] [Google Scholar]
- 73.Tuske S, Sarafianos S, Clark A, Ding J, Naeger L, Miller M, Gibbs C, Jerina D, Hughes S, Arnold E. Crystal structure of HIV-1 RT with template-primer terminated with acyclic nucleotide RT inhibitor tenofovir suggests mechanisms of evading resistance. 9th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. Feb 24–28; 2002. [Google Scholar]
- 74.Van Rampay K, Cherrington J, Marthas M, Berardi C, Mulato A, Spinner A, Tarara R, Canfield D, Telm S, Bischofberger N, Pedersen N. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McColl D, Margot N, Wulfsohn M, Coakley D, Cheng A, Miller M. Patterns of resistance emerging in HIV-1 from antiretroviral-experienced patients undergoing intensification therapy with tenofovir disoproxil fumarate. J Acquir Immune Defic Syndr. 2004;37:1340–1350. doi: 10.1097/00126334-200411010-00002. [DOI] [PubMed] [Google Scholar]
- 76.Wainberg M, Miller M, Quan Y, Salomon H, Mulato A, Lamy P, Margot N, Anton K, Cherrington J. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir Ther. 1999;4:87–94. doi: 10.1177/135965359900400205. [DOI] [PubMed] [Google Scholar]
- 77.Meyer P, Matsuura S, So A, Scott W. Unblocking of chain-terminated primer by HIV- reverse transcriptase through a nucleotide-dependent mechanism. Proc Natl Acad Sci. 1998;95:13471–13476. doi: 10.1073/pnas.95.23.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh Y, Palombo M, Sinko Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem. 2008;15:1802–1826. doi: 10.2174/092986708785132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kratz F, Muller I, Ryppa C, Warnecke A. Prodrug strategies in anticancer chemotherapy. Chem Med Chem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 80.Meares C. American Chemical Society. Washington DC: 1993. Perspectives in Bioconjugate Chemistry; pp. 1–8. [Google Scholar]
- 81.Sinko P, Kunta J, Usansky H, Perry B. Differentiation of gut and hepatic first pass metabolism and secretion of saquinavir in ported rabbits. J Pharmacol Exp Ther. 2004;46:359–366. doi: 10.1124/jpet.103.064394. [DOI] [PubMed] [Google Scholar]
- 82.Su Y, Zhang X, Sinko P. Human organic anion-transporting polypeptide OATP-A (SLC21α3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of saquinavir in Hep G2 cells. Mol Pharm. 2004;1:49–56. doi: 10.1021/mp0340136. [DOI] [PubMed] [Google Scholar]
- 83.Usansky H, Hu P, Sinko P. Differential roles of P-glycoprotein multidrug resistance associated protein 2, and CYP3A on saquinavir oral absorption in Sprague-Dawley rats. Drug Metab Dispos. 2008;36:863–869. doi: 10.1124/dmd.107.017483. [DOI] [PubMed] [Google Scholar]
- 84.Williams G, Liu A, Knipp G, Sinko P. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporters (MRP2) Antimicrob Agents Chemother. 2002;46:3456–3462. doi: 10.1128/AAC.46.11.3456-3462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farese-Di Giorgio A, Rouquayrol M, Greiner J, Aubertin A, Vierling P, Guedj R. Synthesis and anti-HIV activity of prodrugs derived from saquinavir and indinavir. Antivir Chem Chemother. 2000;11:97–110. doi: 10.1177/095632020001100202. [DOI] [PubMed] [Google Scholar]
- 86.Rouquayrol M, Gaucher B, Greiner J, Aubertin A, Vierling P, Guedj R. Synthesis and anti-HIV activity of glucose-containing prodrugs derived from saquinavir, indinavir and nelfinavir. Carbohydr Res. 2001;336:161–180. doi: 10.1016/s0008-6215(01)00260-9. [DOI] [PubMed] [Google Scholar]
- 87.Gunaseelan S, Debrah O, Wan L, Leibowitz M, Rabson A, Stein S, Sinko P. Synthesis of poly(ethylene glycol)-based saquinavir prodrug conjugates and assessment of release and anti-HIV-1 bioactivity using a novel protease inhibition assay. Bioconj Chem. 2004;15:1322–1333. doi: 10.1021/bc0498875. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Wan L, Pooyan S, Su Y, Gardner C, Leibowitz M, Sinko P. Quantitative assessment of the cell penetrating properties of R.I.-Tat-9: evidence for a cell type-specific barrier at the plasma membrane of epithelial cells. Mol Pharm. 2004;1:145–155. doi: 10.1021/mp034014y. [DOI] [PubMed] [Google Scholar]
- 89.Ramanathan S, Pooyan S, Stein S, Prasad P, Wang J, Leibowitz M, Ganapathy V, Sinko P. Targeting the sodium-dependent multivitamin transporter (SMVT) for improving the oral absorption properties of a retro-inverso Tat nonapeptide. Pharm Res. 2001;18:950–956. doi: 10.1023/a:1010932126662. [DOI] [PubMed] [Google Scholar]
- 90.Ramanathan S, Qiu B, Pooyan S, Zhang G, Stein S, Leibowitz M, Sinko P. Targeted PEG-based bioconjugates enhance the cellular uptake and transport of HIV-1 TAT nonapeptide. J Control Release. 2001;77:199–212. doi: 10.1016/s0168-3659(01)00474-6. [DOI] [PubMed] [Google Scholar]
- 91.Wan L, Zhang X, Gunaseelan S, Pooyan S, Debrah O, Leibowitz M, Rabson A, Stein S, Sinko P. Novel multi-component nanopharmaceuticals derived from poly(ethylene)glycol, retro-inverso- Tat nonapeptide and saquinavir demonstrate combined anti-HIV effects. AIDS Res Ther. 2006;3:12–26. doi: 10.1186/1742-6405-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schuitemaker H, Kootstra N, De Goede R, De Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weissleder R, Kelly K, Sun E, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;11:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 94.Auger M, Ross J. In: The Natural Immune System: The Macrophage. Lewis CE, McGee JO, editors. Oxford University Press; New York: 1992. pp. 2–74. [Google Scholar]
- 95.Fridkin M, Tsuberry H, Tzehoval E, Vonsover A, Biondi L, Filira R, Rocchi R. Tuftsin-AZT conjugate: potential macrophage targeting for AIDS therapy. J Peptide Sci. 2005;11:37–44. doi: 10.1002/psc.587. [DOI] [PubMed] [Google Scholar]
- 96.Pooyan S, Qiu B, Chan M, Fong D, Sinko P, Leibowitz M, Stein S. Conjugates bearing multiple formyl-methionyl peptides display enhanced binding to but not activation of phagocytic cells. Bioconj Chem. 2002;13:216–223. doi: 10.1021/bc0100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prossnitz E, Ye R. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 98.Wan L, Zhang X, Pooyan S, Palombo M, Leibowitz M, Stein S, Sinko P. Optimizing size and copy number for PEG-fMLF (N-formyl- methionyl-leucyl-phenylalanine) nanocarrier uptake by macrophages. Bioconj Chem. 2008;19:28–38. doi: 10.1021/bc070066k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakai T, Kanamori T, Sando S, Ayoma Y. Remarkably size-regulated cell invasion by artificial viruses: saccharide-dependent self-aggregation of glycoviruses and its consequence in glycoviral gene delivery. J Am Chem Soc. 2003;125:8465–8475. doi: 10.1021/ja035636f. [DOI] [PubMed] [Google Scholar]
- 100.Osaki F, Kanomori T, Sando S, Sera T, Ayoma Y. A quantum dot conjugated sugar ball and its cellular uptake: on the size effects of endocytosis in the subviral region. J Am Chem Soc. 2004;126:6520–6521. doi: 10.1021/ja048792a. [DOI] [PubMed] [Google Scholar]
- 101.Desai M, Labhesetwar V, Walter E, Levy R, Amidon G. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res. 1997;14:1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 102.Prabha S, Zhou W, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection with fractioned nanoparticles. Int J Pharm. 2002;244:105–115. doi: 10.1016/s0378-5173(02)00315-0. [DOI] [PubMed] [Google Scholar]
- 103.Wan L, Pooyan S, Hu S, Leibowitz M, Stein S, Sinko P. Peritoneal macrophage uptake, pharmacokinetics and biodistribution of macrophage-targeted PEG-fMLF (N-formyl-methionyl-leucyl-phenylalanine) nanocarriers for improving HIV drug delivery. Pharm Res. 2007;24:2100–2119. doi: 10.1007/s11095-007-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]